Abstract
Understanding how clonal plants modulate their reproductive strategies under environmental fluctuations is critical for assessing their resilience amid rapid global change. Phragmites australis, a dominant clonal plant species in coastal wetlands worldwide, provides vital ecological and agricultural services. As coastal wetlands are currently impacted by sea level rise, P. australis faces both salinity and tidal level changes. However, the effects of the combined influences of these two abiotic factors on the reproductive strategy of P. australis remain unclear. We conducted a mesocosm experiment to examine how P. australis allocates resources between clonal and sexual reproduction under different salinity and tidal level conditions. We found increased salinity negatively impacted both sexual and clonal reproductive metrics and shifted reproductive allocation toward clonal reproduction; increasing tidal level had positive effects on the sexual reproductive metrics, but negatively affected the clonal reproductive metrics, leading to a shift toward greater allocation in sexual reproduction. Higher tidal levels could reduce the negative impact of salinity on the plant’s most reproductive metrics. These results highlighted the flexibility of P. australis in adapting its reproductive strategies to environmental changes, suggesting that it could be a promising component for sustainable wetland agriculture, offering significant economic value amid rapid global change.
1. Introduction
Understanding how clonal plants modulate their reproductive strategies under environmental fluctuations is fundamental for assessing their resilience in an era of rapid global change. Phragmites australis, a clonal plant species, is a dominant component of coastal regions worldwide [1], making it crucial to both ecological and agricultural systems [2]. It plays a vital role in sustaining ecosystem functions, including organic material production [3], nutrient cycling [4], decomposition [5], and carbon sequestration [6]. Its extensive root network stabilizes soil and water, serving as a natural barrier in coastal and saline-alkaline land restoration, significantly reducing soil erosion risks in coastal areas [7]. As a high-yield cellulose plant, P. australis provides valuable raw materials for traditional industries like weaving and papermaking [8], while also gaining attention in modern biomass energy development as a clean alternative to fossil fuels [9]. Additionally, it aids in water purification by absorbing nitrogen and phosphorus pollutants [10] and offers high-quality forage for livestock [11]. The synergy between its ecological services and economic benefits makes P. australis important for wetland agriculture [2,12].
Clonal plants are capable of both clonal and sexual reproduction [13]. Clonal reproduction, through vegetative propagation via tillering, stolons, tubers, or rhizome, enables plants to rapidly colonize habitats while preserving the beneficial genotypes of the parent plant [14]. The primary advantage of this strategy lies in its efficient resource utilization: no energy is expended on flowering, pollination, or seed formation, allowing energy allocation to be focused on vegetative growth [15]. Additionally, clonal reproduction bypasses the compatibility barriers inherent in sexual reproduction, ensuring successful reproduction even in environments with limited pollinators or isolated habitats [16]. However, a major drawback of clonal reproduction is genetic homogeneity. Clonal populations, lacking genetic variation, are more susceptible to damage from pests, diseases, or drastic environmental changes [17]. Prolonged clonal reproduction may also lead to the accumulation of harmful mutations that cannot be eliminated through recombination, ultimately reducing the evolutionary potential of the population [18,19].
Sexual reproduction, through genetic recombination, produces genetically diverse offspring and is a core strategy for plants to cope with environmental heterogeneity [20]. Its greatest advantage lies in enhancing the evolutionary plasticity of the population: the randomness of offspring gene combinations can generate new phenotypes that are better adapted to fluctuations in temperature, pests, or soil conditions [21]. Furthermore, seeds produced through sexual reproduction possess dormancy, allowing them to survive in soil seed banks for years, thus ensuring the population’s recovery after disturbances [22]. However, this strategy comes at a high cost, as plants must allocate significant energy to flower development, pollinator attraction, and seed protection [23]. The success of fertilization is also dependent on pollinator efficiency and pollen availability [24]. Additionally, seed dispersal carries high risks, as most seeds may fail to germinate due to predation, pathogens, or unsuitable microhabitats, leading to a significant increase in the uncertainty between reproductive investment and return [25].
Clonal plants may adjust their reproductive strategies in response to environmental changes, including variations in nutrients [26], light [27], temperature [28], water availability [29], etc. To ensure population persistence and growth under fluctuating conditions, these plants may modify their reliance on sexual reproduction, reallocate resources towards clonal reproduction, and balance the two modes of reproduction [30]. Such strategic adjustments are vital not only for the survival and reproductive success of individual plants, but also for maintaining population structure and ecosystem function [13]. Therefore, investigating how clonal plants mediate their reproductive strategies in the face of environmental changes is crucial for understanding how they sustain population survival and thrive in a rapidly changing world.
Coastal wetlands are increasingly threatened by the impacts of rising sea levels due to global climate change [31], resulting in significant alterations to soil conditions [32]. From 1901 to 2018, the global average sea level increased by 0.2 ± 0.05 m [33], with projections indicating that this rate will accelerate in the coming century [34]. As sea levels continue to rise, tidal levels will also increase, leading to more frequent flooding in coastal regions [35]. Moreover, the aggregate effects of rising sea level, storm surge, reduced river flow, and extreme tidal events are expected to intensify saltwater intrusion [36], especially in low-salinity wetlands within the fresher areas of estuaries [37,38]. Given the varying environmental conditions in coastal regions under global change [39], the clonal plant P. australis might adjust the balance between clonal and sexual reproduction. However, the effects of the combined influences of increasing salinity and tidal level on the reproductive strategies of P. australis remain poorly understood.
In this study, we investigated how P. australis adjusts its reproductive strategies in response to environmental changes, with a particular focus on the combined effects of rising salinity and tidal level in coastal regions. By examining how P. australis reallocates resources between clonal and sexual reproduction under varying salinity and tidal conditions, we aim to better understand its reproductive adaptability in the face of global change. This insight is critical for understanding the long-term sustainability of P. australis populations and their ecological and agricultural roles in coastal regions. Furthermore, this study could inform management strategies for coastal ecosystem restoration and conservation, supporting efforts to preserve the ecological and agricultural benefits provided by P. australis amid ongoing global change.
2. Materials and Methods
2.1. Plant Material and Experimental Design
In early March 2023, we collected healthy spring buds of P. australis along with their attached rhizomes from a freshwater wetland (38°39′ N, 117°34′ E) in the coastal area of Tianjin, China. At higher elevations of the wetland, the mean water level is approximately 5 cm below the soil surface, while at lower elevations, the water level fluctuates between 5 cm below and 5 cm above the soil surface. With sea level rise and saltwater intrusion, high tidal levels at lower elevations are expected to reach about 10 cm above the soil surface, while the salinity of the wetland is projected to increase to approximately 10 PSU by the middle of the 21st century. The rhizomes measured approximately 3 cm in length and 0.5 cm in diameter. Before the experiment, the spring buds collected from various locations were randomized to ensure an unbiased selection.
We conducted the mesocosm experiment in the glasshouse at Tianjin Normal University, which was open to the surrounding atmosphere, allowing sunlight and temperature to closely resemble outdoor conditions (25 ± 5 °C throughout the study). The glass roof of the glasshouse prevented natural rainfall from entering. P. australis ramets were cultivated in PVC pots (20 cm in diameter, 50 cm in height, with sealed bottoms).
Soil was collected from the same wetland location where the P. australis spring buds were obtained, and subsequently, it was thoroughly blended. The resulting mixture (non-saline, with a pH of ~7.0 and organic matter content of ~4.6%) was then added to each pot to a depth of 35 cm.
In the middle of March 2023, we planted P. australis spring buds with their rhizomes approximately 2 cm below the soil surface in individual pots, placing one bud per pot. A total of 50 replicate pots were initially prepared and irrigated with freshwater to promote the growth of the rhizome buds. By the end of April 2023, we selected 30 pots containing healthy P. australis individuals of similar size to reduce variation in initial growth before the experiment.
The 30 pots were randomly assigned across all possible combinations of salinity (freshwater with 0 PSU soil porewater salinity (0 M NaCl L−1) and brackish with 10 PSU (~0.17 M NaCl L−1)) and tidal level treatments (drained, tidal-5 cm, tidal-10 cm), with five replicates for each treatment combination (Figure 1). The pots were spaced approximately 0.5 m apart to minimize shading from neighboring plants. The salinity and tidal level conditions were selected to replicate the early stages of sea level rise in low-salinity tidal wetlands.
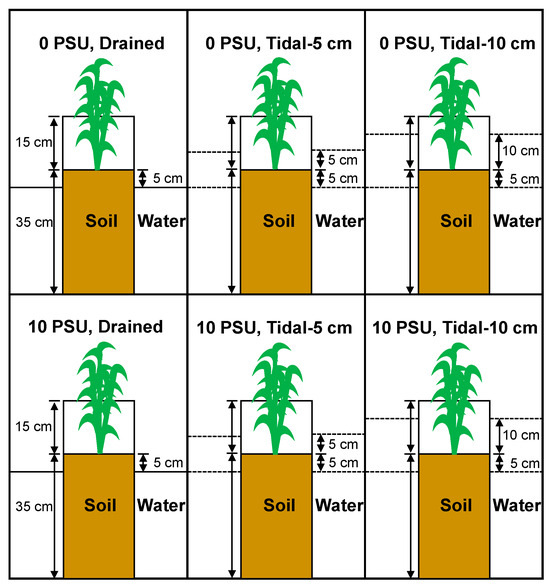
Figure 1.
Diagram of the salinity and tidal level treatments. There were two salinity treatments (0 PSU and 10 PSU) and three tidal level treatments (drained, tidal-5 cm, and tidal-10 cm). Dashed lines represent the high-water and low-water levels.
Starting on 1 May 2023, we implemented the abiotic treatments for the pots. To allow water movement through the pots, four sets of holes (six per set, with a diameter of 0.8 cm) were drilled between the rim and the base of each pot. These holes were covered with mesh to prevent soil loss. In the drained treatment, the water level was kept 5 cm below the soil surface, simulating conditions at higher elevations in the local tidal wetland. For the tidal-5 cm treatment, the tidal cycle consisted of two high-water periods at 5 cm above the soil surface and two low-water periods at 5 cm below the soil surface, resulting in a tidal fluctuation of 10 cm within each 24 h period. This cycle reflected the tidal regime at lower elevations in the local wetland. The tidal-10 cm treatment followed a similar cycle, with two high-water periods at 10 cm above the soil surface and two low-water periods at 5 cm below the soil surface, resulting in a tidal fluctuation of 15 cm within each 24 h period. This cycle represented a projected tidal regime for lower elevations in the local wetland in the future.
The experimental pots, each assigned to a specific combination of salinity and tidal level treatments, were placed into six separate treatment tanks. Each tank was connected to an adjacent reservoir, equipped with a tidal pump to control tidal levels. Tidal cycles were managed using automated timers, with each tank having its own timer. The pumps activated at 8:00 and 20:00 local time, running for six hours each time. During this period, valves in the pipes regulated the inflow and outflow of water to ensure that the target high-water level was achieved after six hours of pumping. Once the pumps stopped, the water in the tanks drained by gravity to the low-water level over the following six hours, and then the next tidal cycle was initiated. For 10 PSU treatments, commercial marine aquarium salt was gradually added to the tanks over 10 days, increasing the salinity in equal increments until the desired soil porewater salinity was reached. Daily monitoring ensured the soil porewater salinity and tidal levels were maintained, while the pots within each treatment tank were rotated regularly to minimize potential bias from light, temperature, and other environmental factors.
At the end of the growing season (October 2023), we carefully harvested both the aboveground and belowground parts of the P. australis plants and assessed several reproductive metrics of P. australis under different experimental treatments, including the number of flowering shoots, proportion of flowering shoots, inflorescence biomass, number of rhizome buds, rhizome biomass, and then calculated the ratio of sexual to clonal biomass allocation. All biomass samples were dried at 60 °C for three days until they reached a constant weight, then weighed.
2.2. Data Analysis
Data analyses were conducted using SPSS 21 (IBM Corp., Chicago, USA). To evaluate the effects of salinity, tidal level, and their interactions on the reproductive metrics of P. australis, we performed ANOVAs followed by post hoc Tukey HSD tests for multiple comparisons, with a significance level of 0.05.
We also assessed the effect size of salinity on the reproductive metrics of P. australis under varying tidal levels. The effect size was calculated using the formula Ln [(mean value of a reproductive metric under 10 PSU conditions)/(mean value of the reproductive metric under 0 PSU conditions)]. A positive effect size indicates a stimulatory effect of salinity on the reproductive metric, whereas a negative effect size suggests an inhibitory effect.
3. Results
3.1. Effects of Salinity and Tidal Level on Number of Flowering Shoots
Figure 2a showed a significant decrease in the number of flowering shoots of P. australis as salinity increased across all tidal levels (Table 1). Regardless of salinity, the number of flowering shoots significantly increased with rising tidal levels (Figure 2a, Table 1). As shown in Figure 2b, the negative effect of salinity on the number of flowering shoots gradually decreased with increasing tidal level, leading to a significant interaction between salinity and tidal level on the number of flowering shoots of P. australis (Table 1).
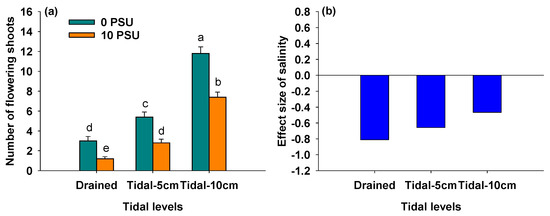
Figure 2.
Number of flowering shoots of P. australis under different salinity and tidal level treatments (a) and the effect size of salinity on number of flowering shoots of P. australis under different tidal level treatments (b). In Panel (a), data are means + SE (n = 5). Shared letters indicate means that are not significantly different from each other (Tukey’s HSD tests, significance level of 0.05).

Table 1.
Summary of ANOVAs examining the effects of salinity, tidal level, and the interaction between them on number of flowering shoots, proportion of flowering shoots, inflorescence biomass, number of rhizome buds, rhizome biomass, ratio of sexual to clonal biomass allocation of P. australis.
3.2. Effects of Salinity and Tidal Level on Proportion of Flowering Shoots
Figure 3a showed that as salinity increased, the proportion of flowering shoots significantly decreased at all tidal levels (Table 1). Regardless of salinity, this proportion increased with rising tidal levels (Figure 3a, Table 1). The negative effect of salinity on the proportion tended to decrease with increasing tidal level (Figure 3b).
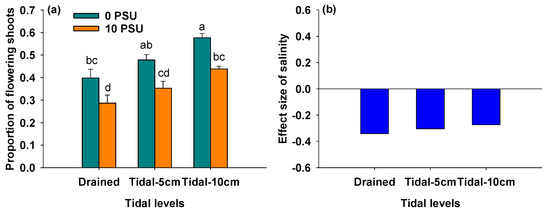
Figure 3.
Proportion of flowering shoots of P. australis under different salinity and tidal level treatments (a) and the effect size of salinity on proportion of flowering shoots of P. australis under different tidal level treatments (b). In Panel (a), data are means + SE (n = 5). Shared letters indicate means that are not significantly different from each other (Tukey’s HSD tests, significance level of 0.05).
3.3. Effects of Salinity and Tidal Level on Inflorescence Biomass
Figure 4a showed that, across all tidal levels, the inflorescence biomass of P. australis was significantly lower under 10 PSU conditions compared to 0 PSU conditions (Table 1). Regardless of salinity, inflorescence biomass increased with rising tidal levels (Figure 4a, Table 1). As shown in Figure 4b, the negative effect of salinity on inflorescence biomass gradually decreased with increasing tidal level, resulting in a significant interaction between salinity and tidal level on the inflorescence biomass of P. australis (Table 1).
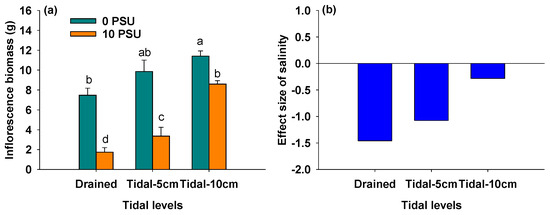
Figure 4.
Inflorescence biomass of P. australis under different salinity and tidal level treatments (a) and the effect size of salinity on inflorescence biomass of P. australis under different tidal level treatments (b). In Panel (a), data are means + SE (n = 5). Shared letters indicate means that are not significantly different from each other (Tukey’s HSD tests, significance level of 0.05).
3.4. Effects of Salinity and Tidal Level on Number of Rhizome Buds
Figure 5a showed that the number of rhizome buds of P. australis significantly decreased with increasing salinity at all tidal levels (Table 1). Regardless of salinity, the number of rhizome buds also significantly decreased with rising tidal levels (Figure 5a, Table 1). As shown in Figure 5b, the negative effect of salinity on the number of rhizome buds gradually decreased as tidal level increased, resulting in a significant interaction between salinity and tidal level on the number of rhizome buds of P. australis (Table 1).
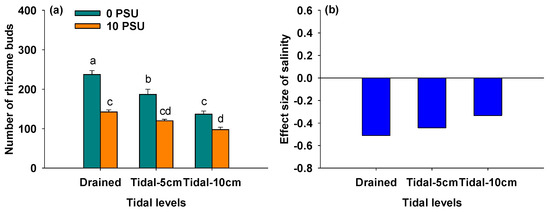
Figure 5.
Number of rhizome buds of P. australis under different salinity and tidal level treatments (a) and the effect size of salinity on number of rhizome buds of P. australis under different tidal level treatments (b). In Panel (a), data are means + SE (n = 5). Shared letters indicate means that are not significantly different from each other (Tukey’s HSD tests, significance level of 0.05).
3.5. Effects of Salinity and Tidal Level on Rhizome Biomass
Figure 6a showed that the rhizome biomass of P. australis significantly decreased with increasing salinity across all tidal levels (Table 1). Regardless of salinity, rhizome biomass also significantly decreased as tidal level increased (Figure 6a, Table 1). As shown in Figure 6b, the negative effect of salinity on rhizome biomass gradually decreased with rising tidal level, resulting in a significant interaction between salinity and tidal level on the rhizome biomass of P. australis (Table 1).
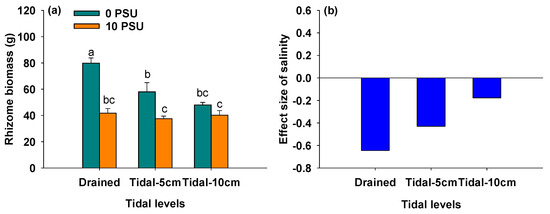
Figure 6.
Rhizome biomass of P. australis under different salinity and tidal level treatments (a) and the effect size of salinity on rhizome biomass of P. australis under different tidal level treatments (b). In Panel (a), data are means + SE (n = 5). Shared letters indicate means that are not significantly different from each other (Tukey’s HSD tests, significance level of 0.05).
3.6. Effects of Salinity and Tidal Level on Ratio of Sexual to Clonal Biomass Allocation
Figure 7a illustrated that the ratio of sexual to clonal biomass allocation in P. australis significantly decreased with increasing salinity across all tidal levels (Table 1). Regardless of salinity, this ratio significantly increased with rising tidal level (Figure 7a, Table 1). As shown in Figure 7b, the negative effect of salinity on the ratio gradually decreased with increasing tidal level, leading to a significant interaction between salinity and tidal level on the ratio of sexual to clonal biomass allocation in P. australis (Table 1).
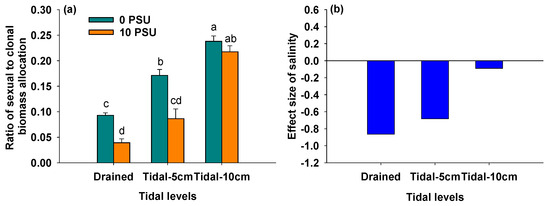
Figure 7.
Ratio of sexual to clonal biomass allocation of P. australis under different salinity and tidal level treatments (a) and the effect size of salinity on ratio of sexual to clonal biomass allocation of P. australis under different tidal level treatments (b). In Panel (a), data are means + SE (n = 5). Shared letters indicate means that are not significantly different from each other (Tukey’s HSD tests, significance level of 0.05).
4. Discussion
4.1. Reproductive Allocation in P. australis Under Varying Salinity Conditions
Our results showed that increased salinity negatively impacted both sexual and clonal reproduction in P. australis, specifically in terms of the number of flowering shoots, proportion of flowering shoots, inflorescence biomass, as well as the bud number and biomass of rhizomes (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6, Table 1). These results indicated that elevated salinity reduced the reproductive capacity of P. australis by exerting stress on its overall growth.
First, increased salinity could cause an imbalance in the plant’s water and nutrient absorption, affecting the root system’s ability to take up water and transport nutrients [40]. For P. australis, the root system is crucial for its growth and reproduction, and high salinity could exacerbate osmotic pressure on the roots, reducing their ability to absorb water [41]. Root damage would not only hamper overall plant growth, but also limit the supply of nutrients and water [42], subsequently affecting the development of inflorescences and the differentiation of rhizomes [43]. This limitation in water and nutrient availability would adversely affect resource allocation for both sexual and clonal reproduction of P. australis.
Moreover, salinity has complex physiological effects on plants. Higher salinity may increase the ion concentration in soil solutions, causing water loss from plant cells and triggering salt stress responses [44]. While P. australis has moderate salt tolerance, it would still experience suppressed growth under elevated salinity, as the accumulation of salts could hinder its normal development [45]. Salt stress could damage leaves, roots, and other essential tissues of P. australis, reducing photosynthetic efficiency and resulting in lower biomass accumulation and impaired reproductive organ formation [46].
Additionally, salinity changes may affect the plant’s endogenous hormone levels, particularly those related to growth and reproduction [47]. Salt stress often induces changes in plant hormone levels, which in turn affects branching and rhizome development [48]. Under higher salinity, P. australis may redirect energy toward coping with salt stress rather than developing reproductive organs [49], leading to the decline in both sexual and clonal reproduction metrics.
Our results demonstrated that elevated salinity significantly reduced the ratio of sexual to clonal biomass allocation in P. australis (Figure 7, Table 1), suggesting a strategic shift in reproductive investment toward clonal reproduction under higher salinity stress. This shift reflected an adaptive response to the challenging conditions imposed by increased salinity. Sexual reproduction, while essential for genetic diversity and long-term population adaptability, is generally more resource intensive and vulnerable to environmental stresses [50]. High salinity conditions could negatively impact flowering, seed development, and seedling establishment of plants due to osmotic stress and ion toxicity [51]. Thus, the observed reduction in the proportion of biomass allocation to sexual reproduction in P. australis might result from physiological constraints limiting the plant’s ability to invest in costly sexual structures and processes. In contrast, clonal reproduction can provide a more resilient and efficient strategy for population persistence, when environmental conditions are unfavorable for sexual propagation [30]. Thus, clonal growth could enable P. australis to bypass the vulnerable seedling stage and exploit existing resources in a relatively stable manner. The observed shift in biomass allocation toward clonal growth in P. australis under elevated salinity might enhance its ability to persist and spread locally, despite the challenging environmental conditions.
4.2. Reproductive Allocation in P. australis Across Different Tidal Levels
Our findings showed that an increasing tidal level had positive effects on the sexual reproduction metrics in P. australis. Specifically, the number of flowering shoots, proportion of flowering shoots, and inflorescence biomass all increased with rising tidal levels (Figure 2, Figure 3 and Figure 4, Table 1). This suggested that elevated tidal levels promoted resource allocation toward sexual reproduction in P. australis. The increase in tidal level might enhance the overall growth of P. australis, particularly by improving water availability [52]. This improvement in water conditions would promote the plant’s photosynthesis and support its vegetative growth [53], allowing it to allocate more biomass to the above-ground parts, where sexual reproductive organs, such as inflorescences, are located. As a result, the inflorescences of P. australis would gain greater access to resources. This, in turn, would boost sexual reproduction and enhance the plant’s evolutionary plasticity.
Our results illustrated that the increase in tidal level negatively affected the clonal reproduction metrics of P. australis, specifically the number of rhizome buds and rhizome biomass (Figure 5 and Figure 6, Table 1). This could be attributed to the altered biomass allocation of P. australis under elevated tidal conditions, where the plant would allocate more biomass to aboveground parts, such as stems and leaves, in response to rising tidal levels [54]. Consequently, less biomass would be available for allocation to the underground rhizomes [55], which are essential for clonal reproduction. Therefore, the clonal reproductive capacity of P. australis was constrained under rising tidal levels.
Our study revealed that the ratio of sexual to clonal biomass allocation in P. australis increased with rising tidal level, indicating a shift towards greater proportion of investment in sexual reproduction (Figure 7, Table 1). This shift was closely tied to the plant’s overall growth strategy, as P. australis also allocated more resources to aboveground structures, such as stems and leaves, under elevated tidal level conditions [54]. This finding indicated that P. australis may become more competitive in terms of aboveground biomass and seed dispersal when invading other coastal wetlands globally under current sea level rise. Our results suggested that reproductive strategies of plants would be influenced by their growth strategies, as the latter provide the material foundation for reproduction [56]. A similar pattern was also observed in another clonal plant species Leersia oryzoides under a flooding condition [57]. Therefore, to better understand how plants adjust their reproductive strategies in response to environmental changes, it is crucial to consider their growth strategies as well.
4.3. Interaction of Salinity and Tidal Level on Reproductive Allocation in P. australis
Our results demonstrated that as tidal levels increased, the effects of salinity on most reproductive metrics of P. australis gradually weakened. Specifically, the number of flowering shoots, inflorescence biomass, number of rhizome buds, rhizome biomass, and the ratio of sexual to clonal biomass allocation all decreased with higher tidal levels (Figure 2, Figure 4, Figure 5, Figure 6 and Figure 7, Table 1). This pattern suggested that, as tidal level rises, tidal conditions would become a more significant factor than salinity in influencing the reproductive strategies of P. australis, thus underscoring the long-term impact of sea level rise on the plant’s reproductive strategies [57]. The results also indicated that in coastal areas affected by drought due to climate change or occasional saltwater intrusion from sea level rise [58], soil salinization might have an even stronger negative impact on both sexual and clonal reproduction of P. australis. This reflected a strategic adjustment in resource allocation, with P. australis prioritizing survival and adaptation to stressful environmental conditions over reproduction [59,60]. Although our results showed that increased tidal levels (tidal-5 cm and tidal-10 cm) alleviated salt stress on reproduction of P. australis to some extent, deeper floods might potentially lead to dieback of P. australis [61]. Our results highlighted the need for a comprehensive understanding of the reproductive strategies of plans in response to environmental changes, considering not only individual factors, but also their interactions in shaping plant performance and reproductive output [62].
5. Conclusions
Our results demonstrated that both salinity and tidal level would significantly influence the reproductive strategies of P. australis, each having distinct effects on sexual and clonal reproduction. Increased salinity reduced both forms of reproduction, causing P. australis to allocate more resources to clonal reproduction as an adaptive survival strategy. In contrast, elevated tidal level promoted sexual reproduction by biomass allocation to aboveground structures (including inflorescences), thereby enhancing the evolutionary plasticity of P. australis. Our study also found significant interactions between salinity and tidal level regarding most reproductive metrics of P. australis. This indicated that higher tidal levels could reduce the negative impact of salinity on the plant’s reproductive metrics. Given the ongoing rise in sea levels, our findings highlighted the increasing significance of tidal level as a crucial factor influencing the reproduction of P. australis. Overall, these results highlighted the flexibility of P. australis in adapting its reproductive strategies to environmental changes, suggesting that it could be a promising component for sustainable wetland agriculture, offering significant economic value amid rapid global change.
Author Contributions
Conceptualization, Y.W. and H.G.; funding acquisition, Y.W. and H.G.; methodology, Y.W. and H.G.; investigation, C.G., X.L., X.T., Y.L., C.Y., and N.L.; formal analysis, Y.W., C.G., X.L., X.T., Y.L., C.Y., N.L., and H.G.; writing—original draft preparation, Y.W., C.G., X.L., X.T., Y.L., C.Y., N.L., and H.G.; writing—review and editing, Y.W., C.G., X.L., X.T., Y.L., C.Y., N.L., and H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by National Natural Science Foundation of China (31300357, 32001179) and Natural Science Foundation of Tianjin City (20JCZDJC00220).
Data Availability Statement
All data supporting the findings of this study were included within the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Milke, J.; Galczynska, M.; Wróbel, J. The importance of biological and ecological properties of Phragmites australis (cav.) Trin. ex Steud., in phytoremendiation of aquatic ecosystems-the review. Water 2020, 12, 1770. [Google Scholar] [CrossRef]
- Haldan, K.; Kuprina, K.; Haase, M.I.; Kieckhäfer, F.; Schade, L.; Schmoldt, J.; Schock, L.S.; Stein, M.; Wille, A.; Schnittler, M.; et al. Choose wisely: Great variation among genotypes of promising paludiculture crop Phragmites australis. Plants 2023, 12, 1045. [Google Scholar] [CrossRef]
- Hansson, P.A.; Fredriksson, H. Use of summer harvested common reed (Phragmites australis) as nutrient source for organic crop production in Sweden. Agric. Ecosyst. Environ. 2004, 102, 365–375. [Google Scholar] [CrossRef]
- Yu, J.H.; Zhong, J.C.; Zhang, Y.S.; Ding, H.; Chen, C.; Zheng, X.Z.; Xu, M.X.; Zhang, Y.L. Fine-scale remobilization of phosphorus by rooted macrophytes (Phragmites australis) growth in lake sediments: Evidence from a holistic growth period simulation study. J. Soils Sediments 2020, 20, 1782–1792. [Google Scholar] [CrossRef]
- Cui, L.J.; Pan, X.; Li, W.; Zhang, X.D.; Liu, G.F.; Song, Y.B.; Yu, F.H.; Prinzing, A.; Cornelissen, J.H.C. Phragmites australis meets Suaeda salsa on the “red beach”: Effects of an ecosystem engineer on salt-marsh litter decomposition. Sci. Total Environ. 2019, 693, 133477. [Google Scholar] [CrossRef]
- Yu, X.Y.; Ye, S.Y.; Olsson, L.; Wei, M.J.; Brix, H. In-situ CO2 partitioning measurements in a Phragmites australis wetland: Understanding carbon loss through ecosystem respiration. Wetlands 2020, 40, 901–914. [Google Scholar] [CrossRef]
- Li, X.Y.; Jin, K.; Qin, P.; Liu, C.X.; Zhu, X.Z.; Zhang, Y.Y.; Zong, Q.L. Enhancement effect of Phragmites australis roots on soil shear strength in the Yellow River Delta. Sustainability 2024, 16, 10657. [Google Scholar] [CrossRef]
- Köbbing, J.F.; Beckmann, V.; Thevs, N.; Peng, H.; Zerbe, S. Investigation of a traditional reed economy (Phragmites australis) under threat: Pulp and paper market, values and netchain at Wuliangsuhai Lake, Inner Mongolia, China. Wetl. Ecol. Manag. 2016, 24, 357–371. [Google Scholar] [CrossRef]
- Vazic, T.; Svircek, Z.; Dulic, T.; Krstic, K.; Obreht, I. Potential for energy production from reed biomass in the Vojvodina region (north Serbia). Renew. Sustain. Energy Rev. 2015, 48, 670–680. [Google Scholar] [CrossRef]
- Rezania, S.; Park, J.; Rupani, P.F.; Darajeh, N.; Xu, X.; Shahrokhishahraki, R. Phytoremediation potential and control of Phragmites australis as a green phytomass: An overview. Environ. Sci. Pollut. Res. 2019, 26, 7428–7441. [Google Scholar] [CrossRef]
- Beyzi, S.B.; Ülger, İ.; Konca, Y. Chemical, fermentative, nutritive and anti-nutritive composition of common reed (Phragmites australis) plant and silage. Waste Biomass Valorization 2023, 14, 927–936. [Google Scholar] [CrossRef]
- He, H.Q.; Li, X.Y.; Li, T.L. The sustainable development of wetlands and agriculture: A literature review. Agronomy 2025, 15, 746. [Google Scholar] [CrossRef]
- Vallejo-Marín, M.; Dorken, M.E.; Barrett, S.C.H. The ecological and evolutionary consequences of clonality for plant mating. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 193–213. [Google Scholar] [CrossRef]
- Yang, P.; Huang, L.; He, S.N.; Zeng, X.H.; Chen, Y.Y.; Wang, H.M. Adaptive strategies employed by clonal plants in heterogeneous patches. Forests 2023, 14, 1648. [Google Scholar] [CrossRef]
- Warren, R.J., II; Mokadam, C. Asexuality and species invasion. Biodivers. Conserv. 2025, 34, 29–43. [Google Scholar] [CrossRef]
- Honnay, O.; Jacquemyn, H. A meta-analysis of the relation between mating system, growth form and genotypic diversity in clonal plant species. Evol. Ecol. 2008, 22, 299–312. [Google Scholar] [CrossRef]
- Lin, C.H.; Miriti, M.N.; Goodell, K. Demographic consequences of greater clonal than sexual reproduction in Dicentra canadensis. Ecol. Evol. 2016, 6, 3871–3883. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Q.H.; Zeng, Z.H.; Zhang, Z.Q.; Cai, J.; Chen, G.; Li, D.Z.; Wang, H.; Zhou, W. Effects of mode of reproduction on genetic polymorphism and divergence in wild yams (Dioscoreaceae: Dioscorea). Plant Divers. 2025, 47, 136–147. [Google Scholar] [CrossRef]
- Barrett, S.C.H. Influences of clonality on plant sexual reproduction. Proc. Natl. Acad. Sci. USA 2015, 112, 8859–8866. [Google Scholar] [CrossRef]
- Becker, A.; Chen, X.; Dresselhaus, T.; Gutsche, N.; Müller-Schüssele, S.J.; Sprunck, S.; Theissen, G.; de Vries, S.; Zachgo, S. Sexual reproduction in land plants: An evolutionary perspective. Plant Reprod. 2025, 38, 12. [Google Scholar] [CrossRef]
- Boavida, L.C.; Vieira, A.M.; Becker, J.D.; Feijó, J.A. Gametophyte interaction and sexual reproduction: How plants make a zygote. Int. J. Dev. Biol. 2005, 49, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Hubert, B.; Leprince, O.; Buitink, J. Sleeping but not defenceless: Seed dormancy and protection. J. Exp. Bot. 2024, 75, 6110–6124. [Google Scholar] [CrossRef] [PubMed]
- Meirmans, S.; Meirmans, P.G.; Kirkendall, L.R. The costs of sex: Facing real-world complexities. Q. Rev. Biol. 2012, 87, 19–40. [Google Scholar] [CrossRef]
- Stenc, J.; Janosík, L.; Matousková, E.; Hadrava, J.; Mikát, M.; Janovsky, Z. Pollinator visitation closely tracks diurnal patterns in pollen release. Am. J. Bot. 2023, 110, e16179. [Google Scholar] [CrossRef]
- Begum, K.; Hasan, N.; Shammi, M. Selective biotic stressors’ action on seed germination: A review. Plant Sci. 2024, 346, 112156. [Google Scholar] [CrossRef]
- Liu, L.; Zuo, S.N.; Ma, M.Y.; Li, J.H.; Guo, L.Z.; Huang, D. Appropriate nitrogen addition regulates reproductive strategies of Leymus chinensis. Glob. Ecol. Conserv. 2021, 27, e01599. [Google Scholar] [CrossRef]
- Kottler, E.J.; Gedan, K.B. Sexual reproduction is light-limited as marsh grasses colonize maritime forest. Am. J. Bot. 2022, 109, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; He, L.; Zhang, H.; Urrutia-Cordero, P.; Ekvall, M.K.; Hollander, J.; Hansson, L.A. Climate warming and heat waves affect reproductive strategies and interactions between submerged macrophytes. Glob. Change Biol. 2017, 23, 108–116. [Google Scholar] [CrossRef]
- Wang, Z.W.; Xie, L.N.; Prather, C.M.; Guo, H.Y.; Han, G.D.; Ma, C.C. What drives the shift between sexual and clonal reproduction of Caragana stenophylla along a climatic aridity gradient? BMC Plant Biol. 2018, 18, 91. [Google Scholar] [CrossRef]
- Silvertown, J. The evolutionary maintenance of sexual reproduction: Evidence from the ecological distribution of asexual reproduction in clonal plants. Int. J. Plant Sci. 2008, 169, 157–168. [Google Scholar] [CrossRef]
- Nevermann, H.; AghaKouchak, A.; Shokri, N. Sea level rise implications on future inland migration of coastal wetlands. Glob. Ecol. Conserv. 2023, 43, e02421. [Google Scholar] [CrossRef]
- Xian, X.X.; Pang, M.Y.; Zhang, J.L.; Zhu, M.K.; Kong, F.L.; Xi, M. Assessing the effect of potential water and salt intrusion on coastal wetland soil quality: Simulation study. J. Soils Sediments 2019, 19, 2251–2264. [Google Scholar] [CrossRef]
- Sadai, S.; Ranganathan, M.; Nauels, A.; Nicholls, Z.; Merner, D.; Dahl, K.; Licker, R.; Ekwurzel, B. Estimating the sea level rise responsibility of industrial carbon producers. Environ. Res. Lett. 2025, 20, 044012. [Google Scholar] [CrossRef]
- IPCC. IPCC Summary for policymakers. In Climate Change 2023; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Talke, S.A.; Jay, D.A. Changing tides: The role of natural and anthropogenic factors. Annu. Rev. Mar. Sci. 2020, 12, 121–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Biemond, B.; van Keulen, D.; Huismans, Y.; van Westen, R.M.; de Swart, H.E.; Dijkstra, H.A.; Kranenburg, W.M. Global increases of salt intrusion in estuaries under future environmental conditions. Nat. Commun. 2025, 16, 3444. [Google Scholar] [CrossRef] [PubMed]
- Solohin, E.; Widney, S.E.; Craft, C.B. Declines in plant productivity drive loss of soil elevation in a tidal freshwater marsh exposed to saltwater intrusion. Ecology 2020, 101, e03148. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Pennings, S.C. Response and recovery of low-salinity marsh plant communities to presses and pulses of elevated salinity. Estuaries Coasts 2019, 42, 708–718. [Google Scholar] [CrossRef]
- Ghirardelli, A.; Straffelini, E.; Park, E.; D’Agostino, V.; Masin, R.; Tarolli, P. Global impact of seawater intrusion on coastal agriculture. Environ. Res. Lett. 2025, 20, 013005. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Gorai, M.; Ennajeh, M.; Khemira, H.; Neffati, M. Influence of NaCl-salinity on growth, photosynthesis, water relations and solute accumulation in Phragmites australis. Acta Physiol. Plant. 2011, 33, 963–971. [Google Scholar] [CrossRef]
- Robin, A.H.K.; Matthew, C.; Uddin, M.J.; Bayazid, K.N. Salinity-induced reduction in root surface area and changes in major root and shoot traits at the phytomer level in wheat. J. Exp. Bot. 2016, 67, 3719–3729. [Google Scholar] [CrossRef] [PubMed]
- Rolletschek, H.; Hartzendorf, T. Effects of salinity and convective rhizome ventilation on amino acid and carbohydrate patterns of Phragmites australis populations in the Neusiedler See region of Austria and Hungary. New Phytol. 2000, 146, 95–105. [Google Scholar] [CrossRef]
- Negrao, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, Z.; Bachofen, C.; Lou, Y.J.; Jiang, M.; Tang, X.G.; Lu, X.G.; Buchmann, N. The effect of saline-alkaline and water stresses on water use efficiency and standing biomass of Phragmites australis and Bolboschoenus planiculmis. Sci. Total Environ. 2018, 644, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Yu, J.B.; Hou, A.X.; Han, G.X.; Wang, G.M.; Qu, F.Z.; Xia, J.B.; Wang, X.H. The ecological adaptability of Phragmites australis to interactive effects of water level and salt stress in the Yellow River Delta. Aquat. Ecol. 2017, 51, 107–116. [Google Scholar] [CrossRef]
- Ryu, H.; Cho, Y.G. Plant hormones in salt stress tolerance. J. Plant Biol. 2015, 58, 147–155. [Google Scholar] [CrossRef]
- Yu, Z.P.; Duan, X.B.; Luo, L.; Dai, S.J.; Ding, Z.J.; Xia, G.M. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Song, H.J.; Guo, X.; Yang, J.C.; Liu, L.L.; Li, M.Y.; Wang, J.F.; Guo, W.H. Phenotypic plasticity variations in Phragmites australis under different plant-plant interactions influenced by salinity. J. Plant Ecol. 2024, 17, rtae035. [Google Scholar] [CrossRef]
- Kobayashi, K. Sexual reproduction and diversity: Connection between sexual selection and biological communities via population dynamics. Popul. Ecol. 2019, 61, 135–140. [Google Scholar] [CrossRef]
- Hualpa-Ramirez, E.; Carrasco-Lozano, E.C.; Madrid-Espinoza, J.; Tejos, R.; Ruiz-Lara, S.; Stange, C.; Norambuena, L. Stress salinity in plants: New strategies to cope with in the foreseeable scenario. Plant Physiol. Biochem. 2024, 208, 108507. [Google Scholar] [CrossRef]
- Pagter, M.; Bragato, C.; Brix, H. Tolerance and physiological responses of Phragmites australis to water deficit. Aquat. Bot. 2005, 81, 285–299. [Google Scholar] [CrossRef]
- Yang, Z.F.; Xie, T.; Liu, Q. Physiological responses of Phragmites australis to the combined effects of water and salinity stress. Ecohydrology 2014, 7, 420–426. [Google Scholar] [CrossRef]
- Guo, H.Y.; Gao, F.L.; Pang, J.L.; Wang, H.H.; Wang, H.D.; Wang, Y.H.; Whitt, A.A.; Ma, C.C. Plant-plant interactions of Phragmites australis and Suaeda salsa as mediated by combined influences of salinity and tidal level changes. Plant Soil. 2022, 474, 141–161. [Google Scholar] [CrossRef]
- Gao, F.L.; Wang, Y.H.; Whitt, A.A.; Wang, H.D.; Ma, C.C.; Guo, H.Y. Belowground responses of Phragmites australis and Suaeda salsa to salinity and water depth changes. Pak. J. Bot. 2018, 50, 853–861. [Google Scholar]
- Obeso, J.R. The costs of reproduction in plants. New Phytol. 2002, 155, 321–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Shao, R.Y.; Zhu, P.C.; Wang, R.Q. The reproductive strategy of the clonal helophyte Leersia oryzoides (L.) Swartz. in response to variable submergence conditions and different harvest times. Evol. Ecol. 2021, 35, 27–40. [Google Scholar] [CrossRef]
- Yu, X.; Xu, B.Y.; Yao, R.J.; Wei, J.H.; Tu, T.B.; Chen, Z. Temporal dynamics of soil salinization due to vertical and lateral saltwater intrusion at an onshore aquaculture farm. Agric. Water Manag. 2024, 306, 109179. [Google Scholar] [CrossRef]
- Kurepa, J.; Smalle, J.A. Plant hormone modularity and the survival-reproduction trade-off. Biology 2023, 12, 1143. [Google Scholar] [CrossRef]
- Pagnussat, G.C.; Gomez-Casati, D.F. Plant development and reproduction in a changing environment. J. Exp. Bot. 2024, 75, 4167–4170. [Google Scholar] [CrossRef]
- Elsey-Quirk, T.; Lynn, A.; Jacobs, M.D.; Diaz, R.; Cronin, J.T.; Wang, L.; Huang, H.; Justic, D. Vegetation dieback in the Mississippi River Delta triggered by acute drought and chronic relative sea-level rise. Nat. Commun. 2024, 15, 3518. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Jiang, L.M.; Chen, Y.D.; Tian, X.M.; Lv, G.H. Abiotic stress-by-competition interactions drive hormone and nutrient changes to regulate Suaeda salsa growth. Glob. Ecol. Conserv. 2021, 31, e01845. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).