Nanoparticles as Potential Eustressors in Plants
Abstract
1. Introduction
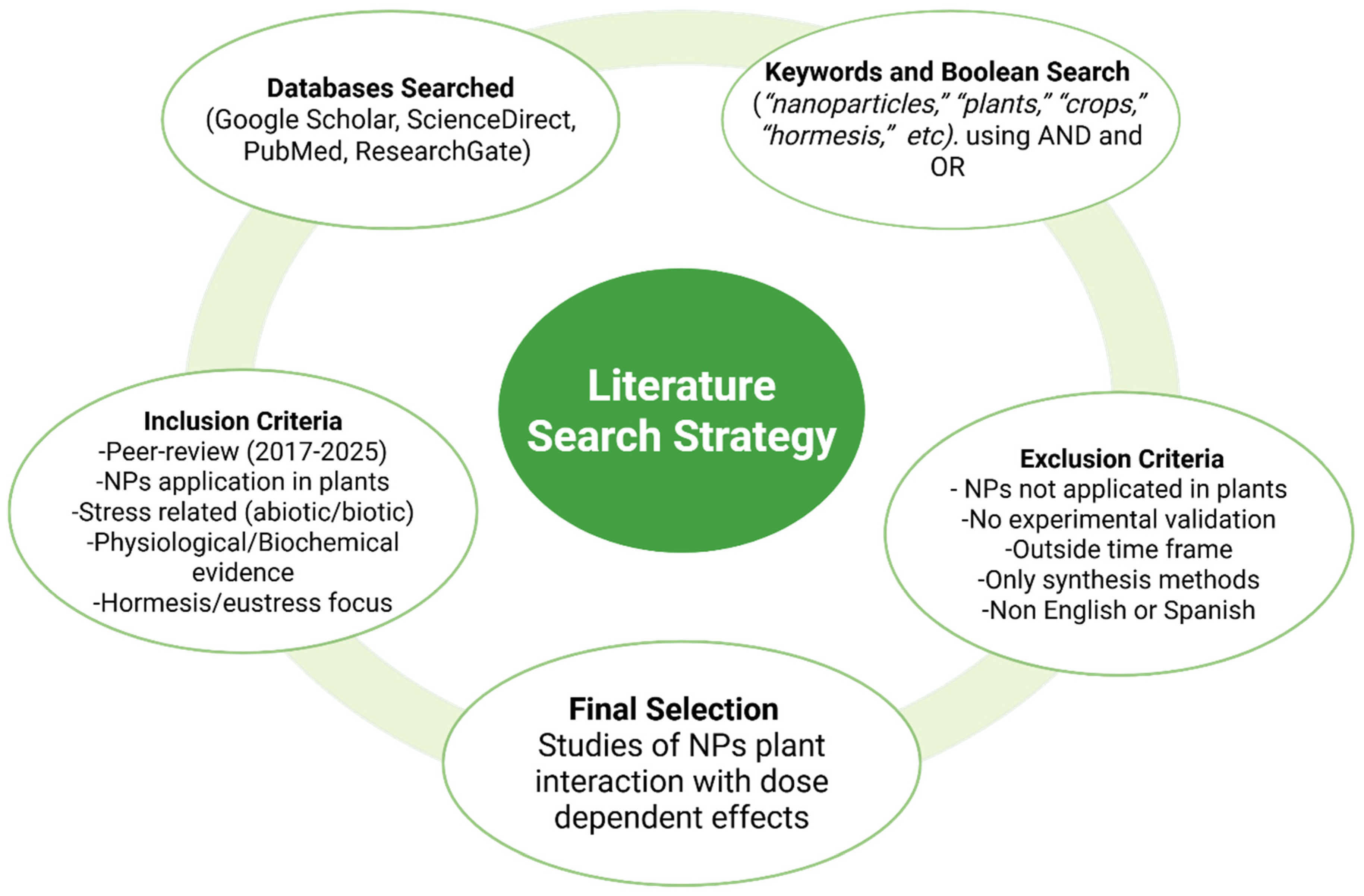
2. Literature Search Strategy
3. Mechanisms of Nanoparticles-Mediated Eustress: From Priming to Stress Alleviation
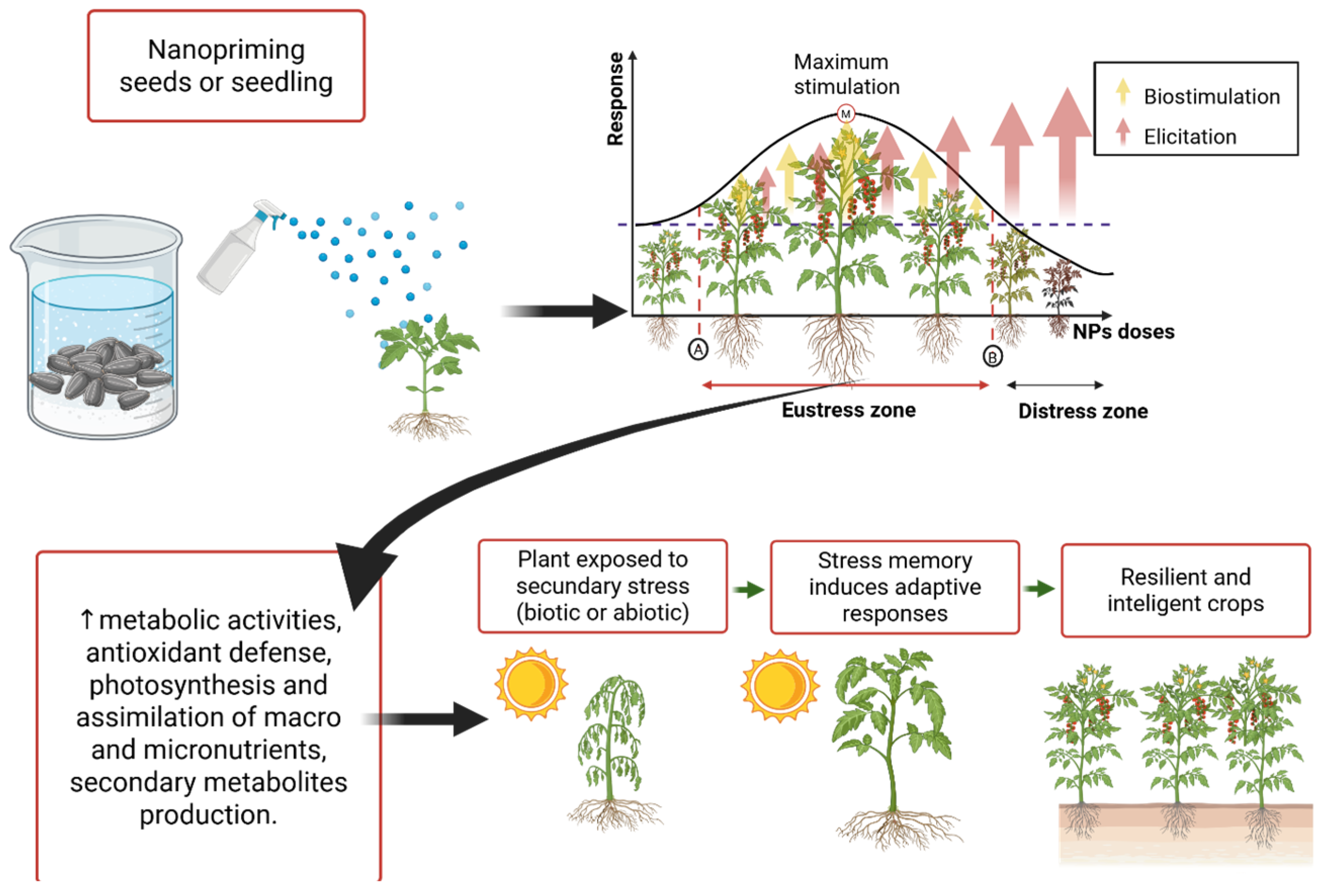
3.1. Nanopriming (Seed and Seedling Priming)
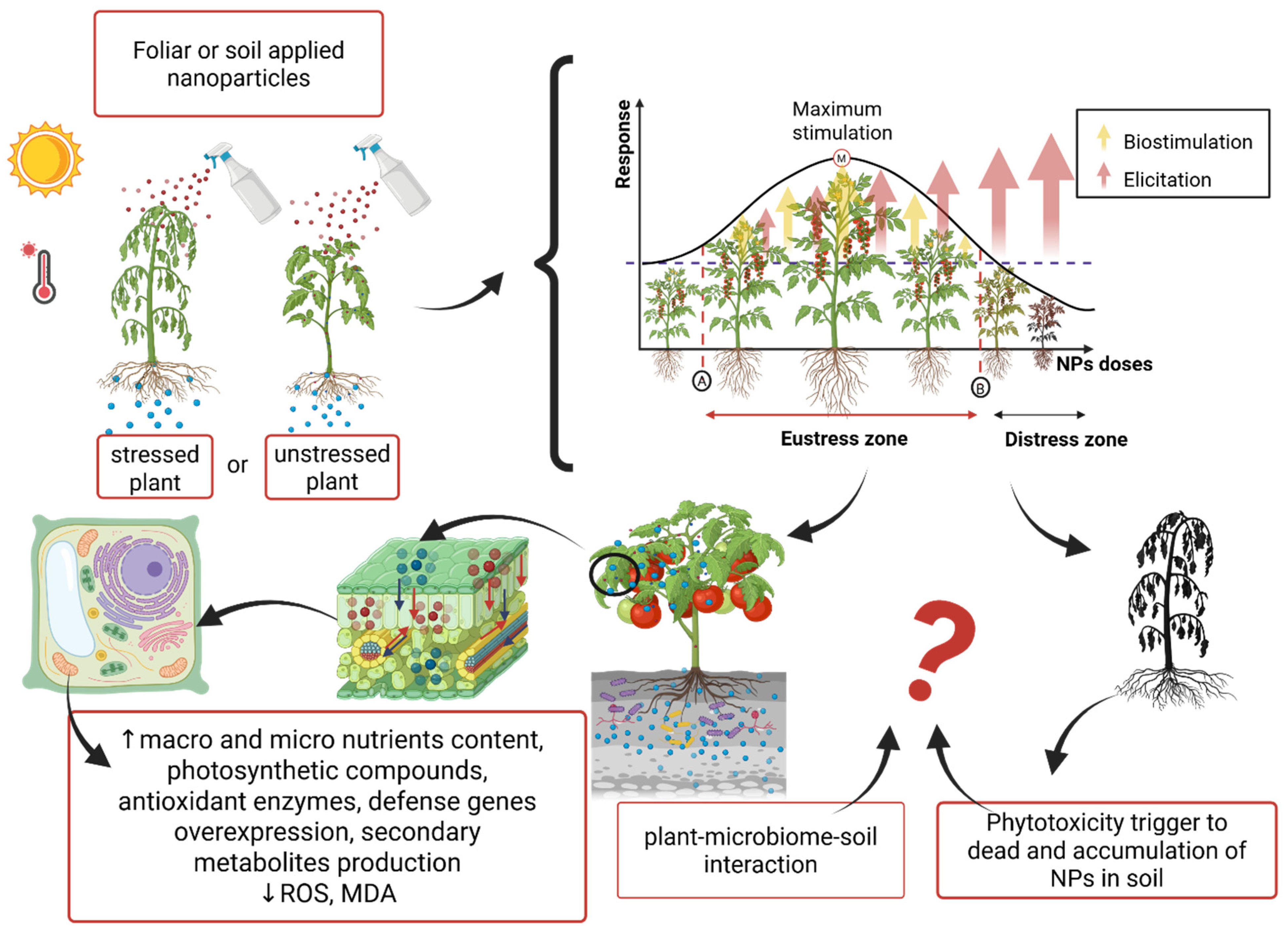
3.2. Nanoparticles as Abiotic/Biotic Stress Alleviators
3.2.1. Growth and Yield Promoting of Plants
3.2.2. Induction of the Antioxidant System
3.2.3. Hormetic Effects in Secondary Metabolites (SM) Production
3.2.4. Disease Suppression
| Nanoparticle and Concentration Applied | Species | Effect | Reference |
|---|---|---|---|
| Cu coated with polyethylene glycol 8000 and ZnO doped with Cu with diethylene glycol (50, 100, 200, 300, 400 μg/mL) and (200, 300, 400, 500, 600 μg/mL) | Lettuce (Lactuca sativa L.) inoculated with S. sclerotiorum or M. javanica. | ↑ antioxidant activity and total phenols. ↓ severity index of S. sclerotiorum, galls and females per gram of root of M. javanica, infestation by fungi and nematodes. | [104] |
| TiO2 * (20, 40, 60, 80 ppm) | Wheat (Triticum aestivum L.) infected with Puccinia striiformis f. sp. Tritici | ↑ SOD, POD, CAT, proline. ↓ total phenols and flavonoids, incidence of disease depending on doses. | [105] |
| TiO2 * (20, 40, 60, 80 ppm) | Wheat (Triticum aestivum L.) infected with Bipolaris sorokiniana | ↑ fresh and dry plant weight, grain yield (weight and number of grains per spike) and total protein, disease resistance. ↓ soluble sugars, phenols and flavonoids, severity disease. | [106] |
| Ag * (25, 50, 75, 100 ppm) | Rice (Oryza sativa L.) infected with Aspergillus flavus | ↑ leaf area, number of leaves, fresh and dry weight. ↓ aflatoxin production. | [107] |
| Iron oxide * (Fe3O4) (0.01, 0.5, 1.5, 2.5, 5, 7.5, 10, 12.5, 15 μg/mL) | Tomato (Solanum Licopersicum L.) infected with Fusarium oxysporum f. sp. Lycopersici. | ↑ PAL, POD, PPO, total phenols, PR-2, PAL, POD, PPO gene expression, plant growth (height, root and shoot length), fresh and dry weight. ↓ F. oxysporum growth and disease severity and incidence. | [108] |
| Chitosan * (1.0%) | Tomato (Solanum lycopersicum L.) infected with Fusarium solani | ↑ total phenols, flavonoids, MDA, CAT, SOD, APX, height, root length, fresh and dry weight, leaf number, total chlorophyll, transcription factors WRKY4, WRKY31 and WRKY37, as well as the other three defense-related genes: glucanase A, defensin and chitinase. ↓ protein and disease severity. | [109] |
| Si * (50, 100, 150, and 200 ppm) | Eggplant (Solanum melongena L.) infected with Meloidogyne incognita | ↑ mean nematode mortality, fresh and dry weight, leaf number, stem diameter, and plant height. ↓ emerged juvenile population and final population of the nematode, number of galls and masses on plants. | [110] |
| Si (10, 100, 500, 1000, 2000, 3000 ppm) | Rice (Oryza sativa L.) infected with Magnaporthe oryzae or under water stress. | ↑ inhibition of fungal growth, gene expression related to the AS pathway: PR1A, PR1B, PR5, PR8, PR10 and PAD, root length. ↓ diseased leaf area, relative fungal growth. | [103] |
| Carbon nanotube (100 ppm) | Tomato (Solanum lycopersicum L.) infected with Alternaria solani | ↑ chlorophyll A, total chlorophyll, WUE, GPX, dry weight, fruit number and yield. ↓ disease incidence and severity. | [111] |
| Cu2O (100 µg/L) | Cucumber (Cucumis sativus L.) infected with F. solani | ↑ CAT, POD, PPO, transcription of PR-1 and LOX-1 genes associated with defense, total chlorophyll, root length, fresh and dry weight, yield increase. | [112] |
| ZnO (50,100,150, 200 mM) | Jalapeño pepper (Capsicum annuum L.) infected or not with the pepper huasteco yellow vein virus. | ↑ weight, height and diameter of fruit, POD and SOD activity. ↓ viral severity, viral levels, infection symptoms, CAT and PAL activity. | [113] |
4. Challenges and Future Perspectives
5. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NPs | Nanoparticles |
| NM | Nanomaterials |
| NT | Nanotechnology |
| ROS | Reactive Oxygen Species |
| PSI | Photosystem I |
| PSII | Photosystem II |
| SOD | Superoxide Dismutase |
| CAT | Catalase |
| POD | Peroxidase |
| APX | Ascorbate Peroxidase |
| GR | Glutathione Reductase |
| GPX | Glutathione Peroxidase |
| PPO | Polyphenol Oxidase |
| PAL | Phenylalanine Ammonia Lyase |
| GPOX | Guaiacol Peroxidase |
| LAA | L-Ascorbic Acid |
| JA | Jasmonic Acid |
| SA | Salicylic Acid |
| ABA | Abscisic Acid |
| IBA | Indole-3-Butyric Acid |
| GSH | Glutathione |
| GSH-Px | Glutathione Peroxidase |
| AsA | Ascorbic Acid |
| ATP | Adenosine Triphosphate |
| MDA | Malondialdehyde |
| DSI | Disease Severity Index |
| DON | Deoxynivalenol |
| REL | Relative Electrolyte Leakage |
| SPAD | Soil–Plant Analysis Development (Chlorophyll Index) |
| CHS | Chalcone Synthase |
| Mn-SOD | Manganese Superoxide Dismutase |
| PR | Pathogenesis-Related proteins |
| WUE | Water Use Efficiency |
| TCA | Tricarboxylic Acid cycle (Krebs cycle) |
| Cd | Cadmium |
| Cr | Chromium |
| Pb | Lead |
| K | Potassium |
| Na | Sodium |
| Ca | Calcium |
| Mg | Magnesium |
| Fe | Iron |
| Zn | Zinc |
| Mn | Manganese |
| Cl | Chlorine |
| P | Phosphorus |
| Si | Silicon |
| Cu | Copper |
| Ag | Silver |
| Au | Gold |
| Se | Selenium |
| TiO2 | Titanium Dioxide |
| Fe2O3 | Iron (III) Oxide |
| Fe3O4 | Magnetite (Iron Oxide) |
| CaO | Calcium Oxide |
| ZnO | Zinc Oxide |
| CuO | Copper (II) Oxide |
| Cu2O | Cuprous Oxide |
| GONPs | Graphene Oxide Nanoparticles |
| MWCNTs | Multi-Walled Carbon Nanotubes |
References
- United Nations. Department of Economic and Social Affairs, Population Division World Population Prospects. 2022, United Nations. World Population Prospects 2024. Online Edition. Available online: https://population.un.org/wpp/downloads?folder=Standard%20Projections&group=Most%20used (accessed on 13 April 2025).
- Lichtenthaler, H.K. Vegetation stress: An introduction to the stress concept in plants. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fichman, Y.; Devireddy, A.R.; Sengupta, S.; Azad, R.K.; Mittler, R. Systemic signaling during abiotic stress combination in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 13810–13820. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. Forty years of stress research: Principal remaining problems and misconceptions. Can. Med. Assoc. J. 1976, 115, 53. [Google Scholar] [PubMed]
- Kupriyanov, R.; Zhdanov, R. The eustress concept: Problems and outlooks. World J. Med. Sci. 2014, 11, 179–185. [Google Scholar] [CrossRef]
- Vázquez-Hernández, M.C.; Parola-Contreras, I.; Montoya-Gómez, L.M.; Torres-Pacheco, I.; Schwarz, D.; Guevara-González, R.G. Eustressors: Chemical and physical stress factors used to enhance vegetables production. Sci. Hortic. 2019, 250, 223–229. [Google Scholar] [CrossRef]
- Godínez-Mendoza, P.L.; Rico-Chávez, A.K.; Ferrusquía-Jimenez, N.I.; Carbajal-Valenzuela, I.A.; Villagómez-Aranda, A.L.; Torres-Pacheco, I.; Guevara-González, R.G. Plant hormesis: Revising of the concepts of biostimulation, elicitation and their application in a sustainable agricultural production. Sci. Total Environ. 2023, 894, 164883. [Google Scholar] [CrossRef]
- Erofeeva, E.A. A method for assessing the frequency of hormetic trade-offs in plants. MethodsX 2022, 9, 101610. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Hormesis in plants: Its common occurrence across stresses. Curr. Opin. Toxicol. 2022, 30, 100333. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Mohamed, I.A.; Khatab, A.; Sherif, A.; Wang, Z.; Zhou, G. Modulation of salinity impact on early seedling stage via nano-priming application of zinc oxide on rapeseed (Brassica napus L.). Plant Physiol. Biochem. 2021, 166, 376–392. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Zivcak, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef]
- Lira-Saldivar, R.H.; Mendez-Arguello, B.; De los Santos-Villarreal, G.; Vera-Reyes, I. Potencial de la nanotecnología en la agricultura. Acta Univ. 2018, 28, 9–24. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Magaña-López, E.; Palos-Barba, V.; Zuverza-Mena, N.; Vázquez-Hernández, M.C.; White, J.C.; Nava-Mendoza, R.; Feregrino-Pérez, A.A.; Torres-Pacheco, I.; Guevara-González, R.G. Nanostructured mesoporous silica materials induce hormesis on chili pepper (Capsicum annuum L.) under greenhouse conditions. Heliyon 2022, 8, e09049. [Google Scholar] [CrossRef]
- Grimsdale, A.C.; Müllen, K. The chemistry of organic nanomaterials. Angew. Chem. Int. Ed. 2005, 44, 5592–5629. [Google Scholar] [CrossRef]
- Paramo, L.A.; Feregrino-Pérez, A.A.; Guevara, R.; Mendoza, S.; Esquivel, K. Nanoparticles in Agroindustry: Applications, Toxicity, Challenges, and Trends. Nanomaterials 2020, 10, 1654. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; AlMutairi, K.A.; Siddiqui, Z.H. Role of nanomaterials in plants under challenging environments. Plant Physiol. Biochem. 2017, 110, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Tiwari, S.; Pandey, J.; Lata, C.; Singh, I.K. Role of nanoparticles in crop improvement and abiotic stress management. J. Biotechnol. 2021, 337, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Singh, A.K.; Kumar, R.; Gupta, A.; Kumar, P.A.; Pandey, A.K. Nanoparticles as modulators of oxidative stress. Nanotechnol. Mod. Anim. Biotechnol. 2019, 3, 29–35. [Google Scholar] [CrossRef]
- Aqeel, U.; Aflab, T.; Khan, M.M.A.; Naeem, M.; Khan, M.N. A comprehensive review of impacts of diverse nanoparticles on growth, development and physiological adjustments in plants under changing environment. Chemosphere 2022, 291, 132672. [Google Scholar] [CrossRef]
- Ahmad, A.; Saman, H.S.; Palma, J.M.; Corpas, F.J. Influence of metallic, metallic oxide, and organic nanoparticles on plant physiology. Chemosphere 2022, 290, 133329. [Google Scholar] [CrossRef]
- Pérez-Labrada, F.; Hernández-Hernández, H.; López-Pérez, M.C.; González-Morales, S.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Nanoparticles in plants: Morphophysiological, biochemical, and molecular responses. In Plant Life Under Changing Environment; Academic Press: Cambridge, MA, USA, 2020; pp. 289–322. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Karuppanapandian, T.; Moon, J.C.; Kim, C.; Manoharan, K.; Kim, W. Reactive oxygen species in plants: Their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop Sci. 2011, 5, 709–725. [Google Scholar]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A Comprehensive Review on the Biological, Agricultural and Pharmaceutical Properties of Secondary Metabolites Based-Plant Origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef]
- Zhan, X.; Chen, Z.; Chen, R.; Shen, C. Environmental and Genetic Factors Involved in Plant Protection-Associated Secondary Metabolite Biosynthesis Pathways. Front. Plant Sci. 2022, 13, 877304. [Google Scholar] [CrossRef]
- Barket, A. Salicylic acid: An efficient elicitor of secondary metabolite production in plants. Biocatal. Agric. Biotechnol. 2021, 31, 101884. [Google Scholar] [CrossRef]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.F.; et al. Production of Plant Secondary Metabolites: Examples, Tips and Suggestions for Biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles Alter Secondary Metabolism in Plants via ROS Burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Fu, C.; Li, J.; Tao, Y.; Li, Y.; Hu, J.; Chen, L.; Khan, Z.; Wu, H.; Li, Z. Seed nanopriming: How do nanomaterials improve seed tolerance to salinity and drought? Chemosphere 2023, 310, 136911. [Google Scholar] [CrossRef]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- García-Locascio, E.; Valenzuela, E.I.; Cervantes-Avilés, P. Impact of seed priming with Selenium nanoparticles on germination and seedlings growth of tomato. Sci. Rep. 2024, 14, 6726. [Google Scholar] [CrossRef]
- Ishtiaq, M.; Mazhar, M.W.; Maqbool, M.; Hussain, T.; Hussain, S.A.; Casini, R.; Abd-ElGawad, A.M.; Elansary, H.O. Seed Priming with the Selenium Nanoparticles Maintains the Redox Status in the Water Stressed Tomato Plants by Modulating the Antioxidant Defense Enzymes. Plants 2023, 12, 1556. [Google Scholar] [CrossRef]
- García-Locascio, E.; Valenzuela, E.I.; Cervantes-Avilés, P. Selenium nanoparticles and maize: Understanding the impact on seed germination, growth, and nutrient interactions. Plant Nano Biol. 2025, 11, 100144. [Google Scholar] [CrossRef]
- Öztürk Erdem, S.; Karakoyun Mutluay, M.; Karaer, M.; Gültaş, H.T. The Effect of Fe3O4 Nanoparticle Applications on Seedling Development in Water-Stressed Strawberry (Fragaria× ananassa ‘Albion’) Plants. Appl. Fruit Sci. 2025, 67, 1–9. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Muzammil, K.; Mohieldin, A.; Dawria, A.; Altijani, A.A.G.; Salih, A.; Ali, O.Y.M.; Elzaki, A.A.M.; et al. Optimizing water relations, gas exchange parameters, biochemical attributes and yield of water-stressed maize plants through seed priming with iron oxide nanoparticles. BMC Plant Biol. 2024, 24, 624. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Kreslavski, V.D.; Shmarev, A.N.; Ivanov, A.A.; Zharmukhamedov, S.K.; Kosobryukhov, A.; Yu, M.; Allakhverdiev, S.I.; Shabala, S. Effects of Iron Oxide Nanoparticles (Fe3O4) on Growth, Photosynthesis, Antioxidant Activity and Distribution of Mineral Elements in Wheat (Triticum aestivum). Plants 2022, 11, 1894. [Google Scholar] [CrossRef] [PubMed]
- Megahed, S.M.; El-Bakatoushi, R.F.; Amin, A.W.; El-Sadek, L.M.; Migahid, M.M. Nanopriming as an approach to induce tolerance against drought stress in wheat cultivars. Cereal Res. Commun. 2025, 53, 1–15. [Google Scholar] [CrossRef]
- Gautam, A.; Sharma, P.; Ashokhan, S.; Yaacob, J.S.; Kumar, V.; Guleria, P. Magnesium oxide nanoparticles improved vegetative growth and enhanced productivity, biochemical potency and storage stability of harvested mustard seeds. Environ. Res. 2023, 229, 116023. [Google Scholar] [CrossRef]
- Ahlawat, Y.K.; Singh, M.; Manorama, K.; Lakra, N.; Zaid, A.; Zulfiqar, F. Plant phenolics: Neglected secondary metabolites in plant stress tolerance. Braz. J. Bot. 2024, 47, 703–721. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Akram, R. Seed priming with Calcium oxide nanoparticles improves germination, biomass, antioxidant defence and yield traits of canola plants under drought stress. S. Afr. J. Bot. 2022, 151, 889–899. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.; Hao, Y.; Shang, H.; Jia, W.; Liang, A.; Xu, X.; Li, C.; Ma, C. Size Effects of Copper Oxide Nanoparticles on Boosting Soybean Growth via Differentially Modulating Nitrogen Assimilation. Nanomaterials 2024, 14, 746. [Google Scholar] [CrossRef]
- Amin, N.; Aziz, K. Copper oxide-based nanoparticles in agro-nanotechnology: Advances and applications for sustainable farming. Agric. Food Secur. 2025, 14, 7. [Google Scholar] [CrossRef]
- Wang, N.; Tian, X.; Song, P.; Guo, W.; Zhang, K.; Li, J.; Ma, Z. The Influence of Cuprous Oxide Nanoparticles on Photosynthetic Efficiency, Antioxidant Responses and Grain Quality throughout the Soybean Life Cycle. Agronomy 2024, 14, 1821. [Google Scholar] [CrossRef]
- Verma, D.K.; Thakor, A.B.; Patel, S. Palladium nanoparticles improved phenotypic characters and alter DNA content in IUCN red listed endangered plant species Pterocarpus santalinus L. Results Chem. 2025, 16, 102353. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, A.; Srivastava, P.K.; Choubey, A.K. Optimizing growth performance of Abelmoschus esculentus (L.) via synergistic effects of biogenic Cu/Ni/Co oxide nanoparticles in conjunction with rice straw and pressmud-based vermicompost. Pedosphere 2025, 35. [Google Scholar] [CrossRef]
- Geremew, A.; Stovall, L.; Woldesenbet, S.; Ma, X.; Carson, L. Nanopriming with zinc oxide: A novel approach to enhance germination and antioxidant systems in amaranth. Front. Plant Sci. 2025, 16, 1599192. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Arshad, A.; Parveen, A.; Azeem, M.; Ishtiaq, M.; Thind, S.; Akram, R.; Maqbool, M.; Mazher, M.; Mahmoud, E.A.; et al. Interaction of arsenic stress and graphene oxide nanoparticle seed priming modulates hormonal signalling to enhance soybean (Glycine max L.) growth and antioxidant defence. Environ. Pollut. Bioavailab. 2025, 37, 2523548. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Priming crops for the future: Rewiring stress memory. Trends Plant Sci. 2022, 27, 699–716. [Google Scholar] [CrossRef]
- Lee, J.H.; Kasote, D.M. Nano-priming for inducing salinity tolerance, disease resistance, yield attributes, and alleviating heavy metal toxicity in plants. Plants 2024, 13, 446. [Google Scholar] [CrossRef]
- Yavari, A.; Ghasemifar, E.; Shahgolzari, M. Seed Nanopriming to Mitigate Abiotic Stresses in Plants. In Interchopen Abiotic Stress in Plants–Adaptations to Climate Change; IntechOpen: London, UK, 2023; pp. 1–16. [Google Scholar] [CrossRef]
- Kalal, P.R.; Tomar, R.S.; Jajoo, A. SiO2 nanopriming protects PS I and PSII complexes in wheat under drought stress. Plant Nano Biol. 2022, 2, 100019. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef]
- Venzhik, Y.; Deryabin, A.; Popov, V.; Dykman, L.; Moshkov, I. Gold nanoparticles as adaptogens increazing the freezing tolerance of wheat seedlings. Environ. Sci. Pollut. Res. 2022, 29, 55235–55249. [Google Scholar] [CrossRef]
- Guha, T.; Gopal, G.; Das, H.; Mukherjee, A.; Kundu, R. Nanopriming with zero-valent iron synthesized using pomegranate peel waste: A “green” approach for yield enhancement in Oryza sativa L. cv. Gonindobhog. Plant Physiol. Biochem. 2021, 163, 261–275. [Google Scholar] [CrossRef]
- Sheikhalipour, M.; Mohammadi, S.A.; Esmaielpour, B.; Spanos, A.; Mahmoudi, R.; Mahdavinia, G.R.; Milani, M.H.; Kahnamoei, A.; Nouraein, M.; Antoniou, C.; et al. Seedling nanopriming with selenium-chitosan nanoparticles mitigates the adverse effects of salt stress by inducing multiple defence pathways in bitter melon plants. Int. J. Biol. Macromol. 2023, 242, 124923. [Google Scholar] [CrossRef]
- Shah, T.; Latif, S.; Saeed, F.; Ali, I.; Ullah, S.; Alsahli, A.A.; Jan, S.; Ahmad, P. Seed priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress. J. King Saud Univ.-Sci. 2021, 33, 101207. [Google Scholar] [CrossRef]
- Gomes, D.G.; Pelegrino, M.T.; Ferreira, A.S.; Bazzo, J.H.; Zucareli, C.; Seabra, A.B.; Oliveira, H.C. Seed priming with copper-loaded chitosan nanoparticles promotes early growth and enzymatic antioxidant defense of maize (Zea mays L.) seedlings. J. Chem. Technol. Biotechnol. 2021, 96, 2176–2184. [Google Scholar] [CrossRef]
- Ochoa-Chaparro, E.H.; Patiño-Cruz, J.J.; Anchondo-Páez, J.C.; Pérez-Álvarez, S.; Chávez-Mendoza, C.; Castruita-Esparza, L.U.; Marquez, E.M.; Sánchez, E. Seed Nanopriming with ZnO and SiO2 Enhances Germination, Seedling Vigor, and Antioxidant Defense Under Drought Stress. Plants 2025, 14, 1726. [Google Scholar] [CrossRef] [PubMed]
- Khairilanwar, K.A.; Yi, S.J.; Bakri, M.D.I.; Nawi, I.H.M.; Azmi, A.A.A.R.; Aik, C.K. Eco-friendly nanopriming: Zinc oxide nanoparticles derived from banana peel extract for improved germination of chili seeds. J. Seed Sci. 2025, 47, e202547006. [Google Scholar] [CrossRef]
- Dong, Q.; Huang, T.; Zhou, C.; Wan, X.; He, X.; Miao, P.; Cheng, H.; Wang, X.; Yu, H.; Hu, M.; et al. Nano-priming with selenium nanoparticles reprograms seed germination, antioxidant defense, and phenylpropanoid metabolism to enhance Fusarium graminearum resistance in maize seedlings. J. Adv. Res. 2025, 75. [Google Scholar] [CrossRef] [PubMed]
- Ulhassan, Z.; Yang, S.; He, D.; Khan, A.R.; Salam, A.; Azhar, W.; Muhammad, S.; Ali, S.; Hamid, Y.; Khan, I.; et al. Seed priming with nano-silica effectively ameliorates chromium toxicity in Brassica napus. J. Hazard. Mater. 2023, 458, 131906. [Google Scholar] [CrossRef]
- Tombuloglu, G.M.; Aldahnem, A.; Tombuloglu, H.; Slimani, Y.; Akhtar, S.; Hakeem, K.R.; Almessiere, M.; Baykal, A.; Ercan, I.; Manikandan, A. Uptake and bioaccumulation of iron oxide nanoparticles (Fe3O4) in barley (Hordeum vulgare L.): Effect of particle-size. Environ. Sci. Pollut. Res. 2024, 31, 22171–22186. [Google Scholar] [CrossRef]
- Nazir, M.M.; Noman, M.; Ahmed, T.; Ali, S.; Ulhassan, Z.; Zeng, F.; Zhang, G. Exogenous calcium oxide nanoparticles alleviate cadmium toxicity by reducing Cd uptake and enhancing antioxidative capacity in barley seedlings. J. Hazard. Mater. 2022, 438, 129498. [Google Scholar] [CrossRef]
- Khan, M.N.; Fu, C.; Liu, X.; Li, Y.; Yan, J.; Yue, L.; Li, J.; Khan, Z.; Nie, L.; Wu, H. Nanopriming with selenium doped carbon dots improved rapeseed germination and seedling salt tolerance. Crop J. 2024, 12, 1333–1343. [Google Scholar] [CrossRef]
- Khan, A.R.; Salam, A.; Li, G.; Iqbal, B.; Ulhassan, Z.; Liu, Q.; Azhar, W.; Liaquat, F.; Shah, I.H.; Hassan, S.S.u.; et al. Nanoparticles and their crosstalk with stress mitigators: A novel approach towards abiotic stress tolerance in agricultural systems. Crop J. 2024, 12, 1280–1298. [Google Scholar] [CrossRef]
- Verma, N.; Kaushal, P.; Sidhu, A.K. Harnessing biological synthesis: Zinc oxide nanoparticles for plant biotic stress management. Front. Chem. 2024, 12, 1432469. [Google Scholar] [CrossRef] [PubMed]
- Gorobets, Y.; Gorobets, S.; Gorobets, O.; Magerman, A.; Sharai, I. Biogenic and Anthropogenic Magnetic Nanoparticles in the Phloem Sieve Tubes of Plants: Magnetic Nanoparticles in Plants. J. Microbiol. Biotechnol. Food Sci. 2023, 12, e5484. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Zia, U.; Rehman, M.; Adrees, M.; Arshad, M.; Qayyum, M.F.; Ali, L.; Hussain, A.; Chatha, S.A.S.; et al. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ Pollut. 2019, 248, 358–367. [Google Scholar] [CrossRef]
- Páramo, L.; Feregrino-Pérez, A.A.; Vega-González, M.; Escobar-Alarcón, L.; Esquivel, K. Medicago sativa L. Plant Response against Possible Eustressors (Fe, Ag, Cu)-TiO2:Evaluation of Physiological Parameters, Total Phenol Content, and Flavonoid Quantification. Plants 2023, 12, 659. [Google Scholar] [CrossRef]
- Younes, N.A.; El-Sherbiny, M.; Alkharpotly, A.A.; Sayed, O.A.; Dawood, A.F.; Hossain, M.A.; Abdelrhim, A.S.; Dawood, M.F. Rice-husks synthesized-silica nanoparticles modulate silicon content, ionic homeostasis, and antioxidants defense under limited irrigation regime in eggplants. Plant Stress 2024, 11, 100330. [Google Scholar] [CrossRef]
- Guo, Y.; Gan, Y.; White, J.C.; Zhang, X.; Wei, D.; Liang, J.; Wang, Y.; Song, C. Fe2O3 nanoparticles enhance soybean resistance to root rot by modulating metabolic pathways and defense response. Pestic. Biochem. Physiol. 2025, 208, 106252. [Google Scholar] [CrossRef]
- Ghouri, F.; Sarwar, S.; Sun, L.; Riaz, M.; Haider, F.U.; Ashraf, H.; Lai, M.; Imran, M.; Liu, J.; Ali, S.; et al. Silicon and iron nanoparticles protect rice against lead (Pb) stress by improving oxidative tolerance and minimizing Pb uptake. Sci. Rep. 2024, 14, 5986. [Google Scholar] [CrossRef]
- Garg, T.; Joshi, A.; Kumar, A.; Kumar, V.; Jindal, N.; Awasthi, A.; Kaur, S. Foliar application of selenium nanoparticles, multiwalled carbon nanotubes and their hybrids stimulates plant growth and yield characters in rice (Oryza sativa L.) under salt stress. Plant Nano Biol. 2025, 11, 100146. [Google Scholar] [CrossRef]
- Nelwamondo, A.M.; Maaza, M.; Mohale, K.C. Symbiotic nitrogen fixation and nutrient acquisition of three groundnut genotypes exposed to different concentrations of magnesium oxide and calcium carbonate nanoparticles. Biocatal. Agric. Biotechnol. 2024, 59, 103246. [Google Scholar] [CrossRef]
- Azizkhani, S.; Javadi, T.; Ghaderi, N.; Farzinpour, A. Replacing conventional iron with cysteine-coated Fe3O4 nanoparticles in soilless culture of strawberry. Sci. Hortic. 2023, 318, 112098. [Google Scholar] [CrossRef]
- Taheri, P.; Puopolo, G.; Santoyo, G. Plant growth-promoting microorganisms: New insights and the way forward. Microbiol. Res. 2025, 318, 128168. [Google Scholar] [CrossRef]
- Ferrusquía-Jiménez, N.I.; González-Arias, B.; Rosales, A.; Esquivel, K.; Escamilla-Silva, E.M.; Ortega-Torres, A.E.; Guevara-González, R.G. Elicitation of Bacillus cereus-Amazcala (B.c-A) with SiO2 Nanoparticles Improves Its Role as a Plant Growth-Promoting Bacteria (PGPB) in Chili Pepper Plants. Plants 2022, 11, 3445. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Kumari, A.; Gupta, A.K.; Sharma, S.; Jadon, V.S.; Sharma, V.; Chun, S.C.; Sivanesan, I. Nanoparticles as a tool for alleviating plant stress: Mechanisms, implications, and challenges. Plants 2024, 13, 1528. [Google Scholar] [CrossRef]
- Almiman, B.; El-Blasy, S.A.; El-Gendy, H.M.; Rashad, Y.M.; Abd El-Hai, K.M.; El-Sayed, S.A. Metallic oxide nanoparticles enhance chickpea resistance to root rot and wilt. Phytopathol. Mediterr. 2024, 63, 407–421. [Google Scholar] [CrossRef]
- Cai, L.; Cai, L.; Jia, H.; Liu, C.; Wang, D.; Sun, X. Foliar Exposure of Fe3O4 Nanoparticles on Nicotiana benthamiana: Evidence for Nanoparticles Uptake, Plant Growth Promoter and Defense Response Elicitor against Plant Virus. J. Hazard. Mater. 2020, 395, 122415. [Google Scholar] [CrossRef]
- Wahid, I.; Kumari, S.; Ahmad, R.; Hussain, S.J.; Alamri, S.; Siddiqui, M.H.; Khan, M.I.R. Silver nanoparticle regulates salt tolerance in wheat through changes in ABA concentration, ion homeostasis, and defense systems. Biomolecules 2020, 10, 1506. [Google Scholar] [CrossRef]
- Sarkar, R.D.; Kalita, M.C. Alleviation of salt stress complications in plants by nanoparticles and the associated mechanisms: An overview. Plant Stress 2023, 7, 100134. [Google Scholar] [CrossRef]
- Ayoobi, A.; Saboora, A.; Asgarani, E.; Efferth, T. Iron oxide nanoparticles (Fe3O4-NPs) elicited Artemisia annua L. in vitro, toward enhancing artemisinin production through overexpression of key genes in the terpenoids biosynthetic pathway and induction of oxidative stress. Plant Cell Tissue Organ Cult. (PCTOC) 2024, 156, 85. [Google Scholar] [CrossRef]
- Inam, M.; Attique, I.; Zahra, M.; Khan, A.K.; Hahim, M.; Hano, C.; Anjum, S. Metal oxide nanoparticles and plant secondary metabolism: Unraveling the game-changer nano-elicitors. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 155, 327–344. [Google Scholar] [CrossRef]
- Hussain, M.; Kaousar, R.; Haq, S.I.U.; Shan, C.; Wang, G.; Rafique, N.; Shizhou, W.; Lan, Y. Zinc-oxide nanoparticles ameliorated the phytotoxic hazards of cadmium toxicity in maize plants by regulating primary metabolites and antioxidants activity. Front. Plant Sci. 2024, 15, 1346427. [Google Scholar] [CrossRef] [PubMed]
- Nayab, N.; Alam, M.A. Chitosan Nanoparticles: A Promising Stimulant for Augmenting Cold Stress Resistance in Safed Velchi Cultivars of Banana Plants. J. Himal. Ecol. Sustain. 2023, 18, 100–115. [Google Scholar]
- Narware, J.; Singh, S.P.; Ranjan, P.; Behera, L.; Das, P.; Manzar, N.; Kashyap, A.S. Enhancing tomato growth and early blight disease resistance through green-synthesized silver nanoparticles: Insights into plant physiology. S. Afr. J. Bot. 2024, 166, 676–689. [Google Scholar] [CrossRef]
- Ansari, M.; Ahmed, S.; Abbasi, A.; Khan, M.T.; Subhan, M.; Bukhari, N.A.; Hatamleh, A.A.; Abdelsalam, N.R. Plant mediated fabrication of silver nanoparticles, process optimization, and impact on tomato plant. Sci. Rep. 2023, 13, 18048. [Google Scholar] [CrossRef]
- Mmereke, K.M.; Venkataraman, S.; Moiketsi, B.N.; Khan, M.R.; Hassan, S.H.; Rantong, G.; Masisi, K.; Kwape, T.E.; Gaobotse, G.; Zulfiqar, F.; et al. Nanoparticle elicitation: A promising strategy to modulate the production of bioactive compounds in hairy roots. Food Res. Int. 2024, 178, 113910. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Alzahrani, H.S. The potential mitigation effect of ZnO nanoparticles on [Abelmoschus esculentus L. Moench] metabolism under salt stress conditions. Saudi J. Biol. Sci. 2020, 27, 3132–3137. [Google Scholar] [CrossRef]
- Torabian, S.; Farhangi-Abriz, S.; Zahedi, M. Efficacy of FeSO4 nano formulations on osmolytes and antioxidative enzymes of sunflower under salt stress. Ind J Plant Physiol. 2018, 23, 305–315. [Google Scholar] [CrossRef]
- Avestan, S.; Ghasemnezhad, M.; Esfahani, M.; Byrt, C.S. Application of Nano-Silicon Dioxide Improves Salt Stress Tolerance in Strawberry Plants. Agronomy 2019, 9, 246. [Google Scholar] [CrossRef]
- Jurkow, R.; Sękara, A.; Pokluda, R.; Smoleń, S.; Kalisz, A. Biochemical Response of Oakleaf Lettuce Seedlings to Different Concentrations of Some Metal (oid) Oxide Nanoparticles. Agronomy 2020, 10, 997. [Google Scholar] [CrossRef]
- Răcuciu, M.; Tecucianu, A.; Oancea, S. Impact of Magnetite Nanoparticles Coated with Aspartic Acid on the Growth, Antioxidant Enzymes Activity and Chlorophyll Content of Maize. Antioxidants 2022, 11, 1193. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, G.; Chen, L.; Gu, J.; Wu, H.; Li, Z. Cerium oxide nanoparticles improve cotton salt tolerance by enabling better ability to maintain cytosolic K+/Na+ ratio. J. Nanobiotechnol. 2021, 19, 153. [Google Scholar] [CrossRef]
- Al-Mokadem, A.Z.; Sheta, M.H.; Mancy, A.G.; Hussein, H.-A.A.; Kenawy, S.K.M.; Sofy, A.R.; Abu-Shahba, M.S.; Mahdy, H.M.; Sofy, M.R.; Al Bakry, A.F.; et al. Synergistic Effects of Kaolin and Silicon Nanoparticles for Ameliorating Deficit Irrigation Stress in Maize Plants by Upregulating Antioxidant Defense Systems. Plants 2023, 12, 2221. [Google Scholar] [CrossRef] [PubMed]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.F. Foliar Application of Zinc Oxide Nanoparticles Promotes Drought Stress Tolerance in Eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef]
- Faizan, M.; Rajput, V.D.; Al-Khuraif, A.A.; Arshad, M.; Minkina, T.; Sushkova, S.; Yu, F. Effect of Foliar Fertigation of Chitosan Nanoparticles on Cadmium Accumulation and Toxicity in Solanum lycopersicum. Biology 2021, 10, 666. [Google Scholar] [CrossRef]
- AlHarethi, A.A.; Abdullah, Q.Y.; AlJobory, H.J.; Anam, A.M.; Arafa, R.A.; Farroh, K.Y. Zinc oxide and copper oxide nanoparticles as a potential solution for controlling Phytophthora infestans, the late blight disease of potatoes. Discov. Nano 2024, 19, 105. [Google Scholar] [CrossRef]
- Mercado-Meza, D.Y.; Guevara-González, R.G.; Esquivel, K.; Carbajal-Valenzuela, I.; Avila-Quezada, G.D. Green silver nanoparticles display protection against Clavibacter michiganensis subsp. michiganensis in tomato plants (Solanum lycopersicum L.). Plant Stress 2023, 10, 100256. [Google Scholar] [CrossRef]
- Du, J.; Liu, B.; Zhao, T.; Xu, X.; Lin, H.; Ji, Y.; Yin, Z.; Ding, X. Silica nanoparticles protect rice against biotic and abiotic stresses. J. Nanobiotechnol. 2022, 20, 197. [Google Scholar] [CrossRef]
- Tryfon, P.; Kamou, N.N.; Ntalli, N.; Mourdikoudis, S.; Karamanoli, K.; Karfaridis, D.; Menkissoglu-Spiroudi, U.; Dendrinou-Samara, C. Coated Cu-doped ZnO and Cu nanoparticles as control agents against plant pathogenic fungi and nematodes. NanoImpact 2022, 28, 100430. [Google Scholar] [CrossRef]
- Satti, S.H.; Raja, N.I.; Ikram, M.; Oraby, H.F.; Mashwani, Z.-U.-R.; Mohamed, A.H.; Singh, A.; Omar, A.A. Plant-based Titanium Dioxide Nanoparticles Trigger Biochemical and Proteome Modifications in Triticum aestivum L. under Biotic Stress of Puccinia striiformis. Molecules 2022, 27, 4274. [Google Scholar] [CrossRef] [PubMed]
- Satti, S.H.; Raja, N.I.; Javed, B.; Akram, A.; Mashwani, Z.-u.-R.; Ahmad, M.S.; Ikram, M. Titanium dioxide nanoparticles elicited agromorphological and physicochemical modifications in wheat plants to control Bipolaris sorokiniana. PLoS ONE 2021, 16, e0246880. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, M.; Raja, N.I.; Mashwani, Z.U.R.; Ahmad, M.S.; Hussain, M.; Iqbal, M. Effect of silver nanoparticles and silver nitrate on growth of rice under biotic stress. IET Nanobiotechnol. 2018, 12, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, H.; Batool, T.; Anjum, T.; Illyas, A.; Li, G.; Naseem, S.; Riaz, S. Antifungal Potential of Green Synthesized Magnetite Nanoparticles Black Coffee–Magnetite Nanoparticles Against Wilt Infection by Ameliorating Enzymatic Activity and Gene Expression in Solanum lycopersicum L. Front. Microbiol. 2022, 13, 754292. [Google Scholar] [CrossRef]
- Abd-Ellatif, S.; Ibrahim, A.A.; Safhi, F.A.; Abdel Razik, E.S.; Kabeil, S.S.A.; Aloufi, S.; Alyamani, A.A.; Basuoni, M.M.; ALshamrani, S.M.; Elshafie, H.S. Green Synthesized of Thymus vulgaris Chitosan Nanoparticles Induce Relative WRKY-Genes Expression in Solanum lycopersicum against Fusarium solani, the Causal Agent of Root Rot Disease. Plants 2022, 11, 3129. [Google Scholar] [CrossRef]
- El-Ashry, R.M.; El-Saadony, M.T.; El-Sobki, A.E.; El-Tahan, A.M.; Al-Otaibi, S.; El-Shehawi, A.M.; Saad, A.M.; Elshaer, N. Biological silicon nanoparticles maximize the efficiency of nematicides against biotic stress induced by Meloidogyne incognita in eggplant. Saudi J. Biol. Sci. 2022, 29, 920–932. [Google Scholar] [CrossRef]
- González-García, Y.; Cadenas-Pliego, G.; Alpuche-Solís, Á.G.; Cabrera, R.I.; Juárez-Maldonado, A. Carbon Nanotubes Decrease the Negative Impact of Alternaria solani in Tomato Crop. Nanomaterials 2021, 11, 1080. [Google Scholar] [CrossRef]
- Kamel, S.M.; Elgobashy, S.F.; Omara, R.I.; Derbalah, A.S.; Abdelfatah, M.; El-Shaer, A.; Al-Askar, A.A.; Abdelkhalek, A.; Abd-Elsalam, K.A.; Essa, T.; et al. Antifungal Activity of Copper Oxide Nanoparticles against Root Rot Disease in Cucumber. J. Fungi. 2022, 8, 911. [Google Scholar] [CrossRef]
- Rivero-Montejo, S.d.J.; Rivera-Bustamante, R.F.; Saavedra-Trejo, D.L.; Vargas-Hernandez, M.; Palos-Barba, V.; Macias-Bobadilla, I.; Guevara-González, R.G.; Rivera-Muñoz, E.M.; Torres-Pacheco, I. Inhibition of pepper huasteco yellow veins virus by foliar application of ZnO nanoparticles in Capsicum annuum L. Plant Physiol. Biochem. 2023, 203, 108074. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, X.; Wu, Z. Impact of TiO2 and ZnO nanoparticles on soil bacteria and the enantioselective transformation of racemic-metalaxyl in agricultural soil with Lolium perenne: A wild greenhouse cultivation. J. Agric. Food Chem. 2020, 68, 11242–11252. [Google Scholar] [CrossRef]
- Liu, D.; Iqbal, S.; Gui, H.; Xu, J.; An, S.; Xing, B. Nano-iron oxide (Fe3O4) mitigates the effects of microplastics on a ryegrass soil–microbe–plant system. ACS Nano 2023, 17, 24867–24882. [Google Scholar] [CrossRef]
- Sun, J.; Yang, W.; Li, M.; Zhang, S.; Sun, Y.; Wang, F. Metagenomic analysis reveals soil microbiome responses to microplastics and ZnO nanoparticles in an agricultural soil. J. Hazard. Mater. 2025, 492, 138164. [Google Scholar] [CrossRef]
| Nanoparticle and Concentration Applied | Species | Effect | Reference |
|---|---|---|---|
| SiO2 (15 ppm) | Wheat (Triticum aestivum L.) | ↑ quantum yield of PSII and PSI, electron transport rate of PSII and PSI, oxidation reduction kinetics. ↓ unregulated energy dissipation. Improved photosynthetic efficiency. | [52] |
| Ag (10, 20 ppm) | Rice (Oryza sativa L. cv. KDML 105) | ↑ germination index, vigor index, α-amylase, total soluble sugars, dehydrogenase activity, SOD, CAT, and ROS in seeds. | [53] |
| ZnO (25, 50, 100 ppm) | Canola (Brassica napus L.) under or not under saline stress. | ↑ chlorophyll, SOD, CAT, POD, K, Zn, carotenoids, stem fresh weight, soluble sugar and total protein, linoleic and linolenic acids, proline, relative BnCAM expression. ↓ MDA, stem and root length, root fresh weight, and Na. | [10] |
| Au (5, 10, 20, 50 µg/mL) | Wheat (Triticum aestivum L.) subject or not to post-germination cold stress. | ↑ percentage of over-survival when exposed to temperatures of −5, −7, and −9 °C, net photosynthetic rate, chlorophyll a and total chlorophyll, sucrose, fructose and glucose. ↓ growth and photosynthesis. | [54] |
| Zero-valent iron at nanoscale (10, 20, 40, 80, 160 ppm) | Rice (Oryza sativa L.) | ↑ percentage germination, water content, amylase and protease activity, CAT, SOD and POD in seed after treatment. ↑ height, root length, vigor index, dehydrogenase activity, relative gene expression of RAmy1A, RAmy3D and PIP1A, leaf area, biomass, yield and S, P, K, Ca, Mg, Mn, Zn, and Fe content. ↓ MDA, proline, CAT, SOD, POD in 14 days seedling. | [55] |
| Se + chitosan (10, 20 ppm) | Bitter melon (Momordica charantia L.) under saline stress. | ↑ photosynthesis, stem and root length, stem and root fresh and dry weight, proline, relative water content, Ca, P, and expression of defense-related genes: WRKY1, SOS1, PM H±ATPase, SKOR, Mc5PTase7, and SOAR1, genes related with secondary metabolism MAP30, α-MMC and polypeptide-P, PAL, POD, CAT, and SOD. ↓ Na, Cl, REL and H2O2 and MDA. | [56] |
| TiO2 (40, 60, 80 ppm) | Maize (Zea mays L.) under saline stress. | ↑ germination percentage, average emergence time, stem length, root, fresh and dry weight, relative water content, proline, total phenols, K, SOD, CAT, and PAL. ↓ vigor index, MDA, Na, and electrolyte leakage. | [57] |
| Chitosan loaded with Cu (0.0625 mmol/L) | Maize (Zea mays L.) | ↑ leaf area, stem dry weight, root length, POD, and SOD. ↓ germination percentage and CAT. | [58] |
| ZnO and SiO2 (100 and 10 ppm respectably) | Jalapeño peppers (Capsicum annuum L.) | ↑ 25% germination percentage when applying ZnO, 34% germination rate when applying SiO2, 42% of seedling fresh weight when applying both, ZnO + SiO2, drought tolerance, osmolite accumulation, antioxidant activity, and water use efficiency. | [59] |
| ZnO * (10, 50, 100, 200, 500, 600 ppm) | Chili (Capsicum annum L.) | ↑ final germination percentage, fresh weight seedling ↓ mean germination time. | [60] |
| Se (5, 25, 50, 75, 100 ppm) | Maize (Zea mays L.) subjected to post-germination stress for Fusarium graminearum | ↑ JA, IBA, SA, germination percentage, biomass, SOD, GSH-PX. ↓ POD, MDA, DSI, DON, ABA, GSH. | [61] |
| SiO2 (400 ppm) | Rapeseed (Brassica napus L.) under and not under Cr stress | ↑ germination percentage, fresh and dry weight, root and shoot length, content of Si, Fe, Zn, P, K, Ca, Mg, carotenoids. SOD, CAT, APX, GR, and their transcript genes. ↓ H2O2, O2∙−, MDA | [62] |
| Fe3O4 (50, 100, 200 ppm) | Barley (Hordeum vulgare L.) | ↑ germination percentage, chlorophyll, carotenoids, H2O2 | [63] |
| CaO (25 mM) | Barley (Hordeum vulgare L.) seedlings under Cd stress | ↑ height, root length, dry weight, Ca content, photosynthetic rate, SOD, APX, GSH and gene expression of HvCu-Zn-SOD, HvCAT, HvcAPX, HvGR1. ↓ Cd content and MDA. | [64] |
| Se-doped carbon dots nanoparticles coated with poly acrylic acid (50 ppm) | Rapeseed (Brassica napus L.) under salt stress. | ↑ endo-β-mannanase enzyme, germination rate, seedling length, fresh weight, total soluble sugar, proteins, respiration rate, ATP content, SOD, POD and CAT activity, K content. ↓ MDA, Na content. | [65] |
| Nanoparticle and Concentration Applied | Species | Effect | Reference |
|---|---|---|---|
| Mesoporous nanostructure silica (20, 50, 100 ppm) | Pepper (Capsicum annuum L.) | ↑ height, stem diameter and cold tolerance, flavonoid phenols and antioxidant capacity, gene expression of CHS, PAL, POD and Mn-SOD associated with plant defense. | [14] |
| Ag * (300 ppm) | Wheat (Triticum aestivum L.) under salinity stress or not under salinity stress. | ↑ germination percentage, K in root and leaf, and antioxidant activity of APX, GPX, AsA, GR and GSH, chlorophyll content, dry weight, and N content. ↓ Na and Cl in root and leaf, H2O2 in plants under salinity stress. | [83] |
| Magnetite * (0.1, 1 mg/mL) | Pea (Pisum sativum L.) | ↑ length stem, roots, and leaves. Plant height, number of roots and leaves. | [68] |
| ZnO alone or in combination with biochar. (50, 75, 100 ppm) | Maize (Zea mays L.) | ↑ height, number of leaves, chlorophyll concentration, inter-gas exchange and enzyme activity. ↓ reduced Cd accumulation. | [69] |
| ZnO * (10 ppm) | Okra (Abelmoschus esculentus L.) | ↑ photosynthetic compound content, SOD and CAT activity. ↓ proline accumulation, total soluble sugars. Moderates the effect of salt stress. | [92] |
| FeSO4 (100 mM) | Sunflower (Helianthus annuus L.) under or not under saline stress. | ↑ growth, seed yield, stem dry weight, leaf Fe concentration, POD, CAT, and PPO enzyme activity, proline and soluble sugar content. ↓ negative impact of salt stress. | [93] |
| SiO2 (50, 100 ppm) | Strawberrie (Fragaria × ananassa) | ↑ root and stem fresh and dry weight, fruit yield, carotenoids, total chlorophyll, and epicuticular wax layer. ↓ Effects due to salinity. | [94] |
| Fe2O3 (0.75, 1.5, 3%) | Lettuce (Lactuca sativa L.) | ↑ stem dry weight, total chlorophyll, APX, GPOX, CAT, GSH, LAA, carotenoids and phenols, Fe content. ↓ stem fresh weight. | [95] |
| Magnetite coated with aspartic acid (0.55, 1.10, 2.20, 4.40, 8.80, 11 ppm) | Maize (Zea mays L.) | ↑ seedling length, chlorophyll synthesis, antioxidant enzyme activity (CAT and POD). | [96] |
| Cerium oxide coated with polyacrylic acid (0.1 mL, 0.9 mM) | Cotton (Gossypium hirsutum L.) under salt stress. | ↑ K, chlorophyll, biomass, photosynthetic yield. ↓ Na, H2O2, O2 and MDA. | [97] |
| SiO2 (1.5, 3 mM) | Maize (Zea mays L.) under or not under water stress. | ↑ plant height, yield, proline, ascorbic acid, phenols, glutathione, and the enzymatic activity of SOD, POX, CAT, APX and GR. ↓ H2O2, OH and in plants under saline stress. | [98] |
| ZnO (50, 100 ppm) | Eggplant (Solanum melongena L.) under or not under water stress. | ↑ K, and P content, leaf number, stem diameter, yield, stem dry weight, chlorophyll, membrane stability index, water productivity and fruit length in water stress treatments only. ↓ Zn, Mn and Fe content in water stress treatments. | [99] |
| Chitosan (100 µg/mL) | Tomato (Solanum lycopersicum L.) under or not under Cd stress. | ↑ stem dry weight, net photosynthetic rate, SPAD, CAT, SOD, POD, GSH, AsA. ↓ MDA, H2O2 proline and Cd in plants under Cd stress ↓ MDA and H2O2 in plants with not stress. | [100] |
| TiO2 doped with (Fe, Ag, Cu) (50, 100, and 500 ppm) | Lucerne (Medicago sativa L.) | ↑ gallic acid, flavonoids, total phenols and antioxidant capacity. ↓ chlorophyll index, stem length and leaf length. | [70] |
| SiO2 (100 ppm) | Pepper (Capsicum annuum L.) | ↑ yield, stem thickness, plant height, number of leaves, CAT, and PAL. | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Jurado, S.; Guevara-González, R.G.; Aguirre-Becerra, H.; Esquivel-Escalante, K.; Feregrino-Pérez, A.A. Nanoparticles as Potential Eustressors in Plants. Agronomy 2025, 15, 2186. https://doi.org/10.3390/agronomy15092186
Rodríguez-Jurado S, Guevara-González RG, Aguirre-Becerra H, Esquivel-Escalante K, Feregrino-Pérez AA. Nanoparticles as Potential Eustressors in Plants. Agronomy. 2025; 15(9):2186. https://doi.org/10.3390/agronomy15092186
Chicago/Turabian StyleRodríguez-Jurado, Susana, Ramón Gerardo Guevara-González, Humberto Aguirre-Becerra, Karen Esquivel-Escalante, and Ana Angélica Feregrino-Pérez. 2025. "Nanoparticles as Potential Eustressors in Plants" Agronomy 15, no. 9: 2186. https://doi.org/10.3390/agronomy15092186
APA StyleRodríguez-Jurado, S., Guevara-González, R. G., Aguirre-Becerra, H., Esquivel-Escalante, K., & Feregrino-Pérez, A. A. (2025). Nanoparticles as Potential Eustressors in Plants. Agronomy, 15(9), 2186. https://doi.org/10.3390/agronomy15092186








