A New Method for Single-Plant Selection of Wheat Genotypes for Tolerance and Resistance to the Root-Lesion Nematode Pratylenchus thornei by Low-Density Sowing
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Site
2.2. Characterisation of the Field Site for Pratylenchus thornei and Plant Available Water
2.3. General Management and Naming Convention of Field Experiments
2.4. Plant Density (PD) Experiments
2.5. Single Plant (SP) Experiments
2.6. In-Season Visual Tolerance Ratings (VTRs) and Normalized Difference Vegetation Index (NDVI) of the Single Plants
2.7. Statistical Software
2.7.1. Statistical Analysis for the Initial Experimental Site Characterization
2.7.2. Statistical Analysis of Plant Density Experiments (PD)
2.7.3. Statistical Analysis of Single-Plant Experiments (SP)
3. Results
3.1. Initial Pratylenchus thornei Population Densities, Plant Available Water (PAW), and In-Crop Rainfall for Each of the Experimental Years
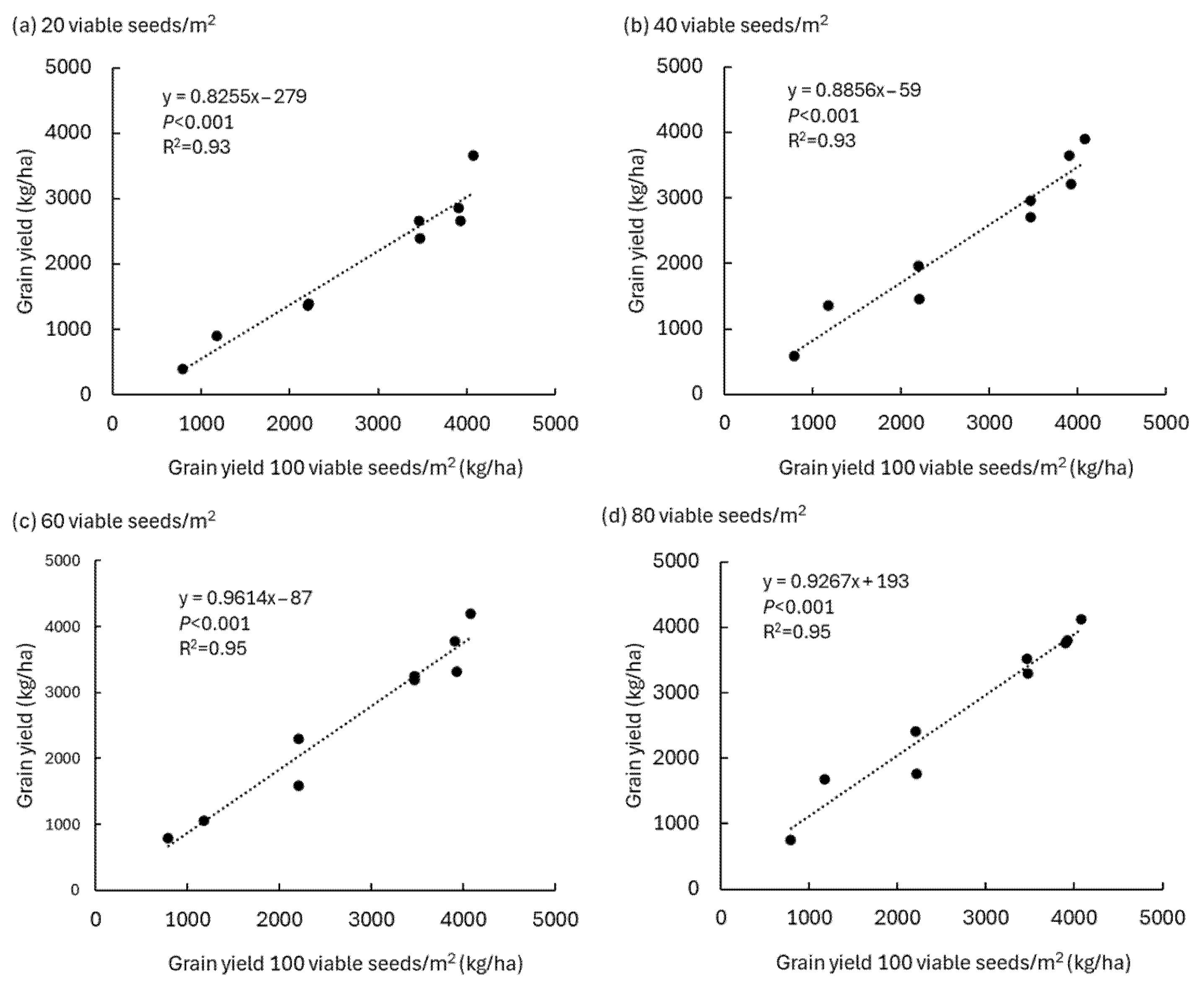
3.2. The 2013 and 2022 Plant Density Experiments on High Population Densities of Pratylenchus thornei
3.3. Single-Plant Assessment of Tolerance to Pratylenchus thornei at 1, 4, 16 and 32 Plants/m2
Single Plant Assessment for Tolerance to Pratylenchus thornei Using Normalized Difference Vegetation Index and Visual Tolerance Ratings in 2022
3.4. Single-Plant Assessment for Resistance to Pratylenchus thornei at 1, 4, 16 and 32 Plants/m2
4. Discussion
4.1. Tolerance Stability at Lower-than-Industry-Recommended Plant Densities
4.2. Evaluating Tolerance and Resistance at the Single-Plant Level Using Low-Density Sowing
4.3. Ultra-Low-Density Sowing for the Selection of Genotypes with Tolerance to Pratylenchus thornei
4.4. Ultra-Low-Density Sowing for the Selection of Genotypes with Resistance to Pratylenchus thornei
4.5. Ultra-Low-Density Sowing for Dual Selection of Tolerance and Resistance to Pratylenchus thornei
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| ANOVA | Analysis of variance |
| BD | Bulk density |
| BTM | Back-transformed mean |
| CLL | Crop lower limit |
| CV | Coefficient of variance |
| DAS | Days after sowing |
| eBLUP | Empirical best linear unbiased prediction |
| FOV | Field of view |
| GRDC | Grains Research and Development Corporation |
| GWC | Gravimetric water content |
| MET | Multi-environment trial |
| LD | Low density |
| NDVI | Normalized difference vegetation index |
| PAW | Plant available water |
| PD | Plant density experiment |
| RLN | Root lesion nematode |
| SP | Single-plant experiment |
| UAV | Unmanned aerial vehicle |
| ULD | Ultra-low-density |
| VTR | Visual tolerance rating |
References
- Buerstmayr, H.; Dreccer, M.F.; Miladinović, D.; Qiu, L.; Rajcan, I.; Reif, J.; Varshney, R.K.; Vollman, J. Plant breeding for increased sustainability: Challenges, opportunities, and progress. Theor. Appl. Genet. 2022, 135, 3679–3683. [Google Scholar] [CrossRef]
- Thompson, J.P.; Sheedy, J.G.; Robinson, N.A.; Clewett, T.G. Tolerance of wheat (Triticum aestivum) genotypes to root-lesion nematode (Pratylenchus thornei) in the subtropical grain region of eastern Australia. Euphytica 2021, 214, 48. [Google Scholar] [CrossRef]
- Roberts, P.A. Concepts and consequences of resistance. In Plant Resistance to Parasitic Nematodes; Starr, J.L., Bridge, J., Cook, R., Eds.; CAB International: Wallingford Oxon, UK, 2002; pp. 23–41. [Google Scholar]
- De Waele, D.; Elsen, A. Migratory endoparasites: Pratylenchus and Radophous species. In Plant Resistance to Parasitic Nematodes; Starr, J.L., Bridge, J., Cook, R., Eds.; CAB International: Wallingford Oxon, UK, 2002; pp. 185–216. [Google Scholar]
- Trudgill, D.L. Resistance to and tolerance of plant parasitic nematodes in plants. Annu. Rev. Phytopathol. 1991, 29, 167–192. [Google Scholar] [CrossRef]
- Thompson, J.P.; Sheedy, J.G.; Clewett, T.G. Advances in breeding wheat for tolerance and resistance to Pratylenchus thornei and P. neglectus for the northern region. In Proceedings of the Tenth Assembly of the Wheat Breeding Society of Australia, Mildura, Australia, 16–21 September 2001; pp. 127–130. [Google Scholar]
- Crespo-Herrera, L.A.; Crossa, J.; Vargas, M.; Bruan, H.-J. Defining target wheat breeding environments. In Wheat Improvement Food Security in a Changing Climate; Reynolds, M.P., Bruan, H.-J., Eds.; Springer: Cham, Switzerland, 2022; pp. 31–46. [Google Scholar]
- Cook, R.; Evans, K. Resistance and tolerance. In Principles and Practice of Nematode Control in Crops; Brown, R.H., Kerry, B.R., Eds.; Academic Press: Sydney, Australia, 1987; pp. 179–231. [Google Scholar]
- Fanning, J.P.; Reeves, K.L.; Forknall, C.R.; McKay, A.C.; Hollaway, G.J. Pratylenchus thornei: The relationship between presowing nematode density and yield loss in wheat and barley. Phytopathology 2020, 110, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Owen, K.J.; Fanning, J.P.; Reeves, K.L.; Hollaway, G.J. Consistent responses of yield and resistance of wheat cultivars to the root-lesion nematode, Pratylenchus thornei, in the Australian northern subtropical region, but not in the temperate southern region. Plant Pathol. 2021, 70, 1790–1806. [Google Scholar] [CrossRef]
- Robinson, N.A.; Sheedy, J.G.; MacDonald, B.J.; Owen, K.J.; Thompson, J.P. Tolerance of wheat cultivars to root-lesion nematode (Pratylenchus thornei) assessed by normalized difference vegetation index is predictive of grain yield. Ann. Appl. Biol. 2019, 174, 388–401. [Google Scholar] [CrossRef]
- Robinson, N.A.; Sheedy, J.G.; Thompson, J.P. Comparison of visual and normalized difference vegetation index (NDVI) assessments to predict the yield tolerance of wheat genotypes to root-lesion nematode Pratylenchus thornei. Agronomy 2024, 14, 3043. [Google Scholar] [CrossRef]
- Smiley, R.W.; Nicol, J.M. Nematodes which challenge global wheat production. In Wheat Science and Trade; Carter, B.F., Ed.; Wiley-Blackwell: Ames, IA, USA, 2009; pp. 171–187. [Google Scholar]
- Thompson, J.P.; Clewett, T.G.; Sheedy, J.G.; Reen, R.A.; O’Reilly, M. Occurrence of root-lesion nematodes (Pratylenchus thornei and P. neglectus) and stunt nematode (Merlinius brevidens) in the northern grain region of Australia. Aust. Plant Pathol. 2010, 39, 254–264. [Google Scholar] [CrossRef]
- van Gundy, S.D.; Perez, B.J.G.; Stolzy, L.H.; Thomason, I.J. A pest management approach to the control of Pratylenchus thornei on wheat in Mexico. J. Nematol. 1974, 6, 107–116. [Google Scholar]
- Murray, G.M.; Brennan, J.P. Estimating disease losses to the Australian wheat industry. Australas. Plant Path. 2009, 38, 558–570. [Google Scholar] [CrossRef]
- Owen, K. A triumph of tolerance: Managing the threat to wheat production by the root lesion nematode Pratylenchus thornei in the subtropical grain region of eastern Australia. In Integrated Nematode Management: State-of-the-Art and Visions for the Future; Sikora, R.A., Desaeger, J., Molendijk, L., Eds.; CABI: Oxon, UK, 2022; pp. 13–19. [Google Scholar]
- Thompson, J.P.; Clewett, T.G.; O’Reilly, M.M. Temperature response of root-lesion nematode (Pratylenchus thornei) reproduction on wheat cultivars has implications for resistance screening and wheat production. Ann. Appl. Biol. 2015, 167, 1–10. [Google Scholar] [CrossRef]
- Whish, J.P.M.; Thompson, J.P.; Clewett, T.G.; Wood, J.; Rostad, H.E. Predicting the slow decline of root lesion nematodes (Pratylenchus thornei) during host-free fallows to improve farm management decisions. Eur. J. Agron. 2017, 91, 44–53. [Google Scholar] [CrossRef]
- Smiley, R.W.; Gourlie, J.A.; Yan, G.; Rhinhart, K.E.L. Resistance and tolerance of landrace wheat in fields infested with Pratylenchus neglectus and P. thornei. Plant Dis. 2014, 98, 797–805. [Google Scholar] [CrossRef] [PubMed]
- GRDC National Variety Trials. 2024 Queensland Winter Sowing Guide. Available online: https://grdc.com.au/resources-and-publications/all-publications/nvt-crop-sowing-guides/qld-winter-crop-sowing-guide (accessed on 2 November 2024).
- Thompson, J.P.; Sheedy, J.G.; Robinson, N.A. Resistance of wheat genotypes to root-lesion nematode (Pratylenchus thornei) can be used to predict final nematode population densities, crop greenness and grain yield in the field. Phytopathology 2020, 110, 505–516. [Google Scholar] [CrossRef]
- Rognoni, B.; Forknall, C.R.; Simpfendorfer, S.; Daniel, R.; Neale, L.; Kelly, A.M. Quantifying the resistance of Australian wheat genotypes to Pratylenchus thornei based on a continuous metric from a factor analytical linear mixed model. Euphytica 2024, 220, 141. [Google Scholar] [CrossRef]
- Kumar, D.; Sharma, S.; Sharma, R.; Pundir, S.; Singh, V.K.; Chaturvedi, D.; Singh, B.; Kumar, S.; Sharma, S. Genome-wide association study in hexaploidy wheat identifies novel genome regions associated with resistance to root lesion nematode (Pratylenchus thornei). Sci. Rep. 2021, 11, 3572. [Google Scholar]
- Sheedy, J.G.; McKay, A.C.; Lewis, J.; Vanstone, V.A.; Fletcher, S.; Kelly, A.; Thompson, J.P. Cereal cultivars can be ranked consistently for resistance to root-lesion nematodes (Pratylenchus thornei & P. neglectus) using diverse procedures. Aust. Plant Pathol. 2015, 44, 175–182. [Google Scholar]
- Zwart, R.S.; Thompson, J.P.; Godwin, I.D. Genetic analysis of resistance to root-lesion nematode (Pratylenchus thornei) in wheat. Plant Breed. 2004, 123, 209–212. [Google Scholar] [CrossRef]
- Tokatlidis, I.S.; Tsialtas, J.T.; Xynias, I.N.; Tamoutsidis, E.; Irakli, M.X. Variation within a bread wheat cultivar for grain yield, protein content, carbon isotope discrimination and ash content. Field Crops Res. 2004, 86, 33–42. [Google Scholar] [CrossRef]
- Tokatlidis, I.S.; Xynias, I.N.; Tsialtas, J.T.; Papadopoulos, I.I. Single plant selection at ultra-low density to improve stability of a bread wheat cultivar. Crop Sci. 2006, 46, 90–97. [Google Scholar] [CrossRef]
- Beaugendre, A.; Mingeot, D.; Visser, M. Complex plant interactions in heterogeneous material require the ecological rethinking of sowing density recommendations for bread wheat. A review. Agron. Sustain. Dev. 2022, 42, 9. [Google Scholar] [CrossRef]
- Fasoula, D.A.; Fasoula, V.A. Competitive ability and plant breeding. In Plant Breeding Reviews; Janick, J., Ed.; Wiley: New York, NY, USA, 1997; pp. 89–138. [Google Scholar]
- Fasoula, V.A.; Fasoula, D.A. Honeycomb breeding: Principles and Applications. In Plant Breeding Reviews; Janick, J., Ed.; Wiley: New York, NY, USA, 2000; Volume 18, pp. 177–250. [Google Scholar]
- Fasoula, V.A.; Fasoula, D.A. Principles underlying genetic improvement for high and stable crop yield potential. Field Crop Res. 2002, 75, 191–209. [Google Scholar] [CrossRef]
- Fasoula, V.A.; Tokatlidis, I.S. Development of crop cultivars by honeycomb breeding. Agron. Sustain. Dev. 2012, 32, 161–180. [Google Scholar] [CrossRef]
- Fischer, R.A. Breeding wheat for increased potential yield: Contrasting ideas from Donald and Fasoulas, and the case for early generation selection under nil competition. Field Crops Res. 2020, 252, 107782. [Google Scholar] [CrossRef]
- Fasoula, D.A.; Ioannides, I.M.; Omirou, M. Phenotyping and plant breeding: Overcoming the barriers. Front. Plant Sci. 2020, 10, 1713. [Google Scholar] [CrossRef] [PubMed]
- Donald, C.M.; Hamblin, J. The biological yield and harvest index of cereals as agronomic and plant breeding criteria. Adv. Agron. 1976, 28, 361–405. [Google Scholar]
- Fischer, R.A.; Rebetzke, G.J. Indirect selection for potential yield in early generation spaced plantings of wheat and other small-grain cereals: A review. Crop Pasture Sci. 2018, 69, 439–459. [Google Scholar] [CrossRef]
- Quail, K.J.; Fischer, R.A.; Wood, J.T. Early generation selection in wheat. I Yield potential. Aust. J. Agric. Res. 1989, 40, 1117–1133. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Chawade, A.; van Ham, J.; Blomquist, H.; Bagge, O.; Alexandersson, E.; Ortiz, R. High-throughput field-phenotyping tools for plant breeding and precision agriculture. Agronomy 2019, 9, 258. [Google Scholar] [CrossRef]
- Furbank, R.; Tester, M. Phenomics—Technologies to relieve the phenotyping bottleneck. Trends Plant Sci. 2011, 16, 635–644. [Google Scholar] [CrossRef]
- Song, P.; Wang, W.; Guo, X.; Yang, W.; Zhao, C. High-throughput phenotyping: Breaking through the bottleneck in future crop breeding. Crop J. 2021, 9, 633–645. [Google Scholar] [CrossRef]
- Isbell, R.F. The Australian Soil Classification, 3rd ed.; CSIRO Publishing: Melbourne, Australia, 2021. [Google Scholar]
- Beckmann, G.C.; Thompson, C.H. Soils and land use in the Kurrawa Area, Darling Downs, Queensland. In CSIRO Division of Soils, Soils and Land Use Series No. 37; CSIRO Publishing: Melbourne, Australia, 1960. [Google Scholar]
- Dang, Y.P.; Balzer, A.; Crawford, M.; Rincon-Florez, V.; Liu, H.; Melland, A.R.; Antille, D.; Kodur, S.; Bell, M.J.; Whish, J.P.M.; et al. Strategic tillage in conservation agricultural systems of north-eastern Australia: Why, where, when and how? Environ. Sci. Poll. Res. 2017, 25, 1000–1015. [Google Scholar] [CrossRef] [PubMed]
- OzForecast Weather Data, Formartin (DDCGI) Weather Station ID11493. Available online: https://ozforecast.com.au/cgi-bin/weatherstation.cgi?station=11493 (accessed on 12 December 2024).
- Whitehead, A.G.; Hemming, J.R. A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann. Appl. Biol. 1965, 55, 25–38. [Google Scholar] [CrossRef]
- Viaene, N.; Hallmann, J.; Molendijk, L. Methods for nematode extraction. In Techniques for Work with Plant and Soil Nematodes; Perry, R.N., Hunt, D.J., Subbotin, S.A., Eds.; CABI: Wallingford, UK, 2021; pp. 12–41. [Google Scholar]
- Fortuner, R. Pratylenchus thornei. In C.I.H. Description of Plant-Parasitic Nematodes, Set 7; Commonwealth Institute of Helminthology: St. Albans, UK, 1997; No. 93. [Google Scholar]
- Peters, B.G. Toxicity tests with vinegar eelworm. Counting and culturing. J. Helminthol. 1952, 26, 97–110. [Google Scholar] [CrossRef]
- Trimble Agriculture Division. Greenseeker Handheld Crop Sensor. 2022. Available online: https://ww2.agriculture.trimble.com/product/greenseeker-handheld-crop-sensor/ (accessed on 4 April 2025).
- VSN International. GenStat for Windows, 23rd ed.; VSN International: Hemel Hempstead, UK, 2021. [Google Scholar]
- Lloveras, J.; Manent, J.; Viudas, J.; López, A.; Santiveri, P. Seeding rate influence on yield and yield components of irrigated winter wheat in a mediterranean climate. Agron. J. 2004, 96, 1258. [Google Scholar] [CrossRef]
- Bastos, L.M.; Carciochi, W.; Lollato, R.P.; Jaenisch, B.R.; Rezende, C.R.; Schwalbert, R.; Vara Prasad, P.V.; Zhang, G.; Fritz, A.K.; Foster, C.; et al. Winter wheat yield response to plant density as a function of yield environment and tillering potential: A review and field studies. Front. Plant Sci. 2020, 5, 54. [Google Scholar] [CrossRef]
- Thompson, J.P.; Clewett, T.G. Impacts of root-lesion nematode (Pratylenchus thornei) on plant nutrition, biomass, grain yield and yield components of susceptible/intolerant wheat genotypes determined by nematicide applications. Agronomy 2021, 11, 296. [Google Scholar] [CrossRef]
- Fischer, R.A.; Moreno Ramos, O.H.; Ortiz Monasterio, I.; Sayre, K.D. Yield response to plant density, row spacing and raised beds in low latitude spring wheat with ample soil resources: An update. Field Crops Res. 2019, 232, 95–105. [Google Scholar] [CrossRef]
- Katsileros, A.; Antonetsis, N.; Gkika, M.G.; Tani, E.; Bebeli, P.J.; Tokatlidis, I. An in-depth presentation of the ‘rhoneycomb’ R package to construct and analyze field experimentation ‘honeycomb selection designs. Agronomy 2023, 13, 2145. [Google Scholar] [CrossRef]
- Ninou, E.; Mylonas, I.; Karagianni, I.; Michailidou, S.; Tsivelikas, A.; Sistanis, I.; Avdikos, I.; Korpetis, E.; Papathanasiou, F. Ultization of intra-cultivar variation for grain yield and protein content within durum wheat cultivars. Agriculture 2022, 12, 661. [Google Scholar] [CrossRef]
- Fasoula, A.C.; Fasoula, V.A. Honeycomb selection designs. In Plant Breeding Reviews; Janick, J., Ed.; Wiley: New York, NY, USA, 1995; Volume 13, pp. 87–139. [Google Scholar]
- Taylor, S.P.; Evans, M.L. Vertical and horizontal distribution of and soil sampling for root lesion nematodes (Pratylenchus neglectus and P. thornei) in South Australia. Australas. Plant Pathol. 1998, 27, 90–96. [Google Scholar] [CrossRef]
- Fanning, J.; Linsell, K.; McKay, A.; Gogel, B.; Munoz Santa, I.; Davey, R.; Hollaway, G. Resistance to the root lesion nematode Pratylenchus thornei and P. neglectus in cereals: Improved assessments in the field. Appl. Soil Ecol. 2018, 132, 146–154. [Google Scholar] [CrossRef]
- Thompson, J.P.; Clewett, T.G.; O’Reilly, M.M. Optimising initial population, growth time and nitrogen nutrition for assessing resistance of wheat cultivars to root-lesion nematode (Pratylenchus thornei). Aust. Plant Pathol. 2015, 44, 133–147. [Google Scholar] [CrossRef]
- Sheedy, J.G.; Thompson, J.P. Resistance to the root-lesion nematode Pratylenchus thornei of Iranian landrace wheat. Aust. Plant Pathol. 2009, 38, 478–489. [Google Scholar] [CrossRef]
- Li, A.; Liu, D.; Yang, W.; Kishii, M.; Mao, L. Synthetic hexaploid wheat: Yesterday, today, and tomorrow. Engineering 2018, 4, 552–558. [Google Scholar] [CrossRef]





| Year | Strip 1 | Strip 2 | Strip 3 | Strip 4 |
|---|---|---|---|---|
| 2012 | Wheat | Sorghum | Experiments | Fallow |
| 2013 | Experiments | Fallow | Sorghum | Wheat |
| 2020 | Wheat | Sorghum | Experiments | Fallow |
| 2021 | Experiments | Fallow | Sorghum | Wheat |
| 2022 | Sorghum | Wheat | Fallow | Experiments |
| 2023 | Fallow | Experiments | Wheat | Sorghum |
| 2024 | Wheat | Sorghum | Experiments | Fallow |
| Year | Experiment Descriptions | Rotation Strip No. | Sowing Date | ||
|---|---|---|---|---|---|
| Experiment Code | Genotypes (n) | Density (Plants/m2) | |||
| 2013 | 13PD | 9 | 20, 40, 60, 80, 100 | 1 | 28 June 2013 |
| 2021 | 21SP04 | 14 | 4 | 1 | 27 July 2021 |
| 21SP16 | 14 | 16 | 1 | 27 July 2021 | |
| 21SP32 | 14 | 32 | 1 | 27 July 2021 | |
| 2022 | 22PD | 9 | 20, 40, 60, 80, 100 | 4 | 15 July 2022 |
| 22SP01 | 15 | 1 | 4 | 27 June 2022 | |
| 22SP04 | 14 | 4 | 4 | 23 June 2022 | |
| 22SP16 | 13 | 16 | 4 | 23 June 2022 | |
| 22SP32 | 14 | 32 | 4 | 23 June 2022 | |
| 2024 | 24SP01 | 15 | 1 | 3 | 19 June 2024 |
| Genotype | Tolerance Rating a | Resistance Rating b | Experiments |
|---|---|---|---|
| Cobalt | T | S | SP |
| Crusader | MI | S | SP |
| Cunningham | MI-I | S | SP |
| EGA Gregory c | T-MT | MS-S | PD, SP |
| EGA Hume | I | S | SP |
| EGA Stampede | VI | S-VS | PD, SP |
| EGA Wylie | T-MT | MS-S | PD |
| Gatcher | VI | S-VS | SP |
| Gauntlet | MT | MR-MS | SP |
| Gladius | I-VI | S-VS | SP |
| GS50a | MT-MI | R-MR | SP |
| Kennedy | MT-MI | S-VS | PD, SP |
| Lang | MI | S | PD |
| Lincoln | VI | S-VS | SP |
| QT8447 | T-MT | MR | PD, SP |
| Strzelecki | I | S-VS | PD, SP |
| Suntop d | T | MR-MS | PD, SP |
| Sunvale | MT | MS-S | PD |
| Year | P. thornei/kg Soil 0–90 cm | Plant Available Water 0–90 cm | Rainfall (mm) d | |||
|---|---|---|---|---|---|---|
| loge(x + 1) a | s.e.m b | BTM c | Mean (mm) a | s.e.m b | ||
| 2013 | 8.96 b | 0.21 | 7777 | 164 ab | 16.38 | 157 |
| 2021 | 7.86 a | 0.12 | 2588 | 121 a | 9.46 | 180 |
| 2022 | 8.83 b | 0.15 | 6835 | 200 b | 11.59 | 249 |
| 2024 | 8.75 b | 0.11 | 6310 | 131 a | 9.09 | 128 |
| ANOVA | F prob | l.s.d e | F prob | l.s.d | c.v. f | |
| Year | >0.001 | 0.43 | >0.001 | 16.75 | 4.9% | |
| Genotype | Seeding Density (Viable Seeds/m2) | Genotype | ||||
|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | Mean | |
| EGA Gregory | 2393 | 2707 | 3241 | 3300 | 3470 | 3022 |
| EGA Stampede | 405 | 584 | 789 | 754 | 792 | 665 |
| EGA Wylie | 2853 | 3651 | 3777 | 3754 | 3903 | 3588 |
| Kennedy | 1362 | 1959 | 2296 | 2410 | 2202 | 2046 |
| Lang | 1398 | 1452 | 1581 | 1763 | 2211 | 1681 |
| QT8447 | 3655 | 3901 | 4197 | 4130 | 4077 | 3992 |
| Strzelecki | 910 | 1361 | 1060 | 1679 | 1177 | 1237 |
| Suntop | 2664 | 3219 | 3321 | 3804 | 3923 | 3386 |
| Sunvale | 2661 | 2968 | 3198 | 3516 | 3463 | 3161 |
| Density mean | 2034 | 2422 | 2607 | 2790 | 2802 | 2531 |
| ANOVA | F prob | l.s.d b | ||||
| Genotypes | <0.001 | 240.1 | ||||
| Density | <0.001 | 179 | ||||
| Geno × dens | ns a | |||||
| Genotype | Seeding Density (Viable Seeds/m2) | Genotype | ||||
|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | Mean | |
| EGA Gregory | 4230 | 5241 | 5144 | 5080 | 5199 | 4979 |
| EGA Stampede | 2231 | 2307 | 2231 | 2968 | 2758 | 2499 |
| EGA Wylie | 3621 | 4162 | 4145 | 4559 | 4613 | 4220 |
| Kennedy | 3207 | 3644 | 4093 | 4775 | 3895 | 3923 |
| Lang | 2773 | 3129 | 3390 | 3462 | 3791 | 3309 |
| QT8447 | 4808 | 5830 | 6651 | 6097 | 6299 | 5937 |
| Strzelecki | 2827 | 3525 | 3747 | 4156 | 4176 | 3686 |
| Suntop | 4310 | 5069 | 5888 | 6116 | 6108 | 5498 |
| Sunvale | 3944 | 4677 | 4214 | 4200 | 4529 | 4312 |
| Density mean | 3550 | 4176 | 4389 | 4601 | 4597 | 2531 |
| ANOVA | F prob | l.s.d b | ||||
| Genotypes | <0.001 | 437.9 | ||||
| Density | <0.001 | 326.4 | ||||
| Geno × dens | ns a | |||||
| Experiment | 22SP01 | 22SP04 | 22SP16 | 22SP32 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Genotypes (n) | 15 | 14 | 13 | 14 | |||||
| Assessment | DAS | r | p | r | p | r | p | r | p |
| NDVI_1 | 80 | 0.73 | 0.002 | 0.69 | 0.006 | 0.54 | 0.059 | 0.50 a | 0.066 |
| NDVI_2 | 86 | 0.66 | 0.007 | 0.77 a | 0.001 | 0.46 | 0.117 | 0.42 a | 0.139 |
| NDVI_3 | 99 | 0.80 | <0.001 | 0.85 | <0.001 | 0.45 | 0.123 | 0.34 a | 0.233 |
| NDVI_4 | 113 | 0.73 | 0.002 | 0.89 | <0.001 | 0.37 a | 0.212 | 0.77 | 0.001 |
| NDVI_5 | 130 | 0.77 | <0.001 | 0.75 | 0.002 | 0.71 a | 0.007 | 0.24 a | 0.408 |
| VTR_1 | 121 | 0.87 | <0.001 | 0.86 | <0.001 | 0.83 | <0.001 | 0.90 | <0.001 |
| VTR_2 | 130 | 0.92 | <0.001 | 0.95 | <0.001 | 0.85 | <0.001 | 0.81 | <0.001 |
| Yield b | 0.83 | <0.001 | 0.90 | <0.001 | 0.85 | <0.001 | 0.80 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, N.A.; Sheedy, J.G.; Zwart, R.S.; Owen, K.J.; Lin, J.; Thompson, J.P. A New Method for Single-Plant Selection of Wheat Genotypes for Tolerance and Resistance to the Root-Lesion Nematode Pratylenchus thornei by Low-Density Sowing. Agronomy 2025, 15, 2049. https://doi.org/10.3390/agronomy15092049
Robinson NA, Sheedy JG, Zwart RS, Owen KJ, Lin J, Thompson JP. A New Method for Single-Plant Selection of Wheat Genotypes for Tolerance and Resistance to the Root-Lesion Nematode Pratylenchus thornei by Low-Density Sowing. Agronomy. 2025; 15(9):2049. https://doi.org/10.3390/agronomy15092049
Chicago/Turabian StyleRobinson, Neil A., Jason G. Sheedy, Rebecca S. Zwart, Kirsty J. Owen, Jing Lin, and John P. Thompson. 2025. "A New Method for Single-Plant Selection of Wheat Genotypes for Tolerance and Resistance to the Root-Lesion Nematode Pratylenchus thornei by Low-Density Sowing" Agronomy 15, no. 9: 2049. https://doi.org/10.3390/agronomy15092049
APA StyleRobinson, N. A., Sheedy, J. G., Zwart, R. S., Owen, K. J., Lin, J., & Thompson, J. P. (2025). A New Method for Single-Plant Selection of Wheat Genotypes for Tolerance and Resistance to the Root-Lesion Nematode Pratylenchus thornei by Low-Density Sowing. Agronomy, 15(9), 2049. https://doi.org/10.3390/agronomy15092049





