Population Fluctuation of Phytophagous Mites and Their Impact on the Quality Properties of Wild and Cultivated Blackberry Fruits (Rubus spp. L.) in Jalisco, Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Study Area
2.2. Mite Sampling and Collection
2.3. Mite Extraction, Mounting, and Identification
2.4. Fruit Quality Evaluation
2.4.1. Physical Variables
2.4.2. Physicochemical Variables
2.5. Statistical Analysis
3. Results
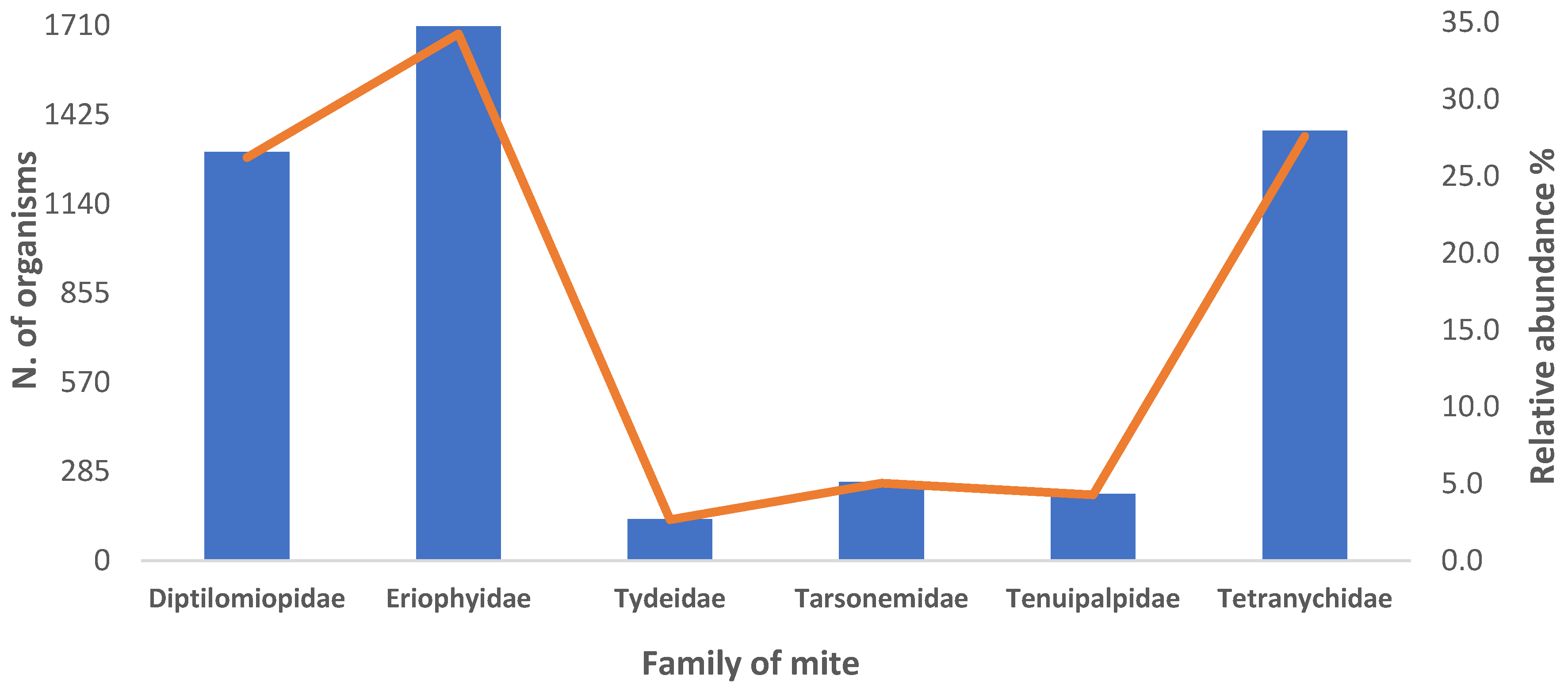
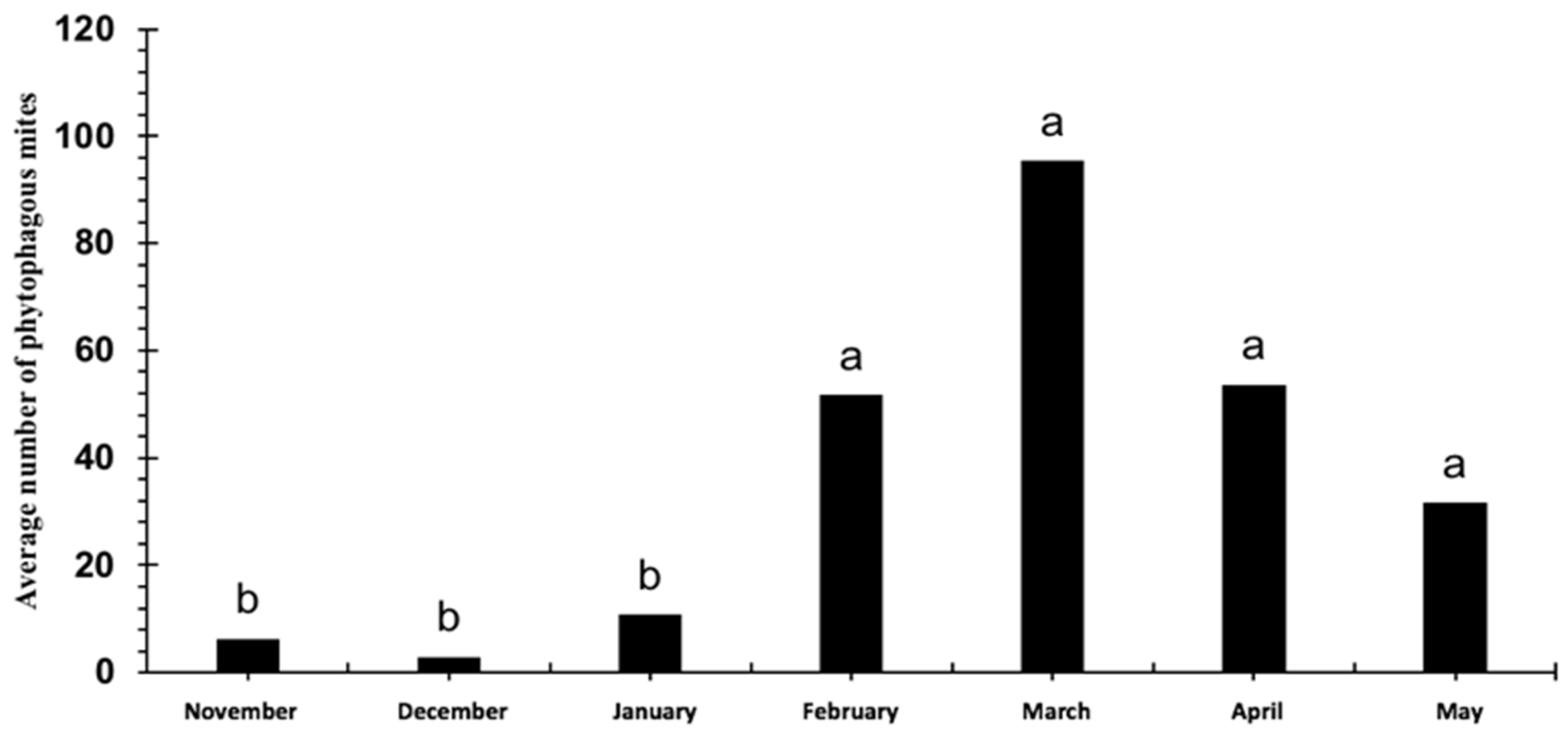
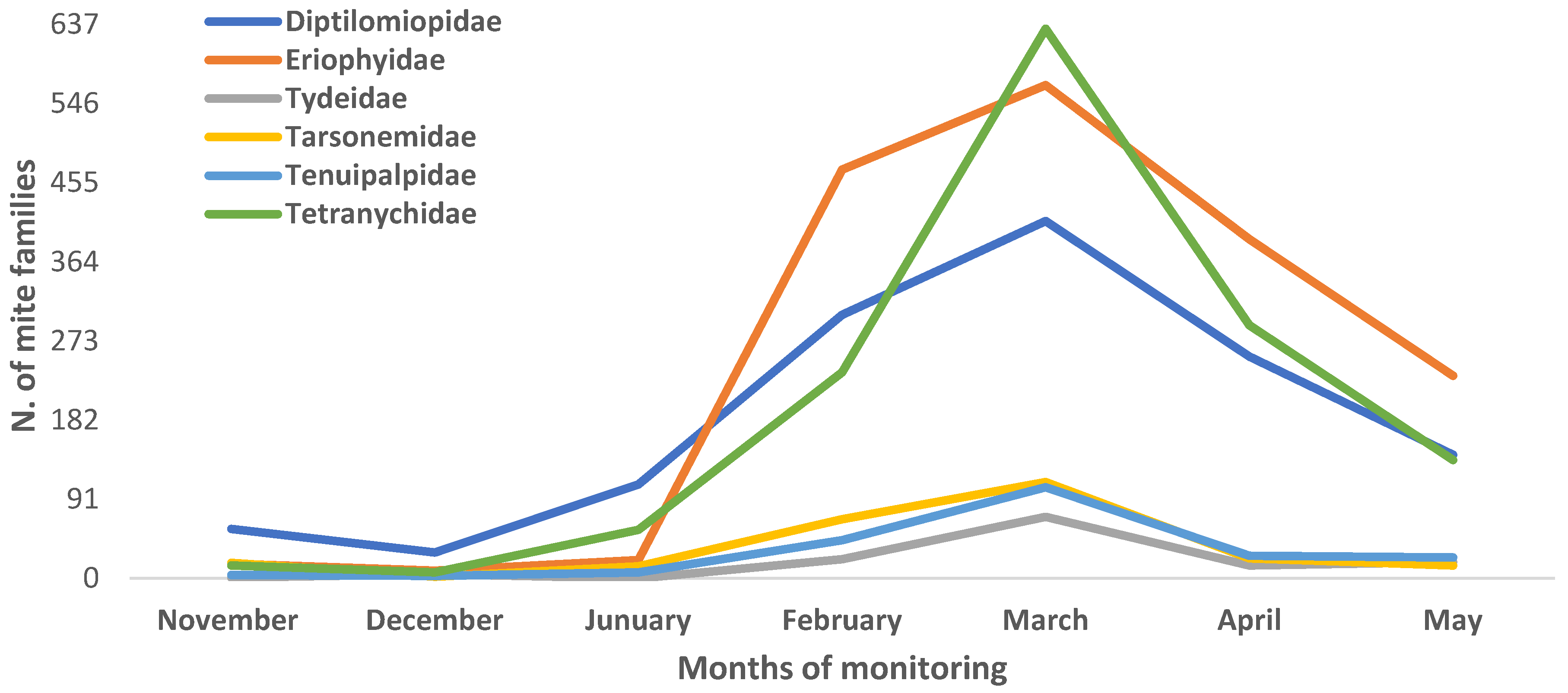
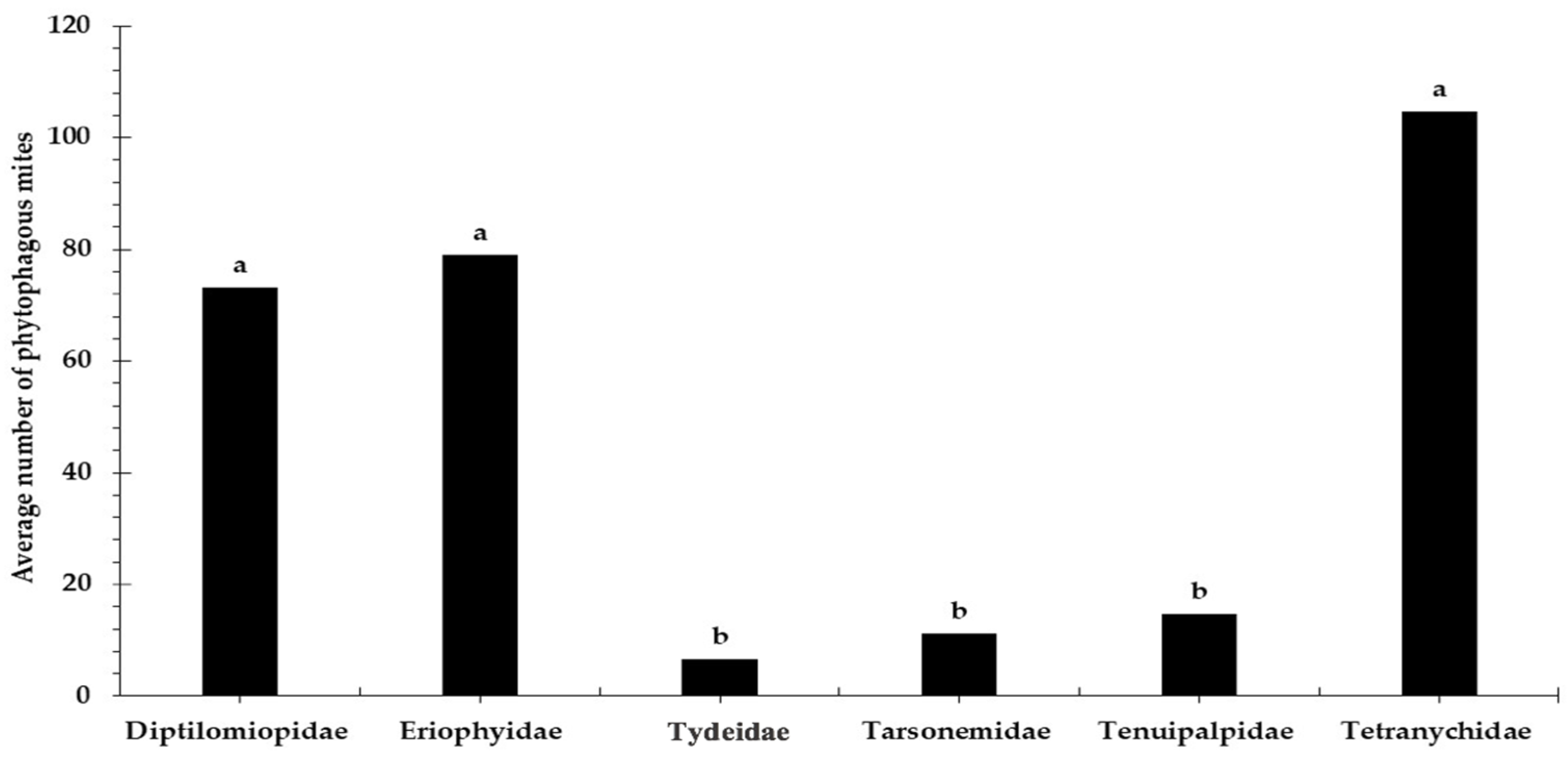
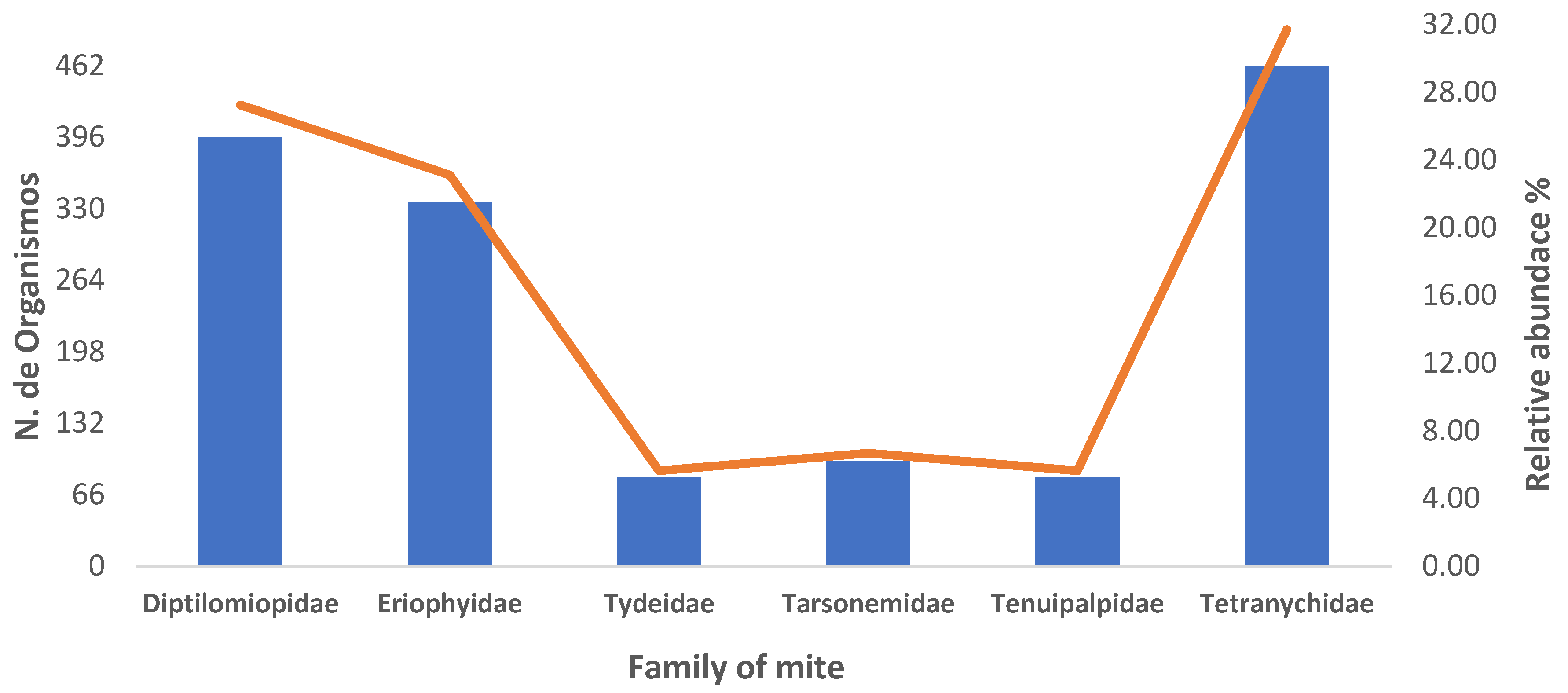
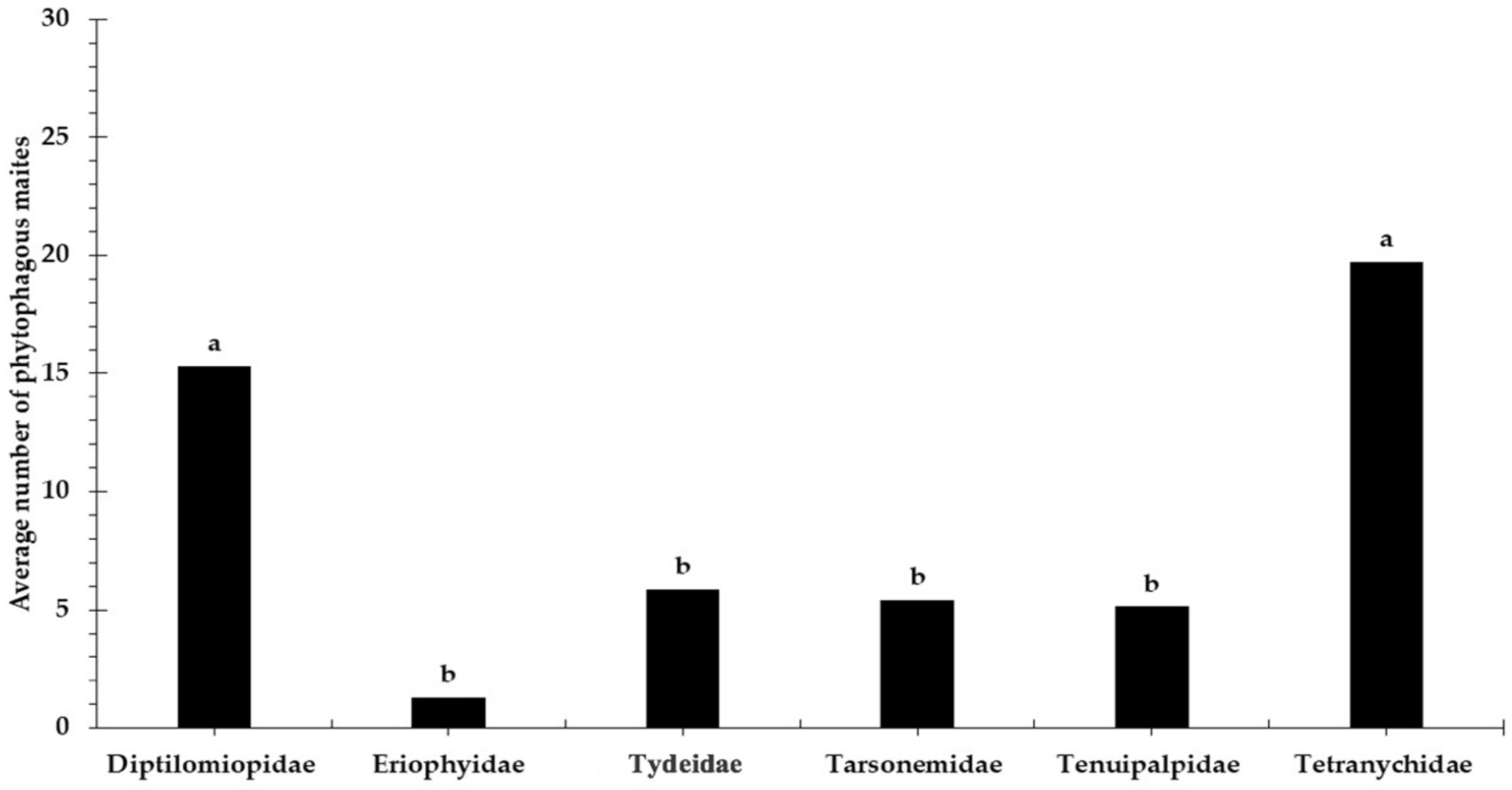
3.1. Relative Abundance and Population Fluctuation of Phytophagous Mite Families in Cultivated Blackberry (Rubus spp. L.)
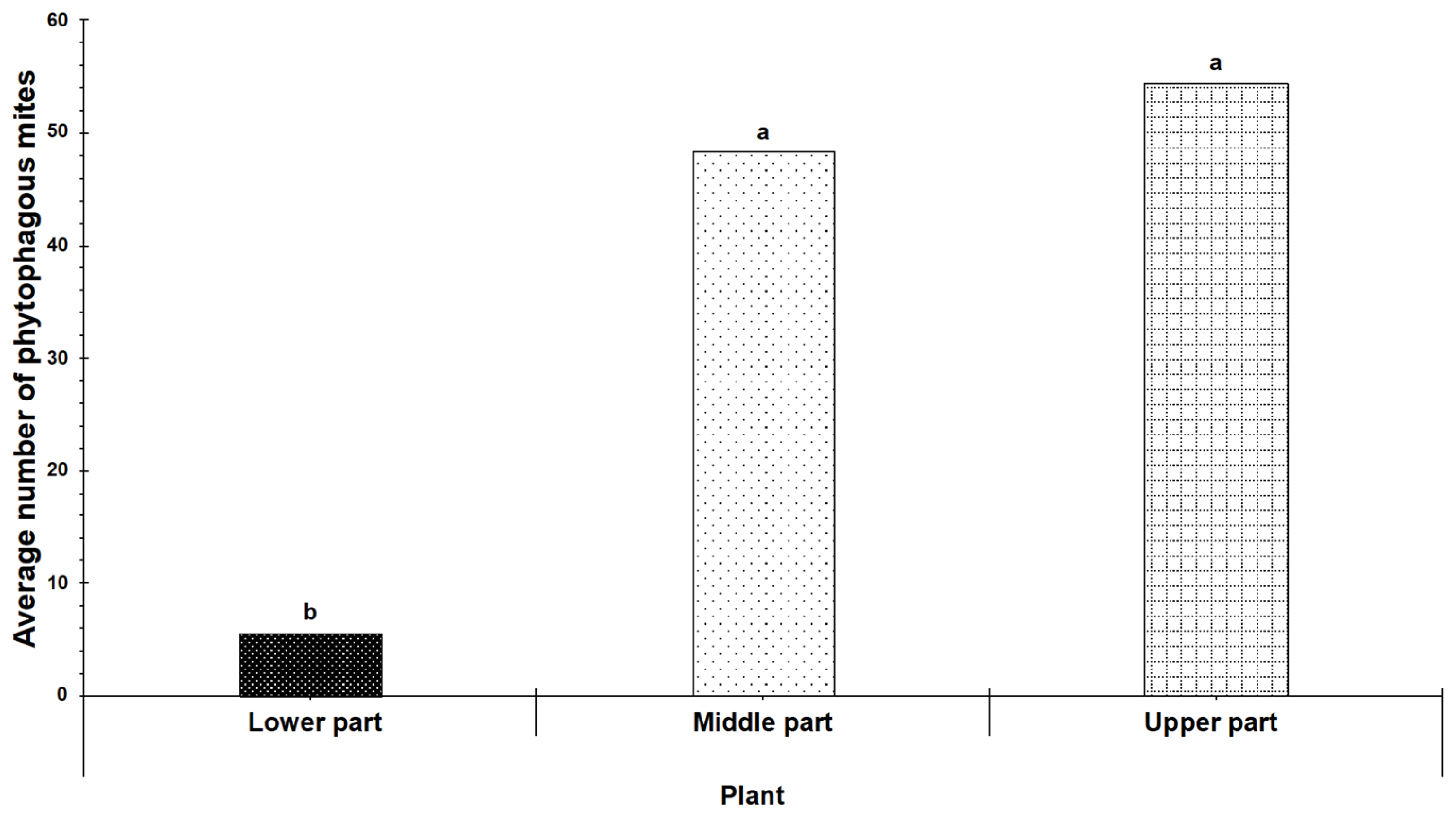
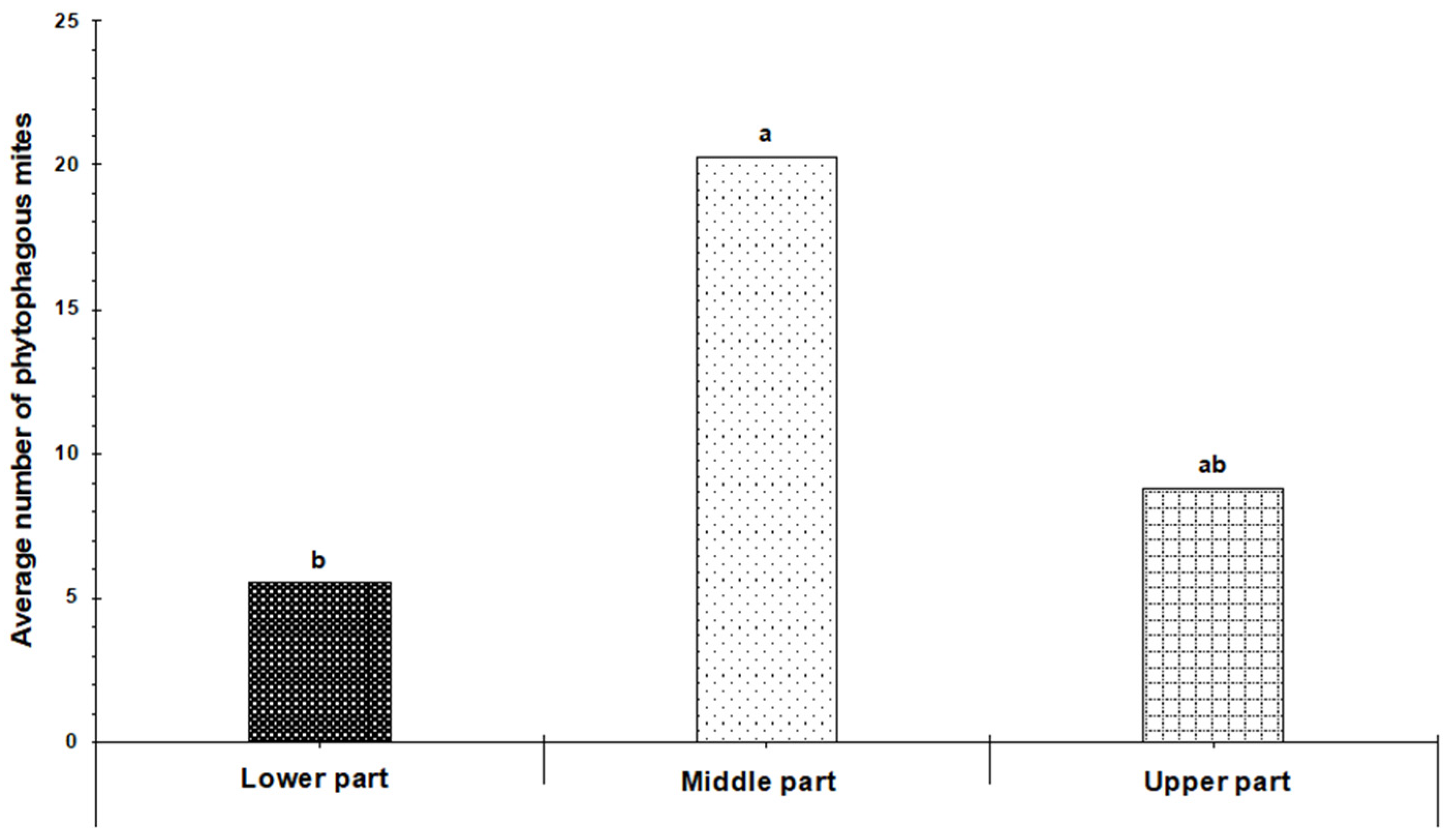
3.2. Spatial Distribution of Phytophagous Mite Families on Cultivated Blackberry Plants
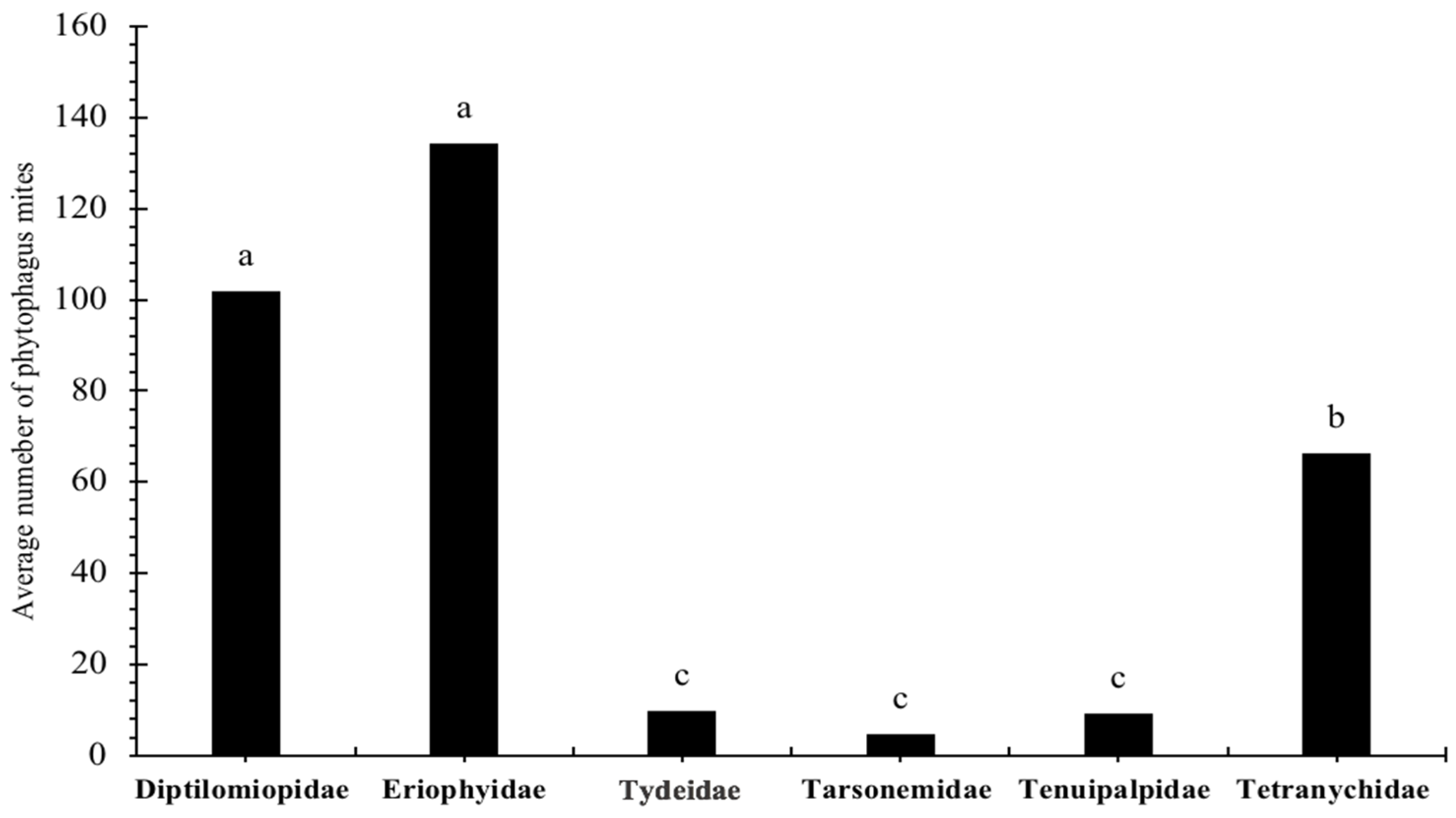
3.3. Relative Abundance and Population Fluctuation of Phytophagous Mite Families on Wild Blackberry (Rubus spp. L.)
3.4. Spatial Distribution of Phytophagous Mite Families on Wild Blackberry Plants
3.5. Determination of Quality Parameters of Cultivated Blackberry (Rubus spp. L.) Fruits
3.5.1. Physical Properties of Cultivated Blackberry Fruits
3.5.2. Physicochemical Properties
3.6. Determination of Quality Parameters of the Fruits of Wild Blackberry (Rubus spp. L.)
3.6.1. Physical Properties of Wild Blackberry Fruits
3.6.2. Physicochemical Properties of Wild Blackberry Fruits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ibarra Morales, L.E.; Romero Vivar, N.G.; Jaime Meuly, R.; Hurtado Bringas, B.A. Estudio de Factibilidad Para La Comercialización de Zarzamora En Mercados Internacionales. Rev. Int. Adm. Finanz. 2013, 6, 57–71. [Google Scholar]
- Servicio de Información Agroalimentaria y Pesquera (SIAP) Cierre de La Producción Agrícola Por Cultivo. Available online: https://nube.agricultura.gob.mx/cierre_agricola/ (accessed on 11 April 2024).
- Doreste, E. Acarología, 2nd ed.; Editorial Universidad de Costa Rica: San José, Costa Rica, 1984. [Google Scholar]
- Gerson, U. The Tenuipalpidae: An under-Explored Family of Plant-Feeding Mites. Syst. Appl. Acarol. 2008, 13, 83. [Google Scholar] [CrossRef]
- De Moraes, G.J.; Flechtmann, C.H.W. Manual de Acarología: Acarología Básica e Ácaros de Plantas Cultivadas no Brasil; Holos Editora: São Paulo, Brazil, 2008. [Google Scholar]
- Walter, D.E.; Lindquist, E.E.; Smith, I.M.; Cook, D.R.; Krantz, G.W. Order Trombidiformes. In A Manual of Acarology; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 233–420. [Google Scholar]
- Zhang, Z.-Q. Mites of Greenhouses: Identification, Biology and Control; Zhang, Z.-Q., Ed.; CABI Publishing: Wallingford, UK, 2003; ISBN 9780851995908. [Google Scholar]
- Lemus-Soriano, B.A. El Ácaro Del Berry Rojo, Una Amenaza En La Producción De Zarzamora; Serie Frutillas; Artículos Técnicos de INTAGRI: Uruapan, México, 2017; Volume 16, p. 2. [Google Scholar]
- Vargas-Madriz, H.; Fabian Grifaldo-Alcantara, P.; Rodríguez-Bautista, G.; Meza-Rodríguez, D.; Jiménez-Camberos, C.; Alberto Acuña-Soto, J.; Azuara-Domínguez, A.; Topete-Corona, C.; Topete-Corona, S. Ácaros En El Cultivo De Zarzamora (Rubus fruticosus, L.) Y Su Importancia Como Plagas Agrícolas. Boletín Soc. Mex. Entomol. (Nueva Ser.) 2022, 8, 1–8. [Google Scholar]
- Ayala-Ortega, J.D.J.; Martínez-Castillo, A.M.; Pineda-Guillermo, S.; Figueroa-De La Rosa, J.I.; Acuña-Soto, J.; Ramos-Lima, M.; Vargas-Sandoval, M. Mites Associated with Blackberry (Rubus sp. Cv. Tupy) in Two Areas of Michoacan, México. Rev. Colomb. Entomol. 2020, 45, e8480. [Google Scholar] [CrossRef]
- Amrine, J.W.; Manson, D.C.M. Preparation, mounting and descriptive study of eriophyoid mites. In Eriophyoid Mites—Their Biology, Natural Enemies and Control; Elsevier: Amsterdam, The Netherlands, 1996; pp. 383–396. [Google Scholar]
- Dhooria, M.S. Fundamentals of Applied Acarology; Springer: Singapore, 2016. [Google Scholar]
- Migeon, A.; Malagnini, M.; Navajas, M.; Duso, C. Notes on the Genus Eotetranychus (Acari: Tetranychidae) in Italy and France with a Redescription of Eotetranychus Fraxini Reck, New Record for Italy and Western Europe. Zootaxa 2007, 1509, 51–60. [Google Scholar] [CrossRef]
- Cardona-Mejía, C.; Mesa-Cobo, N.C. Entomología Económica y Manejo de Plagas; Universidad Nacional de Colombia: Palmira, Colombia, 2015. [Google Scholar]
- Davies, J.T.; Allen, G.R.; Williams, M.A. Intraplant Distribution of Acalitus Essigi (Acari: Eriophyoidea) on Blackberries (Rubus fruticosus Agg.). Exp. Appl. Acarol. 2001, 25, 625–639. [Google Scholar] [CrossRef]
- Marchetti, M.M.; Ferla, N.J. Diversidade e Flutuação Populacional de Ácaros (Acari) Em Amora-Preta (Rubus fruticosus, Rosaceae) No Estado Do Rio Grande Do Sul, Brasil. Iheringia. Ser. Zool. 2011, 101, 43–48. [Google Scholar] [CrossRef]
- Smith, J.F.; Catchot, A.L.; Musser, F.R.; Gore, J. Survey of Twospotted Spider Mite (Acari: Tetranychidae) Host Plants in the Mississippi Delta. J. Entomol. Sci. 2013, 48, 279–290. [Google Scholar] [CrossRef]
- Rodríguez, N.S.; Estébanez, M.L. Acarofauna Asociada a Vegetales de Importancia Agrícola y Económica En México; Universidad Autónoma metropolitana: México City, México, 1996; pp. 1–103. [Google Scholar]
- Walter, D.E. Predation and Mycophagy by Endeostigmatid Mites (Acariformes: Prostigmata). Exp. Appl. Acarol. 1988, 4, 159–166. [Google Scholar] [CrossRef]
- Zhang, Z.-Q. Phylum Arthropoda von Siebold, 1848 In: Zhang, Z.-Q. (Ed.) Animal Biodiversity: An Outline of Higher-Level Classification and Survey of Taxonomic Richness. Zootaxa 2011, 3148, 99–103. [Google Scholar] [CrossRef]
- Mejía-Recamier, B.E.; Palacios-Vargas, J.G. Three New Species of Neoscirula (Prostigmata: Cunaxidae) from a Tropical Dry Forest in Jalisco, Mexico. Zootaxa 2007, 1545, 17–31. [Google Scholar] [CrossRef]
- Acuña-Soto, J.A. Eriophyoidea. In Ácaros de Importancia Agrícola; Estrada-Venegas, E.G., Acuña-Soto, J.A., Chaires-Grijalva, M.P., Equihua-Martínez, A., Eds.; Universidad Autónoma de Chapingo: Texcoco, Mexico, 2012; Volume 1, pp. 158–190. [Google Scholar]
- Vargas-Madriz, H.; Acuña-Soto, J.A.; Rodríguez-Bautista, G.; Grifaldo-Alcantará, P.F.; García-Escamilla, P.; Lázaro-Dzul, M.O. Fluctuación Poblacional de Familias de Ácaros Asociados a Plantas de Zarzamora (Rubus fruticosus). Ecosistemas Recur. Agropecu. 2020, 7, 201–212. [Google Scholar]
- Livinali, E.; Sperotto, R.A.; Ferla, N.J.; de Souza, C.F.V. Physicochemical and Nutritional Alterations Induced by Two-Spotted Spider Mite Infestation on Strawberry Plants. Electron. J. Biotechnol. 2014, 17, 193–198. [Google Scholar] [CrossRef]
- Walter, D.E.; Krantz, G.W. Collection, rearing, and preparing specimens. In A Manual of Acarology; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 83–96. [Google Scholar]
- Castiglioni, E.; Navia, D. Presencia Del Ácaro Del Enrollamiento Del Trigo, Aceria Tosichella Keifer (Prostigmata: Eriophyidae), En Uruguay. Agrociencia 2010, 14, 19–26. [Google Scholar] [CrossRef]
- Walter, D.E.; Proctor, H.C. Mites: Ecology, Evolution & Behaviour; Springer: Dordrecht, The Netherlands, 2013; ISBN 978-94-007-7163-5. [Google Scholar]
- Lindquist, E.E.; Krantz, G.W.; Walter, D.E. Order Mesostigmata. In A Manual of Acarology; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 124–232. [Google Scholar]
- Krantz, G.W. Habits and Habitats. In A Manual of Acarology; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 64–82. [Google Scholar]
- Voss, D.H. Relating Colorimeter Measurement of Plant Color to the Royal Horticultural Society Color Charts. HortScience 1992, 27, 1256–1260. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; AOAC International: Washington, DC, USA, 1990. [Google Scholar]
- Tilman, D. Secondary Succession and the Pattern of Plant Dominance Along Experimental Nitrogen Gradients. Ecol. Monogr. 1987, 57, 189–214. [Google Scholar] [CrossRef]
- Villegas-Elizalde, S.E.; Rodríguez-Maciel, J.C.; Anaya-Rosales, S.; Sánchez-Arroyo, H.; Hernández-Morales, J.; Bujanos-Muñiz, R. Resistencia a Acaricidas En Tetranychus Urticae (Koch) Asociada al Cultivo de Fresa En Zamora, Michoacán, México. Agrociencia 2010, 44, 75–81. [Google Scholar]
- Pye, D.R.L.; De Lillo, E. A Review of the Eriophyoid Mites (Acari: Eriophyoidea) on Rubus spp. in Britain, with a New Species (Diptilomiopidae) and Two New Records. Zootaxa 2010, 2677, 15–26. [Google Scholar] [CrossRef]
- Royalty, R.N.; Perring, T.M. Chapter 3.1 Nature of Damage and Its Assessment. In World Crop Pests; Elsevier: Amsterdam, The Netherlands, 1996; pp. 493–512. [Google Scholar]
- Vargas-Madriz, H.; Lazaro-Dzul, M.O.; Azuara-Domínguez, A.; Acuña-Soto, J.A.; Monteón-Ojeda, A.; Romero-Rosales, T.; Rodríguez-Bautista, G.; Vanegas-Rico, J.M. Distribución Espacial y Fluctuación Poblacional de Familias de Ácaros Asociados a Plantas de Zarzamora Silvestre (Rubusfruticosits L.). Idesia (Arica) 2023, 41, 5–13. [Google Scholar] [CrossRef]
- Mejía-Serrano, I.L. Fluctuación Poblacional y Descripción de Familias de Ácaros Asociados a Plantas de Zarzamora Silvestre (Rubus spp.) En Jalisco, Mexico. Master’s Thesis, Universidad Politécnica de Francisco I. Madero, Tepic, Mexico, 2022. [Google Scholar]
- Fathipour, Y.; Maleknia, B. Mite Predators. In Ecofriendly Pest Management for Food Security; Elsevier: Amsterdam, The Netherlands, 2016; pp. 329–366. [Google Scholar]
- Gulati, R. Eco-Friendly Management of Phytophagous Mites. In Integrated Pest Management; Elsevier: Amsterdam, The Netherlands, 2014; pp. 461–491. [Google Scholar]
- Ibáñez Martínez, A. Caracterización de Zarzamora Silvestre (Rubus spp.) En La Sierra Norte y Nororiente de Puebla, y Sierra Centro de Veracruz. Ph.D. Thesis, Universidad Autónoma Chapingo, Chapingo, Mexico, 2011. [Google Scholar]
- Parra-Quezada, R.A.; Acosta-Rodríguez, G.F.; Arreola-Ávila, J.G. Crecimiento y Producción de Zarzamora Cv. Cheyenne Con Cubiertas Orgánicas. Terra Latinoam. 2005, 23, 233–240. [Google Scholar]
- Rubio Ochoa, E.; Pérez Sánchez, R.E.; Ávila Val, T.C.; Gómez Leyva, J.F.; García Saucedo, P.A. Propiedades Fisicoquímicas de Frutos Silvestres de Rubus Con Potencial Nutracéutico y Alimenticio. Rev. Mex. Cienc. Agric. 2019, 23, 291–301. [Google Scholar] [CrossRef]
- Moreno, B.L.; Deaquiz Oyola, Y.A. Caracterización de Parámetros Fisicoquímicos En Frutos de Mora (Rubus Alpinus Macfad). Acta Agron. 2015, 65, 130–136. [Google Scholar] [CrossRef]
- González-Jiménez, S.L.; Castillo-González, A.M.; García-Mateos, M.D.R.; Valdez-Aguilar, L.A.; Ybarra-Moncada, C.; Avitia-García, E. Respuesta de zarzamora (Rubus spp.) cv. tupy a la salinidad. Rev. Fitotec. Mex. 2020, 43, 299. [Google Scholar] [CrossRef]
- Salgado, A.A.; Clark, J.R. “Crispy” Blackberry Genotypes: A Breeding Innovation of the University of Arkansas Blackberry Breeding Program. HortScience 2016, 51, 468–471. [Google Scholar] [CrossRef]
- Zamorano Montañéz, A.; Morillo Coronado, Y.; Morillo Coronado, A.C.; Vásquez Ameriles, H.D.; Muñoz Flores, J.E. Caracterización Morfológica de Mora En Los Departamentos Del Valle Del Cauca, Cauca y Nariño, de Colombia. Acta Agron. 2007, 56, 51–60. [Google Scholar]
- García Muñoz, M.C. Elaboración de Un Paquete Tecnológico Para Productores, En Manejo Cosecha y Poscosecha de Mora (Rubus Glaucus Benth) Aplicando Ingeniería de Calidad y Determinación de Las Características Nutracéuticas de La Fruta En Precosecha, En El Municipio de Silvania–Cundinamarca. Master’s Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2012. [Google Scholar]
- Rincón Bonilla, C.L.; Moreno Medina, B.L.; Deaquiz Oyola, Y.A. Parámetros Poscosecha En Dos Materiales de Mora (Rubus Glaucus Benth y Rubus Alpinus Macfad). Cult. Científica 2015, 13, 17–25. [Google Scholar]
- Pinzón, I.M.D.P.; Fischer, G.; Corredor, G. Determinación de Los Estados de Madurez Del Fruto de La Gulupa (Passiflora Edulis Sims.). Agron. Colomb. 2007, 25, 83–95. [Google Scholar]
- Meza, M.A.C.; Romo, F.M.G.; Duarte, O.V.R.; Navarro, A.R. La Zarzamora (Rubus sp.), Cultivo Alternativo Para El Estado de Sonora. Rev. Mex. Agronegocios 2013, 33, 600–607. [Google Scholar]
- Sloan, R.C.; Matta, F.B. Performance of Blackberry and Raspberry Cultivars in Northern, 1992–1995. HortTechnology 1996, 21, 1–3. [Google Scholar]
- Amrine, J.A., Jr.; Stasny, T.A. Catalog of the Eriophyoidae (Acarina: Prostigmata) of the World; Indira Publishing House: West Bloomfield, MI, USA, 1994. [Google Scholar]
- Baker, E.W.; Kono, T.; Amrine, J.W., Jr.; Delfinado-Baker, M.; Stasny, T.A. Eriophyoid Mites of the United States; Indira Publishing House: West Bloomfield, MI, USA, 1996. [Google Scholar]
- Trinidad, C.T.O.; Duarte, M.E.; Da Cunha, U.S.; Navia, D. Eriophyoid Mites Associated with the Blackberry in Brazil—A New Species in the Genus Diptacus Keifer 1951 (Diptilomiopidae) and First Report and Supplementary Description of Acalitus Orthomerus (Keifer, 1951) (Eriophyidae). Syst. Appl. Acarol. 2018, 23, 1199. [Google Scholar] [CrossRef]
- Ayala-Ortega, J.D.J.; Acuña-Soto, J.A.; Martínez-Castillo, A.M.; Lara-Chávez, M.B.N.; Vargas-Sandoval, M. Primer Registro de Acalitus Orthomera (Keifer) (Acari: Eriophyidae) Asociado al Cultivo de La Zarzamora (Rubus fruticosus Var. Tupy) En Ziracuaretiro, Michoacán, México. Acta Zoológica Mex. (N.S.) 2019, 35, 1–4. [Google Scholar] [CrossRef]
- Abrego-Alvarez, O. Eriófidos Asociados al Cultivo de Zarzamora (Rubus Subgénero Rubus) y Distribución Intraplanta de *Phyllocoptes Gracilis* (Nalepa) (Acari: Eriophyidae) En Michoacán. Bachelor’s Thesis, Facultad de Estudios Superiores Cuautitlán, Universidad Nacional Autónoma de México, Cuautitlán Izcalli, México, 2023. [Google Scholar]
- Acuña-Soto, J.A.; Vargas-Madriz, H.; Talavera-Villareal, A.; Lázaro-Dzul, O.; Grifaldo-Alcántara, F.; Azuara-Domínguez, A. New Distribution Records of Phytophagous Mites Associated with Cultivated Blackberries at Zapotlan de Vadillo, Jalisco, Mexico. Southwest. Entomol. 2019, 44, 779–784. [Google Scholar] [CrossRef]
- Arreguín-Zavala, J.D.J.; Otero-Colina, G.; Pineda, S.; López-Bautista, E.; Flores-Martínez, B.A.; Rebollar-Alviter, Á. Evaluation of Different Control Strategies for the Management of Redberry Disease Associated with Acalitus Orthomera (Eriophyoidea: Eriophyidae) in Commercial Blackberry Crops. J. Plant Dis. Prot. 2021, 128, 191–202. [Google Scholar] [CrossRef]
- Szendrey, G.; Ilovai, Z.; Lucza, Z. Damage Caused by Blackberry Mite (Acalitus Essigi Hassan) and the Role of Natural Biological Control Agents in Integrated Blackberry Production System in Hungary. IOBC/WPRS Bull. 2003, 226, 133–138. [Google Scholar]
- Cross, J.; Fountain, M.; Harris, A.; Gillian, A. Evaluation of Acaricides for the Control of Blackberry Mite (Acalitus Essigi) to Reduce Redberry Disease on Blackberry; East Malling: Kent, UK, 2012. [Google Scholar]
- Venzon, M.; Oliveira, R.M.; Perez, A.L.; Rodríguez-Cruz Filho, S.M. Lime Sulfur Toxicity to Broad Mite, to Its Host Plants and to Natural Enemies. Pest. Manag. Sci. 2013, 69, 738–743. [Google Scholar] [CrossRef]
- Tuelher, E.S.; Venzon, M.; Guedes, R.N.; Pallini, A. Toxicity of Organic-Coffee-Approved Products to the Southern Red Mite Oligonychus Ilicis and Its Predator Iphiseiodes Zuluagai. Crop Prot. 2013, 55, 28–34. [Google Scholar] [CrossRef]




| Mite Family | Lower Layer | Middle Layer | Upper Layer |
|---|---|---|---|
| Eriophyidae | 214 | 553 | 940 |
| Tetranychidae | 177 | 733 | 464 |
| Diptilomiopidae | 81 | 512 | 713 |
| Tarsonemidae | 138 | 79 | 34 |
| Tenuipalpidae | 45 | 103 | 65 |
| Tydeidae | 17 | 47 | 69 |
| Mite Family | Lower Layer | Middle Layer | Upper Layer |
|---|---|---|---|
| Eriophyidae | 7 | 9 | 320 |
| Tetranychidae | 98 | 138 | 225 |
| Diptilomiopidae | 51 | 107 | 238 |
| Tarsonemidae | 59 | 38 | 0 |
| Tenuipalpidae | 13 | 36 | 33 |
| Tydeidae | 3 | 41 | 38 |
| Variable | Damaged Fruit | Healthy Fruit | p | Damaged Fruit | Healthy Fruit | ||
|---|---|---|---|---|---|---|---|
| LI | LS | LI | LS | ||||
| Physical Variables | |||||||
| Weight (g) | 4.49 ± 1.44 b | 6.1 ± 1.22 a | 0.0001 | 4.15 | 4.83 | 5.81 | 6.38 |
| Firmness (N) | 40.84 ± 19.25 a | 29.81 ± 19.70 b | 0.0002 | 36.31 | 45.36 | 25.18 | 34.44 |
| Length (mm) | 22.28 ± 3.72 b | 26.78 ± 2.48 a | 0.0001 | 21.41 | 23.16 | 26.19 | 27.36 |
| Diameter (mm) | 18.11 ± 2.00 b | 21.19 ± 1.98 a | 0.0001 | 17.64 | 18.58 | 20.73 | 21.65 |
| L (mm) | 31.23 ± 9.23 a | 26.8 ± 6.03 b | 0.0031 | 29.06 | 33.4 | 25.38 | 28.22 |
| Hue (a) | 1.06 ± 1.60 a | 0.82 ± 0.24 b | 0.0187 | 0.69 | 1.44 | 0.77 | 0.68 |
| Chroma (b) | 4.97 ± 1.46 a | 3.97 ± 2.00 b | 0.0014 | 4.63 | 5.31 | 3.5 | 4.44 |
| Physicochemical Variables | |||||||
| °Brix (%) | 0.76 ± 0.22 a | 0.83 ± 0.16 a | 0.1914 | 0.7100 | 0.8200 | 0.8000 | 0.8700 |
| Titratable Acidity (%) | 80.07 ± 19.10 a | 71.54 ± 15.98 b | 0.0177 | 75.580 | 84.560 | 67.790 | 75.300 |
| Vitamin C (mg/100 g) | 4.76 ± 1.53 b | 5.31 ± 0.83 a | 0.0008 | 4.4000 | 5.1200 | 5.1300 | 5.5300 |
| Variable | Damaged Fruit | Healthy Fruit | p | Damaged Fruit | Healthy Fruit | ||
|---|---|---|---|---|---|---|---|
| LI | LS | LI | LS | ||||
| Physical Variables | |||||||
| Weight (g) | 1.41 ± 0.89 b | 2.44 ± 1.03 a | 0.0001 | 1.2 | 1.62 | 2.2 | 2.69 |
| Firmness (N) | 28.98 ± 17.81 a | 26.78 ± 16.24 a | 0.6632 | 24.78 | 33.18 | 22.97 | 30.6 |
| Length (mm) | 13.33 ± 4.01 b | 17.06 ± 3.26 a | 0.0001 | 12.39 | 14.28 | 16.3 | 17.83 |
| Diameter (mm) | 13.31 ± 2.44 b | 15.31 ± 2.10 a | 0.0001 | 12.73 | 13.88 | 14.82 | 15.8 |
| L (mm) | 22.79 ± 4.49 a | 21.85 ± 1.81 a | 0.6034 | 21.74 | 23.85 | 21.42 | 22.27 |
| Hue (a) | 3.75 ± 19.62 a | 0.088 ± 0.41 a | 0.0944 | −0.86 | 8.36 | 0.78 | 0.97 |
| Chroma (b) | 2.64 ± 1.11 a | 2.05 ± 0.73 b | 0.0001 | 2.38 | 2.9 | 1.88 | 2.22 |
| Physicochemical Variables | |||||||
| °Brix (%) | 0.68 ± 0.17 a | 0.68 ± 0.22 a | 0.8672 | 0.6400 | 0.7200 | 0.6200 | 0.7300 |
| Titratable Acidity (%) | 71.82 ± 24.76 a | 64.56 ± 19.68 b | 0.0125 | 66.000 | 77.640 | 59.930 | 69.180 |
| Vitamin C (mg/100 g) | 6.42 ± 2.22 a | 6.54 ± 2.48 a | 0.6361 | 5.9000 | 6.9400 | 5.9500 | 7.1200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Madriz, H.; Azuara-Domínguez, A.; Vargas-Madriz, Á.F.; Topete-Corona, C.; Lázaro-Dzul, M.O.; Acuña-Soto, J.A.; Venegas-Barrera, C.S.; Chávez-Servín, J.L.; Kuri-García, A. Population Fluctuation of Phytophagous Mites and Their Impact on the Quality Properties of Wild and Cultivated Blackberry Fruits (Rubus spp. L.) in Jalisco, Mexico. Agronomy 2025, 15, 1970. https://doi.org/10.3390/agronomy15081970
Vargas-Madriz H, Azuara-Domínguez A, Vargas-Madriz ÁF, Topete-Corona C, Lázaro-Dzul MO, Acuña-Soto JA, Venegas-Barrera CS, Chávez-Servín JL, Kuri-García A. Population Fluctuation of Phytophagous Mites and Their Impact on the Quality Properties of Wild and Cultivated Blackberry Fruits (Rubus spp. L.) in Jalisco, Mexico. Agronomy. 2025; 15(8):1970. https://doi.org/10.3390/agronomy15081970
Chicago/Turabian StyleVargas-Madriz, Haidel, Ausencio Azuara-Domínguez, Ángel Félix Vargas-Madriz, Citlally Topete-Corona, Martha Olivia Lázaro-Dzul, Jesús Alberto Acuña-Soto, Crystian Sadiel Venegas-Barrera, Jorge Luis Chávez-Servín, and Aarón Kuri-García. 2025. "Population Fluctuation of Phytophagous Mites and Their Impact on the Quality Properties of Wild and Cultivated Blackberry Fruits (Rubus spp. L.) in Jalisco, Mexico" Agronomy 15, no. 8: 1970. https://doi.org/10.3390/agronomy15081970
APA StyleVargas-Madriz, H., Azuara-Domínguez, A., Vargas-Madriz, Á. F., Topete-Corona, C., Lázaro-Dzul, M. O., Acuña-Soto, J. A., Venegas-Barrera, C. S., Chávez-Servín, J. L., & Kuri-García, A. (2025). Population Fluctuation of Phytophagous Mites and Their Impact on the Quality Properties of Wild and Cultivated Blackberry Fruits (Rubus spp. L.) in Jalisco, Mexico. Agronomy, 15(8), 1970. https://doi.org/10.3390/agronomy15081970










