1. Introduction
The cultivation of soybeans (
Glycine max (L.) Merr.) has been traced back to China, where they have been planted for over 5000 years [
1]. It is classified under the genus
Glycine and is an annual herbaceous plant. The objectives of modern crop breeders encompass achieving high yields, ensuring excellent quality, maintaining stable production, synchronizing the growth cycle, and adapting to the requirements of modern harvesting [
2]. Elevating the per-unit yield and quality of soybeans constitutes the cardinal strategy for safeguarding soybean food security.
Soybean cyst nematode disease (
Heterodera glycines Ichinohe, SCN) is widely distributed worldwide. The disease was first discovered in Japan in 1915 [
3]. Subsequently, the disease was discovered in the central North American region of the United States. SCN has caused soybean yield losses of up to
$1.2 billion [
4]. In China, SCN is primarily distributed in the Northeast and Huanghuai regions. The disease exhibits differentiation into multiple physiological races. In the Huanghuai region, a major soybean-producing area in China, physiological races SCN 1 and SCN 4 are dominant. In the Northeast region, another major soybean-producing area, physiological races SCN 3 and SCN 4 are primarily found [
5].
J
2 stage SCN causes the most severe damage to soybean root systems. SCN releases cellulase and pectinase through its mouthparts, dissolving the cell walls of plant roots and forming syncytia. Obtaining nutrients from the host soybean [
5], leads to a significant reduction in soybean yield. Yields may decrease by 20% to 30%, and, in severe cases, result in complete crop failure. Currently, the primary measures for controlling SCN include crop rotation, SCN chemical agents, and the development of SCN-resistant varieties. In terms of crop rotation, the corn–soybean–corn rotation system is widely adopted. However, it cannot completely eradicate the SCN pathogen. Chemical agents can control the disease to some extent, but they have a significant impact on the surrounding soil ecosystem. Currently, the SCN antigens widely recognized by the scientific community include PI88788, PI54840 (Peaking), and PI437654 [
6]. However, with the overuse of antigen resistance, the resistance of antigens gradually weakens, and SCN evolves into more potent physiological populations [
7]. The pathogenicity of SCN populations was initially classified as physiological populations based on the responses of four soybean genotypes. Subsequently, seven soybean lines are identified with unique resistance through genotyping methods [
8]. As SCN physiological populations continue to evolve, SCN populations have gradually overcome antigens such as PI88788. Therefore, it is crucial to continue screening for new resistant germplasm and genes from soybean germplasm resources, and to expand the genetic background of superior germplasm resources. Rgh1, located on chromosome 18, exhibits incomplete dominance [
9], while Rgh4, located on chromosome 8, exhibits complete dominance [
10,
11]. Rgh1 and Rgh4 have been extensively validated by quantitative trait locus (QTL) studies and are widely recognized as the primary resistance loci for SCN resistance [
12,
13]. Many studies have focused on developing molecular markers at these loci. However, Rgh1 and Rgh4 can only explain 60% of the genetic variation [
14], and the remaining portion still requires further exploration and development.
ABI transcription factors are widely distributed in plants such as soybeans, corn, wheat, tomatoes, and
Arabidopsis thaliana [
15,
16]. This type of transcription factor contains four conserved domains, namely A, B
1, B
2, and B
3. Among them, the N-terminal A structure is rich in acidic residues; and, in the B
1 domain, there are essential elements necessary for the interaction with
bZIP transcription factors [
17]. The B
2 domain encompasses a putative nuclear-localization signal and is considered to play a crucial function in transcriptional regulation [
18]. The B
3 domain is a highly conserved DNA-binding domain. It is widely involved in the regulation of seed development, participates in stress responses, and affects the growth and development of plants, among other aspects [
19].
ABI3 is widely involved in plant abiotic stress and plays an important regulatory role under conditions such as temperature stress and dehydration stress.
ABI3 mediates the dehydration stress response in non-seed plants and seed plants by regulating downstream genes [
20]. When AtABI3 is absent in
A. thaliana its recovery capacity under dehydration stress is impaired [
21]. The development of seed coats in
Brassica napus is regulated by BnABI3, which plays a crucial role in seed dehydration tolerance [
22]. In response to dehydration stress, ABI3 inhibits the transcription of the
RAV1 gene by suppressing the recruitment of RNA polymerase II. Under stress conditions, the downregulation of
RAV1 gene expression induces a decrease in the expression of ethylene-insensitive 2 (EIN2) and ethylene receptor 1 (ETR1), thereby generating an effective dehydration stress response [
23]. In
A. thaliana, ABI3 transgenic plants exhibit enhanced cold tolerance under short-term ABA treatment, suggesting that the ectopic expression of the
ABI3 gene may regulate cold-induced cold tolerance and confer higher cold temperature tolerance [
24]. The transcription of genes such as
SOS1,
SOS2,
SOS3 sodium/hydrogen ion exchanger, drought stress response 29B, abscisic acid insensitive protein 2, ABI3, MYB15, and δ-1-pyrrolidine-5-carboxylic acid synthase 1 is promoted by salt stress [
25]. The ABI3VP1 protein possesses a B
3 DNA-binding domain and is widely involved in regulation during development and under adverse stress conditions [
26].
In this study, RNA sequencing (RNA-Seq) was conducted on the root systems of highly resistant and susceptible soybean varieties. Analysis of the transcriptome data revealed the differentially expressed gene GmABI3VP1, which was identified between the resistant and susceptible root samples. Overexpression and gene editing vectors were constructed, and transgenic root systems were developed using Agrobacterium-mediated genetic transformation technology. By analyzing the gene expression patterns in both overexpressed and gene-edited roots, the role of this gene in response to SCN stress was established. Additionally, SCN disease resistance to SCN and measurements of root physiological parameters were conducted. This study aims to explore the function of the GmABI3VP1 gene and provide insights into the mechanisms underlying its role in SCN disease resistance.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
The soybean materials used in the experiment included Dongnong 50 (DN50, SCN-susceptible varieties; it is a variety highly susceptible to SCN), Dongnong L10 (SCN-resistant varieties), Suinong14 (SN14,SCN-susceptible varieties), and Heinong 37 (HN 37,SCN-susceptible varieties). Y16 (SCN-resistant varieties; RILF15, Recombinant Inbred LineF15, line 16). All soybean germplasm resources were planted in 2023 in China, Harbin City, Heilongjiang Province (45°45′16.2″ N, 126°54′39.6″ E) in Xiangyang Farm of Northeast Agricultural University. The field experiment was conducted in a randomized block design with 1 m row length, 0.65 m row width, 0.06 m plant spacing, and three replications.
2.2. Preparation of Sequencing Materials
Dongnong 50, which exhibits sensitivity to SCN 3, was sown in black pots to facilitate the propagation of SCN 3. Thirty days after sowing, the intact root systems of the plants were carefully extracted and subsequently rinsed with tap water at a slow flow rate. SCN 3 was isolated using 60-mesh sieves, and the SCN 3 retained on the sieves was meticulously and gently ground by hand. The 40-mesh sieve was then thoroughly rinsed with distilled water, and the collected J4 eggs were transferred to 50 mL centrifuge tubes. After thorough mixing, a fraction of the cyst-containing liquid was retrieved. The J4 eggs were observed under a microscope to determine their quantity, and the solution was adjusted to a final concentration of 1800 J4 eggs per milliliter by appropriate dilution.
Dongnong L10 and Heinong 37 were, respectively, inoculated with SCN 3 to form the experimental groups, while those not inoculated with SCN 3 were set as the control groups. The indoor temperature was set at 25 °C, and the humidity was adjusted to 70%. At the V2 stage of the seedlings, 2 mL of the soybean SCN 3 J4 egg suspension was inoculated into each plant. The root tissues at 0 d and 14 d were collected and stained with acid fuchsin to ensure successful SCN 3 inoculation at 14 d. These root tissues were then used for preparing RNA-Seq sequencing samples.
2.3. RNA Extraction and cDNA Synthesis from Soybean Roots
The soybean variety Dongnong L10, which is resistant to the SCN, was planted in vermiculite. When the soybeans grew to the stage of the second pair of true leaves (V2 stage), the vermiculite on the soybean roots was thoroughly rinsed off with clean water, and then 1.5 g of the soybean roots were cut off by a surgical scalpel. RNA from soybean roots was extracted by using the TRIzol™ reagent (Thermo Fisher Scientific, Waltham, MA, USA). Use 2% agarose gel electrophoresis to detect the quality of the RNA.
RNA was reverse transcribed using the ReverTra Ace® qPCR RT Master Mix (TOYOBO, Osaka, Japan). (1) A total of 4 μL of RNA was heat-denatured at 65 °C for 5 min. After denaturation, it was quickly inserted into crushed ice. (2) Add 2 μL of 4 × DN Master Mix, 2 μL of ddH2O, and 4 μL of the denatured RNA. Remove DNA at 37 °C for 5 min and then take and place it into the denatured solution. (3) Take 2 μL of 5 × RT Master Mix II and add it into the denatured solution in Step 2. Incubate at 37 °C for 15 min, at 50 °C for 5 min, and at 98 °C for 5 min to form cDNA.
2.4. Transcriptome Sequencing and Analysis
In this study, transcriptome sequencing analysis was conducted using the Illumina HiSeq™ 2500 platform to examine the changes in gene expression levels related to synthesis and regulation between soybean varieties exhibiting extreme resistance and susceptibility to SCN. The analysis included both SCN-resistant (Dongnong L10) and SCN-susceptible (Heinong 37) soybean varieties, which were either infected with SCN or not infected.
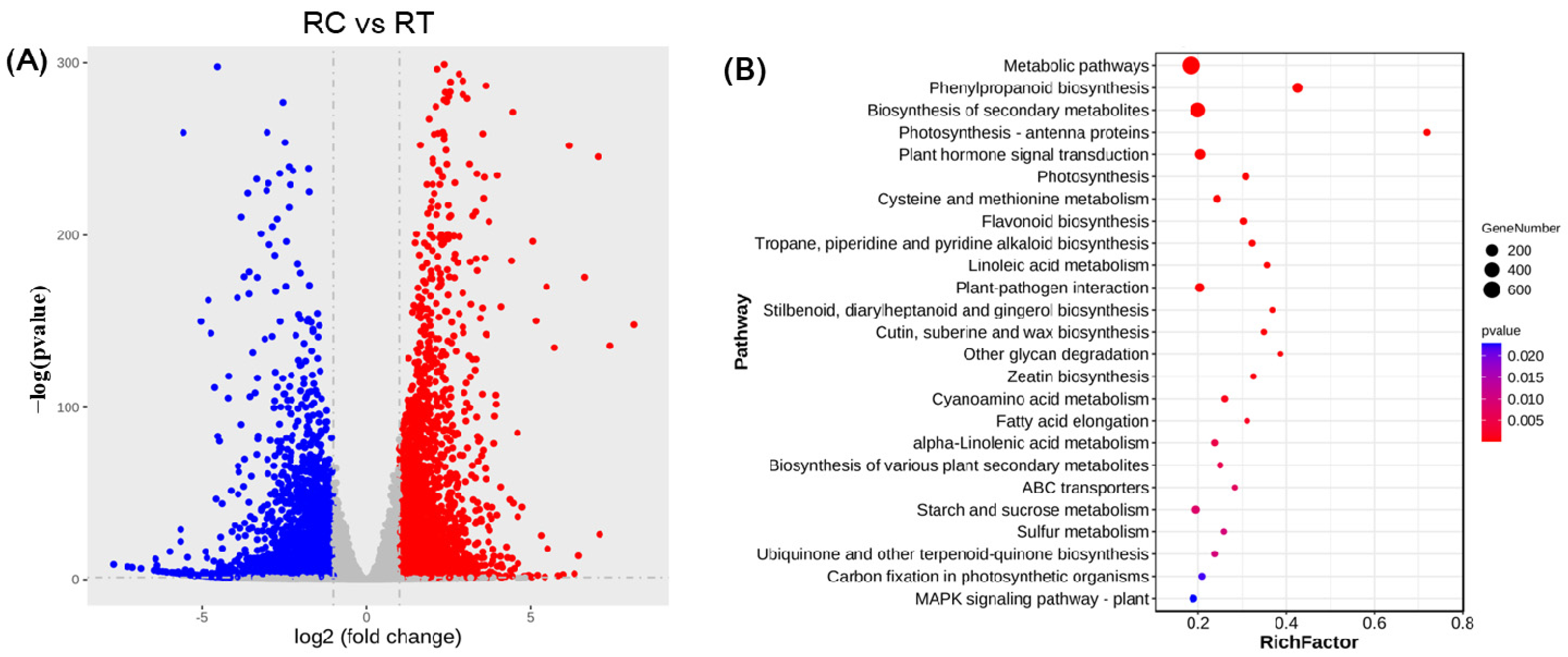
The same quantity of root tissue from identical parts was collected from both the treatment group and the control group. Subsequently, the samples were rapidly frozen in liquid nitrogen and wrapped in aluminum foil. Total RNA was extracted using the TRNzol Universal kit (Thermo Fisher Scientific, Waltham, MA, USA). The cDNA library was constructed with the assistance of the Illumina TruSeq RNA library preparation kit (Illumina, San Diego, CA, USA), and RNA-Seq sequencing of the cDNA was conducted on the Illumina HiSeq™ 2500. The visualization analysis of transcriptome data can be performed using the DESeq2 R package in Rstudio software V2025.05.1+513.pro3. The up-regulated and down-regulated genes in the transcriptome were utilized to screen for candidate genes through fold change analysis of differential expression and KEGG enrichment analysis. Consequently, the candidate gene GmABI3VP1, which exhibited differential expression under SCN 3 stress and non-stress treatments, was identified.
2.5. Fluorescence Quantitative PCR
The PrimerQuest™ Tool (
https://sg.idtdna.com/PrimerQuest/Home/Index accessed on 1 May 2025) was employed for the design of quantitative primers targeting the
GmABI3VP1 candidate gene (see
Table S7). The housekeeping gene selected for this study was
GmActin4 (GenBank accession number: AF049106). The PCR instrument utilized was the Gene ROCGENE quantitative real-time PCR system (Kunpeng, Beijing, China), and the operating software used was Archimed X6 (ver. 201905v1.08, Microvision Instruments, Evry, Île-de-France, France). For specific procedures, experiments were conducted following the operational guidelines provided with the TaKaRa fluorescent quantitative reagent TB Green kit (TaKaRa, Shiga Prefecture, Japan). Relative expression levels were calculated using the 2
−ΔΔCT method. CT values were determined by averaging three biological replicates.
2.6. Cloning of the GmABI3VP1 Gene
Cloning was performed using the cDNA extracted from the roots of Dongnong L10 as a template. A 20 μL reaction mixture was prepared, consisting of the following: 1 μL of cDNA, 10 μL of KOD One Plus (TOYOBO, Osaka, Japan), 1 μL of
GmABI3VP1-F primer, 1 μL of
GmABI3VP1-R primer (refer to
Table S7), and 6 μL of ddH
2O. The PCR program was configured as follows: initial denaturation at 98 °C for 3 min; followed by denaturation at 98 °C for 10 s; annealing at 58 °C; extension at 68 °C for 5 s; and a further extension step at 68 °C for an additional 10 min. The denaturation–extension cycles were repeated for a total of 38 cycles before storing the samples at 4 °C. The position of the target band was assessed through electrophoresis on a gel containing agarose at a concentration of 2%.
2.7. Bioinformatics Analysis of Tools
2.8. Analysis of the Subcellular Localization of GmABI3VP1
Prepare MS solid medium (
Table S2) and pour tobacco seeds into a 1.5 mL sterile EP tube. Add 100 μL of sodium hypochlorite (NaClO) and 900 μL of sterile water. Shake in a vortex oscillator for 8 min, then let it stand still at 25 °C for 4 min. Wash it with 500 μL of sterilized water three times in a sterile operating platform. Use a pipette tip to plant the tobacco seeds in the MS solid medium and cultivate them for 10 d. Transfer the seedlings to black pots filled with a 1:1 mixture of vermiculite and nutrient soil. Conduct the subcellular localization experiment after the tobacco has grown for 28 d. Firstly, pretreat the tobacco. Place the tobacco in the dark for 24 h. Secondly, put the tobacco under normal light conditions 1 h before infection. Then, perform tobacco injection. For the Agrobacterium carrying pCAMBIA1302-
GmABI3VP1 (adjust the tobacco resuspension to an OD
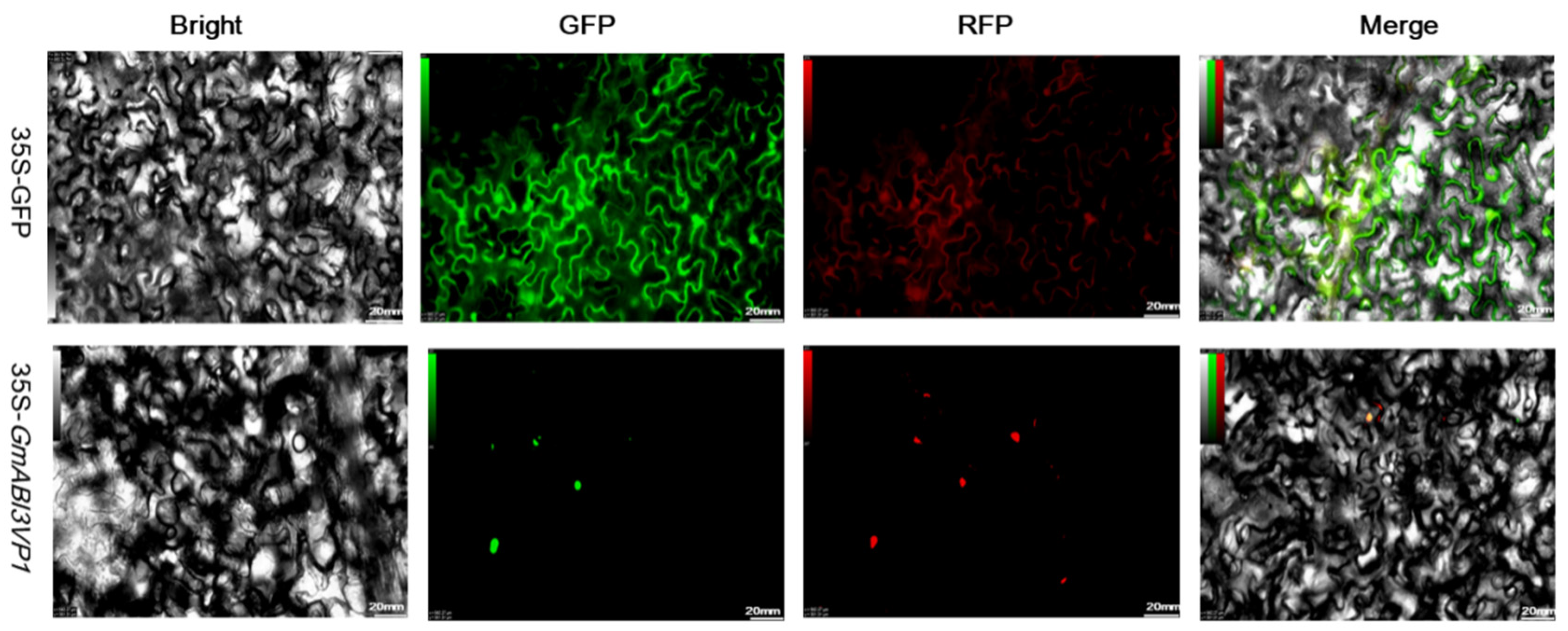
600 value of 0.8), incubate it at a constant temperature for 5 h, then inject it into the abaxial side of tobacco leaves using a sterile syringe. After tobacco injection is completed, place the plants in a dark environment for 12 h, then expose them to light for 24 h. Finally, observe and take pictures under an inverted fluorescence microscope.
2.9. Over-Expression, Gene Editing and Homologous Recombination
The plasmid was linearized by single enzyme digestion. The pCAMBIA3300 vector was linearized with Hind III enzyme (Biolabs New England Biolabs #R3104, Ipswich, MA, USA), the pCAMBIA1302 vector was linearized with Nco Ι enzyme (Biolabs New England Biolabs #R3193, Ipswich, MA, USA), and the pYLCRISPR/Cas9 vector was linearized with Bas I enzyme (Biolabs New England Biolabs #R3733, Ipswich, MA, USA). The PCR program was set as follows: incubation at 37 °C for 5 h, then at 65 °C for 25 min, and, finally, storage at 12 °C. The enzyme digestion system had a total volume of 20 μL, which consisted of 2 μL of Hind III enzyme, 4 μL of enzyme buffer, 10 μL of plasmid (150 ng/μL), and 4 μL of ddH2O.
Use the ClonExpress II One Step Cloning Kit (VAZYME, Nanjing, China) to perform homologous recombination ligation between the cloned DNA and the linearized pCAMBIA3300 and pCAMBIA1302, respectively. The homologous recombination system had a total volume of 20 μL, which consisted of 2 μL of homologous recombination enzyme, 4 μL of buffer, 3.5 μL of the linearized pCAMBIA3300 vector, 1.5 μL of the target fragment, and 9 μL of water. Place it in a water bath at 37 °C, 30 min (
Tables S4 and S5).
Construction of the gene editing vector; for Reaction 1, specific amplification was carried out (including 1 round-3d, 1 round-3b). Based on Reaction 1, Reaction 2 was performed for the second specific PCR amplification (2 rounds-3d, 2 rounds-3b). After the gel purification of the products from the 2 rounds of amplification, they were recombined with the pYLCRISPR/Cas9 vector.
The pCAMBIA3300 and pCAMBIA1302 plasmids were linearized by the method of single enzyme digestion. The results showed that lanes 1–4 carried the flanking target sequences of the pCAMBIA3300 plasmid, and lanes 5–7 carried the flanking target sequences of the pCAMBIA1302 plasmid. After single enzyme digestion, there was a certain electrophoresis distance between the linearized vectors and the plasmids, indicating that the enzyme digestion was complete (
Figure S4C,D). The coding sequence of the target gene was cloned using specific primers. The results demonstrated that an obvious band appeared at 1236 bp (
Figure S4E). The linearized gel products and the target gene gel products were ligated using the ClonExpress II One Step Cloning Kit and then transformed into DH5α. PCR specific amplification was carried out (
Figure S4I), followed by sequencing analysis. The sequencing results indicated that the target gene sequence was successfully recombined into DH5α.
2.10. Agrobacterium-Mediated Hairy Root Transformation
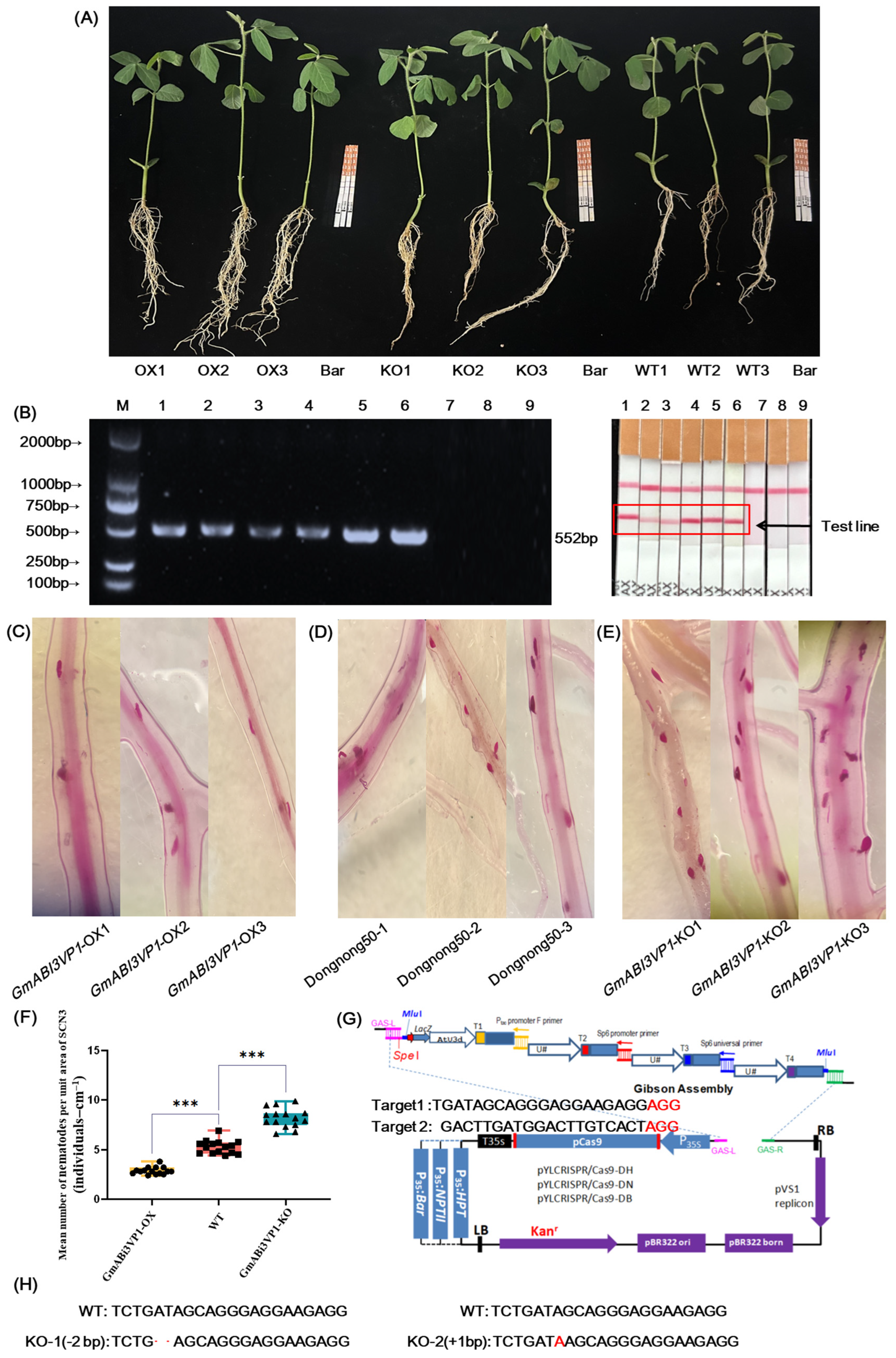
The recipient used Dongnong 50 for the creation of both gene-edited (KO) hairy roots and overexpressed (OX) hairy roots. Soybean Dongnong 50, which is sensitive to SCN 3, was planted in black pots filled with vermiculite. When the soybeans grew to the bud stage (VE stage), the main roots of the soybeans were obliquely cut and then were transferred into Agrobacterium K599 containing the plasmid (7.5 μL of 1M As, and 1 mL of 500 mM MgCl2 with an OD600 value ranging from 0.6 to 0.8). The mixture was placed in a shaker at 28 °C with a rotation speed of 50 rpm/min for 30 min. After that, the soybeans were taken out from the shaker and subjected to vacuum treatment using a vacuum pump. The pressure was set at 5–10 kPa, and the vacuum treatment lasted for 1 min. Then, they were left to stand still for 30 s. This process was repeated three times. For the control group, the soybeans were soaked in distilled water, and the operation process was the same as that of the Agrobacterium-mediated hairy root transformation method. The soybean seedlings in the bacterial solution were reinserted into the vermiculite and were cultured under 80% humidity at 25–28 °C until they reached the second trifoliate leaf stage (V2 stage) for the subsequent identification of SCN.
2.11. Identification of the Disease Phenotype of SCN
The soybeans were extracted from the vermiculite, thoroughly washed with water, and a portion of their root tissues were harvested. Subsequently, both the transgenic plants for validation and the control group plants were transferred into soil inoculated with SCN 3. They were cultured at 25 °C with a soil humidity of 40%. After 14 d, the roots were removed from the infected soil and rinsed extensively with clean water. The identification of SCN 3 in the root tissues of soybeans was conducted using the acid fuchsin staining method. The operational steps are as follows: Firstly, the roots were decolorized by soaking them in a solution of 5% sodium hypochlorite for 80 min. Following this step, they were washed by soaking in double-distilled water (ddH
2O) for an additional 15 min. Next, a working solution of acid fuchsin (1× concentration;
Table S1) was prepared and heated in an induction cooker until boiling for approximately 60 s to firm up the roots. Afterward, the roots were carefully removed from the induction cooker and dried gently using clean paper towels. Finally, for microscopic examination, soybean roots were evenly spread on a transparent plastic pressing plate. The number of SCN present was observed and counted under a stereomicroscope at a magnification of ×20.
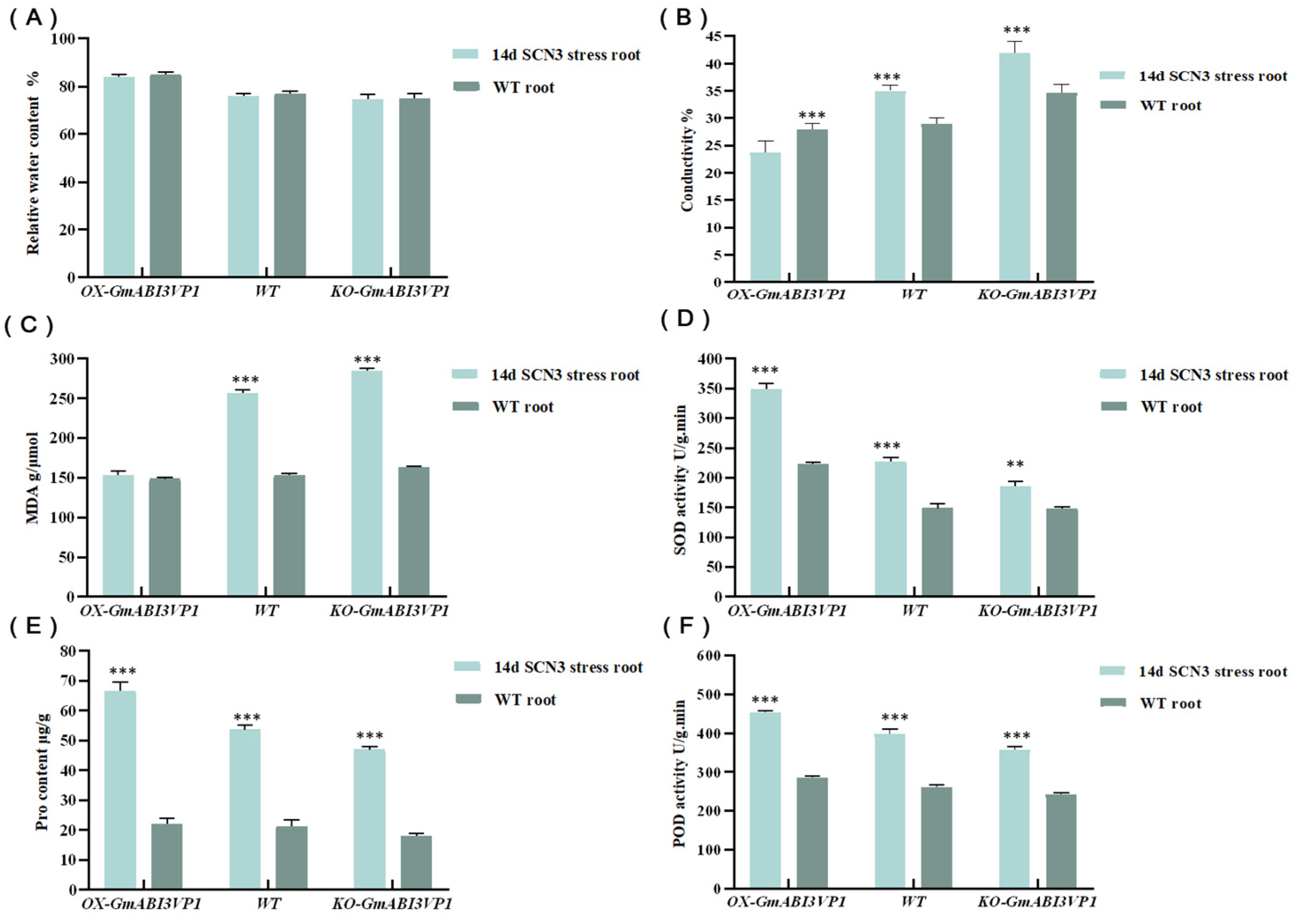
2.12. Measurement of the Indices Related to the Resistance of Roots to Stress Caused by SCN
In this study, roots of transgenic plants stressed by SCN 3 for 14 d and WT plants were rinsed with water. Then, equal-mass lateral roots were selected to measure Peroxidase (POD) and superoxide dismutase (SOD) activities, malondialdehyde (MDA) content, relative water content (RWC), electrical conductivity (EC), and proline synthetase (Pro) activity. The specific determination method was carried out according to the instructions in the manual [
27].
4. Discussion
Currently, most of the research mainly focuses on genes near the major resistance loci, Rgh1 and Rgh4. However, Rgh1 and Rgh4 can only account for 60% of the genetic variation, and a large portion remains to be further explored and developed. Thus, how to identify and discover new genes resistant to SCN disease has become of paramount importance.
ABI3/VP1 was initially detected as being part of the seed-specific transcription factors and was utilized as an intermediary in the regulation of ABA-responsive genes throughout the seed development stage. Nowadays, it is acknowledged to play a role beyond the scope of seed physiology, particularly when non-biological stress occurs. Currently, in the numerous literature, it has been frequently documented that under adverse situations like drought, dehydration, temperature variations and salt-alkali stress, the functions of ABI3/VP1 proteins are activated and engaged. The drought resistance of plants and the photosynthetic efficiency can be enhanced and improved simultaneously when the
AtABI3 gene in
A. thaliana is overexpressed in cotton (
Gossypium spp.) [
28]. The growth and differentiation of poplar (
Populus) plumule leaves can be affected by the overexpression of the
PtABI3 gene [
29]. Through next-generation sequencing of the
ABI3 deletion mutants and wild type, it was indicated in the degradome analysis that the
ABI3 gene might be regulated by the plant-specific
miR536, with desiccation tolerance being potentially affected [
30]. In
Brassica napus L., the seeds can be made to have higher desiccation tolerance under dehydration stress by
BnABI3 through the regulation of seed-coat development so as to adapt to the stressful environment [
31]. In
Brassica napus, it was found that the plant’s cold tolerance system could be triggered by the excessive accumulation of
ABI3 in seeds and pods, and the decomposition of chlorophyll could be effectively promoted by plant embryos and pods even when they were exposed to frost, and the economic value of oil crops could be ensured to a certain extent [
32]. The salt tolerance of the loss-of-function mutants of the
A. thaliana transcription factor DIV2-encoding gene
AtDIV2 was improved, the sensitivity to exogenous ABA was enhanced, and the ABA content was significantly increased. In the
DIV2 mutants, the transcriptional levels of ABA-related genes and stress-related genes such as
ABA1,
ABI3, and
P5CS2 were all up-regulated. It was indicated that the activity of
ABI3 might be appropriately regulated by
AtDIV2 in wild-type plants to participate in the salt stress response [
33,
34].
Although the responsive reactions of the ABI3 gene can be observed under various adverse stress conditions, there have been no relevant reports regarding its role in SCN disease to date. The potential function of this target gene in SCN disease requires further exploration, and ongoing efforts should be made to identify and investigate new genes that confer resistance to SCN disease. Dongnong 50 does not contain the rhg1 and Rhg4 loci, while Dongnong L10 contains both the rhg1 and rhg4 loci. Further research and exploration are still needed on the GmABI3VP1 gene. Currently, whether allelic genotypes exist in the GmABI3VP1 promoter remains to be further investigated, and it is also necessary to explore whether upstream effector factors regulate the GmABI3VP1 protein.
Current management of soybean cyst nematode (SCN) resistance predominantly relies on genes such as Rhg1 and Rhg4, which account for only 60% of the observed genetic variation [
35]. Our study identifies
GmABI3VP1 as a novel contributor to SCN resistance, thereby expanding the genetic toolkit available for breeding efforts. The association of the gene with various stress-responsive pathways, including plant hormone signaling and MAPK pathways, indicates that it may interact with existing resistance loci to synergistically enhance defense mechanisms. Future research should explore its interplay with Rhg1 and Rhg4, as well as its role in resisting other SCN physiological races, to validate its broad applicability. While our results highlight
GmABI3VP1′s role in SCN 3 resistance, several questions remain. The downstream target genes of
GmABI3VP1 in the SCN response pathway require identification, as do the specific molecular mechanisms by which it regulates antioxidant enzyme activity and stress signaling. Additionally, validating its function in stable transgenic soybean lines and under field conditions will be critical for translational applications. Finally, investigating its role in cross-resistance to other pathogens or abiotic stresses could further broaden its utility. In conclusion,
GmABI3VP1 emerges as a key regulator of soybean resistance to SCN 3, acting through transcriptional modulation and physiological adaptation. Its characterization provides a foundation for developing SCN-resistant soybean varieties and deepens our understanding of plant-nematode interactions.
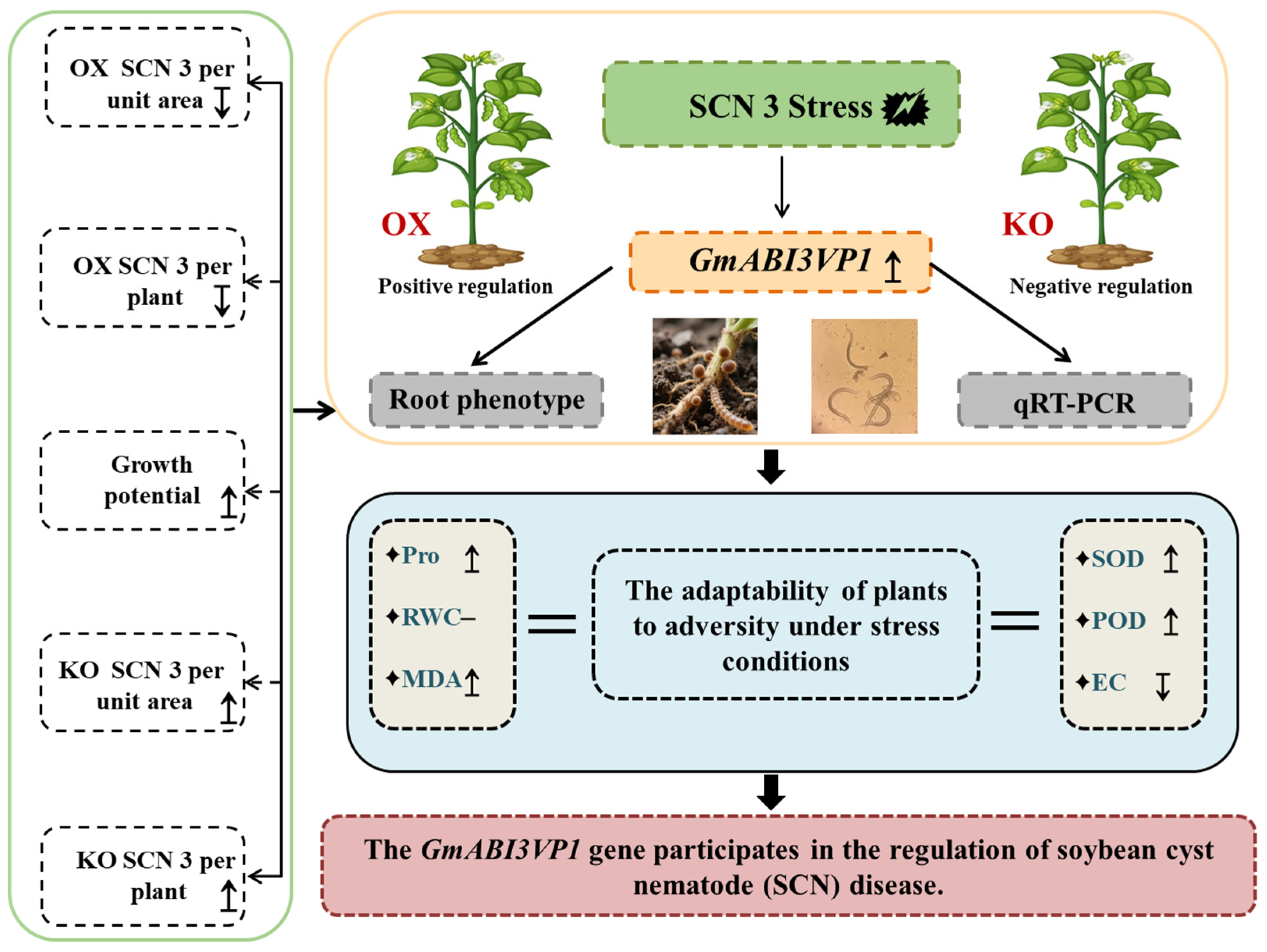
In this study, the Dongnong L10, which is resistant to SCN, and the Heinong 37, which is susceptible to SCN, were adopted. After being subjected to SCN 3 stress for 14 d, the root tissues were sent for RNA-Seq sequencing. The differentially expressed gene
GmABI3VP1 was identified through transcriptome data (RNA-Seq) screening. Then, the overexpression recombinant plasmid pCAMBIA3300-
GmABI3VP1, the subcellular localization recombinant plasmid pCAMBIA1302-
GmABI3VP1, and the gene editing recombinant plasmid pYLCRISPR/Cas9-
GmABI3VP1 were constructed. The successfully constructed recombinant plasmids were, respectively, transferred into the competent cells of
Escherichia coli DH5α and
Agrobacterium tumefaciens K599. Bioinformatics analysis was carried out on the GmABI3VP1 protein, including phosphorylation, secondary and tertiary structures of the protein, and promoter elements. It was shown by the results that multiple gene elements related to responding to adverse stress were contained in this gene, and the plant adverse stress was involved with them. It was also confirmed by the SCN phenotype identification that this gene was involved in resistance to SCN 3, and this gene was classified as a positive regulatory factor. It was preliminarily proved that obvious resistance to SCN 3 disease was possessed by the
GmABI3VP1 gene (
Figure 7).