Effect of Light on the Yield and Nutrient Composition of Selected Mint Species Grown in a Controlled Environment
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results and Discussion
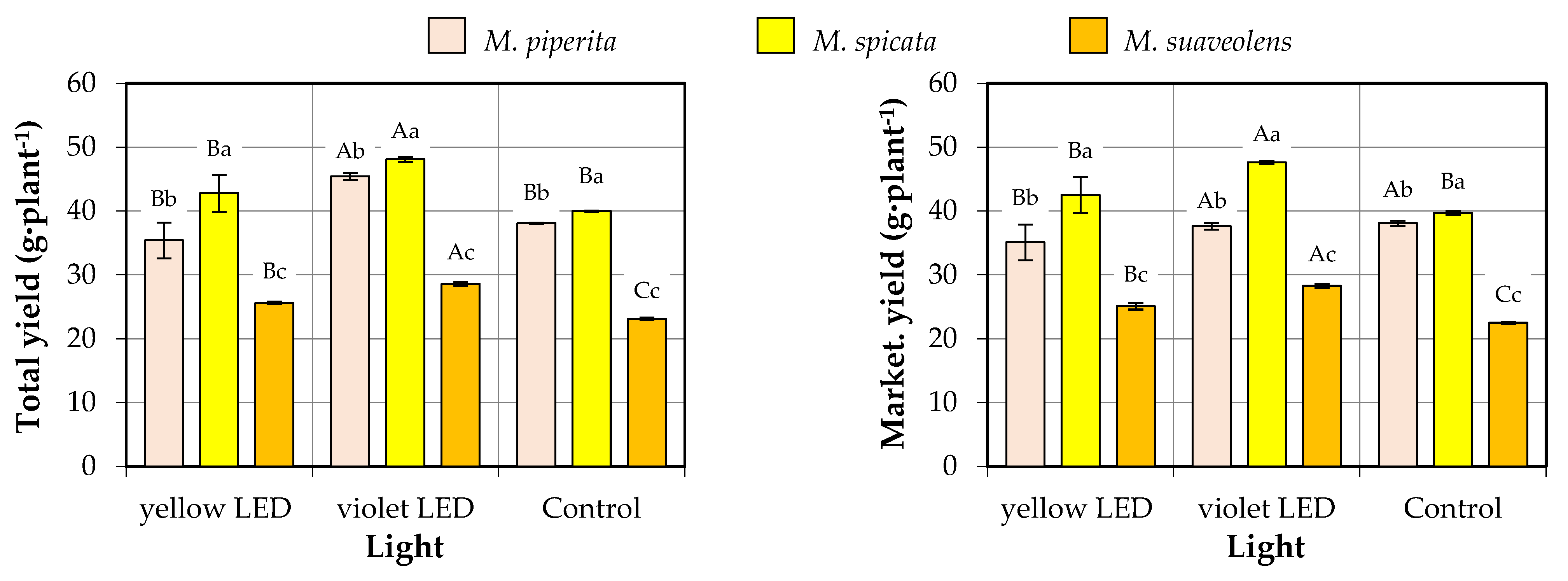
3.1. Mentha Yields
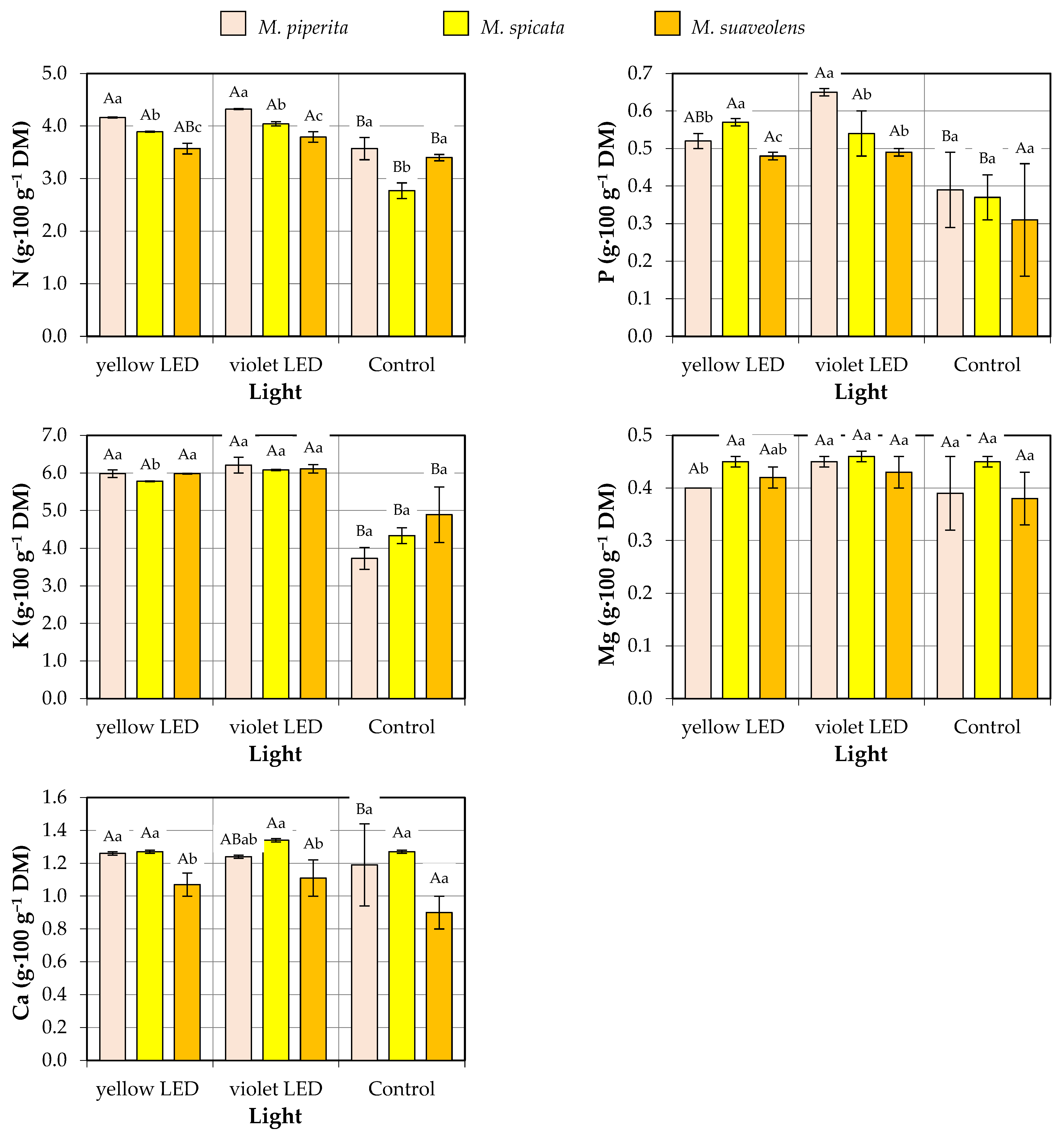
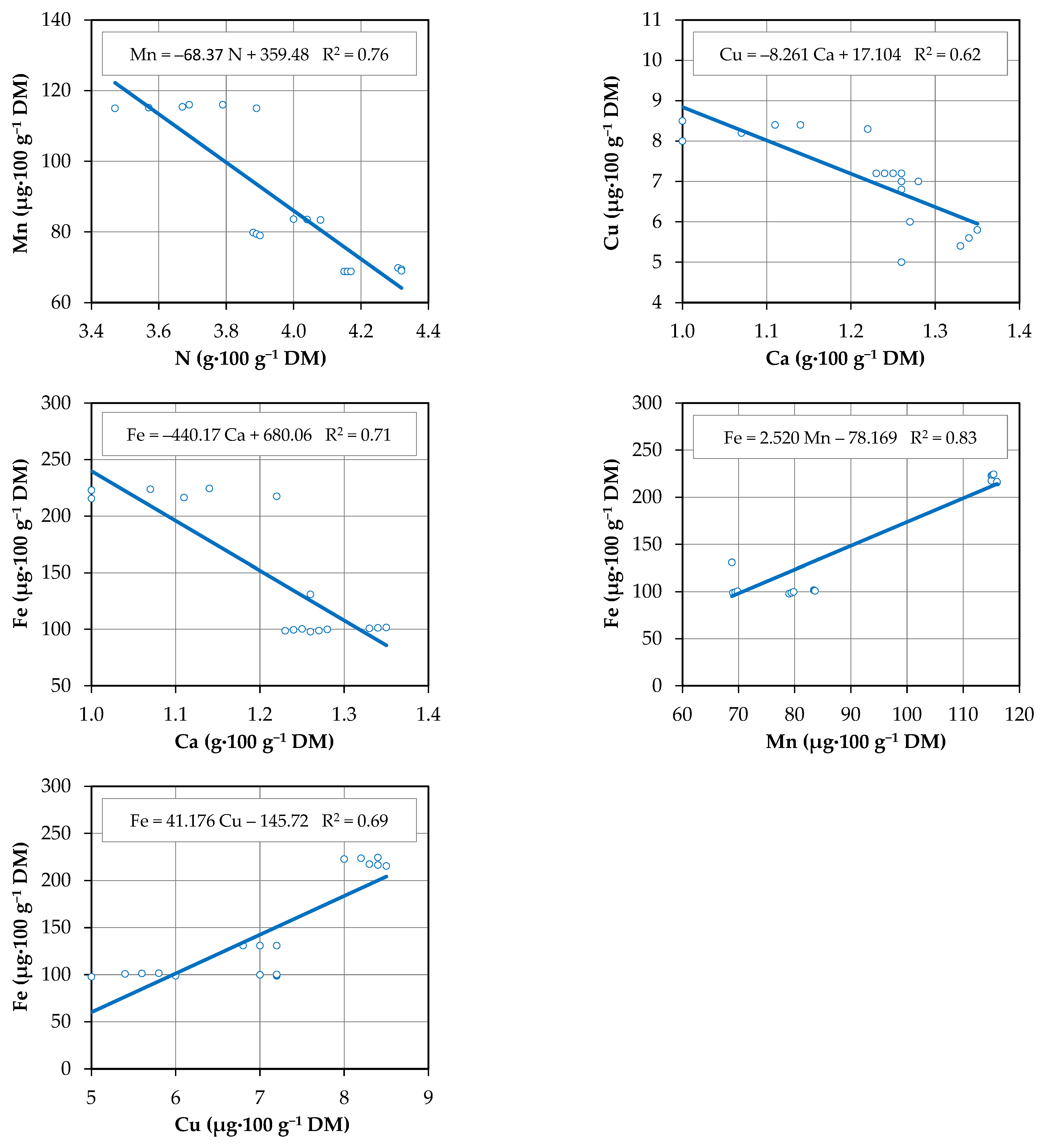
3.2. Macronutrient Concentrations
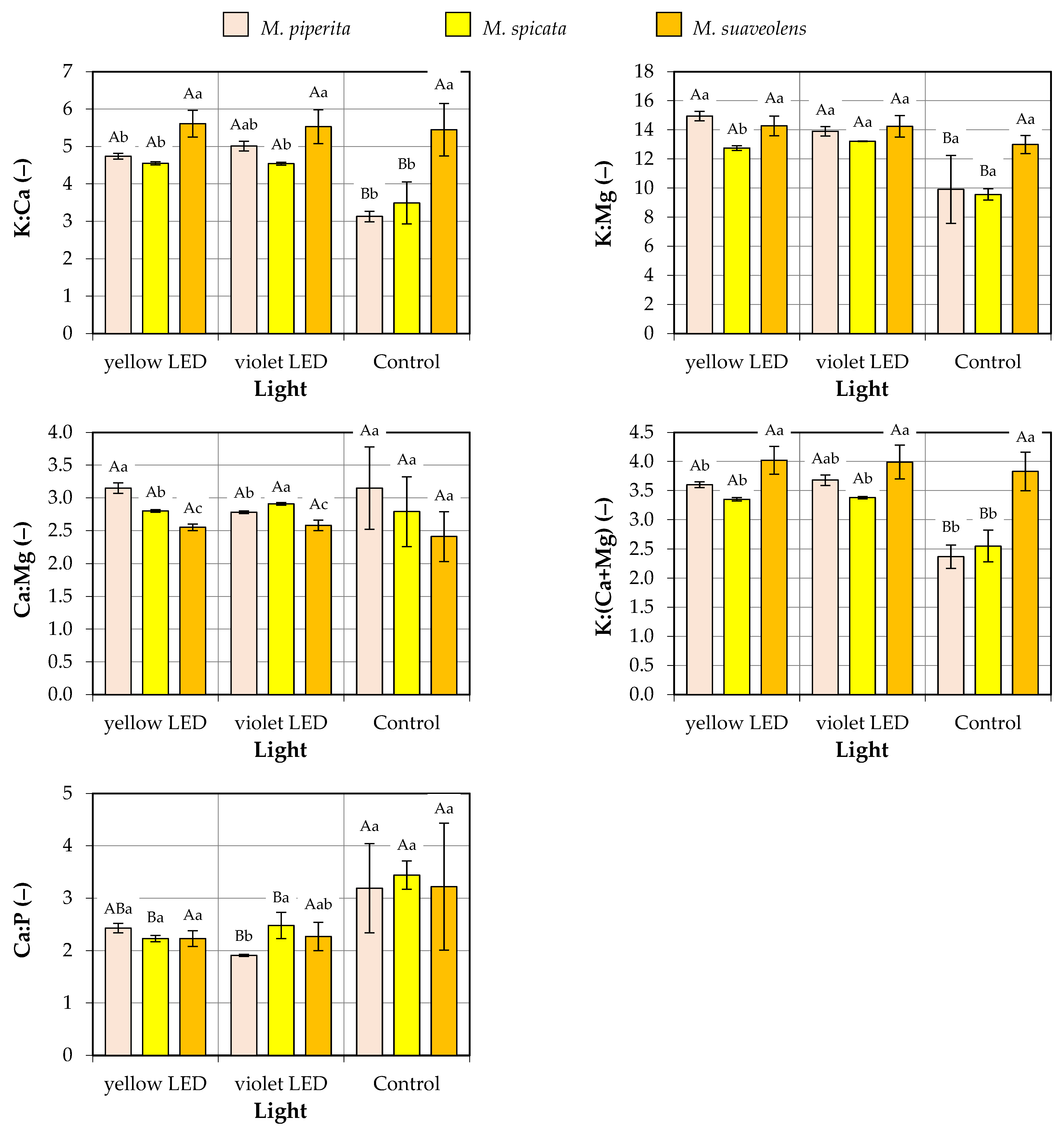
3.3. Macronutrient Ratios
- K:Ca—from 3.13 (M. piperita, control group) to 5.61 (M. suaveolens, yellow LED group);
- K:Mg—from 9.56 (M. spicata, control group) to 14.95 (M. piperita, yellow LED group);
- Ca:Mg—from 2.41 (M. suaveolens, control group) to 3.15 (M. piperita, yellow LED group and control group);
- K:(Ca + Mg)—from 2.37 (M. piperita, control group) to 4.02 (M. suaveolens, yellow LED group);
- Ca:P—from 1.91 (M. piperita, violet LED group) to 3.44 (M. spicata, control group).
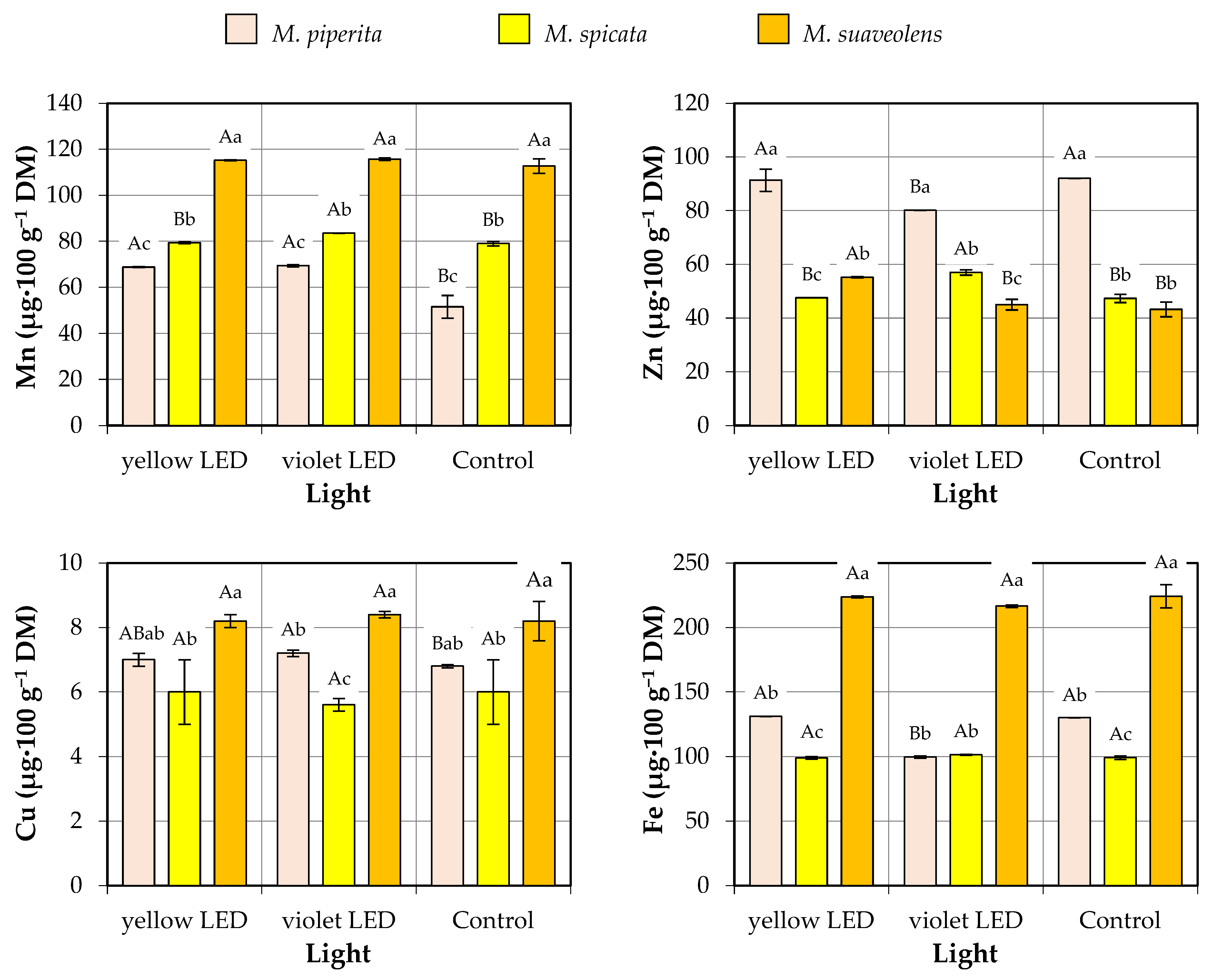
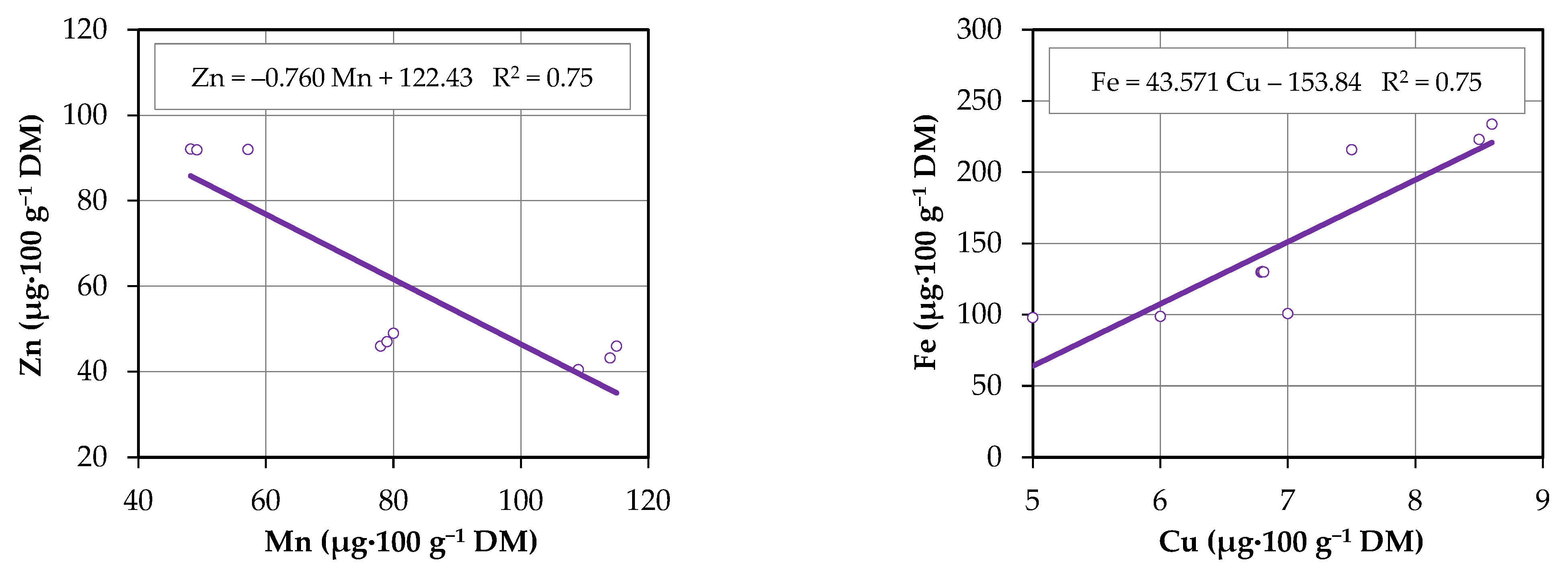
3.4. Micronutrient Content
- Mn—from 51.5 µg·100 g−1 DM (M. piperita, control group) to 115.6 µg·100 g−1 DM (M. suaveolens, violet LED group);
- Zn—from 43.2 µg·100 g−1 DM (M. suaveolens, control group) to 92.0 µg·100 g−1 DM (M. piperita, control group);
- Cu—from 5.6 µg·100 g−1 DM (M. spicata, violet LED group) to 8.4 µg·100 g−1 DM (M. suaveolens, violet LED group);
- Fe—from 99.0 µg·100 g−1 DM (M. spicata, yellow LED group) to 224.2 µg·100 g−1 DM (M. suaveolens, control group).
3.5. Correlations Between Mineral Concentrations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Factor | Source of Variation | Total Yield | Marketable Yield |
|---|---|---|---|
| g·plant−1 | |||
| Species | M. piperita | 39.6 ± 4.7 a | 39.3 ± 4.8 a |
| M. spicata | 43.6 ± 3.8 a | 43.3 ± 3.7 a | |
| M. suaveolens | 25.7 ± 2.4 b | 25.3 ± 2.5 b | |
| Type of light | Yellow LED | 34.6 ± 7.8 a | 34.2 ± 7.8 a |
| Violet LED | 40.7 ± 9.1 a | 40.4 ± 9.1 a | |
| Control | 33.7 ± 8.0 a | 33.3 ± 8.1 a | |
| Factor | Source of Variation | N | P | K | Mg | Ca |
|---|---|---|---|---|---|---|
| (g·100 g−1 DM) | ||||||
| Species | M. piperita | 4.01 ± 0.36 a | 0.52 ± 0.12 a | 5.31 ± 1.20 a | 0.41 ± 0.04 b | 1.23 ± 0.04 a |
| M. spicata | 3.57 ± 0.61 a | 0.49 ± 0.10 a | 5.40 ± 0.82 a | 0.46 ± 0.01 a | 1.29 ± 0.13 a | |
| M. suaveolens | 3.59 ± 0.19 a | 0.43 ± 0.11 a | 5.66 ± 0.69 a | 0.41 ± 0.04 b | 1.03 ± 0.13 b | |
| Type of light | Yellow LED | 3.87 ± 0.26 a | 0.52 ± 0.04 a | 5.91 ± 0.11 a | 0.42 ± 0.03 a | 1.20 ± 0.10 a |
| Violet LED | 4.05 ± 0.23 a | 0.56 ± 0.08 a | 6.13 ± 0.13 a | 0.45 ± 0.02 a | 1.23 ± 0.11 a | |
| Control | 3.24 ± 0.39 b | 0.36 ± 0.10 b | 4.32 ± 0.65 b | 0.41 ± 0.06 a | 1.12 ± 0.22 a | |
| Factor | Source of Variation | K:Ca | K:Mg | Ca:Mg | K:(Ca + Mg) | Ca:P |
|---|---|---|---|---|---|---|
| (–) | ||||||
| Species | M. piperita | 4.3 ± 0.9 b | 12.9 ± 2.6 a | 3.0 ± 0.4 a | 3.2 ± 0.6 b | 2.5 ± 0.7 a |
| M. spicata | 4.2 ± 0.6 b | 11.8 ± 1.7 a | 2.8 ± 0.3 ab | 3.1 ± 0.4 b | 2.7 ± 0.6 a | |
| M. suaveolens | 5.5 ± 0.5 a | 13.8 ± 0.9 a | 2.5 ± 0.2 b | 3.9 ± 0.3 a | 2.6 ± 0.8 a | |
| Type of light | Yellow LED | 5.0 ± 1.0 a | 14.0 ± 1.0 a | 2.8 ± 0.3 a | 3.7 ± 0.3 a | 2.3 ± 0.1 b |
| Violet LED | 5.0 ± 0.5 a | 13.8 ± 0.6 a | 2.8 ± 0.2 a | 3.7 ± 0.3 a | 2.2 ± 0.3 b | |
| Control | 4.0 ± 1.2 b | 10.8 ± 2.0 b | 2.8 ± 0.6 a | 2.9 ± 0.7 b | 3.3 ± 0.8 a | |
| Factor | Source of Variation | Mn | Zn | Cu | Fe |
|---|---|---|---|---|---|
| (μg·100 g−1 DM) | |||||
| Species | M. piperita | 63.2 ± 9.1 c | 87.8 ± 6.2 a | 7.0 ± 0.2 b | 120.3 ± 15.4 b |
| M. spicata | 80.6 ± 2.2 b | 50.6 ± 4.9 b | 5.9 ± 0.7 c | 99.9 ± 1.5 c | |
| M. suaveolens | 114.5 ± 2.2 a | 47.8 ± 5.8 b | 8.3 ± 0.3 a | 221.5 ± 5.9 a | |
| Type of light | Yellow LED | 87.8 ± 21.1 a | 64.7 ± 20.4 a | 7.1 ± 1.1 a | 151.3 ± 56.1 a |
| Violet LED | 89.5 ± 20.5 a | 60.7 ± 15.5 a | 7.1 ± 1.2 a | 139.2 ± 58.0 a | |
| Control | 81.1 ± 26.7 a | 60.9 ± 23.5 a | 7.0 ± 1.1 a | 151.2 ± 56.6 a | |
| Type of Light | Nutrient | Pearson’s Correlation Coefficient | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Mg | Ca | Mn | Zn | Cu | ||
| LED | P | 0.71 | 1 | ||||||
| K | 0.36 | 0.22 | 1 | ||||||
| Mg | 0.21 | 0.41 | 0.18 | 1 | |||||
| Ca | 0.71 | 0.45 | 0.03 | 0.58 | 1 | ||||
| Mn | −0.87 | −0.69 | 0.03 | –0.13 | 0.75 | 1 | |||
| Zn | 0.73 | 0.36 | 0.27 | –0.39 | 0.31 | −0.70 | 1 | ||
| Cu | –0.41 | –0.40 | 0.27 | –0.45 | −0.79 | 0.64 | –0.06 | 1 | |
| Fe | −0.76 | −0.74 | 0.08 | –0.44 | −0.85 | 0.91 | –0.38 | 0.83 | |
| Control | P | 0.14 | 1 | ||||||
| K | –0.09 | 0.20 | 1 | ||||||
| Mg | –0.48 | 0.66 | 0.16 | 1 | |||||
| Ca | –0.38 | 0.53 | –0.24 | 0.43 | 1 | ||||
| Mn | –0.15 | –0.31 | 0.76 | –0.09 | –0.59 | 1 | |||
| Zn | 0.57 | 0.31 | −0.68 | –0.20 | 0.33 | −0.86 | 1 | ||
| Cu | 0.47 | –0.04 | 0.50 | –0.46 | –0.34 | 0.60 | –0.17 | 1 | |
| Fe | 0.50 | –0.24 | 0.58 | –0.48 | −0.75 | 0.76 | –0.35 | 0.87 | |
References
- Chrysargyris, A.; Nikolaidou, E.; Stamatakis, A.; Tzortzakis, N. Vegetative, physiological, nutritional and antioxidant behavior of spearmint (Mentha spicata L.) in response to different nitrogen supply in hydroponics. J. Appl. Res. Med. Aromat. Plants 2017, 6, 52–61. [Google Scholar] [CrossRef]
- Chrapek, A.; Dzida, K. Chemical composition, growing conditions and healing effects of peppermint (Mentha × piperita L.). In Wybrane Zagadnienia z Zakresu Przemysłu Spożywczego oraz Zarządzania i Inżynierii Produkcji, Tom 1; Babicz, N., Kropiwiec-Domańska, K., Eds.; Wydawnictwo Uniwersytetu Przyrodniczego: Lublin, Poland, 2020; pp. 16–23. [Google Scholar] [CrossRef]
- Zheng, L.; He, H.; Song, W. Application of light-emitting diodes and the effect of light quality on horticultural crops: A review. HortScience 2019, 54, 1656–1661. [Google Scholar] [CrossRef]
- Thoma, F.; Somborn-Schulz, A.; Schlehuber, D.; Keuter, V.; Deerberg, G. Effects of light on secondary metabolites in selected leafy greens: A review. Front. Plant Sci. 2020, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Kozai, T. Why LED lighting for urban agriculture? In LED Lighting for Urban Agriculture; Kozai, T., Fujiwara, K., Runkle, E., Eds.; Springer: Singapore, 2016; pp. 3–18. [Google Scholar] [CrossRef]
- Hasan, M.; Bashir, T.; Ghosh, R.; Lee, S.K.; Bae, H. An overview of LEDs’ effects on the production of bioactive compounds and crop quality. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.; Agrawal, S.B. Effects of UV-B radiation on morphological, physiological and biochemical aspects of plants: An overview. J. Sci. Res. 2017, 61, 87–113. [Google Scholar]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Tabbert, J.M.; Schulz, H.; Krähmer, A. Investigation of LED light qualities for peppermint (Mentha x Piperita L.) cultivation focusing on plant quality and consumer safety aspects. Front. Food Sci. Technol. 2022, 2, 852155. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Brito, C.; Ferreira, H.; Dinis, L.-T.; Trindade, H.; Marques, D.; Correia, C.M.; Moutinho-Pereira, J. Different LED light intensity and quality change perennial ryegrass (Lolium perenne L.) physiological and growth responses and water and energy consumption. Front. Plant Sci. 2023, 14, 1160100. [Google Scholar] [CrossRef]
- Behn, H.; Albert, A.; Marx, F.; Noga, G.; Ulbrich, A. Ultraviolet-B and photosynthetically active radiation interactively affect yield and pattern of monoterpenes in leaves of peppermint (Mentha × Piperita L.). J. Agric. Food Chem. 2010, 58, 7361–7367. [Google Scholar] [CrossRef]
- Alvarenga, J.P.; Pacheco, F.V.; Bertolucci, S.K.V.; Silva, S.T.; de Oliveira, T.; Pinto, J.E.B.P. In vitro culture of Mentha viridis: Quality and intensity of light on growth and production of volatiles. Acta Hortic. 2018, 1224, 175–182. [Google Scholar] [CrossRef]
- Kozai, T. Towards sustainable plant factories with artificial lighting (PFALs) for achieving SDGs. Int. Agric. Biol. Eng. 2019, 12, 28–37. [Google Scholar] [CrossRef]
- Soufi, H.R.; Roosta, H.R.; Fatehi, F.; Ghorbanpour, M. Spectral composition of LED light differentially affects biomass, photosynthesis, nutrient profile, and foliar nitrate accumulation of lettuce grown under various replacement methods of nutrient solution. Food Sci. Nutr. 2023, 11, 8143–8162. [Google Scholar] [CrossRef]
- Pinho, P.; Jokinen, K.; Halonen, L. The influence of the LED light spectrum on the growth and nutrient uptake of hydroponically grown lettuce. Light. Res. Technol. 2017, 49, 866–881. [Google Scholar] [CrossRef]
- Kołodziej, B. Uprawa Ziół: Poradnik dla Plantatorów [Growing Herbs. A Grower’s Guide]; PWRiL: Poznań, Poland, 2010. (In Polish) [Google Scholar]
- Majkowska-Gadomska, J.; Kaliniewicz, Z.; Francke, A.; Sałata, A.; Jadwisieńczak, K.K. An evaluation of the biometric parameters and chemical composition of the florets, leaves, and stalks of broccoli plants grown in different soil types. Appl. Sci. 2024, 14, 4411. [Google Scholar] [CrossRef]
- Trivellini, A.; Toscano, S.; Romano, D.; Ferrante, A. LED lighting to produce high-quality ornamental plants. Plants 2023, 12, 1667. [Google Scholar] [CrossRef]
- Eichhorn Bilodeau, S.; Wu, B.-S.; Rufyikiri, A.-S.; MacPherson, S.; Lefsrud, M. An update on plant photobiology and implications for cannabis production. Front. Plant Sci. 2019, 10, 296. [Google Scholar] [CrossRef]
- McCree, K.J. The action spectrum absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the role of red:blue LED lights on resource use efficiency and nutritional properties of indoor grown sweet basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Alrifai, O.; Hao, X.; Marcone, M.F.; Tsao, R. Current review of the modulatory effects of LED lights on photosynthesis of secondary metabolites and future perspectives of microgreen vegetables. J. Agric. Food Chem. 2019, 67, 6075–6090. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, J. The effect of red and violet light emitting diode (LED) treatments on the postharvest quality and biodiversity of fresh-cut pakchoi (Brassica rapa L. Chinensis). Food Sci. Technol. Int. 2022, 28, 297–308. [Google Scholar] [CrossRef]
- Mansoori, I. The effect of plant density and harvesting time on growth and essential oil of peppermint (Mentha piperita L.). J. Med. Bioeng. 2014, 3, 113–116. [Google Scholar] [CrossRef][Green Version]
- Martínez-Moreno, A.; Frutos-Tortosa, A.; Diaz-Mula, H.; Mestre, T.C.; Martínez, V. Effect of the intensity and spectral quality of LED light on growth and quality of spinach indoors. Horticulturae 2024, 10, 411. [Google Scholar] [CrossRef]
- Ruamrungsri, S.; Utrapen, Y.; Tateing, S.; Panjama, K.; Inkham, C. Impact of LED combinations and light intensity on growth and yields of wasabi. Horticulturae 2025, 11, 3. [Google Scholar] [CrossRef]
- Surendran, U.; Chandran, C.; Joseph, E.J. Hydroponic cultivation of Mentha spicata and comparison of biochemical and antioxidant activities with soil-grown plants. Acta Physiol. Plant. 2017, 39, 26. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Z.; Jiang, X.; Ahammed, G.J.; Zhou, Y. Light regulation of horticultural crop nutrient uptake and utilization. Hortic. Plant J. 2021, 7, 367–379. [Google Scholar] [CrossRef]
- Francke, A.; Majkowska-Gadomska, J.; Kaliniewicz, Z.; Jadwisieńczak, K. No effect of biostimulants on the growth, yield and nutritional value of shallots grown for bunch harvest. Agronomy 2022, 12, 1156. [Google Scholar] [CrossRef]
- Skubij, N.; Dzida, K.; Jarosz, Z.; Pitura, K.; Jaroszuk-Sierocińska, M. Nutritional value of savory herb (Satureja hortensis L.) and plant response to variable mineral nutrition conditions in various phases of development. Plants 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Trivellini, A.; Toscano, S.; Romano, D.; Ferrante, A. The role of blue and red light in the orchestration of secondary metabolites, nutrient transport and plant quality. Plants 2023, 12, 2026. [Google Scholar] [CrossRef]
- Sadowska, U.; Domagała-Świątkiewicz, I.; Żabiński, A. Biochar and its effects on plant-soil macronutrient cycling during a three-year field trial on sandy soil with peppermint (Mentha piperita L.). Part I: Yield and macro element content in soil and plant biomass. Agronomy 2020, 10, 1950. [Google Scholar] [CrossRef]
- Nishioka, N.; Nishimura, T.; Ohyama, K.; Sumino, M.; Malayeri, S.H.; Goto, E.; Inagaki, N.; Morota, T. Light quality affected growth and contents of essential oil components of Japanese mint plants. Acta Hortic. 2008, 797, 431–436. [Google Scholar] [CrossRef]
- Zhai, J.; Gao, Y.; Zhang, X.W.; Han, L.J.; Bi, H.G.; Li, Q.M.; Ai, X.Z. Effects of silicon and calcium on photosynthesis yield and quality of cucumber in solar-greenhouse. Acta Hortic. Sin. 2019, 46, 701–713. [Google Scholar] [CrossRef]
- Oivukkamäki, J.; Atherton, J.; Xu, S.; Riikonen, A.; Zhang, C.; Hakala, T.; Honkavaara, E.; Porcar-Castell, A. Investigating foliar macro- and micronutrient variation with chlorophyll fluorescence and reflectance measurements at the leaf and canopy scales in potato. Remote Sens. 2023, 15, 2498. [Google Scholar] [CrossRef]
- Jarnuszewski, G.; Meller, E. Mineral element ratios in plants grown on post-bog soils fertilised with zinc and copper. Folia Pomer. Univ. Technol. Stetin. Agric. Aliment. Piscaria Zootech. 2013, 304, 25–32. (In Polish) [Google Scholar]
- Rosanoff, A.; Capron, E.; Barak, P.; Mathews, B.; Nielsen, F. Edible plant tissue and soil calcium:magnesium ratios: Data too sparse to assess implications for human health. Crop Pasture Sci. 2015, 66, 1265–1277. [Google Scholar] [CrossRef]
- Kızıl, S.; Hasımı, N.; Tolan, V.; Kılınc, E.; Yuksel, U. Mineral content, essential oil components and biological activity of two mentha species (M. piperita L., M. spicata L.). Turk. J. Field Crops 2010, 15, 148–153. [Google Scholar]
- Hudz, N.; Kobylinska, L.; Pokajewicz, K.; Horčinová Sedláčková, V.; Fedin, R.; Voloshyn, M.; Myskiv, I.; Brindza, J.; Wieczorek, P.P.; Lipok, J. Mentha piperita: Essential oil and extracts, their biological activities, and perspectives on the development of new medicinal and cosmetic products. Molecules 2023, 28, 7444. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.O. Spectral effects of artificial light on plant physiology and secondary metabolism: A review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Weng, X.; Li, H.; Zhou, Y.; Ren, C.; Zhang, S.; Liu, L. Relative availability of nitrogen and calcium regulates the growth of poplar seedlings due to transcriptome changes. Forests 2023, 14, 1899. [Google Scholar] [CrossRef]
- Bhat, M.A.; Mishra, A.K.; Shah, S.N.; Bhat, M.A.; Jan, S.; Rahman, S.; Baek, K.-H.; Jan, A.T. Soil and mineral nutrients in plant health: A prospective study of iron and phosphorus in the growth and development of plants. Curr. Issues Mol. Biol. 2024, 46, 5194–5222. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Gupta, S.; Singh, A.P. Review: Nutrient-nutrient interactions governing underground plant adaptation strategies in a heterogeneous environment. Plant Sci. 2024, 342, 112024. [Google Scholar] [CrossRef]
- Xing, Y.; Feng, Z.-Q.; Zhang, X.; Cao, H.-X.; Liu, C.-L.; Qin, H.-H.; Jiang, H.; Zhu, Z.-L.; Ge, S.-F.; Jiang, Y.-M. Nitrogen reduces calcium availability by promoting oxalate biosynthesis in apple leaves. Hortic. Res. 2024, 11, uhae208. [Google Scholar] [CrossRef] [PubMed]
- David, E.F.S.; Mischan, M.M.; Marques, M.O.M.; Boaro, C.S.F. Physiological indexese macro- and micronutrients in plant tissue and essential oil of Mentha piperita L. grown in nutrient solution with variation in N, P, K and Mg levels. Rev. Bras. Plantas Med. 2014, 16, 97–106. [Google Scholar] [CrossRef]
| Factor | Total Yield | Marketable Yield |
|---|---|---|
| Species (A) | <0.001 | <0.001 |
| Type of light (B) | 0.176 | 0.170 |
| Interaction (A × B) | 0.002 | 0.003 |
| Factor | N | F | K | Mg | Ca |
|---|---|---|---|---|---|
| Species (A) | 0.056 | 0.231 | 0.703 | 0.013 | <0.001 |
| Type of light (B) | <0.001 | <0.001 | <0.001 | 0.098 | 0.301 |
| Interaction (A × B) | <0.001 | 0.346 | 0.010 | 0.464 | 0.537 |
| Factor | K:Ca | K:Mg | Ca:Mg | K:(Ca + Mg) | Ca:P |
|---|---|---|---|---|---|
| Species (A) | <0.001 | 0.098 | 0.004 | 0.002 | 0.808 |
| Type of light (B) | 0.022 | <0.001 | 0.902 | 0.004 | <0.001 |
| Interaction (A × B) | 0.006 | 0.018 | 0.495 | 0.001 | 0.768 |
| Factor | Mn | Zn | Cu | Fe |
|---|---|---|---|---|
| Species (A) | <0.001 | <0.001 | <0.001 | <0.001 |
| Type of light (B) | 0.714 | 0.892 | 0.990 | 0.876 |
| Interaction (A × B) | <0.001 | <0.001 | 0.700 | <0.001 |
| Type of Light | Nutrient | N | P | K | Mg | Ca | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|
| LED | P | + | 1 | ||||||
| K | ns | ns | 1 | ||||||
| Mg | ns | ns | ns | 1 | |||||
| Ca | + | ns | ns | + | 1 | ||||
| Mn | ++ | + | ns | ns | + | 1 | |||
| Zn | + | ns | ns | ns | ns | + | 1 | ||
| Cu | ns | ns | ns | ns | + | + | ns | 1 | |
| Fe | + | + | ns | ns | ++ | ++ | ns | ++ | |
| Control | P | ns | 1 | ||||||
| K | ns | ns | 1 | ||||||
| Mg | ns | ns | ns | 1 | |||||
| Ca | ns | ns | ns | ns | 1 | ||||
| Mn | ns | ns | + | ns | ns | 1 | |||
| Zn | ns | ns | + | ns | ns | ++ | 1 | ||
| Cu | ns | ns | ns | ns | ns | ns | ns | 1 | |
| Fe | ns | ns | ns | ns | + | + | ns | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadwisieńczak, K.K.; Kaliniewicz, Z.; Majkowska-Gadomska, J.; Mikulewicz, E.; Francke, A.; Marks, M.; Choszcz, D.J. Effect of Light on the Yield and Nutrient Composition of Selected Mint Species Grown in a Controlled Environment. Agronomy 2025, 15, 1959. https://doi.org/10.3390/agronomy15081959
Jadwisieńczak KK, Kaliniewicz Z, Majkowska-Gadomska J, Mikulewicz E, Francke A, Marks M, Choszcz DJ. Effect of Light on the Yield and Nutrient Composition of Selected Mint Species Grown in a Controlled Environment. Agronomy. 2025; 15(8):1959. https://doi.org/10.3390/agronomy15081959
Chicago/Turabian StyleJadwisieńczak, Krzysztof K., Zdzisław Kaliniewicz, Joanna Majkowska-Gadomska, Emilia Mikulewicz, Anna Francke, Marek Marks, and Dariusz J. Choszcz. 2025. "Effect of Light on the Yield and Nutrient Composition of Selected Mint Species Grown in a Controlled Environment" Agronomy 15, no. 8: 1959. https://doi.org/10.3390/agronomy15081959
APA StyleJadwisieńczak, K. K., Kaliniewicz, Z., Majkowska-Gadomska, J., Mikulewicz, E., Francke, A., Marks, M., & Choszcz, D. J. (2025). Effect of Light on the Yield and Nutrient Composition of Selected Mint Species Grown in a Controlled Environment. Agronomy, 15(8), 1959. https://doi.org/10.3390/agronomy15081959






