Transcriptomics and Metabolomics Analyses Reveal How Rhizobacteria Acinetobacter calcoaceticus Enhance the Growth and Stress Tolerance in Lespedeza davurica
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Plant Material
2.2. Bacterial Inoculum Preparation
2.3. Plant Growth Assay and Experimental Design
2.4. Biochemical Assays
2.5. Transcriptome Sequencing and Analysis
2.6. Metabolomic Analysis
2.7. Statistical Analysis
3. Results
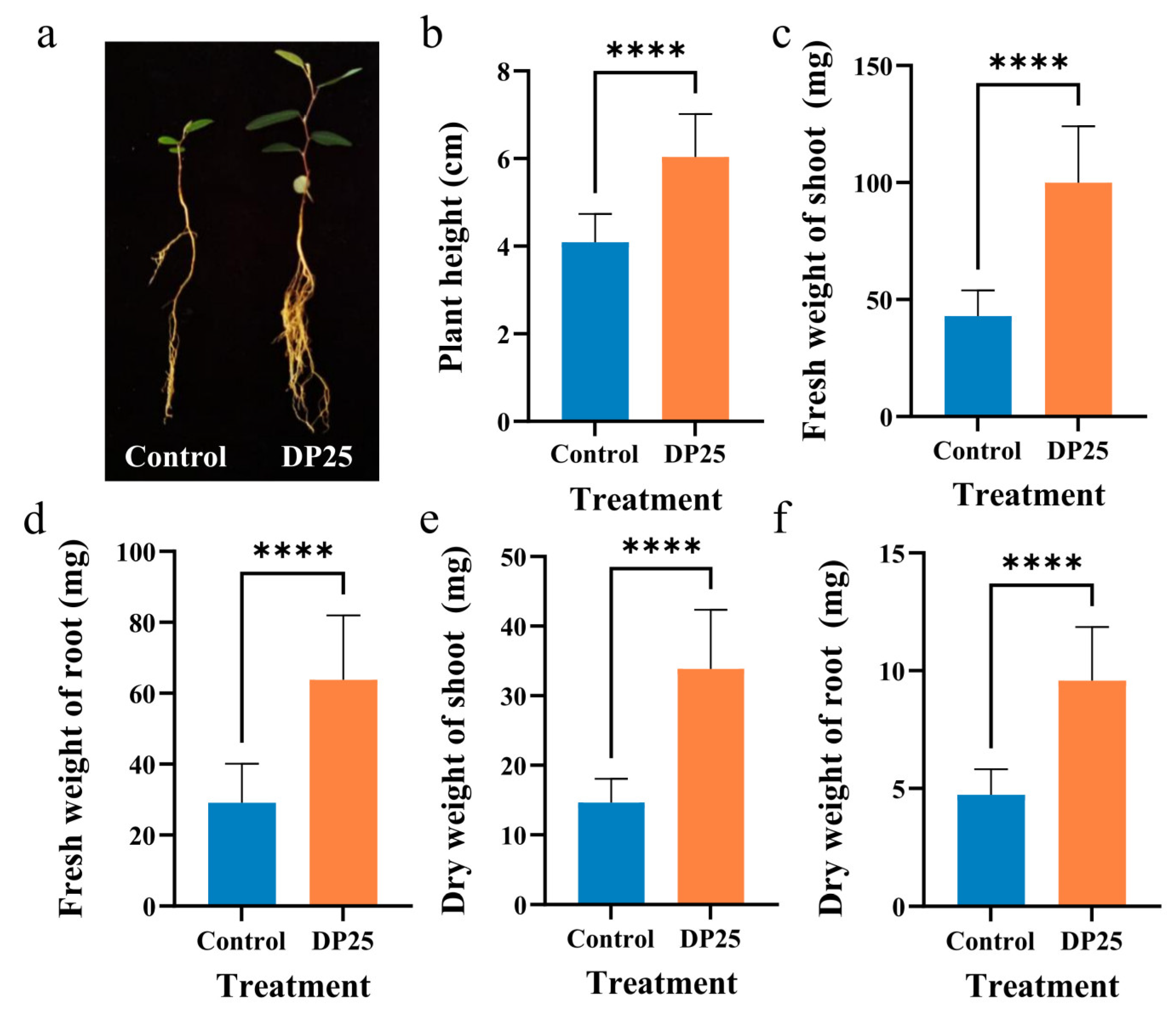
3.1. A. calcoaceticus DP25 Inoculation Significantly Enhances Growth Performance of L. davurica
3.2. A. calcoaceticus DP25 Treatment Modulates Antioxidant Defense Systems and Stress-Related Metabolites
3.3. Transcriptomic Profiling of A. calcoaceticus DP25-Mediated Growth Promotion in L. davurica
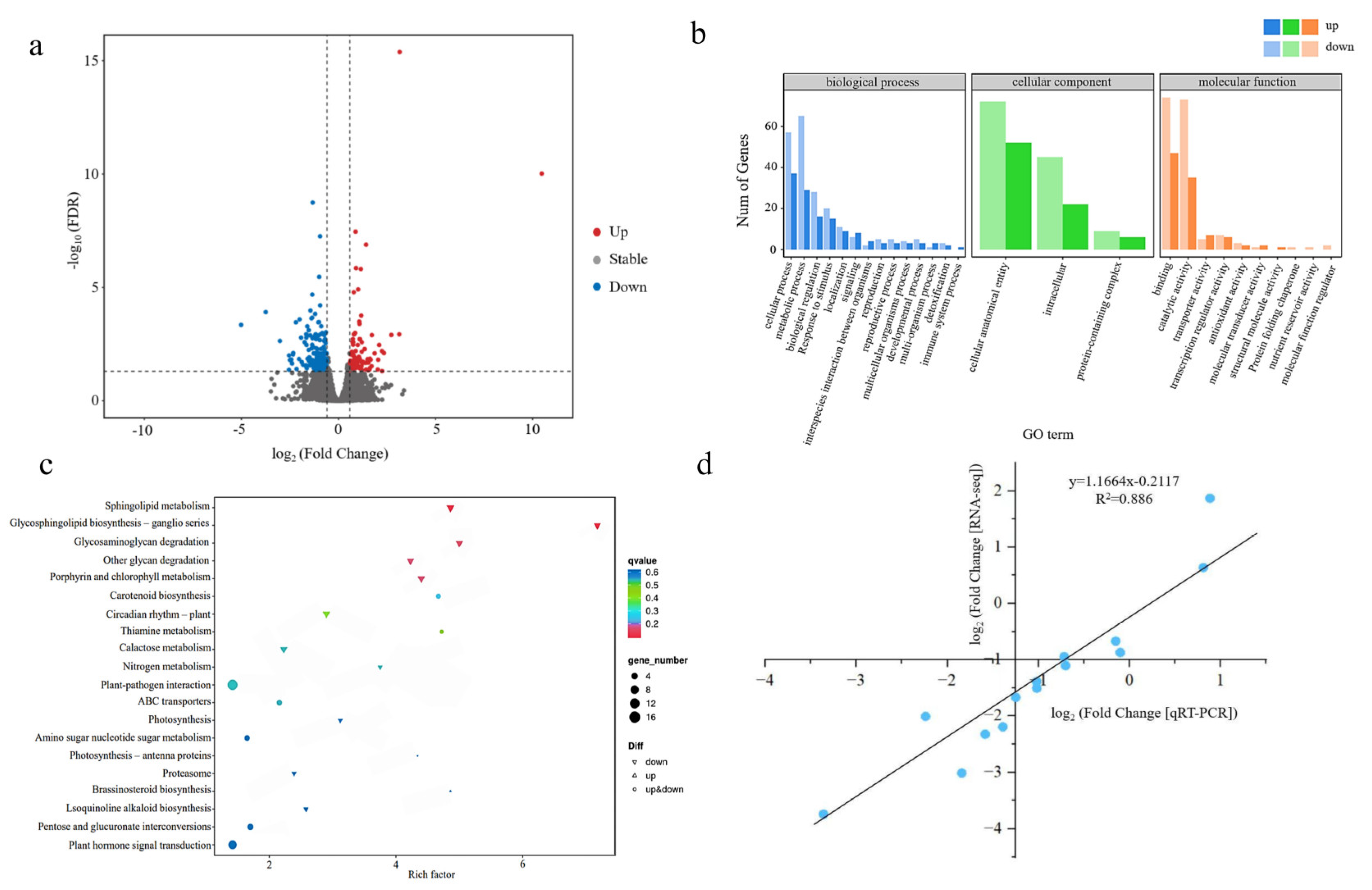
3.3.1. Transcriptome Sequencing and Identification of Differentially Expressed Genes (DEGs)
3.3.2. GO Functional Enrichment Analysis of DEGs
3.3.3. Functional Enrichment Analysis of DEGs Using the Kyoto Encyclopedia of Genes and Genomes (KEGG)
3.3.4. Verification of DEGs by qRT-PCR
3.4. Metabolomic Analysis of the Growth Promotion Effect of DP25 on L. davurica
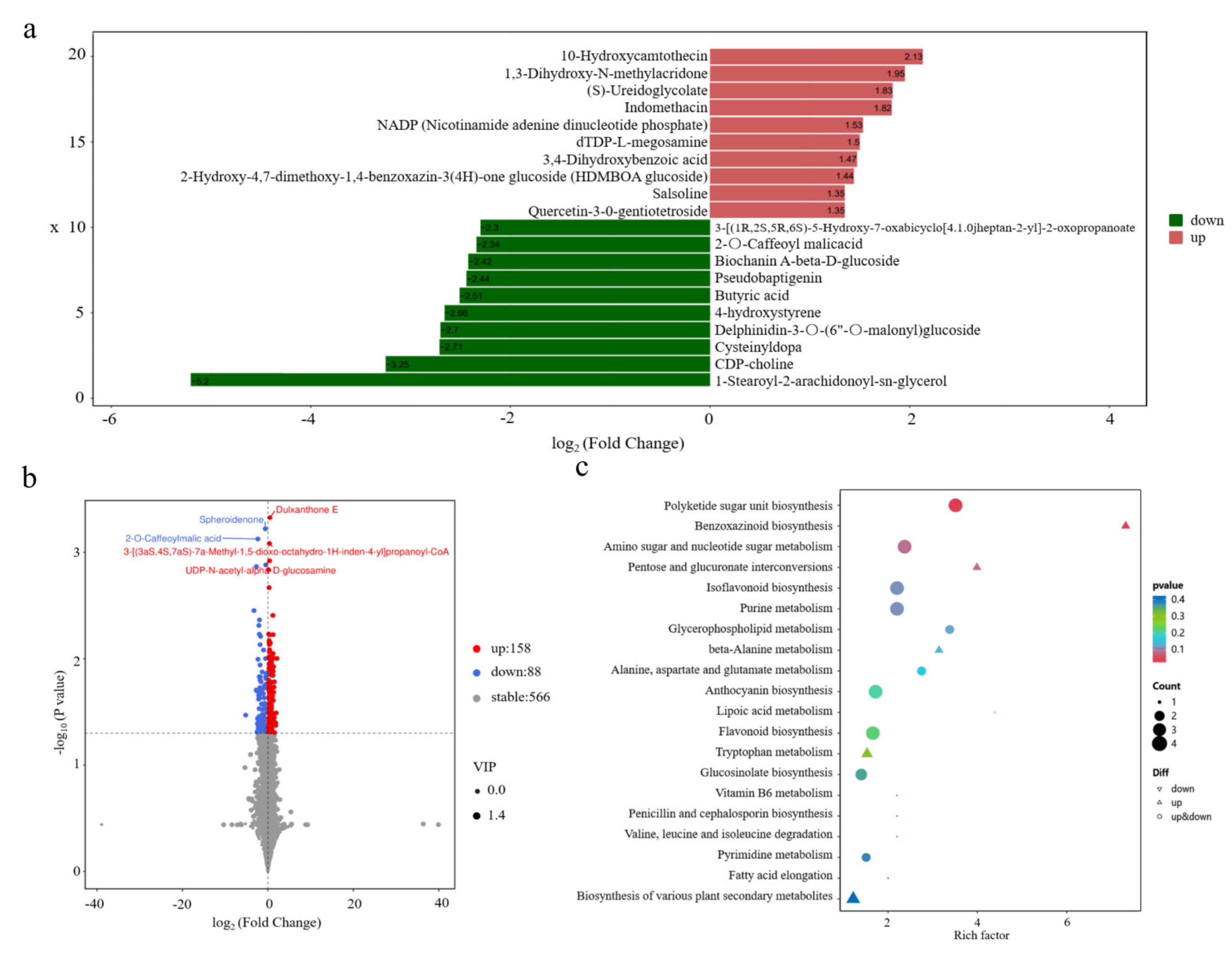
3.4.1. Metabolite Annotation
3.4.2. Statistical Characterization of Significantly Altered Metabolites
3.4.3. Functional Enrichment Analysis of DAMs Using KEGG
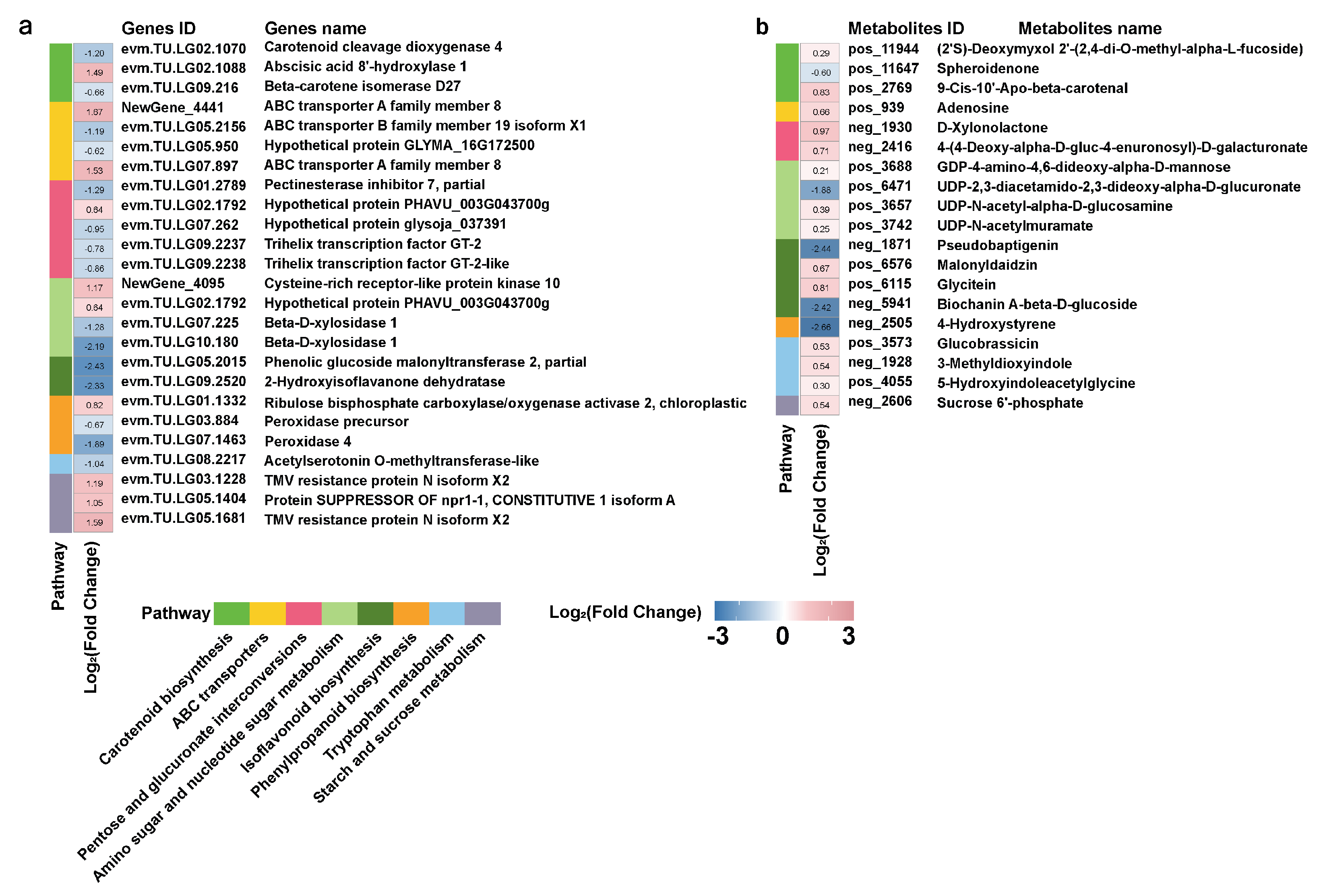
3.5. Transcriptome and Metabolomics Integration Analysis of the Growth Promoting Effect of DP25 on L. davurica
4. Discussion
4.1. PGPR-Mediated Enhancement of Plant Growth and Stress Tolerance
4.2. Molecular Mechanisms Underlying PGPR–Plant Interactions
4.3. Secondary Metabolite Biosynthesis and Plant Defense
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Wei, W.; Fu, B.; Lu, Y. Soil and water conservation on the Loess Plateau in China: Review and perspective. Prog. Phys. Geogr. 2007, 31, 3547–3554. [Google Scholar] [CrossRef]
- Zhao, G.; Mu, X.; Wen, Z.; Wang, F. Soil erosion, conservation, and eco-environment changes in the Loess Plateau of china. Land Degrad. Dev. 2013, 24, 499–510. [Google Scholar] [CrossRef]
- Lirong Tong, J.W. Morphological study on the differentiation of flower buds and the embryological stages of male and female floral organs in Lespedeza davurica (Laxm.) Schindl. cv. JinNong (Fabaceae). Plants 2024, 13, 1661. [Google Scholar] [CrossRef]
- Wang, F.; Shi, L.; Zhang, R.; Xu, W.; Xu, W.; Bo, Y.; Bo, Y. Effects of nitrogen addition and Bothriochloa ischaemum and Lespedeza davurica mixture on plant chlorophyll fluorescence and community production in semi-arid grassland. Front. Plant Sci. 2024, 15, 1400309. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Chen, J.; Xiong, P.; Wang, Z.; Zhang, H.; Xu, B. Responses of soil respiration to rainfall pulses in a natural grassland community on the semi-arid Loess Plateau of China. Catena 2019, 178, 199–208. [Google Scholar] [CrossRef]
- Ma, J.; Chen, Y.; Zhou, J.; Wang, K.; Wu, J. Soil quality should be accurate evaluated at the beginning of lifecycle after land consolidation for eco-sustainable development on the Loess Plateau. J. Clean. Prod. 2020, 267, 122244. [Google Scholar] [CrossRef]
- Xu, B.; Xu, W.; Wang, Z.; Chen, Z.; Palta, J.; Chen, Y. Accumulation of N and P in the legume Lespedeza davurica in controlled mixtures with the grass bothriochloa ischaemum under varying water and fertilization conditions. Front. Plant Sci. 2018, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Gao, Z.; Wang, J.; Xu, W.; Palta, J.; Chen, Y. N:P ratio of the grass Bothriochloa ischaemum mixed with the legume Lespedeza davurica under varying water and fertilizer supplies. Plant Soil 2016, 400, 67–79. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of plant growth promoting rhizobacteria in agricultural sustainability—A Review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.-S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Singh, A.; Yadav, V.K.; Chundawat, R.S.; Soltane, R.; Awwad, N.S.; Ibrahium, H.A.; Yadav, K.K.; Vicas, S.I. Enhancing plant growth promoting rhizobacterial activities through consortium exposure: A review. Front. Bioeng. Biotechnol. 2023, 11, 1099999. [Google Scholar] [CrossRef] [PubMed]
- Balemi, T.; Negisho, K. Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: A review. J. Soil Sci. Plant Nutr. 2012, 12, 547–562. [Google Scholar] [CrossRef]
- Khan, M.; Asaf, S.; Khan, A.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.M.; Lee, I.J. Plant growth-promoting endophytic bacteria augment growth and salinity tolerance in rice plants. Plant Biol. 2020, 22, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Sharif, M.; Shah, F.; Ali, A.; Adnan, M.; Shah, S.; Danish, S.; Ali, M.A.; Ahmed, N.; Arslan, H. Mycorrhiza and phosphate solubilizing bacteria: Potential bioagents for sustainable phosphorus management in agriculture. Phyton 2022, 91, 257. [Google Scholar] [CrossRef]
- Palaniappan, P.; Chauhan, P.S.; Saravanan, V.S.; Anandham, R.; Sa, T. Isolation and characterization of plant growth promoting endophytic bacterial isolates from root nodule of Lespedeza sp. Biol. Fertil. Soils 2010, 46, 807–816. [Google Scholar] [CrossRef]
- Li, C.; Jia, Z.; Peng, X.; Zhai, L.; Zhang, B.; Liu, X.; Zhang, J. Functions of mineral-solubilizing microbes and a water retaining agent for the remediation of abandoned mine sites. Sci. Total Environ. 2021, 761, 143215. [Google Scholar] [CrossRef]
- Ishizawa, H.; Ogata, Y.; Hachiya, Y.; Tokura, K.-I.; Kuroda, M.; Inoue, D.; Toyama, T.; Tanaka, Y.; Mori, K.; Morikawa, M.; et al. Enhanced biomass production and nutrient removal capacity of duckweed via two-step cultivation process with a plant growth-promoting bacterium, Acinetobacter calcoaceticus P23. Chemosphere 2020, 238, 124682. [Google Scholar] [CrossRef]
- Laha, A.; Sarkar, S.; Sengupta, S.; Das, A.; Paul, S.; Bhattacharyya, S. Unraveling the potential of Acinetobacter calcoaceticus for arsenic resistance and plant growth promotion in contaminated lentil field. S. Afr. J. Bot. 2024, 168, 61–70. [Google Scholar] [CrossRef]
- Dutta, P.; Muthukrishnan, G.; Gopalasubramaiam, S.K.; Dharmaraj, R.; Karuppaiah, A.; Loganathan, K.; Periyasamy, K.; Pillai, M.A.; Upamanya, G.K.; Boruah, S.; et al. Plant growth-promoting rhizobacteria (PGPR) and its mechanisms against plant diseases for sustainable agriculture and better productivity. Biocell 2022, 46, 1843–1859. [Google Scholar] [CrossRef]
- Ríos-Ruiz, W.F.; Jave-Concepción, H.G.; Torres-Chávez, E.E.; Rios-Reategui, F.; Padilla-Santa-Cruz, E.; Guevara-Pinedo, N.E. Plant-growth-promoting microorganisms: Their impact on crop quality and yield, with a focus on rice. Int. J. Plant Biol. 2025, 16, 9. [Google Scholar] [CrossRef]
- Yin, H.; Jing, Y.; Lin, Y.; Song, N.; Zong, H.; Wang, F.; Li, S.; Song, X.; Hou, H.; Guan, Y.S.; et al. Phosphorus and selenium compounding mitigates Cr stress in peanut seedlings by enhancing growth homeostasis and antioxidant properties. Environ. Sci. Pollut. Res. 2024, 31, 50929–50941. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Nagpal, S.; Sharma, P. Potential of plant growth-promoting rhizobacteria-plant interactions in mitigating salt stress for sustainable agriculture: A review. Pedosphere 2022, 32, 223–245. [Google Scholar] [CrossRef]
- Ullah, S.; Ashraf, M.; Asghar, H.N.; Iqbal, Z.; Ali, R. Plant growth promoting rhizobacteria mediated amelioration of drought in crop plants: A Review. Soil Environ. 2019, 38, 1–20. [Google Scholar] [CrossRef]
- Kanghong, F.; Ping, S.; Youyuan, C.; Jiameng, G.; Kanghong, F.; Ping, S.; Youyuan, C.; Jiameng, G. Endophytic bacteria promote the growth of Suaeda glauca in saline-alkali stress: Regulation of osmotic pressure and antioxidative defense system. J. Ocean Univ. China 2023, 22, 1109–1118. [Google Scholar] [CrossRef]
- Abogadallah, G.M. Antioxidative defense under salt stress. Plant Signal. Behav. 2010, 5, 369–374. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, R.; Gheith, E.M.S.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci. Rep. 2021, 11, 3195. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, R.; Liu, L.; Zhang, W.; Yin, Z.; Guo, R.; Wang, D.; Guo, C. Integrated physiological, transcriptomic and metabolomic analyses of the response of rice to aniline toxicity. Int. J. Mol. Sci. 2025, 26, 582. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, M.; Mahmood, A.; Alawadi, H.F.N.; Adiba, A.; Javaid, M.M.; Khan, B.A.; Wahid, A.; Abdullah, F.; Seleiman, M.F. Impact of boron on Glycine max L. to mitigate salt stress by modulating the morpho-physiological and biochemical responses. BMC Plant Biol. 2025, 25, 286. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Chen, Y. Transcriptomics: Advances and approaches. Sci. China Life Sci. 2013, 56, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Rychlik, I.; Martin, G.; Methner, U.; Lovell, M.; Cardova, L.; Sebkova, A.; Sevcik, M.; Damborsky, J.; Barrow, P.A. Identification of Salmonella enterica serovar Typhimurium genes associated with growth suppression in stationary-phase nutrient broth cultures and in the chicken intestine. Arch. Microbiol. 2002, 178, 411–420. [Google Scholar] [CrossRef]
- De Leersnyder, I.; De Gelder, L.; Van Driessche, I.; Vermeir, P. Influence of growth media components on the antibacterial effect of silver ions on Bacillus subtilis in a liquid growth medium. Sci. Rep. 2018, 8, 9325. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Bai, J.; Cai, Z.; Ouyang, F. Optimization of a cultural medium for bacteriocin production by Lactococcus lactis using response surface methodology. J. Biotechnol. 2002, 93, 27–34. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yong-Sheng, L.; Gao, X.-F. A new method for accurate determination of peroxidase activity based on fluorescence decrease of guaiacol. Chin. J. Anal. Chem. 2015, 43, 1040–1046. [Google Scholar]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Georgiou, C.D.; Grintzalis, K.; Zervoudakis, G.; Papapostolou, I. Mechanism of Coomassie brilliant blue G-250 binding to proteins: A hydrophobic assay for nanogram quantities of proteins. Anal. Bioanal. Chem. 2008, 391, 391–403. [Google Scholar] [CrossRef]
- Loewus, F.A. Improvement in anthrone method for determination of carbohydrates. Anal. Chem. 1952, 24, 219. [Google Scholar] [CrossRef]
- Ábrahám, E.; Cabassa, C.; Erdei, L.; Szabados, L. Methods for determination of proline in plants. Methods Mol. Biol. 2010, 639, 317–331. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Ali, M.B.; Hahn, E.-J.; Paek, K.-Y. Effects of light intensities on antioxidant enzymes and malondialdehyde content during short-term acclimatization on micropropagated Phalaenopsis plantlet. Environ. Exp. Bot. 2005, 54, 109–120. [Google Scholar] [CrossRef]
- Gan, P.; Li, P.; Zhang, X.; Li, H.; Ma, S.; Zong, D.; He, C. Comparative Transcriptomic and Metabolomic Analyses of Differences in Trunk Spiral Grain in Pinus yunnanensis. Int. J. Mol. Sci. 2023, 24, 14658. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Q.; Chen, Y.; Huang, X.; Feng, Y.; Shi, Y.; Li, R.; Yin, X.; Song, X.; Liang, Y.; et al. Ferritinophagy mediates adaptive resistance to EGFR tyrosine kinase inhibitors in non-small cell lung cancer. Nat. Commun. 2024, 15, 4195. [Google Scholar] [CrossRef]
- Yan, F.; Wei, T.; Yang, C.; Yang, Y.; Luo, Z.; Jiang, Y. Combined analysis of untargeted metabolomics and transcriptomics revealed seed germination and seedling establishment in Zelkova schneideriana. Genes 2024, 15, 488. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Meng, D.; Wang, Y.; Fernie, A.R.; Bai, C.; He, X.; Tao, J.; Zhou, X.; Ren, Y.; Zuo, J.; et al. Chlorine dioxide affects metabolism of harvested cauliflower. Postharvest Biol. Technol. 2025, 230, 113735. [Google Scholar] [CrossRef]
- Rattan, B.; Banerjee, A.; Dhobale, K.V.; Garg, A.; Sreedeep, S.; Sahoo, L. Examining the soil bacterial community under the combined influence of water-absorbing polymer and plant subjected to drought stress. Plant Soil 2024, 504, 763–777. [Google Scholar] [CrossRef]
- Freschet, G.T.; Violle, C.; Bourget, M.Y.; Scherer-Lorenzen, M.; Fort, F. Allocation, morphology, physiology, architecture: The multiple facets of plant above- and below-ground responses to resource stress. New Phytol. 2018, 219, 1338–1352. [Google Scholar] [CrossRef]
- Li, H.; Testerink, C.; Zhang, Y. How roots and shoots communicate through stressful times. Trends Plant Sci. 2021, 26, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Mehla, N.; Sindhi, V.; Josula, D.; Bisht, P.; Wani, S.H. An introduction to antioxidants and their roles in plant stress tolerance. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation Under Abiotic Stress; Khan, M.I.R., Khan, N.A., Eds.; Springer: Singapore, 2017; pp. 1–23. [Google Scholar]
- Mir, R.A.; Khah, M.A. Chapter 10—Recent progress in enzymatic antioxidant defense system in plants against different environmental stresses. In Improving Stress Resilience in Plants; Ahanger, M.A., Bhat, J.A., Ahmad, P., John, R., Eds.; Academic Press: Cambridge, MA, USA., 2024; pp. 203–224. [Google Scholar]
- Samaras, A.; Kamou, N.; Tzelepis, G.; Karamanoli, K.; Menkissoglu-Spiroudi, U.; Karaoglanidis, G.S. Root transcriptional and metabolic dynamics induced by the plant growth promoting rhizobacterium (PGPR) Bacillus subtilis Mbi600 on Cucumber Plants. Plants 2022, 11, 1218. [Google Scholar] [CrossRef] [PubMed]
- Mall, M.S.; Shah, S.; Singh, S.; Singh, N.; Singh, N.; Vaish, S.; Gupta, D. Genome-wide identification and characterization of ABC transporter superfamily in the legume Cajanus cajan. J. Appl. Genet. 2023, 64, 615–644. [Google Scholar] [CrossRef]
- Ke, D.; Xie, Y.; Li, H.; Hu, L.; He, Y.; Guo, C.; Zhai, Y.; Guo, J.; Li, K.; Chu, Z.; et al. Anchorene, a carotenoid-derived growth regulator, modulates auxin homeostasis by suppressing GH3-mediated auxin conjugation. J. Integr. Plant Biol. 2024, 66, 2490–2504. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Zhao, W.; Tian, S.; Wang, D.; Wu, H.; Wang, C.; Zhu, J.; Li, N.; Zhu, X.; Mu, S.; et al. Exploring the mechanisms underlying petal pigmentation differences in two cultivars of Physalis philadelphica based on HPLC and NGS. Horticulturae 2024, 10, 507. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, Y.; Jiang, X.; Li, H.; Liu, X.; Deng, L.; Wang, G. Integrated transcriptomics and metabolomics analysis of volatiles and variations in carotenoid biosynthesis during different developmental stages of Camellia huana. BMC Plant Biol. 2025, 25, 734. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. Phenylpropanoid derivatives and their role in plants’ health and as antimicrobials. Curr. Microbiol. 2023, 80, 380. [Google Scholar] [CrossRef] [PubMed]
- Trush, K.; Pal’ove-Balang, P. Biosynthesis and role of isoflavonoids in legumes under different environmental conditions. Plant Stress 2023, 8, 100153. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Luo, K. Biosynthesis and metabolic engineering of isoflavonoids in model plants and crops: A review. Front. Plant Sci. 2024, 15, 1384091. [Google Scholar] [CrossRef]
- Liu, W.-C.; Song, R.-F.; Zheng, S.-Q.; Li, T.-T.; Zhang, B.-L.; Gao, X.; Lu, Y.-T. Coordination of plant growth and abiotic stress responses by tryptophan synthase β subunit 1 through modulation of tryptophan and ABA homeostasis in Arabidopsis. Mol. Plant 2022, 15, 973–990. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, X.; Gong, B.; Yan, Y.; Shi, Q. Proteomics and metabolomics analysis of tomato fruit at different maturity stages and under salt treatment. Food Chem. 2020, 311, 126009. [Google Scholar] [CrossRef]
- Bar-Peled, M.; O’Neill, M.A. Plant nucleotide sugar formation, interconversion, and salvage by sugar recycling. Annu. Rev. Plant Biol. 2011, 62, 127–155. [Google Scholar] [CrossRef]
- Figueroa, C.M.; Lunn, J.E.; Iglesias, A.A. Nucleotide-sugar metabolism in plants: The legacy of Luis F. Leloir. J. Exp. Bot. 2021, 72, 4053–4067. [Google Scholar] [CrossRef]
- Chen, L.; Meng, Y.; Bai, Y.; Yu, H.; Qian, Y.; Zhang, D.; Zhou, Y. Starch and sucrose metabolism and plant hormone signaling pathways play crucial roles in aquilegia salt stress adaption. Int. J. Mol. Sci. 2023, 24, 3948. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, D.; Wang, B.; Hou, X.; Zhang, R.; Yan, M.; Liao, W. Changes of starch and sucrose content and related gene expression during the growth and development of Lanzhou lily bulb. PLoS ONE 2022, 17, e0262506. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, L.H.; Tun, W.; Jeon, J.S.; An, G. Sucrose signaling in higher plants. Plant Sci. 2021, 302, 110703. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Du, Y.; Na, X.; Wang, M.; Qu, Y.; Ge, L.; Wang, Y.; Gao, L.; Bai, W.; Bi, Y.; et al. Integrative transcriptome and metabolome revealed the molecular mechanism of Bacillus megaterium BT22-mediated growth promotion in Arabidopsis thaliana. J. Plant Physiol. 2023, 285, 153995. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Jiang, L.; Zhang, Y.; Guo, Z.; Han, L.; Gao, P.; Zhao, X.; Zhao, X. Transcriptomics and Metabolomics Analyses Reveal How Rhizobacteria Acinetobacter calcoaceticus Enhance the Growth and Stress Tolerance in Lespedeza davurica. Agronomy 2025, 15, 1992. https://doi.org/10.3390/agronomy15081992
Liang Y, Jiang L, Zhang Y, Guo Z, Han L, Gao P, Zhao X, Zhao X. Transcriptomics and Metabolomics Analyses Reveal How Rhizobacteria Acinetobacter calcoaceticus Enhance the Growth and Stress Tolerance in Lespedeza davurica. Agronomy. 2025; 15(8):1992. https://doi.org/10.3390/agronomy15081992
Chicago/Turabian StyleLiang, Yinping, Lin Jiang, Yining Zhang, Zhanchao Guo, Linjuan Han, Peng Gao, Xiaoyan Zhao, and Xiang Zhao. 2025. "Transcriptomics and Metabolomics Analyses Reveal How Rhizobacteria Acinetobacter calcoaceticus Enhance the Growth and Stress Tolerance in Lespedeza davurica" Agronomy 15, no. 8: 1992. https://doi.org/10.3390/agronomy15081992
APA StyleLiang, Y., Jiang, L., Zhang, Y., Guo, Z., Han, L., Gao, P., Zhao, X., & Zhao, X. (2025). Transcriptomics and Metabolomics Analyses Reveal How Rhizobacteria Acinetobacter calcoaceticus Enhance the Growth and Stress Tolerance in Lespedeza davurica. Agronomy, 15(8), 1992. https://doi.org/10.3390/agronomy15081992




