Substrate–Genotype Interaction Influences Growth and Phytochemical Composition of Wild and Commercial Purslane (Portulaca oleracea L.) Microgreens
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
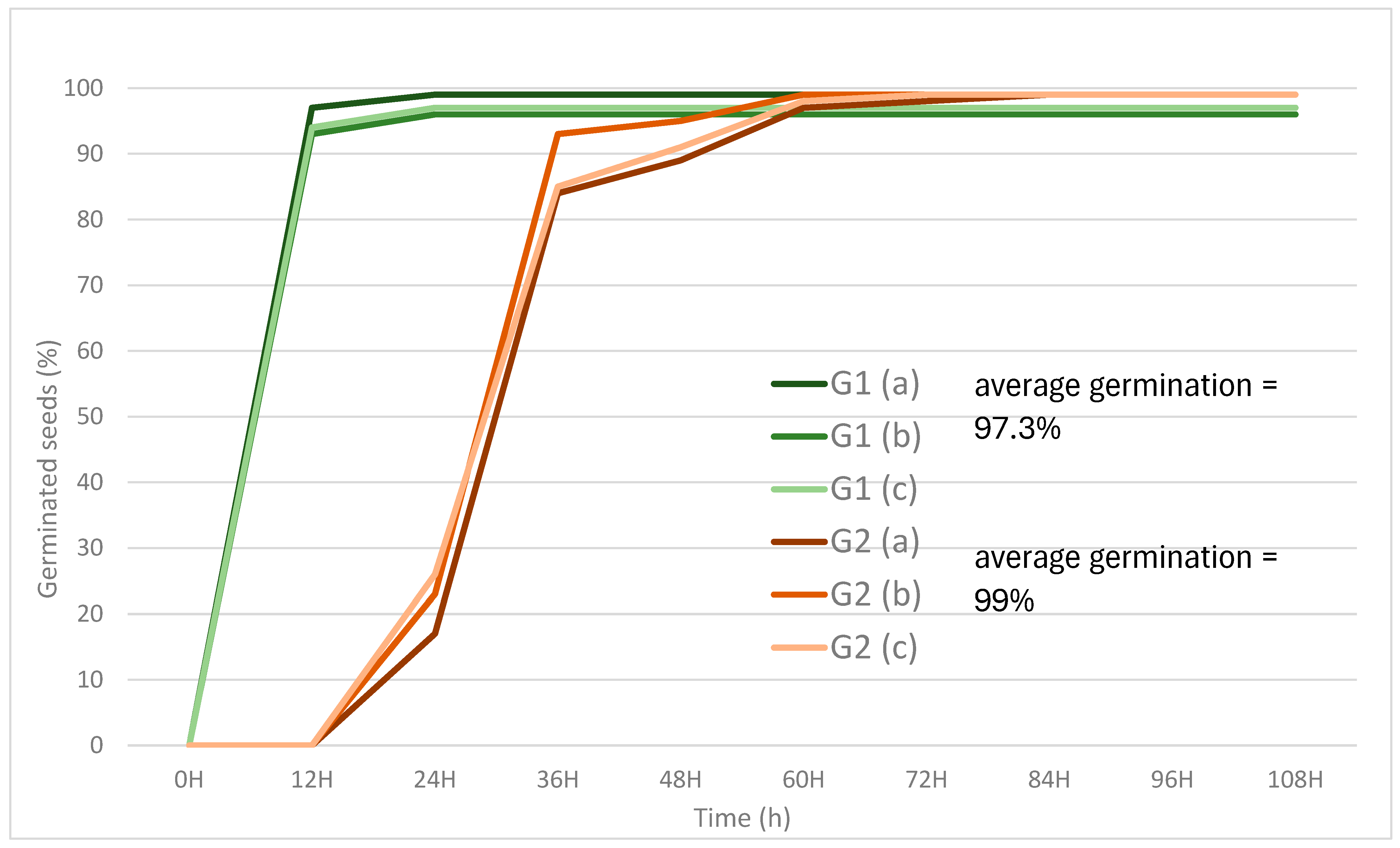
2.1.1. Germination Test
2.1.2. Plant Cultivation
2.1.3. Plant Harvest and Postharvest Processing
2.2. Genetic Analysis
2.3. Determination of Yield Parameters
2.4. Phytochemical and Nutritional Analysis
2.4.1. Chlorophyll a and b Content
- A665 = absorbance at 665 nm;
- A649 = absorbance at 649 nm;
- df = dilution factor (if needed);
- w = sample weight.
2.4.2. Total Carotenoid Content
- A450 = absorbance at 450 nm;
- V1 = extraction volume (cm3);
- V2 = dilution volume (if applicable) (cm3);
- V3 = pipetted volume at dilution (cm3);
- d = cuvette path length (1 cm);
- M = average molecular weight of carotenoids (548 g/mol);
- ε = specific average absorbance of carotenoids (135,310 L/mol/cm);
- w = sample weight (g).
2.4.3. Anthocyanin Content
2.4.4. Antioxidant Activity
2.4.5. Statistical Analysis
3. Results and Discussion
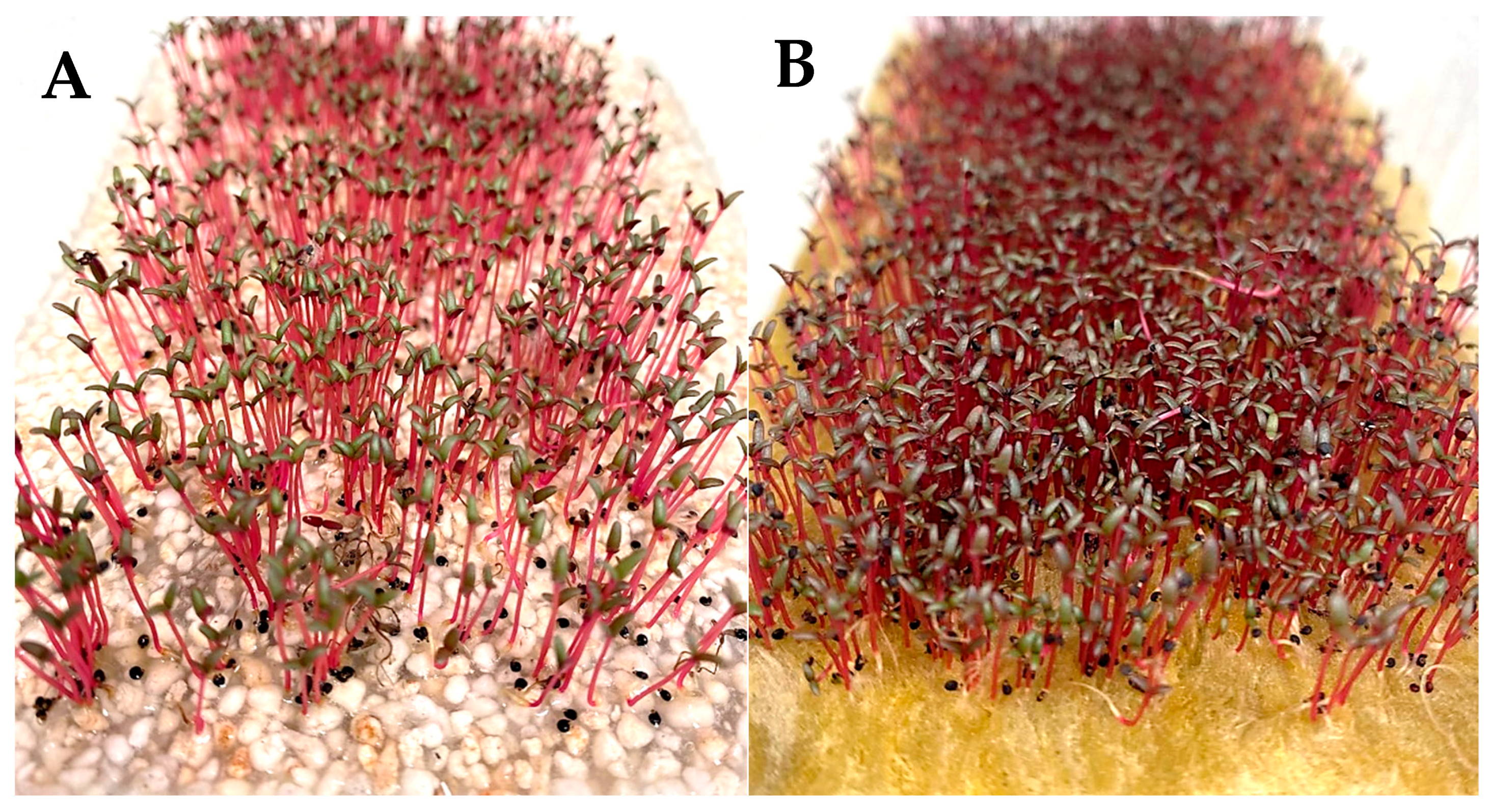
3.1. Germination Test
3.2. Genetic Analysis
3.3. Yield Parameters
3.4. Phytochemical and Nutritional Parameters
3.4.1. Chlorophyll a and b Content
Chlorophyll a/b Ratio
3.4.2. Total Carotenoid Content
3.4.3. Anthocyanin Content
3.4.4. Antioxidant Activity
Correlation Between Antioxidant Activity Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- ReferencesAkbar, S. Handbook of 200 Medicinal Plants: A Comprehensive Review of Their Traditional Medical Uses and Scientific Justifications; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; ISBN 9783030168070. [Google Scholar]
- Srivastava, R.; Srivastava, V.; Singh, A. Multipurpose Benefits of an Underexplored Species Purslane (Portulaca oleracea L.): A Critical Review. Environ. Manag. 2023, 72, 309–320. [Google Scholar] [CrossRef]
- Saffaryazdi, A.; Ganjeali, A.; Farhoosh, R.; Cheniany, M. Variation in Phenolic Compounds, α-Linolenic Acid and Linoleic Acid Contents and Antioxidant Activity of Purslane (Portulaca oleracea L.) during Phenological Growth Stages. Physiol. Mol. Biol. Plants 2020, 26, 1519–1529. [Google Scholar] [CrossRef]
- Abdel-Massih, R.M.; El Beyrouthy, M. Plants Used in Lebanon and the Middle East as Antimicrobials. In Medicinal Plants as Anti-Infectives; Academic Press: Cambridge, MA, USA, 2022; pp. 59–101. [Google Scholar] [CrossRef]
- Petropoulos, S.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Phytochemical Composition and Bioactive Compounds of Common Purslane (Portulaca oleracea L.) as Affected by Crop Management Practices. Trends Food Sci. Technol. 2016, 55, 1–10. [Google Scholar] [CrossRef]
- Kumar, A.; Sreedharan, S.; Kashyap, A.K.; Singh, P.; Ramchiary, N. A Review on Bioactive Phytochemicals and Ethnopharmacological Potential of Purslane (Portulaca oleracea L.). Heliyon 2022, 8, e08669. [Google Scholar] [CrossRef]
- Carrascosa, A.; Pascual, J.A.; Ros, M.; Petropoulos, S.A.; Alguacil, M.d.M. Agronomical Practices and Management for Commercial Cultivation of Portulaca oleracea as a Crop: A Review. Plants 2023, 12, 1246. [Google Scholar] [CrossRef] [PubMed]
- Montoya-García, C.O.; García-Mateos, R.; Becerra-Martínez, E.; Toledo-Aguilar, R.; Volke-Haller, V.H.; Jesús Magdaleno-Villar, J. Bioactive Compounds of Purslane (Portulaca oleracea L.) According to the Production System: A Review. Sci. Hortic. 2023, 308, 111584. [Google Scholar] [CrossRef]
- De Souza, P.G.; Rosenthal, A.; Ayres, E.M.M.; Teodoro, A.J. Potential Functional Food Products and Molecular Mechanisms of Portulaca oleracea L. on Anticancer Activity: A Review. Oxid. Med. Cell. Longev. 2022, 2022, 7235412. [Google Scholar] [CrossRef]
- Purslane, Raw. USDA. Available online: https://fdc.nal.usda.gov/food-details/169274/nutrients (accessed on 22 June 2025).
- Barut Gök, S.; Özdüven, F.; Eryilmaz Açikgöz, F. The Effect of Different Harvest Times on Phenolic Content and Antioxidant Activity in Some Microgreens. Kahramanmaraş Sütçü İmam Üniversitesi Tarım Ve Doğa Derg. 2024, 27, 417–422. [Google Scholar] [CrossRef]
- Hassama, P.; Htwe, N.M.P.S.; Rattanaphan, T.; Promwee, A.; Ruangra, E. Effect of Monosodium Glutamate on the Growth and Quality of Sunflower Microgreens. ASEAN J. Sci. Technol. Rep. 2025, 28, e255735. [Google Scholar] [CrossRef]
- Mallor, C.; Bertolín, J.R.; Paracuellos, P.; Juan, T. Nutraceutical Potential of Leafy Vegetables Landraces at Microgreen, Baby, and Adult Stages of Development. Foods 2023, 12, 3173. [Google Scholar] [CrossRef]
- Rizvi, A.; Sharma, M.; Saxena, S. Microgreens: A Next Generation Nutraceutical for Multiple Disease Management and Health Promotion. Genet. Resour. Crop Evol. 2023, 70, 311–332. [Google Scholar] [CrossRef]
- Singh, N.; Aditika; Rani, S.; Chaurasia, O.P. Vegetable Microgreens Farming in High-Altitude Region of Trans-Himalayas to Maintain Nutritional Diet of Indian Troops. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 743–752. [Google Scholar] [CrossRef]
- Du, M.; Xiao, Z.; Luo, Y. Advances and Emerging Trends in Cultivation Substrates for Growing Sprouts and Microgreens toward Safe and Sustainable Agriculture. Curr. Opin. Food Sci. 2022, 46, 100863. [Google Scholar] [CrossRef]
- Koley, T.K.; Pandey, V. Microgreens from Vegetables: More Nutrition for Better Health. In Vegetables for Nutrition and Entrepreneurship; Springer Nature: Singapore, 2023; pp. 103–113. [Google Scholar] [CrossRef]
- Lone, J.K.; Pandey, R.; Gayacharan. Microgreens on the Rise: Expanding Our Horizons from Farm to Fork. Heliyon 2024, 10, e25870. [Google Scholar] [CrossRef]
- Kováčik, P.; Ducsay, L.; Varga, L. Pestovateľské Substráty (Cultivation Substrates); Slovenská Poľnohospodárska Univerzita v Nitre: Nitra, Slovakia, 2001; ISBN 80-7137-875-5. [Google Scholar]
- Gustianty, L.R.; Zulia, C. Muhammad Yoga Growth and Results of Some Microgreens of Order Caryophyllales on Different Plant Media. J. Sci. Res. Educ. Technol. (JSRET) 2023, 2, 1452–1460. [Google Scholar] [CrossRef]
- Sukewijaya, M.I.; Dwiyani, R.; Bimantara, P.O. Optimization of Growing Media to Support Microgreens Growth and Nutritional Profile. Agro Bali Agric. J. 2025, 8, 102–113. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Seed Germination Ecology of Portulaca oleracea L.: An Important Weed of Rice and Upland Crops. Ann. Appl. Biol. 2009, 155, 61–69. [Google Scholar] [CrossRef]
- Dahlquist, R.M.; Prather, T.S.; Stapleton, J.J. Time and Temperature Requirements for Weed Seed Thermal Death. Weed Sci. 2007, 55, 619–625. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph; Open Agrar Repositorium: Bonn, Germany, 2018. [Google Scholar] [CrossRef]
- Collard, B.C.Y.; Mackill, D.J. Start Codon Targeted (SCoT) Polymorphism: A Simple, Novel DNA Marker Technique for Generating Gene-Targeted Markers in Plants. Plant Mol. Biol. Report. 2009, 27, 86–93. [Google Scholar] [CrossRef]
- Hegedűsová, A.; Šlosár, M.; Mezeyová, I.; Hegedűs, O.; Andrejiová, A.; Szarka, K. Methods for Estimation of Selected Biologically Active Substances; Slovak university of Agriculture: Nitra, Slovakia, 2018; ISBN 978-80-552-1928-8. [Google Scholar]
- Biehler, E.; Mayer, F.; Hoffmann, L.; Krause, E.; Bohn, T. Comparison of 3 Spectrophotometric Methods for Carotenoid Determination in Frequently Consumed Fruits and Vegetables. J. Food Sci. 2010, 75, C55–C61. [Google Scholar] [CrossRef]
- Amirul Alam, M.; Juraimi, A.S.; Rafii, M.Y.; Hamid, A.A.; Kamal Uddin, M.; Alam, M.Z.; Latif, M.A. Genetic Improvement of Purslane (Portulaca oleracea L.) and Its Future Prospects. Mol. Biol. Rep. 2014, 41, 7395–7411. [Google Scholar] [CrossRef]
- Aouadi, M.; Guenni, K.; Abdallah, D.; Louati, M.; Chatti, K.; Baraket, G.; Salhi Hannachi, A. Conserved DNA-Derived Polymorphism, New Markers for Genetic Diversity Analysis of Tunisian Pistacia vera L. Physiol. Mol. Biol. Plants 2019, 25, 1211–1223. [Google Scholar] [CrossRef]
- Corrado, G.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Zarrelli, A.; Giannini, P.; Ritieni, A.; De Pascale, S.; Kyriacou, M.C.; Rouphael, Y. Productive and Morphometric Traits, Mineral Composition and Secondary Metabolome Components of Borage and Purslane as Underutilized Species for Microgreens Production. Horticulturae 2021, 7, 211. [Google Scholar] [CrossRef]
- Plocek, G.; Kathi, S.; Simpson, C. Effects of Eustress Induced by Low Concentrations of Salinity on Broccoli (Brassica oleracea) and Purslane (Portulaca oleracea) Microgreens. Technol. Hortic. 2023, 3, 4. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Pintimalli, L.; Malorgio, F. Selenium Biofortification of Three Wild Species, Rumex acetosa L., Plantago coronopus L., and Portulaca oleracea L., Grown as Microgreens. Agronomy 2021, 11, 1155. [Google Scholar] [CrossRef]
- Giménez, A.; Martínez-Ballesta, M.D.C.; Egea-Gilabert, C.; Gómez, P.A.; Artés-Hernández, F.; Pennisi, G.; Orsini, F.; Crepaldi, A.; Fernández, J.A. Combined Effect of Salinity and Led Lights on the Yield and Quality of Purslane (Portulaca oleracea L.) Microgreens. Horticulturae 2021, 7, 180. [Google Scholar] [CrossRef]
- Bonasia, A.; Lazzizera, C.; La Rotonda, P.; Santoro, A.M.; Botticella, L.; Elia, A.; Conversa, G. Productive and Qualitative Profile of Unexploited Microgreen Genotypes from Brassicaceae, Chenopodiaceae, Asteraceae and Portulacaceae Families. ITALUS HORTUS 2024, 31, 110–128. [Google Scholar] [CrossRef]
- Srivastava, R. Physicochemical, Antioxidant Properties of Carotenoids and Its Optoelectronic and Interaction Studies with Chlorophyll Pigments. Sci. Rep. 2021, 11, 18365. [Google Scholar] [CrossRef]
- Martins, T.; Novo Barros, A.; Rosa, E.; Antunes, L. Enhancing Health Benefits through Chlorophylls and Chlorophyll-Rich Agro-Food: A Comprehensive Review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Babani, F. Contents of Photosynthetic Pigments and Ratios of Chlorophyll a/b and Chlorophylls to Carotenoids (A+b)/(X+c) in C4 Plants as Compared to C3 Plants. Photosynthetica 2022, 60, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mena, A.; Ochoa-Martínez, L.A.; González-Herrera, S.M.; Rutiaga-Quiñones, O.M.; González-Laredo, R.F.; Olmedilla-Alonso, B. Natural Pigments of Plant Origin: Classification, Extraction and Application in Foods. Food Chem. 2023, 398, 133908. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Genotype-Specific Modulatory Effects of Select Spectral Bandwidths on the Nutritive and Phytochemical Composition of Microgreens. Front. Plant Sci. 2019, 10, 1501. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Sajiv, G.; Muruganandam, C.; Rameshkumar, S. Physicochemical Evaluation of Common Purslane (Portulaca oleracea L.) Accessions through Correlation and Regression. J. Appl. Nat. Science. 2023, 15, 356–364. [Google Scholar] [CrossRef]
- Dabbou, S.; Lahbib, K.; Pandino, G.; Dabbou, S.; Lombardo, S. Evaluation of Pigments, Phenolic and Volatile Compounds, and Antioxidant Activity of a Spontaneous Population of Portulaca oleracea L. Grown in Tunisia. Agriculture 2020, 10, 353. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Microgreens: Production, Shelf Life, and Bioactive Components. Crit. Rev. Food Sci. Nutr. 2017, 57, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of the Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]






| Gene | Primer | Primer Sequence (5′ to 3′) | Length | Reference |
|---|---|---|---|---|
| WRKY | F | TGGCGSAAGTACGGCCAG | 18 | [25] |
| R1 | GTGGTTGTGCTTGCC | 15 | ||
| R2 | GCCCTCGTASGTSGT | 15 | ||
| R3 | GCASGTGTGCTCGCC | 15 |
| Genotype | Substrate | FW (g per 0.5 g Seeds) | Dry Matter Content (%) | Shoot Length (cm) | Root Length (cm) | Total Plant Length (cm) |
|---|---|---|---|---|---|---|
| G1 | agar + perlite | 1.93 ± 0.22 b | 10.12 ± 1.10 ab | 1.78 ± 0.11 c | 3.02 ± 1.55 c | 4.8 ± 1.53 c |
| G2 | agar + perlite | 1.23 ± 0.18 a | 13.63 ± 0.41 c | 0.92 ± 0.17 a | 1.87 ± 0.65 b | 2.79 ± 0.64 ab |
| G1 | rock wool | 2.49 ± 0.29 c | 8.21 ± 0.86 a | 1.91 ± 0.41 c | 1.2 ± 0.52 ab | 3.11 ± 0.40 b |
| G2 | rock wool | 2.02 ± 0.31 b | 10.68 ± 0.52 b | 1.17 ± 0.13 b | 1.03 ± 0.30 a | 2.2 ± 0.34 a |
| p-value (substrate) | 0.0000 *** | 0.0066 ** | 0.0165 * | 0.0000 *** | 0.0003 *** | |
| p-value (genotype) | 0.0000 *** | 0.0027 ** | 0.0000 *** | 0.0284 * | 0.0000 *** | |
| p-value (interaction substrate × genotype) | 0.3227 | 0.3955 | 0.4353 | 0.0906 | 0.0536 | |
| Genotype | Substrate | Chlorophyll a (mg kg−1 FW) | Chlorophyll b (mg kg−1 FW) | Total Carotenoids (mg kg−1 FW) | Cyanidin-3-G (µg g−1 DW) |
|---|---|---|---|---|---|
| G1 | agar + perlite | 458.20 ± 24.30 a | 176.55 ± 7.72 b | 196.23 ± 0.56 b | 26.13 ± 0.28 c |
| G2 | agar + perlite | 610.05 ± 42.41 b | 229.07 ± 2.40 c | 269.79 ± 0.57 d | 15.93 ± 0.18 b |
| G1 | rock wool | 503.42 ± 3.65 a | 175.42 ± 3.70 b | 187.58 ± 0.34 a | 16.19 ± 0.63 b |
| G2 | rock wool | 452.84 ± 21.91 a | 154.79 ± 5.27 a | 235.08 ± 0.38 c | 13.22 ± 0.48 a |
| p-value (substrate) | 0.2991 | 0.0734 | 0.0138 * | 0.0006 *** | |
| p-value (genotype) | 0.3429 | 0.3831 | 0.0001 *** | 0.0005 *** | |
| p-value (interaction substrate × genotype) | 0.0060 ** | 0.0006 *** | 0.0000 *** | 0.0000 *** | |
| Genotype | Substrate | DPPH (µmol TE g−1 DW) | ABTS (µmol TE g−1 DW) | FRAP (µmol TE g−1 DW) |
|---|---|---|---|---|
| G1 | agar + perlite | 62.26 ± 3.00 b | 97.64 ±1.19 c | 44.37 ± 3.78 c |
| G2 | agar + perlite | 55.93 ± 4.49 a | 89.36 ±6.69 b | 41.89 ±2.30 bc |
| G1 | rock wool | 54.25 ± 2.19 a | 83.56 ±0.99 a | 35.84 ± 1.44 a |
| G2 | rock wool | 56.86 ± 4.11 a | 93.11 ± 2.47 b | 39.0 ± 1.82 b |
| p-value (substrate) | 0.0527 | 0.0455 * | 0.0001 *** | |
| p-value (genotype) | 0.2926 | 0.7967 | 0.7749 | |
| p-value (interaction substrate × genotype) | 0.0060 ** | 0.0000 *** | 0.0121 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kollárová, I.; Mezeyová, I.; Galovičová, L.; Žiarovská, J.; Farkasová, S.; Pencák, P.; Golian, M. Substrate–Genotype Interaction Influences Growth and Phytochemical Composition of Wild and Commercial Purslane (Portulaca oleracea L.) Microgreens. Agronomy 2025, 15, 2141. https://doi.org/10.3390/agronomy15092141
Kollárová I, Mezeyová I, Galovičová L, Žiarovská J, Farkasová S, Pencák P, Golian M. Substrate–Genotype Interaction Influences Growth and Phytochemical Composition of Wild and Commercial Purslane (Portulaca oleracea L.) Microgreens. Agronomy. 2025; 15(9):2141. https://doi.org/10.3390/agronomy15092141
Chicago/Turabian StyleKollárová, Ivana, Ivana Mezeyová, Lucia Galovičová, Jana Žiarovská, Silvia Farkasová, Peter Pencák, and Marcel Golian. 2025. "Substrate–Genotype Interaction Influences Growth and Phytochemical Composition of Wild and Commercial Purslane (Portulaca oleracea L.) Microgreens" Agronomy 15, no. 9: 2141. https://doi.org/10.3390/agronomy15092141
APA StyleKollárová, I., Mezeyová, I., Galovičová, L., Žiarovská, J., Farkasová, S., Pencák, P., & Golian, M. (2025). Substrate–Genotype Interaction Influences Growth and Phytochemical Composition of Wild and Commercial Purslane (Portulaca oleracea L.) Microgreens. Agronomy, 15(9), 2141. https://doi.org/10.3390/agronomy15092141






