Lignin-Modified Petrochemical-Source Polyester Polyurethane Enhances Nutrient Release Performance of Coated Urea
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Lignin-Based Polyol
2.2.2. Preparation of Different CRFs
2.3. Measurement Indicators
2.3.1. Determination of the Composition of Lignin Liquefaction Products
2.3.2. Determination of Nitrogen Release Rate
2.3.3. Determination of Fertilizer Membrane Shell Performance
2.3.4. Determination of the Hardness of Fertilizer Granules
2.4. Statistical Analysis
3. Results and Discussion
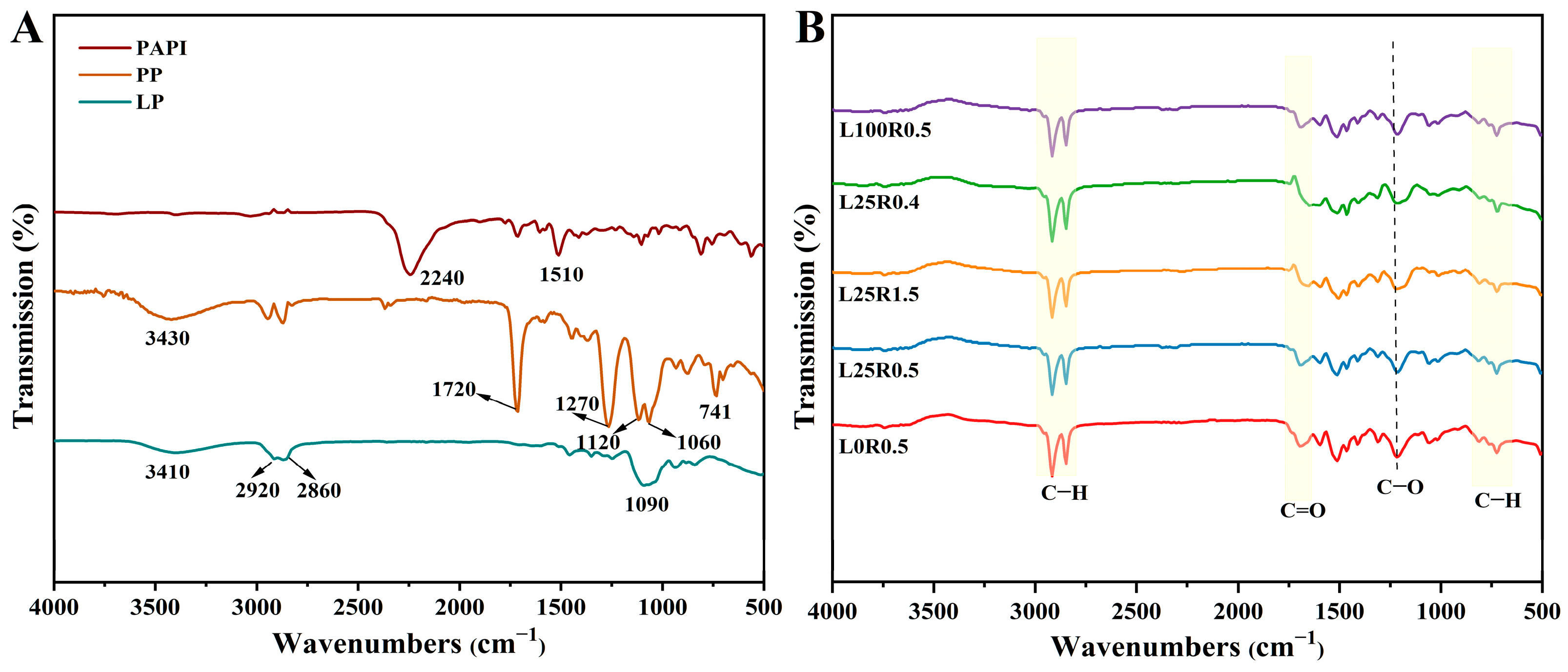
3.1. Compositional Properties of the Coating Material
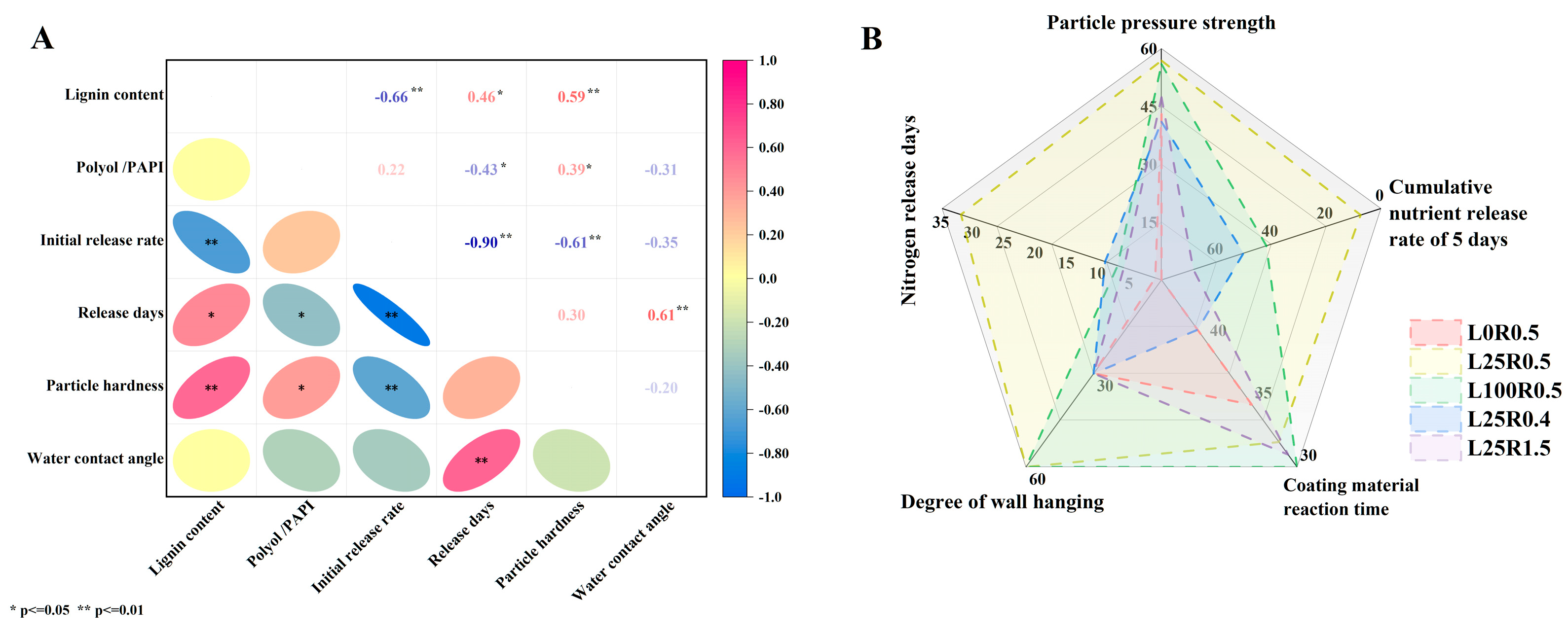
3.2. Nitrogen Release Behavior of CRUs Coated with Different Materials
3.3. Microstructure of Different Coating Materials
3.4. Physical Properties of CRUs Coated with Different Materials
3.5. Thermal Stability Analysis of Coating Materials
3.6. Comprehensive Analysis of Coating Materials
3.7. Feasibility of the Research and Its Limitations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Timilsena, Y.P.; Adhikari, R.; Casey, P.; Muster, T.; Gill, H.; Adhikari, B. Enhanced efficiency fertilizers: A review of formulation and nutrient release patterns. J. Sci. Food Agric. 2015, 95, 1131–1142. [Google Scholar] [CrossRef]
- Duan, Q.; Jiang, S.; Chen, F.; Li, Z.; Ma, L.; Song, Y.; Yu, X.; Chen, Y.; Liu, H.; Yu, L. Fabrication, evaluation methodologies, and models of slow-release fertilizers: A review. Ind. Crops Prod. 2023, 192, 116075. [Google Scholar] [CrossRef]
- Yao, Z.; Zheng, X.; Dong, H.; Wang, R.; Mei, B.; Zhu, J. A 3-year record of N2O and CH4 emissions from a sandy loam paddy during rice seasons as affected by different nitrogen application rates. Agric. Ecosyst. Environ. 2012, 152, 1–9. [Google Scholar] [CrossRef]
- Faizan, M.; Karabulut, F.; Khan, I.; Akhtar, M.S.; Alam, P. Emergence of nanotechnology in efficient fertilizer management in soil. S. Afr. J. Bot. 2024, 164, 242–249. [Google Scholar] [CrossRef]
- Li, H.F.; An, S.D.; Zhang, L.; Peng, H.; Ma, W.; Meng, X.; Ye, H.M. Urea fertilizer with precisely regulable slow-release performance by complexing with a random copolyester. J. Environ. Chem. Eng. 2021, 9, 105120. [Google Scholar] [CrossRef]
- Mikula, K.; Izydorczyk, G.; Skrzypczak, D.; Mironiuk, M.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Controlled release micronutrient fertilizers for precision agriculture—A review. Sci. Total Environ. 2020, 712, 136365. [Google Scholar] [CrossRef] [PubMed]
- Zafar, N.; Niazi, M.B.K.; Sher, F.; Khalid, U.; Jahan, Z.; Shah, G.A.; Zia, M. Starch and polyvinyl alcohol encapsulated biodegradable nanocomposites for environment-friendly slow release of urea fertilizer. Chem. Eng. J. Adv. 2021, 7, 100123. [Google Scholar] [CrossRef]
- Qiao, D.; Li, J.; Zhang, S.; Yang, X. Controlled release fertilizer with temperature-responsive behavior coated using polyether polyol (PPG)/polycaprolactone (PCL) blend-based polyurethane performs smart nutrient release. Mater. Today Chem. 2022, 26, 101249. [Google Scholar] [CrossRef]
- Park, W.J.; Hwangbo, M.; Chu, K.H. Plastisphere and microorganisms involved in polyurethane biodegradation. Sci. Total Environ. 2023, 886, 163932. [Google Scholar] [CrossRef]
- Han, Y.; Chen, S.; Yang, M.; Zou, H.; Zhang, Y. Inorganic matter modified water-based copolymer prepared by chitosan-starch-CMC-Na-PVAL as an environment-friendly coating material. Carbohydr. Polym. 2020, 234, 115925. [Google Scholar] [CrossRef]
- Wei, B.; Jiang, J.; Gao, C.; Zhang, L.; Zhan, Y.; Jiang, S.; Li, Y.; Sun, S.; Xie, J.; Fan, X. Revealing channel-controlled nutrient release mechanism of bio-oil polymer-coated controlled-release fertilizer. Ind. Crops Prod. 2021, 173, 114096. [Google Scholar] [CrossRef]
- Tian, H.; Li, Z.; Lu, P.; Wang, Y.; Jia, C.; Wang, H.; Liu, Z.; Zhang, M. Starch and castor oil mutually modified, cross-linked polyurethane for improving the controlled release of urea. Carbohydr. Polym. 2021, 251, 117060. [Google Scholar] [CrossRef]
- Liang, D.; Wang, Y.; Shi, H.; Luo, Z.; Quirino, R.L.; Lu, Q.; Zhang, C. Controllable release fertilizer with low coating content enabled by superhydrophobic castor oil-based polyurethane nanocomposites prepared through a one-step synthetic strategy. Ind. Crops Prod. 2022, 189, 115803. [Google Scholar] [CrossRef]
- Cregut, M.; Bedas, M.; Durand, M.J.; Thouand, G. New insights into polyurethane biodegradation and realistic prospects for the development of a sustainable waste recycling process. Biotechnol. Adv. 2013, 31, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, H.; Zhang, L.; Li, Z.; Xue, Y.; Wang, R.; Fan, X.; Sun, S. Green construction and release mechanism of lignin-based double-layer coated urea. Biotechnol. Biofuels Bioprod. 2023, 16, 97. [Google Scholar] [CrossRef]
- El Bouchtaoui, F.Z.; Ablouh, E.H.; Mhada, M.; Kassem, I.; Salim, M.H.; Mouhib, S.; Kassab, Z.; Sehaqui, H.; El Achaby, M. Methylcellulose/lignin biocomposite as an eco-friendly and multifunctional coating material for slow-release fertilizers: Effect on nutrients management and wheat growth. Int. J. Biol. Macromol. 2022, 221, 398–415. [Google Scholar] [CrossRef]
- Ni, K.; Pan, H.; Shen, Y. Preparation and Properties of Lignin-Based Graded Porous Carbon Composite Phase Change Materials. J. Funct. Mater. 2022, 53, 1175–1180. [Google Scholar]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Villafan-Vidales, H.I.; Longoria, A.; Sebastian, P.J.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Okoye, P.U. A review on trends in lignin extraction and valorization of lignocellulosic biomass for energy applications. J. Clean. Prod. 2021, 293, 126123. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Ghosh, T.; Purohit, S.D.; Prasannavenkadesan, V.; Rhim, J.W. Lignin as a sustainable and functional material for active food packaging applications: A review. J. Clean. Prod. 2024, 469, 143151. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Li, P.; He, C. Conversion of biomass lignin to high-value polyurethane: A review. J. Bioresour. Bioprod. 2020, 5, 163–179. [Google Scholar] [CrossRef]
- Abbas, A.; Wang, Z.; Zhang, Y.; Peng, P.; She, D. Lignin-based controlled release fertilizers: A review. Int. J. Biol. Macromol. 2022, 222, 1801–1817. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, L.; Wang, M.; Li, L.; Cao, B.; Wang, H.; Huang, W. Preparation and properties of starch-based polyurethane/montmorillonite composite coatings for controlled-release fertilizer. Polym. Compos. 2021, 42, 2293–2304. [Google Scholar] [CrossRef]
- Mulder, W.J.; Gosselink, R.J.A.; Vingerhoeds, M.H.; Harmsen, P.F.H.; Eastham, D. Lignin-based controlled release coatings. Ind. Crops Prod. 2011, 34, 915–920. [Google Scholar] [CrossRef]
- Morales-Cerrada, R.; Tavernier, R.; Caillol, S. Fully bio-based thermosetting polyurethanes from bio-based polyols and isocyanates. Polymers 2021, 13, 1255. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Song, S.; Yang, Z.; Liu, Y.; He, Z.; Zhou, C.; Du, L.; Sun, D.; Li, P. Hydrophobic modification of castor oil-based polyurethane coated fertilizer to improve the controlled release of nutrients with polysiloxane and halloysite. Prog. Org. Coat. 2022, 165, 106756. [Google Scholar] [CrossRef]
- Lawrencia, D.; Wong, S.K.; Low, D.Y.S.; Goh, B.H.; Tang, S.Y. Controlled release fertilizers: A review on coating materials and mechanism of release. Plants 2021, 10, 238. [Google Scholar] [CrossRef]
- Yilgor, I.; Eynur, T.; Yilgor, E.; Wilkes, G.L. Contribution of soft segment entanglement on the tensile properties of silicone–urea copolymers with low hard segment contents. Polymer 2009, 50, 4432–4437. [Google Scholar] [CrossRef]
- Bortoletto-Santos, R.; Cavigelli, M.A.; Montes, S.E.; Schomberg, H.H.; Le, A.; Thompson, A.I.; Kramer, M.; Polito, W.L.; Ribeiro, C. Oil-based polyurethane-coated urea reduces nitrous oxide emissions in a corn field in a Maryland loamy sand soil. J. Clean. Prod. 2020, 249, 119329. [Google Scholar] [CrossRef]
- Barratt, S.R.; Ennos, A.R.; Greenhalgh, M.; Robson, G.D.; Handley, P.S. Fungi are the predominant microorganisms responsible for the degradation of soil-buried polyester polyurethane over a range of soil water holding capacities. J. Appl. Microbiol. 2003, 95, 78–85. [Google Scholar] [CrossRef]
- Tosin, M.; Barbale, M.; Chinaglia, S.; Degli-Innocenti, F. Disintegration and mineralization of mulch films and leaf litter in soil. Polym. Degrad. Stab. 2020, 179, 109309. [Google Scholar] [CrossRef]
- Yildirim, E.; Yurtsever, M.; Yilgör, E.; Yilgör, I.; Wilkes, G.L. Temperature-dependent changes in the hydrogen-bonded hard-segment network and microphase morphology in a model polyurethane: Experimental and simulation studies. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 182–192. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.; Hou, L.; Chen, Y.; Xu, T. Effect of polyether/polyester polyol ratio on properties of waterborne two-component polyurethane coatings. Prog. Org. Coat. 2020, 141, 105545. [Google Scholar] [CrossRef]
- Pietrzak, K.; Kirpluks, M.; Cabulis, U.; Ryszkowska, J. Effect of the addition of tall oil-based polyols on the thermal and mechanical properties of ureaurethane elastomers. Polym. Degrad. Stab. 2014, 108, 201–211. [Google Scholar] [CrossRef]
- Luo, W.; Ying. Hydrogen-bonding properties of segmented polyether poly (urethane urea) copolymer. Macromolecules 1997, 30, 4405–4409. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, S.; Zhang, W.; Zhang, S.; Han, Y. Camellia meal-based adhesive with synergistic crosslinking of physical and chemical interaction for preparing aldehyde-free, anti-mildew, water-resistant wood-based composites. J. Clean. Prod. 2024, 451, 142091. [Google Scholar] [CrossRef]
- Garrett, J.T.; Runt, J.; Lin, J.S. Microphase separation of segmented poly (urethane urea) block copolymers. Macromolecules 2000, 33, 6353–6359. [Google Scholar] [CrossRef]
- Chen, S.; Yan, P.; Yu, X.; Zhu, W.; Wang, H. Conversion of lignin to high yields of aromatics over Ru–ZnO/SBA-15 bifunctional catalysts. Renew. Energy 2023, 215, 118919. [Google Scholar] [CrossRef]
- Ye, J.; Chen, S.; Feng, L.; Zhang, J.; Liu, Y.; Zhu, D. Multi-responsive self-healing behavior of polyurethane modified with photothermal and microwave conversion nanoparticles. Eur. Polym. J. 2024, 213, 113080. [Google Scholar] [CrossRef]
- Jakhmola, S.; Das, S.; Dutta, K. Emerging research trends in the field of polyurethane and its nanocomposites: Chemistry, Synthesis, Characterization, Application in coatings and Future perspectives. J. Coat. Technol. Res. 2024, 21, 137–172. [Google Scholar] [CrossRef]
- Senichev, V.Y.; Pogoreltsev, E.V. Relationship between the Abrasion Resistance of Urethane-Containing Elastomers and Their Structure. Polym. Sci. Ser. D 2023, 16, 549–552. [Google Scholar] [CrossRef]
- Ransom, C.J.; Jolley, V.D.; Blair, T.A.; Sutton, L.E.; Hopkins, B.G. Nitrogen release rates from slow-and controlled-release fertilizers influenced by placement and temperature. PLoS ONE 2020, 15, e0234544. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, M.; Li, Z.; Zhang, C.; Wan, C.; Zhang, Y.; Lee, D.J. Inhibition of urease activity by humic acid extracted from sludge fermentation liquid. Bioresour. Technol. 2019, 290, 121767. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Z.; Zhang, A. Water retention and fertilizer slow release integrated superabsorbent synthesized from millet straw and applied in agriculture. Ind. Crops Prod. 2021, 160, 113126. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Mu, L.; Wu, J.; Matsakas, L.; Chen, M.; Rova, U.; Christakopoulos, P.; Zhu, J.; Shi, Y. Two important factors of selecting lignin as an efficient lubricating additive in poly (ethylene glycol): Hydrogen bond and molecular weight. Int. J. Biol. Macromol. 2019, 129, 564–570. [Google Scholar] [CrossRef]
- Sun, Y.; Sheng, D.; Wu, H.; Tian, X.; Xie, H.; Shi, B.; Liu, X.; Yang, Y. Bio-based vitrimer-like polyurethane based on dynamic imine bond with high-strength, reprocessability, rapid-degradability and antibacterial ability. Polymer 2021, 233, 124208. [Google Scholar] [CrossRef]
- Li, Y.; Jia, C.; Zhang, X.; Jiang, Y. Synthesis and performance of bio-based epoxy coated urea as controlled release fertilizer. Prog. Org. Coat. 2018, 119, 50–56. [Google Scholar] [CrossRef]
- Zeller, M.; Garbev, K.; Weigel, L.; Saatzer, T.; Merz, D.; Tavakkol, S.; Stapf, D. Thermogravimetric studies, kinetic modeling and product analysis of the pyrolysis of model polymers for technical polyurethane applications. J. Anal. Appl. Pyrolysis 2023, 171, 105976. [Google Scholar] [CrossRef]
- Liu, W.; Fang, C.; Wang, S.; Huang, J.; Qiu, X. High-performance lignin-containing polyurethane elastomers with dynamic covalent polymer networks. Macromolecules 2019, 52, 6474–6484. [Google Scholar] [CrossRef]
- Sitthisuwannakul, K.; Boonpavanitchakul, K.; Wirunmongkol, T.; Muthitamongkol, P.; Kangwansupamonkon, W. A tunable controlled-release urea fertilizer coated with a biodegradable polyurethane-nanoclay composite layer. J. Coat. Technol. Res. 2023, 20, 635–646. [Google Scholar] [CrossRef]
- Tang, C.; Han, M.; Yang, X.; Shen, T.; Gao, Y.; Wang, Y.; Li, Y.C. Gene expression, enzyme activity, nitrogen use efficiency, and yield of rice affected by controlled-release nitrogen. ACS Omega 2023, 8, 23772–23781. [Google Scholar] [CrossRef] [PubMed]
- Serrano, L.; Rincón, E.; García, A.; Rodríguez, J.; Briones, R. Bio-degradable polyurethane foams produced by liquefied polyol from wheat straw biomass. Polymers 2020, 12, 2646. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, C.C.; Yuan, Z.; Wei, Q. Synthesis of bio-based polyurethane foams with liquefied wheat straw: Process optimization. Biomass Bioenergy 2018, 111, 134–140. [Google Scholar] [CrossRef]
- Conti Silva, J.A.; Patel, P.; Pazymino, I.; Inglesby, S.; Kirk, T.; Kazen, R.; Quirino, R.L. Effective Lignin-Based Binders for Particle Boards. ACS Sustain. Resour. Manag. 2024, 1, 1225–1237. [Google Scholar] [CrossRef]
- Henrik-Klemens, Å.; Caputo, F.; Ghaffari, R.; Westman, G.; Edlund, U.; Olsson, L.; Larsson, A. The glass transition temperature of isolated native, residual, and technical lignin. Holzforschung 2024, 78, 216–230. [Google Scholar] [CrossRef]









| Code | L | R |
|---|---|---|
| 1 | 0 | 1:2.5 |
| 2 | 0.25 | 1:2 |
| 3 | 0.5 | 1:1.5 |
| 4 | 0.75 | 1:1 |
| 5 | 1 | 1.5:1 |
| Treatment | Area (%) | HBI | DPS | |
|---|---|---|---|---|
| Free Carbonyl Group | Hydrogen-Bonded Carbonyl Group | |||
| L0R0.5 | 56.49 | 43.51 | 0.7721 | 0.4351 |
| L25R0.5 | 26.44 | 73.56 | 2.7984 | 0.7356 |
| L100R0.5 | 40.16 | 59.84 | 1.4939 | 0.5984 |
| L25R0.4 | 33.66 | 64.68 | 1.9403 | 0.6583 |
| L25R1.5 | 44.08 | 55.92 | 1.2714 | 0.5592 |
| Equation | Parameter | L25R0.5 | L100R0.5 | L25R0.4 | L25R1.5 |
|---|---|---|---|---|---|
| Mi/M∞ = Kt n | R2 | 0.9885 | 0.9920 | 0.9958 | 0.9420 |
| K | 4.60 | 14.64 | 13.97 | 39.67 | |
| n | 0.8924 | 0.8907 | 0.8994 | 0.5388 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Liu, B.; Chen, S.; Chen, Q.; Chen, H.; Dong, J.; Zhang, K.; Wang, J.; Zhang, M.; Liu, Z. Lignin-Modified Petrochemical-Source Polyester Polyurethane Enhances Nutrient Release Performance of Coated Urea. Agronomy 2025, 15, 2030. https://doi.org/10.3390/agronomy15092030
Hu X, Liu B, Chen S, Chen Q, Chen H, Dong J, Zhang K, Wang J, Zhang M, Liu Z. Lignin-Modified Petrochemical-Source Polyester Polyurethane Enhances Nutrient Release Performance of Coated Urea. Agronomy. 2025; 15(9):2030. https://doi.org/10.3390/agronomy15092030
Chicago/Turabian StyleHu, Xiaomin, Baishan Liu, Siyu Chen, Qi Chen, Heping Chen, Jingjing Dong, Kexin Zhang, Junxi Wang, Min Zhang, and Zhiguang Liu. 2025. "Lignin-Modified Petrochemical-Source Polyester Polyurethane Enhances Nutrient Release Performance of Coated Urea" Agronomy 15, no. 9: 2030. https://doi.org/10.3390/agronomy15092030
APA StyleHu, X., Liu, B., Chen, S., Chen, Q., Chen, H., Dong, J., Zhang, K., Wang, J., Zhang, M., & Liu, Z. (2025). Lignin-Modified Petrochemical-Source Polyester Polyurethane Enhances Nutrient Release Performance of Coated Urea. Agronomy, 15(9), 2030. https://doi.org/10.3390/agronomy15092030





