Biochar Application Methods Matter: Biochemical and Enological Responses of an Italian Field-Grown Grapevine (Vitis vinifera L.) Using Solid and Liquid Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar
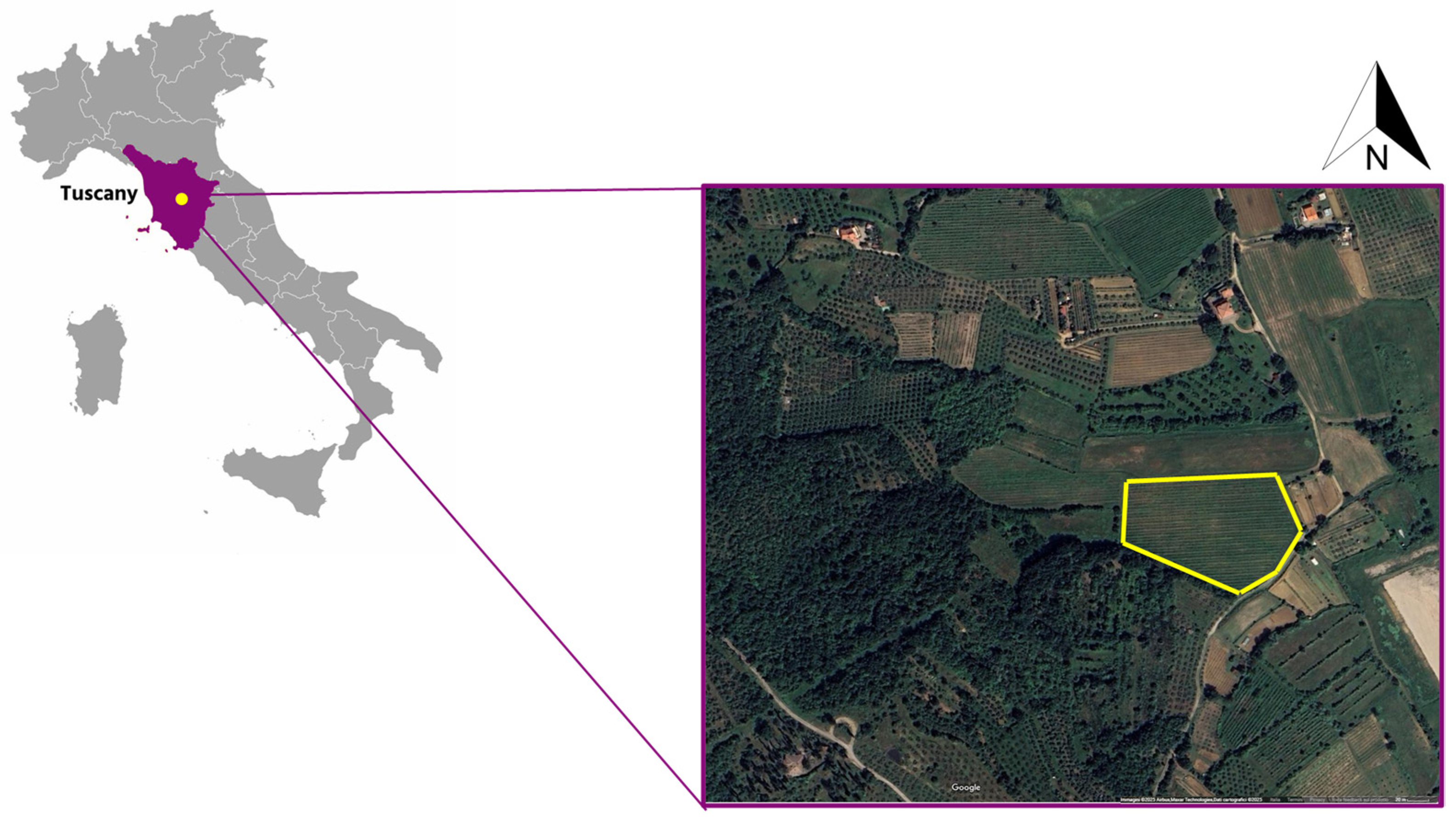
2.2. Site Description and Experimental Design
- i.
- A control (indicated as “NB—No Biochar”), where no formulation of biochar was applied to the soil;
- ii.
- A solid biochar (indicated as “SB”), where solid biochar was applied to the soil only once at the beginning of the experiment (November 2022);
- iii.
- A liquid biochar (indicated as “LB”), where liquid biochar was applied five times (in November, April, May, June, and July) during both the 2022–2023 and 2023–2024 growing seasons.
2.3. Leaf Material
2.3.1. Assimilation Pigments
2.3.2. Malondialdehyde
2.3.3. Proline
2.3.4. Soluble Proteins
2.3.5. Antioxidants
2.3.6. Minerals
2.4. Berry Skin Material
2.4.1. Antioxidants
2.4.2. Starch
2.4.3. Proteins
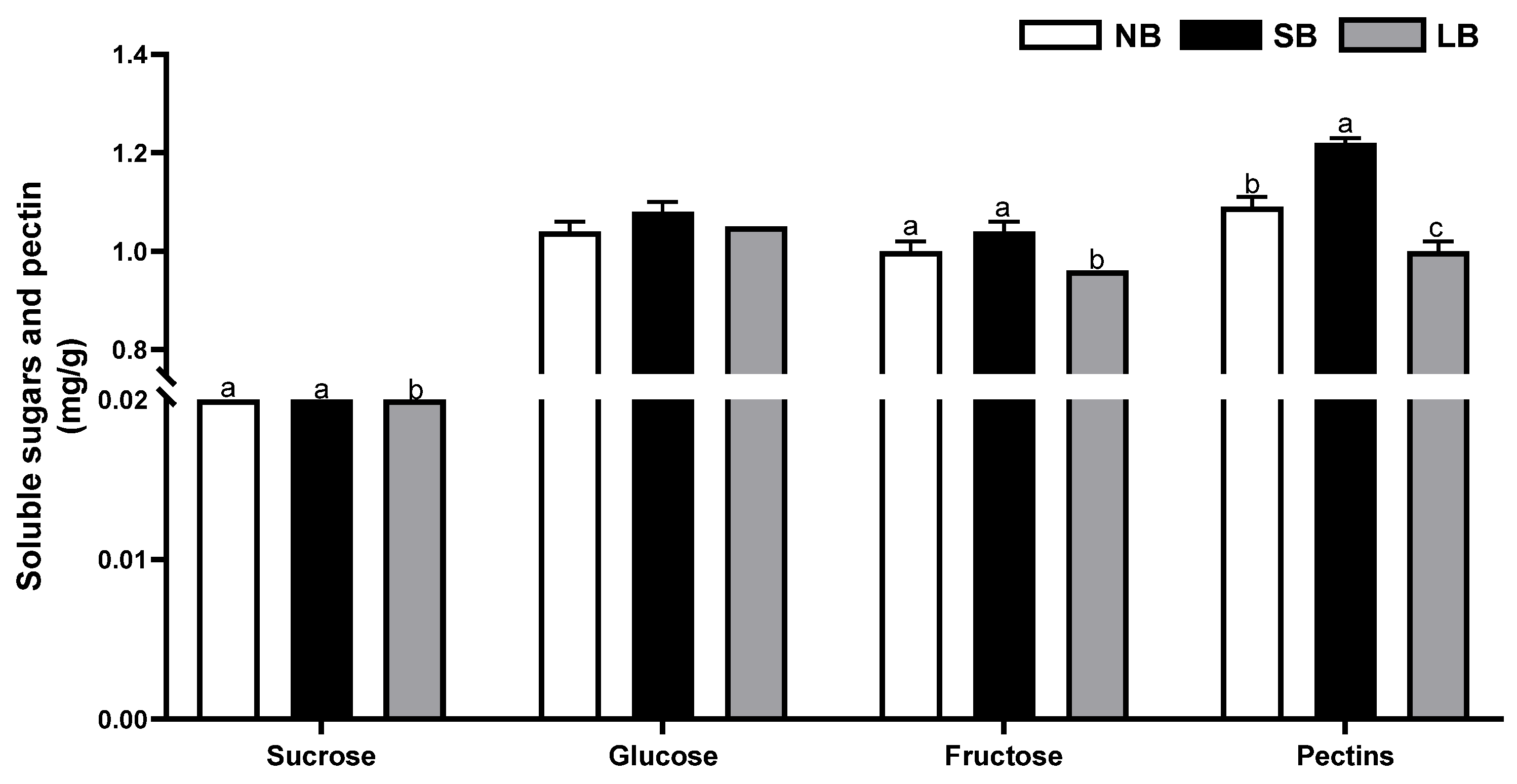
2.4.4. Soluble Sugars and Pectin
2.4.5. Mineral Content
2.5. Must Material
2.5.1. Antioxidants
2.5.2. Quality Parameters
2.6. Statistical Analysis
3. Results
3.1. Leaves
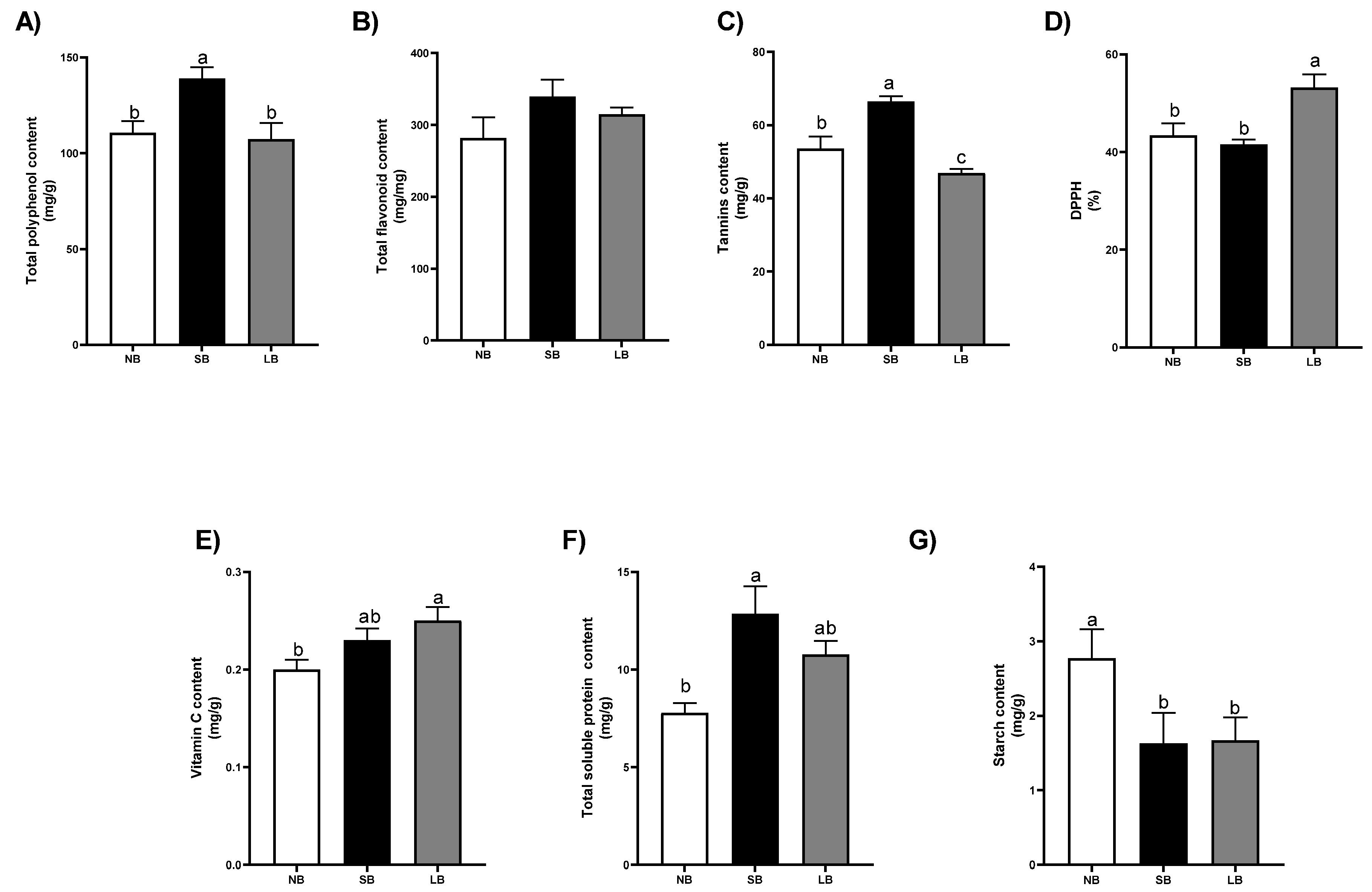
3.2. Berry Skins
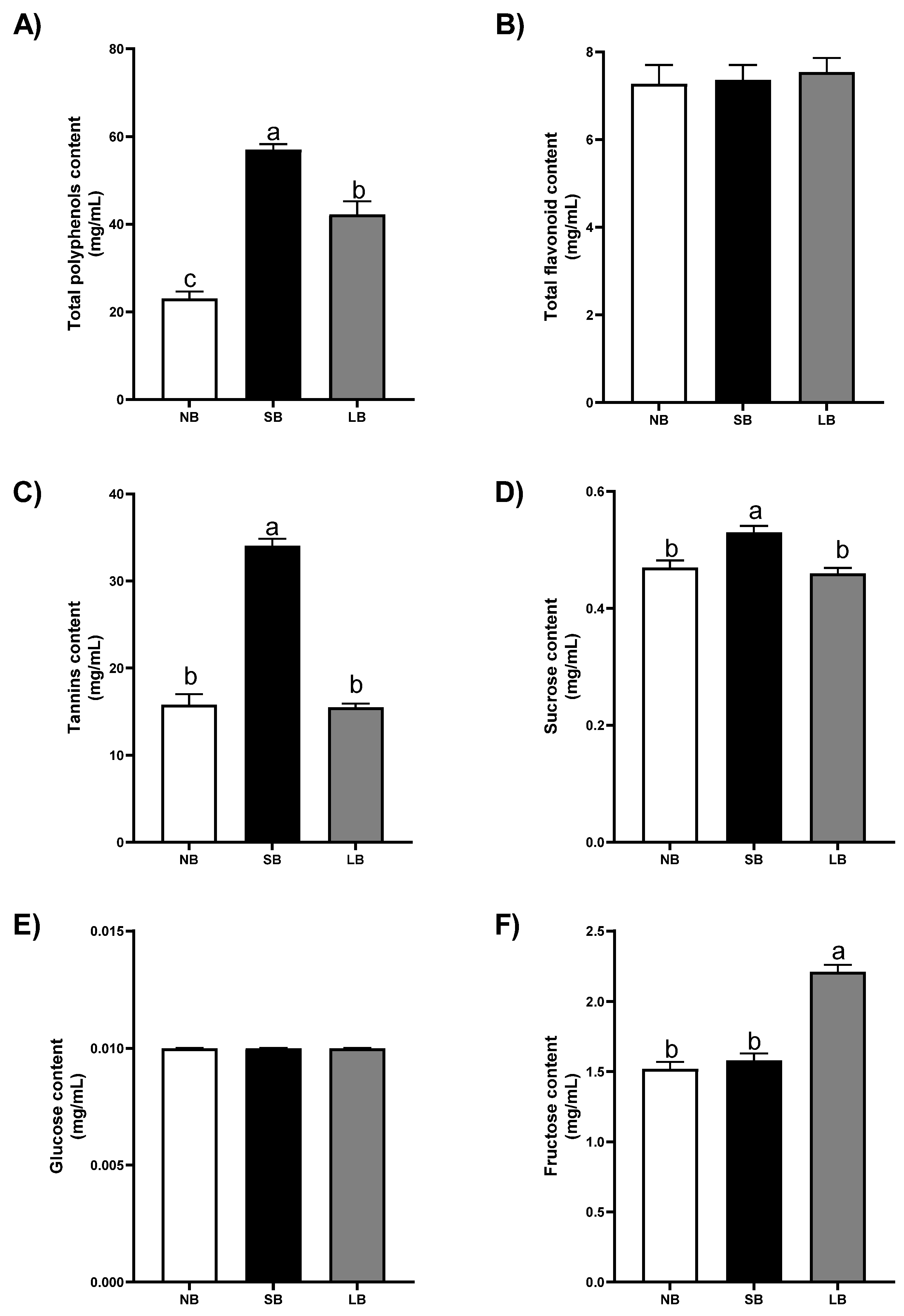
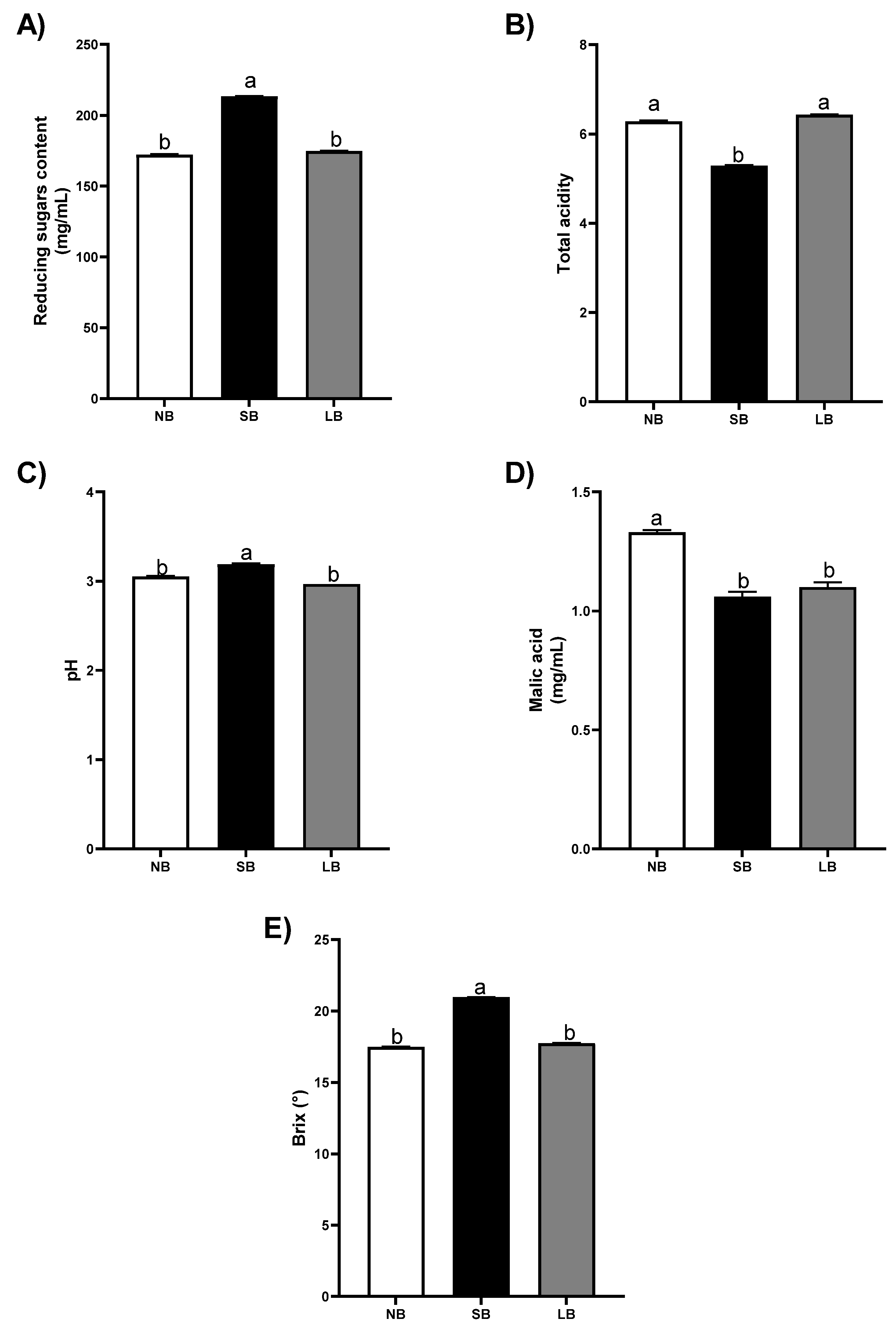
3.3. Musts
4. Discussion
4.1. Leaves
4.2. Skins
4.3. Musts
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eurostat. Organic Farming Statistics. 2022. Available online: https://ec.europa.eu/eurostat (accessed on 30 June 2025).
- Eurostat. Sustainable Use of Pesticides Statistics. 2021. Available online: https://ec.europa.eu/eurostat (accessed on 30 June 2025).
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Kuo, W.H.J.; Chung, C.L.; Juang, K.W.; Tung, C.W.; Liu, L.Y.D. Challenges to agriculture production under climate change. In Agricultural Nutrient Pollution and Climate Change: Challenges and Opportunities; Springer Nature Switzerland: Cham, Switzerland, 2025; pp. 29–56. [Google Scholar]
- European Commission. Farm to Fork. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 14 August 2025).
- European Commission. European Green Deal. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 14 August 2025).
- Garibaldi, L.A.; Gemmill-Herren, B.; D’Annolfo, R.; Graeub, B.E.; Cunningham, S.A.; Breeze, T.D. Farming approaches for greater biodiversity, livelihoods, and food security. Trends Ecol. Evol. 2017, 32, 68–80. [Google Scholar] [CrossRef]
- Michaliszyn-Gabryś, B.; Krupanek, J.; Kalisz, M.; Smith, J. Challenges for sustainability in packaging of fresh vegetables in organic farming. Sustainability 2022, 14, 5346. [Google Scholar] [CrossRef]
- Fedeli, R.; Fiaschi, T.; Angiolini, C.; Maccherini, S.; Loppi, S.; Fanfarillo, E. Dose-Dependent and Species-Specific Effects of Wood Distillate Addition on the Germination Performance of Threatened Arable Plants. Plants 2023, 12, 3028. [Google Scholar] [CrossRef]
- Khan, M.T.; Aleinikovienė, J.; Butkevičienė, L.M. Innovative organic fertilizers and cover crops: Perspectives for sustainable agriculture in the era of climate change and organic agriculture. Agronomy 2024, 14, 2871. [Google Scholar] [CrossRef]
- Mamatha, B.; Mudigiri, C.; Ramesh, G.; Saidulu, P.; Meenakshi, N.; Prasanna, C.L. Enhancing soil health and fertility management for sustainable agriculture: A review. Asian J. Soil Sci. Plant Nutr. 2024, 10, 182–190. [Google Scholar] [CrossRef]
- Sani, M.N.H.; Yong, J.W. Harnessing synergistic biostimulatory processes: A plausible approach for enhanced crop growth and resilience in organic farming. Biology 2021, 11, 41. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A.; Salvi, B.L. Comprehensive review on production and utilization of biochar. SN Appl. Sci. 2019, 1, 168. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Nichols, K.A. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef]
- Gopal, M.; Gupta, A.; Hameed, K.S.; Sathyaseelan, N.; Rajeela, T.H.K.; Thomas, G.V. Biochars produced from coconut palm biomass residues can aid regenerative agriculture by improving soil properties and plant yield in humid tropics. Biochar 2020, 2, 211–226. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Hu, T.; Mahmoud, A.; Li, J.; Zhu, R.; Jing, P. A quantitative review of the effects of biochar application on rice yield and nitrogen use efficiency in paddy fields: A meta-analysis. Sci. Total Environ. 2022, 830, 154792. [Google Scholar] [CrossRef]
- Hu, Q.; Jung, J.; Chen, D.; Leong, K.; Song, S.; Li, F.; Wang, C.H. Biochar industry to circular economy. Sci. Total Environ. 2021, 757, 143820. [Google Scholar] [CrossRef]
- Harty, M.; Forrestal, P.; Watson, C.; McGeough, K.; Carolan, R.; Elliot, C.; Krol, D.; Laughlin, R.; Richards, K.; Lanigan, G.J. Reducing nitrous oxide emissions by changing N fertiliser use from calcium ammonium nitrate (CAN) to urea based formulations. Sci. Total Environ. 2016, 563, 576–586. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Bilias, F.; Kalderis, D.; Richardson, C.; Barbayiannis, N.; Gasparatos, D. Biochar application as a soil potassium management strategy: A review. Sci. Total Environ. 2023, 858, 159782. [Google Scholar] [CrossRef]
- Scott, H.L.; Ponsonby, D.; Atkinson, C.J. Biochar: An improver of nutrient and soil water availability—What is the evidence? CABI Rev. 2014, 1–19. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the ‘charosphere’—Does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Diatta, A.A.; Fike, J.H.; Battaglia, M.L.; Galbraith, J.M.; Baig, M.B. Effects of biochar on soil fertility and crop productivity in arid regions: A review. Arab. J. Geosci. 2020, 13, 595. [Google Scholar] [CrossRef]
- Khan, S.; Irshad, S.; Mehmood, K.; Hasnain, Z.; Nawaz, M.; Rais, A.; Ibrar, D. Biochar production and characteristics, its impacts on soil health, crop production, and yield enhancement: A review. Plants 2024, 13, 166. [Google Scholar] [CrossRef]
- Singh, R.; Singh, P.; Singh, H.; Raghubanshi, A.S. Impact of sole and combined application of biochar, organic and chemical fertilizers on wheat crop yield and water productivity in a dry tropical agro-ecosystem. Biochar 2019, 1, 229–235. [Google Scholar] [CrossRef]
- Lou, Y.; Joseph, S.; Li, L.; Graber, E.R.; Liu, X.; Pan, G. Water extract from straw biochar used for plant growth promotion: An initial test. BioResources 2016, 11, 249–266. [Google Scholar] [CrossRef]
- Bian, X.; Wang, K.; Gong, H. Biochar-enhanced agricultural application of liquid digestate from food waste anaerobic digestion for celery cultivation. Sci. Total Environ. 2023, 869, 161562. [Google Scholar] [CrossRef]
- Sixt, T.; Pacholski, A.; Winkhart, F.; Jaufmann, E.; Schmid, H.; Hülsbergen, K.J. Does woody biochar mixed with liquid organic fertilizer reduce ammonia volatilization following field application? Nutr. Cycl. Agroecosyst. 2025, 131, 165–184. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Sbraci, S.; Storchi, P.; Mattii, G.B. Sustainable viticulture: Effects of soil management in Vitis vinifera. Agronomy 2020, 10, 1949. [Google Scholar] [CrossRef]
- Lavee, S. Grapevine (Vitis vinifera) growth and performance in warm climates. In Temperate Fruit Crops in Warm Climates; Springer: Dordrecht, The Netherlands, 2000; pp. 343–366. [Google Scholar] [CrossRef]
- Torregrosa, L.; Vialet, S.; Adivèze, A.; Iocco-Corena, P.; Thomas, M.R. Grapevine (Vitis vinifera L.). In Agrobacterium Protocols: Volume 2; Wang, K., Ed.; Humana Press: New York, NY, USA, 2015; pp. 177–194. [Google Scholar] [CrossRef]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.P.; Tavares, R.M.; Sousa, M.J.; Gerós, H. Biochemical changes throughout grape berry development and fruit and wine quality. Phytochemistry 2007, 68, 1343–1355. [Google Scholar] [CrossRef]
- Ferrandino, A.; Lovisolo, C. Abiotic stress effects on grapevine (Vitis vinifera L.): Focus on abscisic acid-mediated consequences on secondary metabolism and berry quality. Environ. Exp. Bot. 2014, 103, 138–147. [Google Scholar] [CrossRef]
- Biodea. Available online: https://biodea.bio/scheda-colturale/vigneto/ (accessed on 15 July 2025).
- Fedeli, R.; Celletti, S.; Alexandrov, D.; Nafikova, E.; Loppi, S. Biochar-mediated bioremediation: A sustainable strategy to increase Avena sativa L. tolerance to crude oil soil contamination. Environ. Sci. Pollut. Res. 2024, 31, 52774–52783. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Borella, M.; Baghdadi, A.; Bertoldo, G.; Della Lucia, M.C.; Chiodi, C.; Nardi, S. Transcriptomic and physiological approaches to decipher cold stress mitigation exerted by brown-seaweed extract application in tomato. Front. Plant Sci. 2023, 14, 1232421. [Google Scholar] [CrossRef]
- Mendes, R.; Cardoso, C.; Pestana, C. Measurement of malondialdehyde in fish: A comparison study between HPLC methods and the traditional spectrophotometric test. Food Chem. 2009, 112, 1038–1045. [Google Scholar] [CrossRef]
- Azarnejad, N.; Celletti, S.; Ghorbani, M.; Fedeli, R.; Loppi, S. Dose-dependent effects of a corn starch-based bioplastic on basil (Ocimum basilicum L.): Implications for growth, biochemical parameters, and nutrient content. Toxics 2024, 12, 80. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Fedeli, R.; Zhatkanbayeva, Z.; Marcelli, R.; Zhatkanbayev, Y.; Desideri, S.; Loppi, S. Mitigation of cadmium and copper stress in lettuce: The role of biochar on metal uptake, oxidative stress, and yield. Plants 2025, 14, 2255. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Fedeli, R.; Mazza, I.; Perini, C.; Salerni, E.; Loppi, S. New frontiers in the cultivation of edible fungi: The application of biostimulants enhances the nutritional characteristics of Pleurotus eryngii (DC.) Quél. Agriculture 2024, 14, 1012. [Google Scholar] [CrossRef]
- Al-Duais, M.; Müller, L.; Böhm, V.; Jetschke, G. Antioxidant capacity and total phenolics of Cyphostemma digitatum before and after processing: Use of different assays. Eur. Food Res. Technol. 2009, 228, 813–821. [Google Scholar] [CrossRef]
- Fedeli, R.; Cruz, C.; Loppi, S.; Munzi, S. Hormetic effect of wood distillate on hydroponically grown lettuce. Plants 2024, 13, 447. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Fedeli, R.; Loppi, S.; Cruz, C.; Munzi, S. Evaluating seawater and wood distillate for sustainable hydroponic cultivation: Implications for crop growth and nutritional quality. Sustainability 2024, 16, 7186. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Fedeli, R.; Di Lella, L.A.; Loppi, S. Suitability of XRF for routine analysis of multi-elemental composition: A multi-standard verification. Methods Protoc. 2024, 7, 53. [Google Scholar] [CrossRef]
- Fedeli, R.; Zhatkanbayeva, Z.; Loppi, S. Soil amendment with biochar from slaughterhouse waste bones enhances soil quality and promotes the growth of crop plants. Plant Biosyst. 2025, 159, 378–386. [Google Scholar] [CrossRef]
- Fedeli, R.; Zhatkanbayeva, Z.; Loppi, S. Wood distillate as a solution for growing crops under water deficiency. Crops 2025, 5, 22. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C.L.W.T. Kinetics and mechanisms of antioxidant activity using the DPPH. free radical method. LWT-Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Carullo, G.; Borghini, F.; Fusi, F.; Saponara, S.; Fontana, A.; Pozzetti, L.; Fedeli, R.; Panti, A.; Gorelli, B.; Aquino, G.; et al. Traceability and authentication in agri-food production: A multivariate approach to the characterization of the Italian food excellence elephant garlic (Allium ampeloprasum L.), a vasoactive nutraceutical. Food Chem. 2024, 444, 138684. [Google Scholar] [CrossRef]
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. J. Funct. Foods 2011, 3, 44–49. [Google Scholar] [CrossRef]
- Et-Tazy, L.; Fedeli, R.; Khibech, O.; Lamiri, A.; Challioui, A.; Loppi, S. Effects of Monoterpene-Based Biostimulants on Chickpea (Cicer arietinum L.) Plants: Functional and Molecular Insights. Biology 2025, 14, 657. [Google Scholar] [CrossRef]
- Fedeli, R.; Celletti, S.; Loppi, S.; Vannini, A. Comparison of the Effect of Solid and Liquid Digestate on the Growth of Lettuce (Lactuca sativa L.) Plants. Agronomy 2023, 13, 782. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Grattacaso, M.; Loppi, S. Wood distillate (pyroligneous acid) boosts nutritional traits of potato tubers. Ann. Appl. Biol. 2023, 183, 135–140. [Google Scholar] [CrossRef]
- Bevin, C.J.; Dambergs, R.G.; Fergusson, A.J.; Cozzolino, D. Varietal discrimination of Australian wines by means of mid-infrared spectroscopy and multivariate analysis. Anal. Chim. Acta 2008, 621, 19–23. [Google Scholar] [CrossRef]
- CoHort Software, version 6.4; Costat Statistical Software; CoHort Software: Monterey, CA, USA, 2025. Available online: https://www.cohortsoftware.com (accessed on 14 August 2025).
- Ahmed, N.; Deng, L.; Wang, C.; Shah, Z.U.H.; Deng, L.; Li, Y.; Tu, P. Advancements in biochar modification for enhanced phosphorus utilization in agriculture. Land 2024, 13, 644. [Google Scholar] [CrossRef]
- Elad, Y.; Cytryn, E.; Harel, Y.M.; Lew, B.; Graber, E.R. The biochar effect: Plant resistance to biotic stresses. Phytopathol. Mediterr. 2011, 50, 335–349. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Munera-Echeverri, J.L.; Martinsen, V.; Strand, L.T.; Zivanovic, V.; Cornelissen, G.; Mulder, J. Cation exchange capacity of biochar: An urgent method modification. Sci. Total Environ. 2018, 642, 190–197. [Google Scholar] [CrossRef]
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does biochar improve soil water retention? A systematic review and meta-analysis. Geoderma 2020, 361, 114055. [Google Scholar] [CrossRef]
- Baronti, S.; Vaccari, F.P.; Miglietta, F.; Calzolari, C.; Lugato, E.; Orlandini, S.; Genesio, L. Impact of biochar application on plant water relations in Vitis vinifera (L.). Eur. J. Agron. 2014, 53, 38–44. [Google Scholar] [CrossRef]
- Liu, J.; Huang, S.; Chen, K.; Wang, T.; Mei, M.; Li, J. Preparation of biochar from food waste digestate: Pyrolysis behavior and product properties. Bioresour. Technol. 2020, 302, 122841. [Google Scholar] [CrossRef]
- Vaccari, F.P.; Maienza, A.; Miglietta, F.; Baronti, S.; Di Lonardo, S.; Giagnoni, L.; Genesio, L. Biochar stimulates plant growth but not fruit yield of processing tomato in a fertile soil. Agric. Ecosyst. Environ. 2015, 207, 163–170. [Google Scholar] [CrossRef]
- Wang, C.; Luo, D.; Zhang, X.; Huang, R.; Cao, Y.; Liu, G.; Wang, H. Biochar-based slow-release of fertilizers for sustainable agriculture: A mini review. Environ. Sci. Ecotechnol. 2022, 10, 100167. [Google Scholar] [CrossRef]
- Genesio, L.; Miglietta, F.; Baronti, S.; Vaccari, F.P. Biochar increases vineyard productivity without affecting grape quality: Results from a four years field experiment in Tuscany. Agric. Ecosyst. Environ. 2015, 201, 20–25. [Google Scholar] [CrossRef]
- Dubey, R.S. Photosynthesis in plants under stressful conditions. In Handbook of Photosynthesis, 3rd ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 629–649. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Tripathy, B.C.; Pattanayak, G.K. Chlorophyll biosynthesis in higher plants. In Photosynthesis: Plastid Biology, Energy Conversion and Carbon Assimilation; Springer: Dordrecht, The Netherlands, 2011; pp. 63–94. [Google Scholar]
- Zulfiqar, F.; Moosa, A.; Nazir, M.M.; Ferrante, A.; Ashraf, M.; Nafees, M.; Chen, J.; Darras, A.; Siddique, K.H. Biochar: An emerging recipe for designing sustainable horticulture under climate change scenarios. Front. Plant Sci. 2022, 13, 1018646. [Google Scholar] [CrossRef]
- Cong, M.; Hu, Y.; Sun, X.; Yan, H.; Yu, G.; Tang, G.; Siddique, K.H. Long-term effects of biochar application on the growth and physiological characteristics of maize. Front. Plant Sci. 2023, 14, 1172425. [Google Scholar] [CrossRef]
- Zhou, Y.; Zou, Z.; Wang, M.; Wang, Y.; Li, J.; Qiu, L.; Dai, Z. Biochar and nano-ferric oxide synergistically alleviate cadmium toxicity of muskmelon. Environ. Sci. Pollut. Res. 2023, 30, 57945–57959. [Google Scholar] [CrossRef] [PubMed]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Fiorentino, N.; Sánchez-Monedero, M.A.; Lehmann, J.; Enders, A.; Fagnano, M.; Cayuela, M.L. Interactive priming of soil N transformations from combining biochar and urea inputs: A 15N isotope tracer study. Soil Biol. Biochem. 2019, 131, 166–175. [Google Scholar] [CrossRef]
- Sharifi, M.; Hajiaghaei-Kamrani, M. Biochar–compost mixture and cover crop effects on soil carbon and nitrogen dynamics, yield, and fruit quality in an irrigated vineyard. Can. J. Soil Sci. 2022, 103, 200–212. [Google Scholar] [CrossRef]
- Agegnehu, G.; Jemal, K.; Abebe, A.; Lulie, B. Plant growth and oil yield response of lemon grass (Cymbopogon citratus L.) to biochar application. Ethiop. J. Agric. Sci. 2019, 29, 1–12. [Google Scholar]
- Pontin, M.A.; Piccoli, P.N.; Francisco, R.; Bottini, R.; Martinez-Zapater, J.M.; Lijavetzky, D. Transcriptome changes in grapevine (Vitis vinifera L.) cv. Malbec leaves induced by ultraviolet-B radiation. BMC Plant Biol. 2010, 10, 224. [Google Scholar] [CrossRef]
- Schmidt, H.P.; Kammann, C.; Niggli, C.; Evangelou, M.W.; Mackie, K.A.; Abiven, S. Biochar and biochar-compost as soil amendments to a vineyard soil: Influences on plant growth, nutrient uptake, plant health and grape quality. Agric. Ecosyst. Environ. 2014, 191, 117–123. [Google Scholar] [CrossRef]
- Rouxinol, M.I.; Martins, M.R.; Barroso, J.M.; Rato, A.E. Wine grapes ripening: A review on climate effect and analytical approach to increase wine quality. Appl. Biosci. 2023, 2, 347–372. [Google Scholar] [CrossRef]
- Alem, H.; Rigou, P.; Schneider, R.; Ojeda, H.; Torregrosa, L. Impact of agronomic practices on grape aroma composition: A review. J. Sci. Food Agric. 2019, 99, 975–985. [Google Scholar] [CrossRef] [PubMed]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Herderich, M.J.; Smith, P.A. Analysis of grape and wine tannins: Methods, applications and challenges. Aust. J. Grape Wine Res. 2005, 11, 205–214. [Google Scholar] [CrossRef]
- Frąc, M.; Sas-Paszt, L.; Sitarek, M. Influence of biochar on the vegetative and generative growth of ‘Meredith’ peach trees. Acta Sci. Pol. Hortorum Cultus 2022, 21, 61–69. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Roby, J.P.; De Rességuier, L. Soil-related terroir factors: A review. OENO One 2018, 52, 173–188. [Google Scholar] [CrossRef]
- Bely, M.; Sablayrolles, J.M.; Barre, P. Automatic detection of assimilable nitrogen deficiencies during alcoholic fermentation in oenological conditions. J. Ferment. Bioeng. 1990, 70, 246–252. [Google Scholar] [CrossRef]
- Kontoudakis, N.; Esteruelas, M.; Fort, F.; Canals, J.M.; De Freitas, V.; Zamora, F. Influence of the heterogeneity of grape phenolic maturity on wine composition and quality. Food Chem. 2011, 124, 767–774. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine polyphenol content and its influence on wine quality and properties: A review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
| NB | SB | LB | |
|---|---|---|---|
| Chla (mg/g) | 0.56 ± 0.03 a | 0.58 ± 0.02 a | 0.42 ± 0.02 b |
| Chlb (mg/g) | 1.20 ± 0.04 a | 1.21 ± 0.03 a | 0.91 ± 0.04 b |
| Carotenoids (mg/g) | 0.19 ± 0.02 a | 0.15 ± 0.02 b | 0.15 ± 0.01 b |
| MDA (µg/g) | 4.61 ± 0.15 a | 3.87 ± 0.37 b | 4.48 ± 0.39 a |
| Proline (µmol/g) | 19.5 ± 1.0 a | 20.1 ± 0.3 a | 18.5 ± 1.3 a |
| Total soluble proteins(mg/g) | 3.94 ± 0.25 c | 6.52 ± 0.73 a | 5.50 ± 0.35 b |
| TPC (mg/g) | 1.57 ± 0.23 b | 2.47 ± 0.49 a | 1.40 ± 0.37 b |
| TFC (mg/g) | 604 ± 34 b | 610 ± 13 b | 682 ± 26 a |
| Tannins (mg/g) | 522 ± 5 | 521 ± 6 | 509 ± 6 |
| P (mg/kg) | 1582 ± 75 a | 1622 ± 137 a | 1181 ± 47 b |
| S (mg/kg) | 1682 ± 79 a | 1738 ± 152 a | 1352 ± 52 b |
| K (mg/kg) | 6825 ± 260 a | 5988 ± 503 b | 6612 ± 271 a |
| Ca (mg/kg) | 18,293 ± 863 a | 15,396 ± 1353 b | 14,400 ± 575 b |
| Mn (mg/kg) | 63.6 ± 5.0 a | 51.0 ± 9.0 b | 57.6 ± 5.1 b |
| Fe (mg/kg) | 152 ± 6 a | 150 ± 15 a | 124 ± 5 b |
| Cu (mg/kg) | 93.1 ± 2.7 a | 41.0 ± 2.8 c | 65.0 ± 2.1 b |
| Zn (mg/kg) | 21.3 ± 0.6 a | 17.6 ± 0.8 b | 16.3 ± 0.6 b |
| NB | SB | LB | |
|---|---|---|---|
| P | 653 ± 53 ab | 703 ± 30 a | 575 ± 16 b |
| S | 241 ± 20 | 220 ± 7 | 211 ± 8 |
| K | 5496 ± 255 a | 4844 ± 208 b | 4064 ± 89 c |
| Ca | 395 ± 26 | 425 ± 37 | 406 ± 13 |
| Mn | 19.9 ± 4.4 a | 10.2 ± 1.2 b | 9.0 ± 1.5 b |
| Fe | 11.3 ± 0.8 | 12.0 ± 1.1 | 10.2 ± 0.9 |
| Cu | 9.2 ± 0.4 a | 4.2 ± 0.3 b | 3.8 ± 0.1 b |
| Zn | 26 ± 1.6 a | 3.6 ± 0.2 b | 4.5 ± 0.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedeli, R.; Celletti, S.; Loppi, S. Biochar Application Methods Matter: Biochemical and Enological Responses of an Italian Field-Grown Grapevine (Vitis vinifera L.) Using Solid and Liquid Formulations. Agronomy 2025, 15, 2124. https://doi.org/10.3390/agronomy15092124
Fedeli R, Celletti S, Loppi S. Biochar Application Methods Matter: Biochemical and Enological Responses of an Italian Field-Grown Grapevine (Vitis vinifera L.) Using Solid and Liquid Formulations. Agronomy. 2025; 15(9):2124. https://doi.org/10.3390/agronomy15092124
Chicago/Turabian StyleFedeli, Riccardo, Silvia Celletti, and Stefano Loppi. 2025. "Biochar Application Methods Matter: Biochemical and Enological Responses of an Italian Field-Grown Grapevine (Vitis vinifera L.) Using Solid and Liquid Formulations" Agronomy 15, no. 9: 2124. https://doi.org/10.3390/agronomy15092124
APA StyleFedeli, R., Celletti, S., & Loppi, S. (2025). Biochar Application Methods Matter: Biochemical and Enological Responses of an Italian Field-Grown Grapevine (Vitis vinifera L.) Using Solid and Liquid Formulations. Agronomy, 15(9), 2124. https://doi.org/10.3390/agronomy15092124









