Characterization of Hemp Hurd-Derived Biochar for Potential Agricultural Applications
Abstract
1. Introduction
2. Materials and Methods
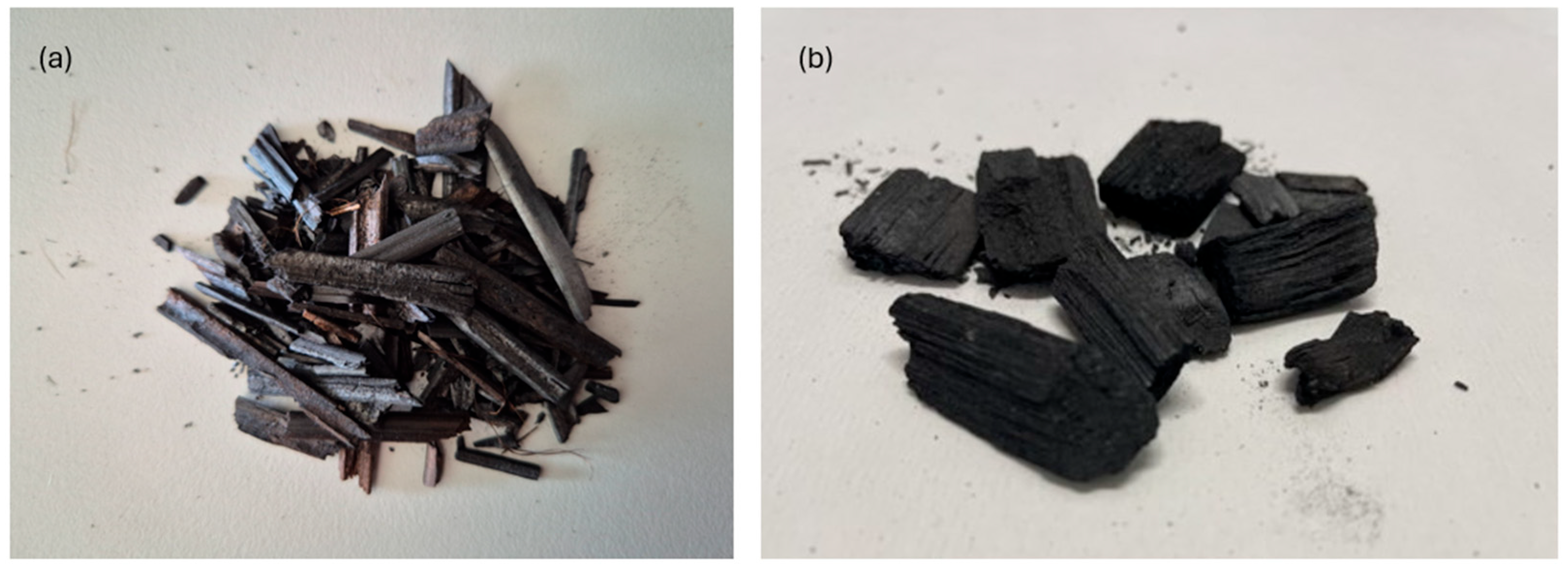
2.1. Feedstock
2.2. Pyrolysis, Biochar Production, and Characterization
- Liquid (tars, bio-oil, and water);
- Solid (biochar and ash);
- Gas (syngas).
2.3. Germination Test
2.4. Statistical Analysis
3. Results and Discussions
3.1. Biomass Characterization
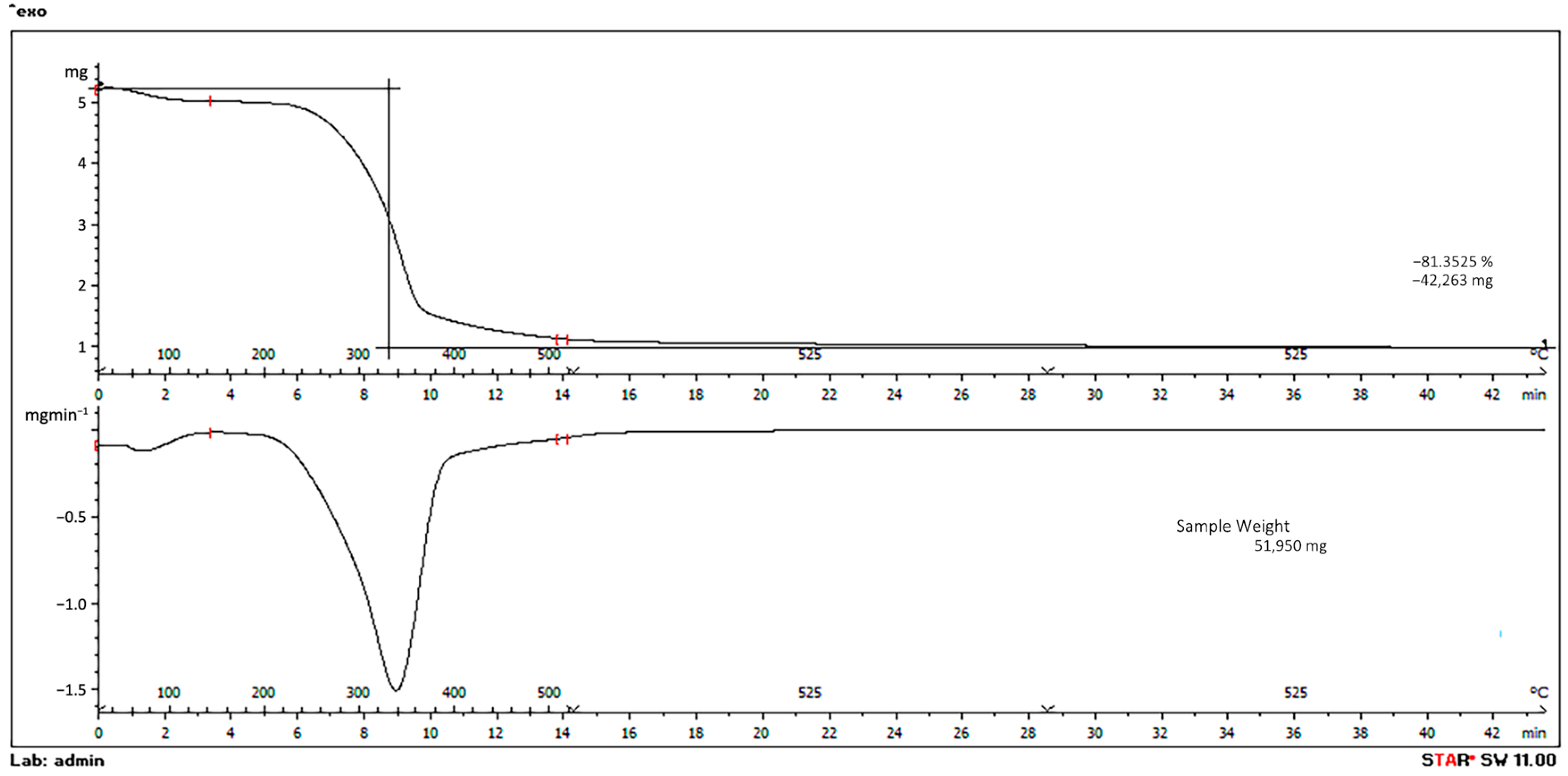
3.2. Biochar Characterization
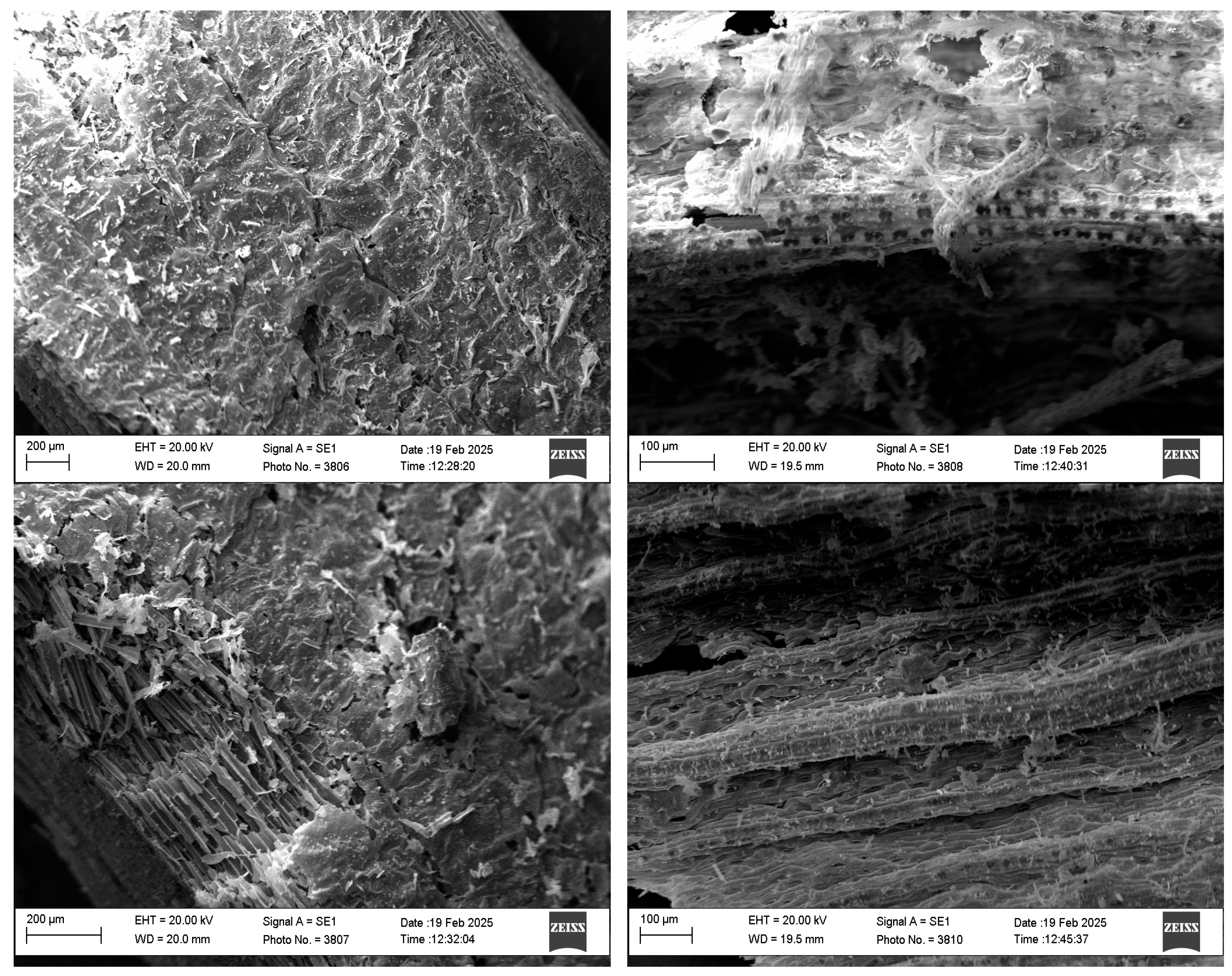
3.3. Qualitative and Quantitative Analysis with SEM-EDS
3.4. Pyrolysis and By-Products Yields
- Liquid (tars, bio-oil, and water) = 25.2%;
- Solid (biochar and ash) = 40.3%;
- Gas (syngas) = 34.6%.
3.5. Results on Germination Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BET | Brunauer–Emmett–Teller |
| CREA-IT | Council for Agricultural Research and Economics, Research Center for Engineering and Agro-Food processing |
| CDR | Carbon Dioxide Removal |
| DIMA | Department of Mechanical and Aerospace Engineering-Sapienza University of Rome |
| FC | Fixed Carbon |
| SEM-EDS | Scanning Electron Microscope-Energy-Dispersive X-ray Spectroscopy |
| TGA | Thermogravimetric Analyzer |
| VM | Volatile Matter |
| WHC | Water Holding Capacity |
References
- Baldini, M.; Ferfuia, C.; Piani, B.; Sepulcri, A.; Dorigo, G.; Zuliani, F.; Danuso, F.; Cattivello, C. The Performance and Potentiality of Monoecious Hemp (Cannabis sativa L.) Cultivars as a Multipurpose Crop. Agronomy 2018, 8, 162. [Google Scholar] [CrossRef]
- Rehman, M.; Fahad, S.; Du, G.; Cheng, X.; Yang, Y.; Tang, K.; Liu, L.; Liu, F.-H.; Deng, G. Evaluation of Hemp (Cannabis sativa L.) as an Industrial Crop: A Review. Environ. Sci. Pollut. Res. Int. 2021, 28, 52832–52843. [Google Scholar] [CrossRef] [PubMed]
- Fidan, M.; Süzerer, V.; Onay, A. Cannabis sativa L.: Origin, Distribution, Taxonomy and Biology. Türk Bilimsel Derlemeler Derg. 2023, 16, 10–28. [Google Scholar]
- Petit, J.; Salentijn, E.M.J.; Paulo, M.-J.; Denneboom, C.; Trindade, L.M. Genetic Architecture of Flowering Time and Sex Determination in Hemp (Cannabis sativa L.): A Genome-Wide Association Study. Front. Plant Sci. 2020, 11, 569958. [Google Scholar] [CrossRef]
- Salentijn, E.M.J.; Zhang, Q.; Amaducci, S.; Yang, M.; Trindade, L.M. New Developments in Fiber Hemp (Cannabis sativa L.) Breeding. Ind. Crops Prod. 2015, 68, 32–41. [Google Scholar] [CrossRef]
- Tang, K.; Struik, P.C.; Yin, X.; Thouminot, C.; Bjelková, M.; Stramkale, V.; Amaducci, S. Comparing Hemp (Cannabis sativa L.) Cultivars for Dual-Purpose Production under Contrasting Environments. Ind. Crops Prod. 2016, 87, 33–44. [Google Scholar] [CrossRef]
- Tang, K.; Struik, P.C.; Yin, X.; Calzolari, D.; Musio, S.; Thouminot, C.; Bjelková, M.; Stramkale, V.; Magagnini, G.; Amaducci, S. A Comprehensive Study of Planting Density and Nitrogen Fertilization Effect on Dual-Purpose Hemp (Cannabis sativa L.) Cultivation. Ind. Crops Prod. 2017, 107, 427–438. [Google Scholar] [CrossRef]
- Tănase Apetroaei, V.; Pricop, E.M.; Istrati, D.I.; Vizireanu, C. Hemp Seeds (Cannabis sativa L.) as a Valuable Source of Natural Ingredients for Functional Foods—A Review. Molecules 2024, 29, 2097. [Google Scholar] [CrossRef]
- Pari, L.; Baraniecki, P.; Kaniewski, R.; Scarfone, A. Harvesting Strategies of Bast Fiber Crops in Europe and in China. Ind. Crops Prod. 2015, 68, 90–96. [Google Scholar] [CrossRef]
- Iucolano, F.; Boccarusso, L.; Langella, A. Hemp as Eco-Friendly Substitute of Glass Fibres for Gypsum Reinforcement: Impact and Flexural Behaviour. Compos. Part B Eng. 2019, 175, 107073. [Google Scholar] [CrossRef]
- Dayo, A.Q.; Luengrojanakul, P.; Boonnao, N.; Charoensuk, K.; Argunam, H.; Ahn, C.-H.; Rimdusit, S. Development of Flame Retardant and Thermally Stable Acoustic Green Composites from Waste Hemp Fibers Reinforcement in Fully Biobased Epoxy and Benzoxazine Hybrid Thermosets. Int. J. Lightweight Mater. Manuf. 2024, 8, 658–668. [Google Scholar] [CrossRef]
- Gencel, O.; Güler, O.; Ustaoğlu, A.; Erdoğmuş, E.; Sarı, A.; Hekimoğlu, G.; Boztoprak, Y.; Subaşı, S. Characteristics of Cement-Based Thermo-Concretes Containing Capric Acid Impregnated Hemp for Thermal Energy Storage and Sound Isolation in Buildings. Energy 2025, 329, 136837. [Google Scholar] [CrossRef]
- Nawawithan, N.; Kittisakpairach, P.; Nithiboonyapun, S.; Ruangjirakit, K.; Jongpradist, P. Design and Performance Simulation of Hybrid Hemp/Glass Fiber Composites for Automotive Front Bumper Beams. Compos. Struct. 2024, 335, 118003. [Google Scholar] [CrossRef]
- Umair, M.; Khan, R.M.W.U. Fibers for Sports Textiles. In Fibers for Technical Textiles; Ahmad, S., Rasheed, A., Nawab, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 93–115. ISBN 978-3-030-49224-3. [Google Scholar]
- Baldini, M.; Ferfuia, C.; Zuliani, F.; Danuso, F. Suitability Assessment of Different Hemp (Cannabis sativa L.) Varieties to the Cultivation Environment. Ind. Crops Prod. 2020, 143, 111860. [Google Scholar] [CrossRef]
- Carus, M.; Meridionale, C.; Kauffmann, A.; Hobson, J.; Bertucelli, S. The European Hemp Industry: Cultivation, Processing and Applications for Fibres, Shives and Seeds. Available online: https://pdf.sciencedirectassets.com/271144/1-s2.0-S0926669016X00052/1-s2.0-S0926669016302436/main.pdf?X-Amz-Security-T (accessed on 3 June 2025).
- Dudziec, P.; Warmiński, K.; Stolarski, M.J. Industrial Hemp As a Multi-Purpose Crop: Last Achievements and Research in 2018−2023. J. Nat. Fibers 2024, 21, 2369186. [Google Scholar] [CrossRef]
- Kołodziej, J.; Kicińska-Jakubowska, A. Utilization of Hemp Shives for Various Purposes—A Review. J. Nat. Fibers 2025, 22, 2448016. [Google Scholar] [CrossRef]
- Borhan, M.D.S.; Rahman, S.; Hammer, C. Water Absorption Capacity of Flax and Pine Horse Beddings and Gaseous Concentrations in Bedded Stalls. J. Equine Vet. Sci. 2014, 34, 611–618. [Google Scholar] [CrossRef]
- Small, E.; Marcus, D. Hemp: A New Crop with New Uses for North America; Reprinted from: Trends in New Crops and New, Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002. [Google Scholar]
- del Carmen Recio-Ruiz, M.; Ruiz-Rosas, R.; García-Mateos, F.J.; Valero-Romero, M.J.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. An Integrated Approach to the Valorization of Pyrolysis Products from Lignocellulosic Residues and By-Products. Biomass Bioenergy 2025, 196, 107676. [Google Scholar] [CrossRef]
- Amalina, F.; Syukor Abd Razak, A.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Advanced Techniques in the Production of Biochar from Lignocellulosic Biomass and Environmental Applications. Clean. Mater. 2022, 6, 100137. [Google Scholar] [CrossRef]
- Zhu, X.; Labianca, C.; He, M.; Luo, Z.; Wu, C.; You, S.; Tsang, D.C.W. Life-Cycle Assessment of Pyrolysis Processes for Sustainable Production of Biochar from Agro-Residues. Bioresour. Technol. 2022, 360, 127601. [Google Scholar] [CrossRef]
- Tiwari, M.; Dirbeba, M.J.; Lehmusto, J.; Yrjas, P.; Vinu, R. Analytical and applied pyrolysis of challenging biomass feedstocks: Effect of pyrolysis conditions on product yield and composition. J. Anal. Appl. Pyrolysis 2024, 177, 106355. [Google Scholar] [CrossRef]
- Jerzak, W.; Acha, E.; Li, B. Comprehensive Review of Biomass Pyrolysis: Conventional and Advanced Technologies, Reactor Designs, Product Compositions and Yields, and Techno-Economic Analysis. Energies 2024, 17, 5082. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Liu, P.; Wang, Z.; Huhe, T.; Chen, Z.; Wu, Y.; Lei, T. A Study on the Pyrolysis Behavior and Product Evolution of Typical Wood Biomass to Hydrogen-Rich Gas Catalyzed by the Ni-Fe/HZSM-5 Catalyst. Catalysts 2024, 14, 200. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A Review of Biochar and Its Use and Function in Soil. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2010; Volume 105, pp. 47–82. ISBN 978-0-12-381023-6. [Google Scholar]
- Pinna, M.V.; Lauro, G.P.; Diquattro, S.; Garau, M.; Senette, C.; Castaldi, P.; Garau, G. Softwood-Derived Biochar as a Green Material for the Recovery of Environmental Media Contaminated with Potentially Toxic Elements. Water Air Soil Pollut. 2022, 233, 152. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential Mechanisms for Achieving Agricultural Benefits from Biochar Application to Temperate Soils: A Review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Gallucci, F.; Palma, A.; Vincenti, B.; Carnevale, M.; Paris, E.; Ancona, V.; Migliarese Caputi, M.V.; Borello, D. Fluidized Bed Gasification of Biomass from Plant Assisted Bioremediation (PABR): Lab-Scale Assessment of the Effect of Different Catalytic Bed Material on Emissions. Fuel 2022, 322, 124214. [Google Scholar] [CrossRef]
- Abideen, Z.; Koyro, H.-W.; Huchzermeyer, B.; Ansari, R.; Zulfiqar, F.; Gul, B. Ameliorating Effects of Biochar on Photosynthetic Efficiency and Antioxidant Defence of Phragmites Karka under Drought Stress. Plant Biol. 2020, 22, 259–266. [Google Scholar] [CrossRef]
- Hansen, V.; Müller-Stöver, D.; Ahrenfeldt, J.; Holm, J.K.; Henriksen, U.B.; Hauggaard-Nielsen, H. Gasification Biochar as a Valuable By-Product for Carbon Sequestration and Soil Amendment. Biomass Bioenergy 2015, 72, 300–308. [Google Scholar] [CrossRef]
- Hansen, V.; Müller-Stöver, D.; Imparato, V.; Krogh, P.H.; Jensen, L.S.; Dolmer, A.; Hauggaard-Nielsen, H. The Effects of Straw or Straw-Derived Gasification Biochar Applications on Soil Quality and Crop Productivity: A Farm Case Study. J. Environ. Manag. 2017, 186, 88–95. [Google Scholar] [CrossRef]
- Liao, W.; Thomas, S.C. Biochar Particle Size and Post-Pyrolysis Mechanical Processing Affect Soil pH, Water Retention Capacity, and Plant Performance. Soil Syst. 2019, 3, 14. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Routledge: London, UK, 2015; ISBN 978-0-203-76226-4. [Google Scholar]
- Ajien, A.; Idris, J.; Md Sofwan, N.; Husen, R.; Seli, H. Coconut Shell and Husk Biochar: A Review of Production and Activation Technology, Economic, Financial Aspect and Application. Waste Manag. Res. 2023, 41, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Seow, Y.X.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Khalid, M.; Ibrahim, M.L.; Ghasemi, M. A Review on Biochar Production from Different Biomass Wastes by Recent Carbonization Technologies and Its Sustainable Applications. J. Environ. Chem. Eng. 2022, 10, 107017. [Google Scholar] [CrossRef]
- Wang, Z.; Li, T.; Song, B.; Liu, S.; Li, S. Thermochemical Conversion of Waste Hemp Textiles into Hierarchical Porous Carbons for CO2 Capture: Kinetic Insights and Structure–Property Relationships. AIP Adv. 2025, 15, 055107. [Google Scholar] [CrossRef]
- Puglia, M.; Morselli, N.; Lumi, M.; Santunione, G.; Pedrazzi, S.; Allesina, G. Assessment of Hemp Hurd-Derived Biochar Produced through Different Thermochemical Processes and Evaluation of Its Potential Use as Soil Amendment. Heliyon 2023, 9, e14698. [Google Scholar] [CrossRef]
- Marrot, L.; Candelier, K.; Valette, J.; Lanvin, C.; Horvat, B.; Legan, L.; DeVallance, D.B. Valorization of Hemp Stalk Waste Through Thermochemical Conversion for Energy and Electrical Applications. Waste Biomass Valor. 2022, 13, 2267–2285. [Google Scholar] [CrossRef]
- Bergman, R.D.; Zhang, H.; Englund, K.; Windell, K.; Gu, H. Estimating GHG Emissions from the Manufacturing of Field-Applied Biochar Pellets. In Proceedings of the 59th International Convention of Society of Wood Science and Technology, Curitiba, Brazil, 6–10 March 2016; pp. 1–11. [Google Scholar]
- Bergman, R.; Sahoo, K.; Englund, K.; Mousavi-Avval, S.H. Lifecycle Assessment and Techno-Economic Analysis of Biochar Pellet Production from Forest Residues and Field Application. Energies 2022, 15, 1559. [Google Scholar] [CrossRef]
- Mohammadi, A. Overview of the Benefits and Challenges Associated with Pelletizing Biochar. Processes 2021, 9, 1591. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Rubio-Clemente, A.; Pérez, J.F. Analysis of Biochars Produced from the Gasification of Pinus Patula Pellets and Chips as Soil Amendments. Maderas. Cienc. Tecnol. 2022, 24. [Google Scholar] [CrossRef]
- Assirelli, A.; Fischetti, E.; Santangelo, E.; Civitarese, V.; Palma, A.; Vincenti, B.; Salerno, M.; Paris, E.; Carnevale, M.; Gallucci, F. Technical and Energetic Characterization of the Different Products Obtainable from Industrial Hemp. In Proceedings of the European Biomass Conference and Exhibition Proceedings 2024, 32nd EUBCE-Marseille 2024, Marseille, France, 24–29 June 2024; pp. 582–586. [Google Scholar] [CrossRef]
- ASTM D 3175-89; Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 1989; p. 305.
- Gallucci, F.; Paris, E.; Palma, A.; Vincenti, B.; Carnevale, M.; Ancona, V.; Borello, D. Fluidized Bed Gasification of Biomass from Plant-Assisted Bioremediation: Fate of Contaminants. Sustain. Energy Technol. Assess. 2022, 53, 102458. [Google Scholar] [CrossRef]
- Demirbas, A. Pyrolysis of Biomass for Fuels and Chemicals. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 31, 1028–1037. [Google Scholar] [CrossRef]
- Mustafa, A.; Calay, R.K.; Mustafa, M.Y. A Techno-Economic Study of a Biomass Gasification Plant for the Production of Transport Biofuel for Small Communities. Energy Procedia 2017, 112, 529–536. [Google Scholar] [CrossRef]
- UNI EN ISO 16948:2015; Solid Biofuels—Determination of Total Content of Carbon, Hydrogen and Nitrogen. ISO: Geneva, Switzerland, 2015.
- UNI EN ISO 16967:2015; Solid Biofuels—Determination of Major Elements—Al, Ca, Fe, Mg, P, K, Si, Na and Ti. ISO: Geneva, Switzerland, 2015.
- UNI EN ISO 16968:2015; Solid Biofuels—Determination of Minor Elements. ISO: Geneva, Switzerland, 2015.
- UNI EN 13037:2012; Soil Improvers and Growing Media—Determination of pH. ISO: Geneva, Switzerland, 2012.
- Hassan, M.; Liu, Y.; Naidu, R.; Parikh, S.J.; Du, J.; Qi, F.; Willett, I.R. Influences of Feedstock Sources and Pyrolysis Temperature on the Properties of Biochar and Functionality as Adsorbents: A Meta-Analysis. Sci. Total Environ. 2020, 744, 140714. [Google Scholar] [CrossRef]
- Wiersma, W.; Van Der Ploeg, M.J.; Sauren, I.J.M.H.; Stoof, C.R. No Effect of Pyrolysis Temperature and Feedstock Type on Hydraulic Properties of Biochar and Amended Sandy Soil. Geoderma 2020, 364, 114209. [Google Scholar] [CrossRef]
- ISO 17126:2024(En); Soil Quality—Determination of the Effects of Pollutants on Soil Flora—Screening Test for Emergence of Lettuce Seedlings (Lactuca sativa L.). ISO: Geneva, Switzerland, 2024. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:17126:ed-2:v1:en (accessed on 19 May 2025).
- Amaducci, S.; Zatta, A.; Pelatti, F.; Venturi, G. Influence of Agronomic Factors on Yield and Quality of Hemp (Cannabis sativa L.) Fibre and Implication for an Innovative Production System. Field Crops Res. 2008, 107, 161–169. [Google Scholar] [CrossRef]
- Musio, S.; Müssig, J.; Amaducci, S. Optimizing Hemp Fiber Production for High Performance Composite Applications. Front. Plant Sci. 2018, 9, 1702. [Google Scholar] [CrossRef]
- Nordin, A. Chemical Elemental Characteristics of Biomass Fuels. Biomass Bioenergy 1994, 6, 339–347. [Google Scholar] [CrossRef]
- Wongsiriamnuay, T.; Tippayawong, N. Thermogravimetric Analysis of Giant Sensitive Plants under Air Atmosphere. Bioresour. Technol. 2010, 101, 9314–9320. [Google Scholar] [CrossRef] [PubMed]
- Di Blasi, C. Modeling Chemical and Physical Processes of Wood and Biomass Pyrolysis. Prog. Energy Combust. Sci. 2008, 34, 47–90. [Google Scholar] [CrossRef]
- Ferdous, D.; Dalai, A.K.; Bej, S.K.; Thring, R.W. Pyrolysis of Lignins: Experimental and Kinetics Studies. Energy Fuels 2002, 16, 1405–1412. [Google Scholar] [CrossRef]
- Jung, S.-H.; Kang, B.-S.; Kim, J.-S. Production of Bio-Oil from Rice Straw and Bamboo Sawdust under Various Reaction Conditions in a Fast Pyrolysis Plant Equipped with a Fluidized Bed and a Char Separation System. J. Anal. Appl. Pyrolysis 2008, 82, 240–247. [Google Scholar] [CrossRef]
- Mansaray, K.G.; Ghaly, A.E. Agglomeration Characteristics of Alumina Sand-Rice Husk Ash Mixtures at Elevated Temperatures. Energy Sources 1997, 19, 1005–1025. [Google Scholar] [CrossRef]
- González-Prieto, Ó.; Ortiz Torres, L.; Vazquez Torres, A. Comparison of Waste Biomass from Pine, Eucalyptus, and Acacia and the Biochar Elaborated Using Pyrolysis in a Simple Double Chamber Biomass Reactor. Appl. Sci. 2024, 14, 1851. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential Role of Biochars in Decreasing Soil Acidification—A Critical Review. Sci. Total Environ. 2017, 581, 601–611. [Google Scholar] [CrossRef]
- Malabadi, R.B.; Kolkar, K.P.; Chalannavar, R.K.; Acharya, M.; Mudigoudra, B.S. Industrial Cannabis Sativa-Hemp: Biochar Applications and Disadvantages. World J. Adv. Res. Rev. 2023, 20, 371–383. [Google Scholar] [CrossRef]
- Conte, P.; Schmidt, H.-P.; Cimò, G. Research and Application of Biochar in Europe. In SSSA Special Publications; Guo, M., He, Z., Uchimiya, S.M., Eds.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 2015; pp. 409–422. ISBN 978-0-89118-967-1. [Google Scholar]
- Saikia, R.; Chutia, R.S.; Kataki, R.; Pant, K.K. Perennial Grass (Arundo donax L.) as a Feedstock for Thermo-Chemical Conversion to Energy and Materials. Bioresour. Technol. 2015, 188, 265–272. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Deng, X.; Zhao, J.; Luo, Y.; Novak, J.; Herbert, S.; Xing, B. Characteristics and Nutrient Values of Biochars Produced from Giant Reed at Different Temperatures. Bioresour. Technol. 2013, 130, 463–471. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Gundale, M.J.; MacKenzie, M.D.; Jones, D.L. Biochar Effects on Soil Nutrient Transformations. In Biochar for Environmental Management; Routledge: Oxford, UK, 2015; ISBN 978-0-203-76226-4. [Google Scholar]
- Cheng, H.; Jones, D.L.; Hill, P.; Bastami, M.S.; Tu, C. long Influence of Biochar Produced from Different Pyrolysis Temperature on Nutrient Retention and Leaching. Arch. Agron. Soil Sci. 2018, 64, 850–859. [Google Scholar] [CrossRef]
- Baronti, S.; Vaccari, F.P.; Miglietta, F.; Calzolari, C.; Lugato, E.; Orlandini, S.; Pini, R.; Zulian, C.; Genesio, L. Impact of Biochar Application on Plant Water Relations in Vitis vinifera (L.). Eur. J. Agron. 2014, 53, 38–44. [Google Scholar] [CrossRef]
- Saracini, C. Decreto Legislativo 75/2010. Disciplina Fertilizzanti—Consolidato. Available online: https://www.certifico.com/haccp/264-legislazione-chemicals-food/15653-decreto-legislativo-75-2010-disciplina-fertilizzanti-consolidato (accessed on 23 May 2025).
- Braghiroli, F.L.; Bouafif, H.; Neculita, C.M.; Koubaa, A. Influence of Pyro-Gasification and Activation Conditions on the Porosity of Activated Biochars: A Literature Review. Waste Biomass Valor. 2020, 11, 5079–5098. [Google Scholar] [CrossRef]
- Fahmi, A.H.; Samsuri, A.W.; Jol, H.; Singh, D. Physical Modification of Biochar to Expose the Inner Pores and Their Functional Groups to Enhance Lead Adsorption. RSC Adv. 2018, 8, 38270–38280. [Google Scholar] [CrossRef]
- Liu, R.; Liu, G.; Yousaf, B.; Abbas, Q. Operating Conditions-Induced Changes in Product Yield and Characteristics during Thermal-Conversion of Peanut Shell to Biochar in Relation to Economic Analysis. J. Clean. Prod. 2018, 193, 479–490. [Google Scholar] [CrossRef]
- Mui, E.L.K.; Cheung, W.H.; McKay, G. Tyre Char Preparation from Waste Tyre Rubber for Dye Removal from Effluents. J. Hazard. Mater. 2010, 175, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An Overview on Engineering the Surface Area and Porosity of Biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Huang, H.; Reddy, N.G.; Huang, X.; Chen, P.; Wang, P.; Zhang, Y.; Huang, Y.; Lin, P.; Garg, A. Effects of Pyrolysis Temperature, Feedstock Type and Compaction on Water Retention of Biochar Amended Soil. Sci. Rep. 2021, 11, 7419. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, M.; Zhang, Z.; Leong, Y.-K.; Zhang, Y.; Zhang, D. Rheological Behaviour and Stability Characteristics of Biochar-Water Slurry Fuels: Effect of Biochar Particle Size and Size Distribution. Fuel Process. Technol. 2017, 156, 27–32. [Google Scholar] [CrossRef]
- Sun, F.; Lu, S. Biochars Improve Aggregate Stability, Water Retention, and Pore-Space Properties of Clayey Soil. J. Plant Nutr. Soil Sci. 2014, 177, 26–33. [Google Scholar] [CrossRef]
- Wang, D.; Li, C.; Parikh, S.J.; Scow, K.M. Impact of Biochar on Water Retention of Two Agricultural Soils—A Multi-Scale Analysis. Geoderma 2019, 340, 185–191. [Google Scholar] [CrossRef]
- Xie, T.; Reddy, K.R.; Wang, C.; Yargicoglu, E.; Spokas, K. Characteristics and Applications of Biochar for Environmental Remediation: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 939–969. [Google Scholar] [CrossRef]
- Sun, H.; Hockaday, W.C.; Masiello, C.A.; Zygourakis, K. Multiple Controls on the Chemical and Physical Structure of Biochars. Ind. Eng. Chem. Res. 2012, 51, 3587–3597. [Google Scholar] [CrossRef]
- Valenzuela-Calahorro, C.; Bernalte-Garcia, A.; Gómez-Serrano, V.; Bernalte-García, M.J. Influence of Particle Size and Pyrolysis Conditions on Yield, Density and Some Textural Parameters of Chars Prepared from Holm-Oak Wood. J. Anal. Appl. Pyrolysis 1987, 12, 61–70. [Google Scholar] [CrossRef]
- Zhang, J.; Amonette, J.E.; Flury, M. Effect of Biochar and Biochar Particle Size on Plant-Available Water of Sand, Silt Loam, and Clay Soil. Soil Tillage Res. 2021, 212, 104992. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2018; ISBN 978-0-12-813040-7. [Google Scholar]
- Pari, L.; Alfano, V.; Scafone, A. An innovative harvesting system for multipurpose hemp. In Proceedings of the 24th European Biomass Conference and Exhibition, Amsterdam, The Netherlands, 6–9 June 2016; pp. 356–358. [Google Scholar]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed Germination Test for Toxicity Evaluation of Compost: Its Roles, Problems and Prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Subramanian, S.; Tanga, C.M. Nutrient Quality and Maturity Status of Frass Fertilizer from Nine Edible Insects. Sci. Rep. 2022, 12, 7182. [Google Scholar] [CrossRef]
- Kataya, G.; El Charif, Z.; Badran, A.; Cornu, D.; Bechelany, M.; Hijazi, A.; Sukkariyah, B.; Issa, M. Evaluating the impact of different biochar types on wheat germination. Sci. Rep. 2024, 14, 28663. [Google Scholar] [CrossRef] [PubMed]
- Rogovska, N.; Laird, D.; Cruse, R.M.; Trabue, S.; Heaton, E. Germination tests for assessing biochar quality. J. Environ. Qual. 2012, 41, 1014–1022. [Google Scholar] [CrossRef] [PubMed]





| Hemp Stem | Hemp Hurd | |
|---|---|---|
| Diameter Class (mm) | Length Class (cm) | Length Class (cm) |
| 2 < x < 4 | 90 < x < 100 | 25 < x < 40 |
| 4 < x < 6 | 100 < x < 110 | 41 < x < 55 |
| 6 < x < 8 | 110 < x < 120 | 56 < x < 70 |
| 8 < x < 10 | 120 < x < 130 | 70 < x < 80 |
| Parameter | |
|---|---|
| Biomass | 80 g |
| N2 flow | 5 NL min−1 |
| Reactor temperature | 550 °C |
| Pyrolysis time | 25 min |
| Heating rate | 21 °C min−1 |
| Hemp Stem | |||
|---|---|---|---|
| Diameter Class (mm) | Number of Pieces | Length Class (cm) | Number of Pieces |
| 2 < x < 4 | 9 | 90 < x < 100 | 36 |
| 4 < x < 6 | 57 | 100 < x < 110 | 47 |
| 6 < x < 8 | 24 | 110 < x < 120 | 15 |
| 8 < x < 10 | 10 | 120 < x < 130 | 2 |
| Hemp Hurd | |
|---|---|
| Length Class (cm) | Number of Pieces |
| 25 < x < 40 | 44 |
| 41 < x < 55 | 39 |
| 56 < x < 70 | 13 |
| 70 < x < 80 | 4 |
| Parameters | Hemp Hurd * | Hemp Hurd Biochar |
|---|---|---|
| C% | 45.71 ± 0.68 | 76.22 ± 4.66 |
| H% | 5.25 ± 0.77 | 2.36 ± 0.32 |
| N% | 0.45 ± 0.13 | 0.97 ± 0.10 |
| S% | 2.17 ± 0.03 | 0.55 ± 0.07 |
| O% | 42.07 ± 1.74 | 19.89 ± 4.44 |
| pH | - | 9.63 ± 0.05 |
| H:C | - | 0.37 |
| O:C | - | 0.19 |
| Macro-Elements (g kg−1) | |
|---|---|
| Na | 0.36 ± 25.00 |
| Mg | 2.34 ± 91.42 |
| K | 18.23 ± 245.05 |
| Ca | 2.88 ± 17.68 |
| Micro-Elements (mg kg−1) | |
|---|---|
| B | 86.46 ± 5.05 |
| Cr | 0.54 ± 3.41 |
| Mn | 334.52 ± 29.73 |
| Fe | 3683.10 ± 236.65 |
| Co | 2.34 ± 0.35 |
| Ni | 16.49 ± 2.08 |
| Cu | 33.55 ± 5.77 |
| Zn | 143.97 ± 47.66 |
| Al | 221.63 ± 56.2 |
| As | 2.85 ± 0.78 |
| Mo | 1.33 ± 0.57 |
| Ag | 0.20 ± 0.06 |
| Cd | 0.17 ± 0.03 |
| Sn | 2.19 ± 0.81 |
| Ba | 13.96 ± 1.25 |
| Pb | 1.02 ± 0.11 |
| Dimensional Classes (mm) | n. Pieces |
|---|---|
| 23–30 | 13 |
| 31–40 | 36 |
| 41–50 | 29 |
| 51–60 | 12 |
| 61–72 | 10 |
| Element | Mass% |
|---|---|
| C | 94.28 |
| K | 3.19 |
| O | 1.54 |
| Al | 0.99 |
| X2 | df | p | |
|---|---|---|---|
| N° of germinated seeds | 2.26 | 3 | 0.521 |
| Epicotyl length (cm) | 7.26 | 3 | 0.064 |
| N° of secondary rootlets | 2.09 | 3 | 0.555 |
| Epicotyl length (cm) | 6.81 | 3 | 0.078 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assirelli, A.; Fischetti, E.; Scarfone, A.; Santangelo, E.; Carnevale, M.; Paris, E.; Palma, A.; Gallucci, F. Characterization of Hemp Hurd-Derived Biochar for Potential Agricultural Applications. Agronomy 2025, 15, 2136. https://doi.org/10.3390/agronomy15092136
Assirelli A, Fischetti E, Scarfone A, Santangelo E, Carnevale M, Paris E, Palma A, Gallucci F. Characterization of Hemp Hurd-Derived Biochar for Potential Agricultural Applications. Agronomy. 2025; 15(9):2136. https://doi.org/10.3390/agronomy15092136
Chicago/Turabian StyleAssirelli, Alberto, Elisa Fischetti, Antonio Scarfone, Enrico Santangelo, Monica Carnevale, Enrico Paris, Adriano Palma, and Francesco Gallucci. 2025. "Characterization of Hemp Hurd-Derived Biochar for Potential Agricultural Applications" Agronomy 15, no. 9: 2136. https://doi.org/10.3390/agronomy15092136
APA StyleAssirelli, A., Fischetti, E., Scarfone, A., Santangelo, E., Carnevale, M., Paris, E., Palma, A., & Gallucci, F. (2025). Characterization of Hemp Hurd-Derived Biochar for Potential Agricultural Applications. Agronomy, 15(9), 2136. https://doi.org/10.3390/agronomy15092136









