Raman Spectroscopy as a Tool for Early Identification of Tan Spot Disease and Assessment of Fungicide Response in Wheat
Abstract
1. Introduction
1.1. Role of Pyrenophora tritici-repentis and Propiconazole
1.2. Raman Spectroscopy in Agricultural Diagnostics and Objectives of the Study
2. Materials and Methods
2.1. Study Objectives
2.2. Scope of the Study
2.3. Nature of the Study
2.4. Tan Spot Disease in Wheat: Understanding the Epidemic and Pesticide Application
2.5. Critical Issue 1: Pyrenophora tritici-repentis, Inoculum Sources, Toxins, and Wheat Stages of Infection
2.6. Critical Issue 2: The Role of Fungicide Application in Managing Pyrenophora tritici-repentis
2.7. Critical Issue 3: Pyrenophora tritici-repentis, the Dilemma of Fungicide Use, and the Role of the Primary and Secondary Source of Inoculum
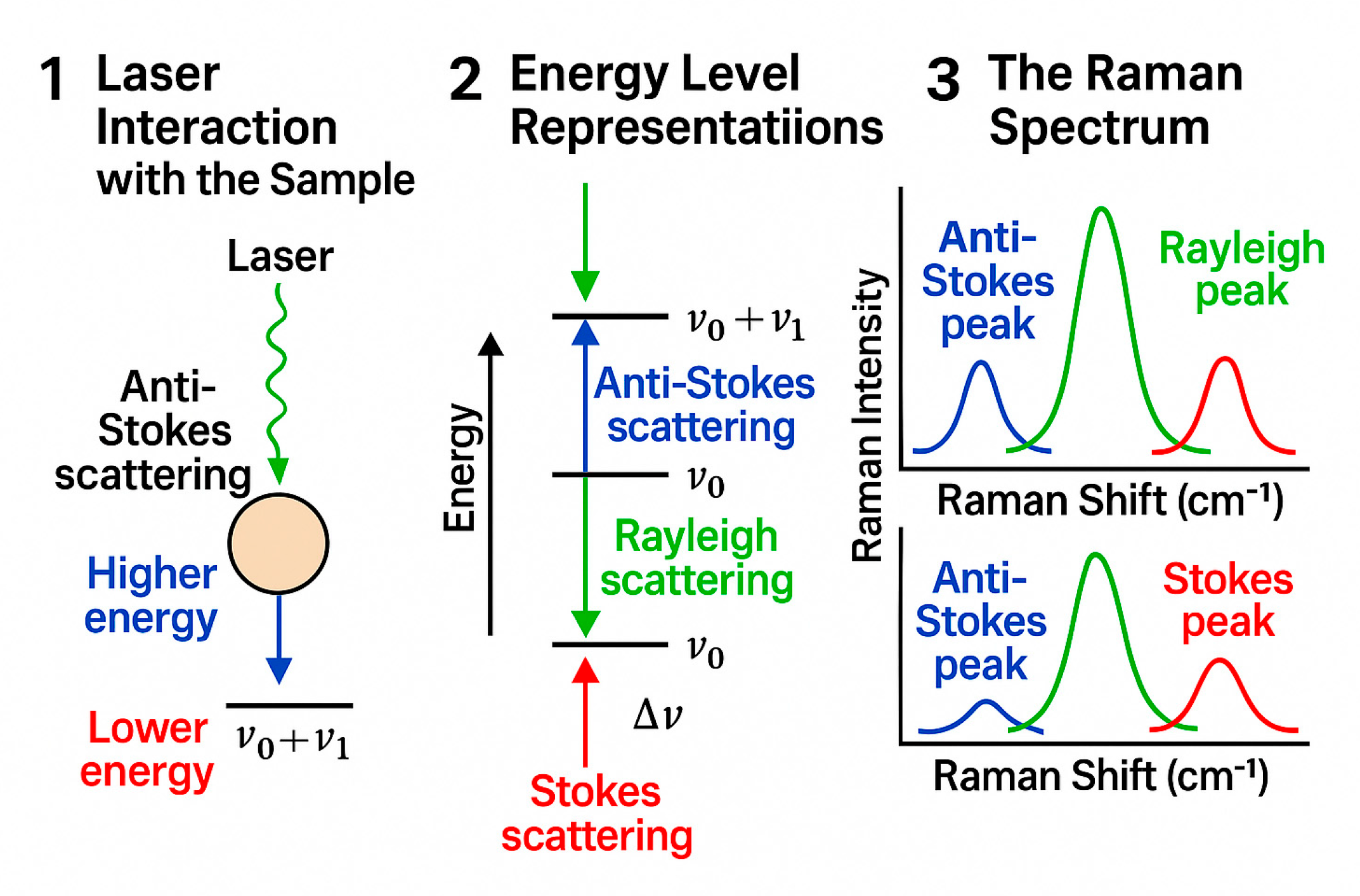
2.8. Methodology Statement for Figure Creation
- Panel 1: Laser interaction with sample and scattered signal depiction;
- Panel 2: Energy level transitions with Raman shift representations;
- Panel 3: Raman spectrum with labeled peaks corresponding to the scattering types.
2.9. No Laboratory Setup
2.10. Comparative Analysis
3. Theoretical Results and Simulated Insights
3.1. Principles of Raman Spectroscopy—Raman Parameters
3.1.1. Addressing the Study Objectives (Critical Issues 1–3) Through Raman Spectroscopy
3.1.2. Fundamental Principles of Raman Spectroscopy
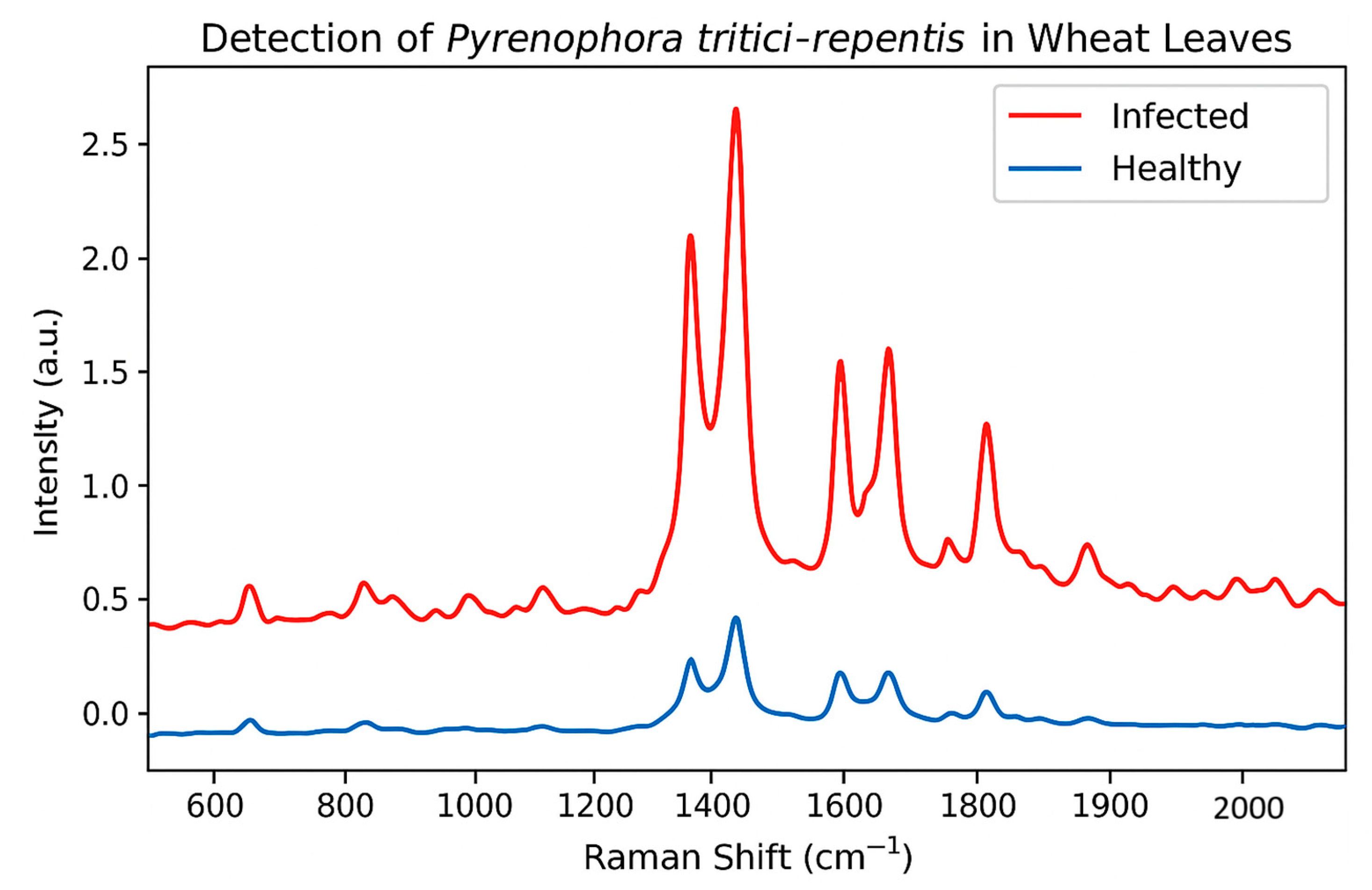
3.2. Detection of Fungal Biomarkers Such as Ergosterol and Chitin via SERS
3.2.1. Raman Spectroscopy for Identifying Plant Pathogens and Diagnosing Diseases
3.2.2. Challenges of SERS vs. Raman Spectroscopy in Detecting Plant Fungal Pathogens
- Higher Sensitivity: SERS can detect extremely low concentrations of pathogens due to its signal enhancement mechanism, making it ideal for early-stage detection.
- Overcomes Fluorescence Interference: Many biological samples, including plant tissues, exhibit fluorescence that can overwhelm Raman signals. SERS mitigates this issue by quenching fluorescence.
- Rapid and Non-Destructive: SERS allows for fast detection without damaging the sample, which is crucial for real-time monitoring in agriculture.
- Specific Molecular Fingerprinting: SERS provides highly specific spectral signatures, enabling precise identification of pathogens.
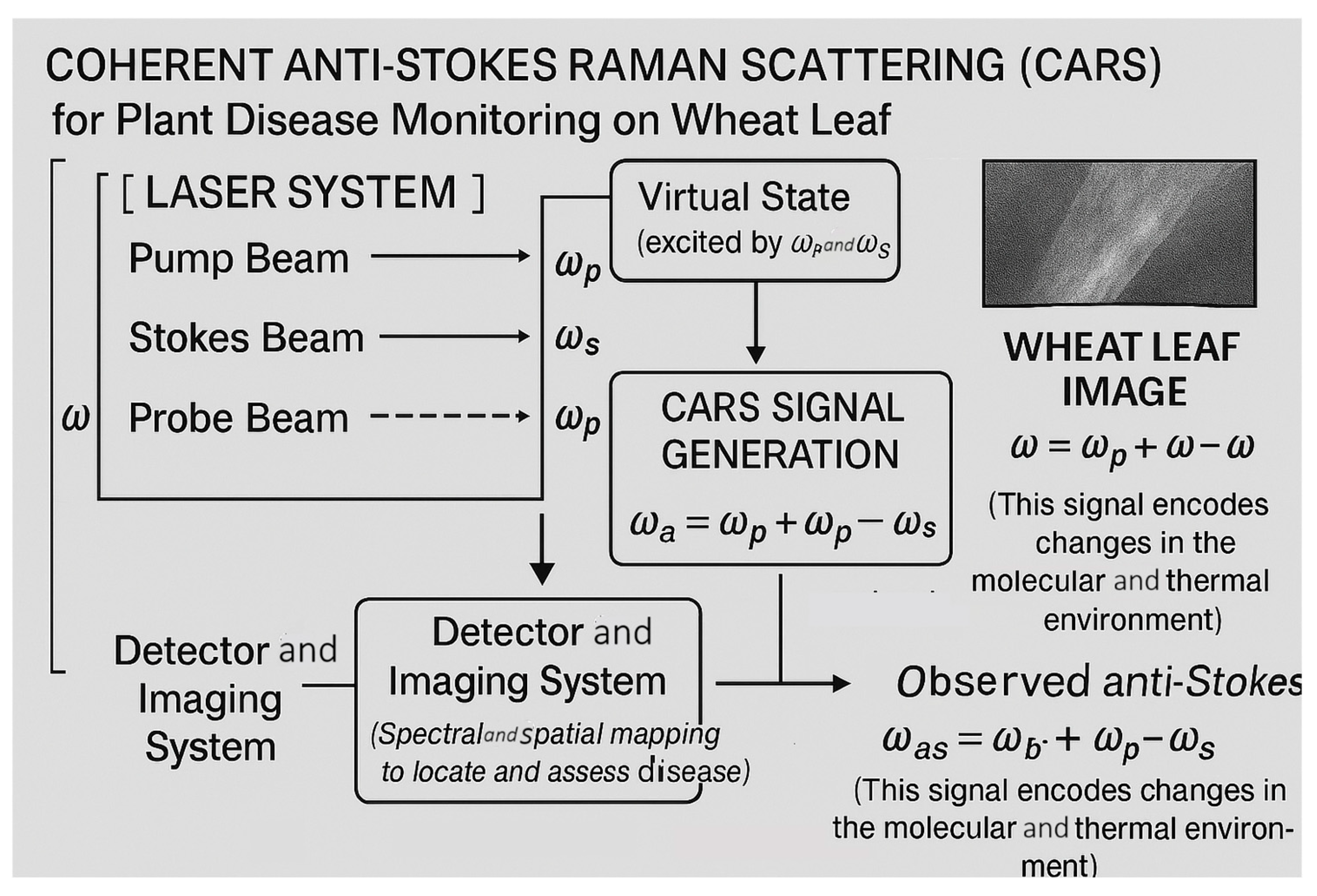
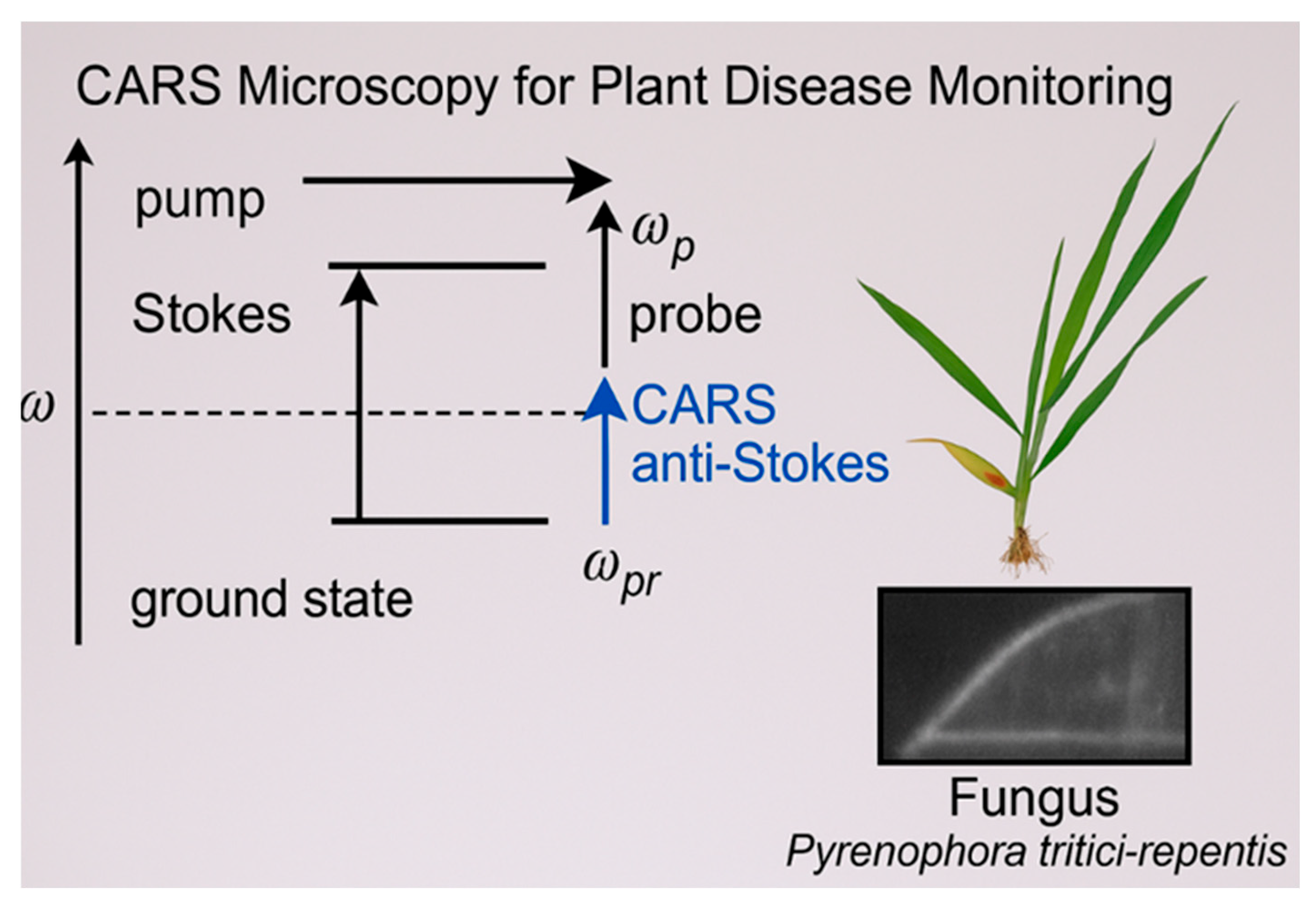
3.3. Coherent Anti-Stokes Raman Scattering (CARS)
3.3.1. Temperature Variations in Plant Tissues and Their Impact on Virulence Expression During Pathogen Infection
3.3.2. Coherent Anti-Stokes Raman Scattering (CARS) Spectroscopy: Principles and Applications
3.4. Identification of Fungal Toxins and Effectors
Raman Spectroscopy for Detecting Fungal Toxins (Mycotoxins) and Effector Molecules
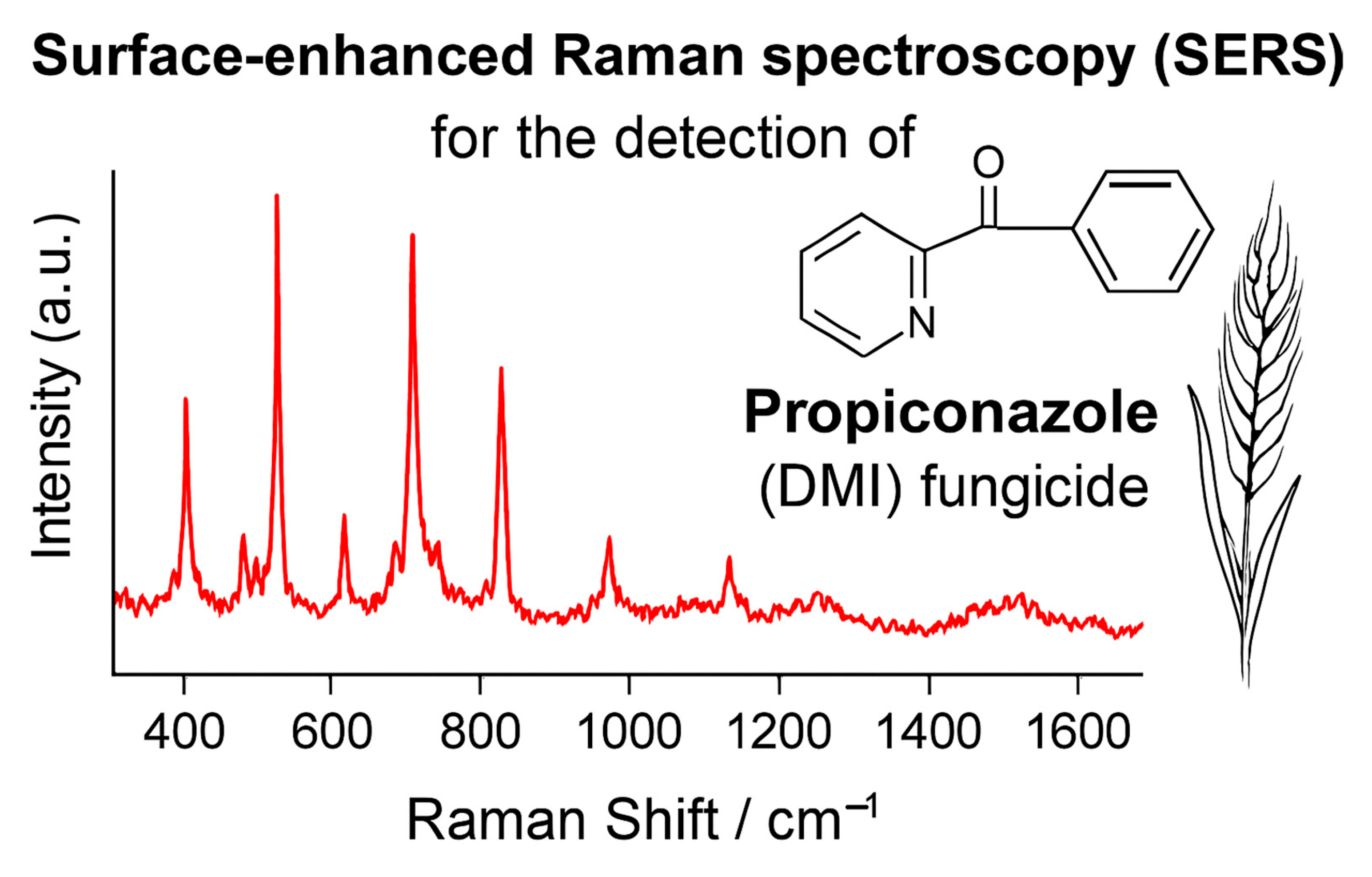
3.5. Detection of Propiconazole via SERSRaman Spectroscopy and SERS for Fungicide Detection: Advances and Applications
3.5.1. Raman Spectroscopy for Detecting Fungicides, Fungal Growth, and Mycotoxins in Cereals
3.5.2. Challenges of SERS and qPCR for Fungicides, Fungi and Mycotoxins Detection
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meena, A.K.; Varma, S.; Kumar, V.; Sharma, R. Impact of climate change on plant diseases and management strategies: A review. Int. J. Chem. Stud. 2020, 8, 2968–2973. [Google Scholar] [CrossRef]
- Lahlali, R.; Taoussi, M.; Laasli, S.-E.; Gachara, G.; Ezzouggari, R.; Belabess, Z.; Aberkani, K.; Assouguem, A.; Meddich, A.; El Jarroudi, M.; et al. Effects of climate change on plant pathogens and host-pathogen interactions. Crop Environ. 2024, 3, 159–170. [Google Scholar] [CrossRef]
- Ávila-Quezada, G.D.; Esquivel, J.F.; Silva-Rojas, H.V.; Leyva-Mir, S.G.; García-Ávila, C.D.; Noriega-Orozco, L.; Rivas-Valencia, P.; Ojeda-Barrios, D.L.; Castillo, A.M. Emerging plant diseases under a changing climate scenario: Threats to our global food supply. Emir. J. Food Agric. 2018, 30, 443–450. [Google Scholar] [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Mironenko, N.V.; Baranova, O.A.; Kovalenko, N.M.; Mikhailova, L.A.; Rosseva, L.P. Genetic structure of the Russian populations of Pyrenophora tritici-repentis, determined by using microsatellite markers. Russ. J. Genet. 2016, 52, 771–779. [Google Scholar] [CrossRef]
- Kremneva, O.Y.; Mironenko, N.V.; Volkova, G.V.; Baranova, O.A.; Kim, Y.S.; Kovalenko, N.M. Resistance of winter wheat varieties to tan spot in the North Caucasus region of Russia. Saudi J. Biol. Sci. 2020, 28, 1787–1794. [Google Scholar] [CrossRef]
- Hýsek, J.; Vavera, R.; Růžek, P. Influence of temperature, precipitation, and cultivar characteristics on changes in the spectrum of pathogenic fungi in winter wheat. Int. J. Biometeorol. 2017, 61, 967–975. [Google Scholar] [CrossRef]
- Porras, R.; Miguel-Rojas, C.; Lorite, I.J.; Pérez-De-Luque, A.; Sillero, J.C. Characterization of Durum Wheat Resistance against Septoria Tritici Blotch under Climate Change Conditions of Increasing Temperature and CO2 Concentration. Agronomy 2023, 13, 2638. [Google Scholar] [CrossRef]
- See, P.T.; Moffat, C.S.; Morina, J.; Oliver, R.P. Evaluation of a Multilocus Indel DNA Region for the Detection of the Wheat Tan Spot Pathogen Pyrenophora tritici-repentis. Plant Dis. 2016, 100, 2215–2225. [Google Scholar] [CrossRef]
- Guo, J.; Shi, G.; Kalil, A.; Friskop, A.; Elias, E.M.; Xu, S.S.; Faris, J.D.; Liu, Z. Pyrenophora tritici-repentis race 4 isolates cause disease on tetraploid wheat. Phytopathology 2020, 110, 1781–1790. [Google Scholar] [CrossRef]
- Kumarbayeva, M.; Kokhmetova, A.; Kovalenko, N.; Atishova, M.; Keishilov, Z.; Aitymbetova, K. Characterization of Pyrenophora tritici-repentis (tan spot of wheat) races in Kazakhstan. Phytopathol. Mediterr. 2022, 16, 243–257. [Google Scholar] [CrossRef]
- Mangel, D.; Bruce, M.; Noller, J.R. Race Structure of Pyrenophora tritici-repentis in the Kansas wheat pathogen population. Plant Dis. 2024, 109, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Vagelas, I. Effective Strategies for Managing Wheat Diseases: Mapping Academic Literature Utilizing VOSviewer and Insights from Our 15 Years of Research. Agrochemicals 2025, 4, 4. [Google Scholar] [CrossRef]
- Friesen, T.L.; Ali, S.; Stack, R.W.; Francl, L.J.; Rasmussen, J.B. Rapid and efficient production of the Pyrenophora tritici-repentis teleomorph. Can. J. Bot. 2003, 81, 890–895. [Google Scholar] [CrossRef]
- Duveiller, E.; Kandel, Y.R.; Sharma, R.C.; Shrestha, S.M. Epidemiology of foliar blights (spot blotch and tan spot) of wheat in the plains bordering the himalayas. Phytopathology 2005, 95, 248–256. [Google Scholar] [CrossRef]
- See, P.T.; Schultz, N.; Moffat, C.S. Evaluation of Pyrenophora tritici-repentis Infection of Wheat Heads. Agriculture 2020, 10, 417. [Google Scholar] [CrossRef]
- Manning, V.A.; Hardison, L.K.; Ciuffetti, L.M. Ptr ToxA interacts with a chloroplast-localized protein. Mol. Plant-Microbe Interact. 2007, 20, 168–177. [Google Scholar] [CrossRef]
- Mironenko, N.V.; Baranova, O.A.; Kovalenko, N.M. The role of the sexual process in preserving the alien translocation of the ToxA gene in the genome of Pyrenophora tritici-repentis. Mikol. Fitopatol. 2019, 53, 115–123. [Google Scholar] [CrossRef]
- Aboukhaddour, R.; Kim, Y.M.; Strelkov, S.E. RNA-mediated gene silencing of ToxB in Pyrenophora tritici-repentis. Mol. Plant Pathol. 2012, 13, 318–326. [Google Scholar] [CrossRef]
- Kaņeps, J.; Bankina, B.; Moročko-Bičevska, I. Virulence of Pyrenophora tritici-repentis: A minireview. Res. Rural Dev. 2021, 21–28. [Google Scholar] [CrossRef]
- Shi, G.; Kariyawasam, G.; Liu, S.; Leng, Y.; Zhong, S.; Ali, S.; Moolhuijzen, P.; Moffat, C.S.; Rasmussen, J.B.; Friesen, T.L.; et al. A conserved hypothetical gene is required but not sufficient for Ptr ToxC production in Pyrenophora tritici-repentis. Mol. Plant-Microbe Interact. 2022, 35, 336–348. [Google Scholar] [CrossRef]
- Laribi, M.; Yahyaoui, A.H.; Abdedayem, W.; Kouki, H.; Sassi, K.; Ben M’barek, S. Characterization of Mediterranean Durum Wheat for Resistance to Pyrenophora tritici-repentis. Genes 2022, 13, 336. [Google Scholar] [CrossRef]
- See, P.T.; Marathamuthu, K.A.; Iagallo, E.M.; Oliver, R.P.; Moffat, C.S. Evaluating the importance of the tan spot ToxA–Tsn1 interaction in Australian wheat varieties. Plant Pathol. 2018, 67, 1066–1075. [Google Scholar] [CrossRef]
- Ciuffetti, L.M.; Manning, V.A.; Pandelova, I.; Betts, M.F.; Martinez, J.P. Host-selective toxins, Ptr ToxA and Ptr ToxB, as necrotrophic effectors in the Pyrenophora tritici-repentis–wheat interaction. New Phytol. 2010, 187, 911–919. [Google Scholar] [CrossRef]
- Moolhuijzen, P.M.; See, P.T.; Oliver, R.P.; Moffat, C.S.; Wilson, R.A. Genomic distribution of a novel Pyrenophora tritici-repentis ToxA insertion element. PLoS ONE 2018, 13, e0206586. [Google Scholar] [CrossRef]
- See, P.T.; Iagallo, E.M.; Marathamuthu, K.A.; Wood, B.; Aboukhaddour, R.; Moffat, C.S. A New ToxA haplotype in the wheat fungal pathogen Bipolaris sorokiniana. Phytopathology 2024, 114, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, L.; Olsen, L. Control of tan spot (Drechslera tritici-repentis) using cultivar resistance, tillage methods and fungicides. Crop. Prot. 2007, 26, 1606–1616. [Google Scholar] [CrossRef]
- Vagelas, I.; Cavalaris, C.; Karapetsi, L.; Koukidis, C.; Servis, D.; Madesis, P. Protective Effects of Systiva® Seed Treatment Fungicide for the Control of Winter Wheat Foliar Diseases Caused at Early Stages Due to Climate Change. Agronomy 2022, 12, 2000. [Google Scholar] [CrossRef]
- Carignano, M.; Staggenborg, S.A.; Shroyer, J.P. Management Practices to Minimize Tan Spot in a Continuous Wheat Rotation. Agron. J. 2008, 100, 145–153. [Google Scholar] [CrossRef]
- Reynoso, A.; Sautua, F.; Carmona, M.; Chulze, S.; Palazzini, J. Tan spot of wheat: Can biological control interact with actual management practices to counteract this global disease? Eur. J. Plant Pathol. 2023, 166, 27–38. [Google Scholar] [CrossRef]
- Bugingo, C.; Ali, S.; Yabwalo, D.; Byamukama, E. Optimizing Fungicide Seed Treatments for Early Foliar Disease Management in Wheat Under Northern Great Plains Conditions. Agronomy 2025, 15, 291. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, R.P.; Duveiller, E.; Mergoum, M.; Adhikari, T.B.; Elias, E.M. Genetics of wheat–Pyrenophora tritici-repentis interactions. Euphytica 2009, 171, 1–13. [Google Scholar] [CrossRef]
- Bhathal, J.; Loughman, R.; Speijers, J. Yield Reduction in Wheat in Relation to Leaf Disease From Yellow (tan) Spot and Septoria Nodorum Blotch. Eur. J. Plant Pathol. 2003, 109, 435–443. [Google Scholar] [CrossRef]
- Bouras, N.; Strelkov, S.E. The anthraquinone catenarin is phytotoxic and produced in leaves and kernels of wheat infected by Pyrenophora tritici-repentis. Physiol. Mol. Plant Pathol. 2008, 72, 87–95. [Google Scholar] [CrossRef]
- Bouras, N.; Holtz, M.D.; Aboukhaddour, R.; Strelkov, S.E. Influence of nitrogen sources on growth and mycotoxin production by isolates of Pyrenophora tritici-repentis from wheat. Crop J. 2016, 4, 119–128. [Google Scholar] [CrossRef]
- Colson, E.S.; Platz, G.J.; Usher, T.R. Fungicidal control of Pyrenophora tritici-repentis in wheat. Australas. Plant Pathol. 2003, 32, 241–246. [Google Scholar] [CrossRef]
- Vagelas, I. Important Foliar Wheat Diseases and their Management: Field Studies in Greece. Mod. Concepts Dev. Agron. 2021, 8, 783–786. [Google Scholar] [CrossRef]
- Kaņeps, J.; Bankina, B.; Moročko-Bičevska, I.; Apsīte, K.; Roga, A.; Fridmanis, D. Sensitivity Analysis of Pyrenophora tritici-repentis to Quinone-Outside Inhibitor and 14α-Demethylase Inhibitor Fungicides in Latvia. Pathogens 2024, 13, 1060. [Google Scholar] [CrossRef]
- Sautua, F.J.; Carmona, M.A. Detection and characterization of QoI resistance in Pyrenophora tritici-repentis populations causing tan spot of wheat in Argentina. Plant Pathol. 2021, 70, 2125–2136. [Google Scholar] [CrossRef]
- Sautua, F.J.; Carmona, M.A. Baseline sensitivity of QoI-resistant isolates of Pyrenophora tritici-repentis from Argentina to fenpicoxamid. Eur. J. Plant Pathol. 2022, 164, 583–591. [Google Scholar] [CrossRef]
- Bankina, B.; Bimšteine, G.; Arhipova, I.; Kaņeps, J.; Stanka, T. Importance of Agronomic Practice on the Control of Wheat Leaf Diseases. Agriculture 2018, 8, 56. [Google Scholar] [CrossRef]
- Bankina, B.; Bimšteine, G.; Arhipova, I.; Kaņeps, J.; Darguža, M. Impact of Crop Rotation and Soil Tillage on the Severity of Winter Wheat Leaf Blotches. Rural Sustain. Res. 2021, 45, 21–27. [Google Scholar] [CrossRef]
- Mazzilli, S.R.; Ernst, O.R.; de Mello, V.P.; Pérez, C.A. Yield losses on wheat crops associated to the previous winter crop: Impact of agronomic practices based on on-farm analysis. Eur. J. Agron. 2016, 75, 99–104. [Google Scholar] [CrossRef]
- Kauffmann, T.H.; Kokanyan, N.; Fontana, M.D. Use of Stokes and anti-Stokes Raman scattering for new applications. J. Raman Spectrosc. 2018, 50, 418–424. [Google Scholar] [CrossRef]
- Maher, R.C.; Cohen, L.F.; Le Ru, E.C.; Etchegoin, P.G. A study of local heating of molecules under surface enhanced raman scattering (SERS) conditions using the anti-Stokes/Stokes ratio. Faraday Discuss. 2006, 132, 77–83, discussion 85–94. [Google Scholar] [CrossRef] [PubMed]
- Herman, I.P. Peak temperatures from Raman Stokes/anti-Stokes ratios during laser heating by a Gaussian beam. J. Appl. Phys. 2011, 109, 016103. [Google Scholar] [CrossRef]
- Saletnik, A.; Saletnik, B.; Zaguła, G.; Puchalski, C. Raman Spectroscopy for Plant Disease Detection in Next-Generation Agriculture. Sustainability 2024, 16, 5474. [Google Scholar] [CrossRef]
- Farber, C.; Kurouski, D. Raman Spectroscopy and Machine Learning for Agricultural Applications: Chemometric Assessment of Spectroscopic Signatures of Plants as the Essential Step Toward Digital Farming. Front. Plant Sci. 2022, 13, 887511. [Google Scholar] [CrossRef]
- Farber, C.; Bryan, R.; Paetzold, L.; Rush, C.; Kurouski, D. Non-Invasive Characterization of Single-, Double- and Triple-Viral Diseases of Wheat With a Hand-Held Raman Spectrometer. Front. Plant Sci. 2020, 11, 01300. [Google Scholar] [CrossRef]
- Salbreiter, M.; Frempong, S.B.; Even, S.; Wagenhaus, A.; Girnus, S.; Rösch, P.; Popp, J. Lighting the Path: Raman Spectroscopy’s Journey Through the Microbial Maze. Molecules 2024, 29, 5956. [Google Scholar] [CrossRef]
- Ji, X.; Xue, J.; Shi, J.; Wang, W.; Zhang, X.; Wang, Z.; Lu, W.; Liu, J.; Fu, Y.V.; Xu, N. Noninvasive Raman spectroscopy for the detection of rice bacterial leaf blight and bacterial leaf streak. Talanta 2024, 282, 126962. [Google Scholar] [CrossRef]
- Egging, V.; Nguyen, J.; Kurouski, D. Detection and Identification of Fungal Infections in Intact Wheat and Sorghum Grain Using a Hand-Held Raman Spectrometer. Anal. Chem. 2018, 90, 8616–8621. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.; Pant, S.; Mandadi, K.; Kurouski, D. Raman Spectroscopy vs Quantitative Polymerase Chain Reaction in Early Stage Huanglongbing Diagnostics. Sci. Rep. 2020, 10, 10101. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Hu, X.; Wang, J.; Tang, L.; Li, P.; Zheng, S.; Zheng, L.; Huang, L.; Xin, Z. Advanced Application of Raman Spectroscopy and Surface-Enhanced Raman Spectroscopy in Plant Disease Diagnostics: A Review. J. Agric. Food Chem. 2021, 69, 2950–2964. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Lin, Q.; Zhang, J.; Young, G.M.; Jiang, C.; Zhong, Y.; Zhang, J. Rapid identification of pathogens by using surface-enhanced Raman spectroscopy and multi-scale convolutional neural network. Anal. Bioanal. Chem. 2021, 413, 3801–3811. [Google Scholar] [CrossRef]
- Tahir, M.A.; Dina, N.E.; Cheng, H.; Valev, V.K.; Zhang, L. Surface-enhanced Raman spectroscopy for bioanalysis and diagnosis. Nanoscale 2021, 13, 11593–11634. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Sun, Y.; Wu, H.; Luo, L.; Song, Y. Recent Advances in Surface-Enhanced Raman Scattering for Pathogenic Bacteria Detection: A Review. Sensors 2025, 25, 1370. [Google Scholar] [CrossRef]
- Kissell, L.N.; Liu, H.; Sheokand, M.; Vang, D.; Kachroo, P.; Strobbia, P. Direct Detection of Tobacco Mosaic Virus in Infected Plants with SERS-Sensing Hydrogels. ACS Sens. 2024, 9, 514–523. [Google Scholar] [CrossRef]
- Xia, J.; Li, W.; Sun, M.; Wang, H. Application of SERS in the Detection of Fungi, Bacteria and Viruses. Nanomaterials 2022, 12, 3572. [Google Scholar] [CrossRef]
- Liu, L.; Ma, W.; Wang, X.; Li, S. Recent Progress of Surface-Enhanced Raman Spectroscopy for Bacteria Detection. Biosensors 2023, 13, 350. [Google Scholar] [CrossRef]
- Liu, Y.; Su, G.; Wang, W.; Wei, H.; Dang, L. A novel multifunctional SERS microfluidic sensor based on ZnO/Ag nanoflower arrays for label-free ultrasensitive detection of bacteria. Anal. Methods 2024, 16, 2085–2092. [Google Scholar] [CrossRef]
- Yu, X.; Park, S.; Lee, S.; Joo, S.-W.; Choo, J. Microfluidics for disease diagnostics based on surface-enhanced raman scattering detection. Nano Converg. 2024, 11, 17. [Google Scholar] [CrossRef]
- Wang, X.; Liang, S.; Gan, Q.; Cai, B.; Liu, C. Current status and future perspectives of the diagnostic of plant bacterial pathogens. Front. Plant Sci. 2025, 16, 1547974. [Google Scholar] [CrossRef] [PubMed]
- Peña Quiñones, A.J.; Keller, M.; Salazar Gutierrez, M.R.; Khot, L.; Hoogenboom, G. Comparison between grapevine tissue temperature and air temperature. Sci. Hortic. 2019, 247, 407–420. [Google Scholar] [CrossRef]
- Vagelas, I.; Papadimos, A.; Lykas, C. Pre-Symptomatic Disease Detection in the Vine, Chrysanthemum, and Rose Leaves with a Low-Cost Infrared Sensor. Agronomy 2021, 11, 1682. [Google Scholar] [CrossRef]
- Onaga, G.; Wydra, K.D.; Koopmann, B.; Séré, Y.; von Tiedemann, A. Elevated temperature increases in planta expression levels of virulence related genes in Magnaporthe oryzae and compromises resistance in Oryza sativa cv. Nipponbare. Funct. Plant Biol. 2017, 44, 358–371. [Google Scholar] [CrossRef]
- Vagelas, I.; Manthos, I.; Sotiropoulos, T. Applications of Raman Microscopy/Spectroscopy-Based Techniques to Plant Disease Diagnosis. Appl. Sci. 2024, 14, 5926. [Google Scholar] [CrossRef]
- Eckbreth, A.C. Coherent Anti-Stokes Raman Spectroscopy (CARS). In Laser Diagnostics for Combustion Temperature and Species; Routledge: London, UK, 2022. [Google Scholar] [CrossRef]
- Weissflog, I.; Vogler, N.; Akimov, D.; Dellith, A.; Schachtschabel, D.; Svatos, A.; Boland, W.; Dietzek, B.; Popp, J. Toward in Vivo Chemical Imaging of Epicuticular Waxes. Plant Physiol. 2010, 154, 604–610. [Google Scholar] [CrossRef]
- Pezacki, J.P.; Blake, J.A.; Danielson, D.C.; Kennedy, D.C.; Lyn, R.K.; Singaravelu, R. Chemical contrast for imaging living systems: Molecular vibrations drive CARS microscopy. Nat. Chem. Biol. 2011, 7, 137–145. [Google Scholar] [CrossRef]
- Khmaladze, A.; Jasensky, J.; Zhang, C.; Han, X.; Ding, J.; Seeley, E.; Liu, X.; Smith, G.; Chen, Z. Hyperspectral microscopic imaging by multiplex coherent anti-Stokes Raman scattering (CARS). In Proceedings of the Optical Engineering + Applications, San Diego, CA, USA, 6 September 2011. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Yi, R.; Liu, L.; Qu, J. Coherent Anti-Stokes Raman Scattering Microscopy and Its Applications. Front. Phys. 2020, 8, 598420. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, H.; Huff, T.B.; Shi, R.; Cheng, J. Coherent anti-stokes Raman scattering imaging of myelin degradation reveals a calcium-dependent pathway in lyso-PtdCho-induced demyelination. J. Neurosci. Res. 2007, 85, 2870–2881. [Google Scholar] [CrossRef] [PubMed]
- Pelegati, V.B.; Kyotoku, B.B.C.; Padilha, L.A.; Cesar, C.L. Six-wave mixing coherent anti-Stokes Raman scattering microscopy. Biomed. Opt. Express 2018, 9, 2407–2417. [Google Scholar] [CrossRef] [PubMed]
- Patel, I.I.; Steuwe, C.; Reichelt, S.; Mahajan, S. Coherent anti-Stokes Raman scattering for label-free biomedical imaging. J. Opt. 2013, 15, 094006. [Google Scholar] [CrossRef][Green Version]
- Evans, C.L.; Xie, X.S. Coherent anti-stokes raman scattering microscopy: Chemical imaging for biology and medicine. Annu. Rev. Anal. Chem. 2008, 1, 883–909. [Google Scholar] [CrossRef]
- Khmaladze, A.; Jasensky, J.; Price, E.; Zhang, C.; Boughton, A.; Han, X.; Seeley, E.; Liu, X.; Holl, M.M.B.; Chen, Z. Hyperspectral Imaging and Characterization of Live Cells by Broadband Coherent Anti-Stokes Raman Scattering (CARS) Microscopy with Singular Value Decomposition (SVD) Analysis. Appl. Spectrosc. 2014, 68, 1116–1122. [Google Scholar] [CrossRef]
- Murugkar, S.; Evans, C.; Xie, X.; Anis, H. Chemically specific imaging of cryptosporidium oocysts using coherent anti-Stokes Raman scattering (CARS) microscopy. J. Microsc. 2009, 233, 244–250. [Google Scholar] [CrossRef]
- Wu, F.; Li, S.; Chen, X.; Yue, S.; Hong, W.; Wang, P. High-Sensitive and Background-Free Coherent Anti-Stokes Raman Scattering Microscopy Using Delay Modulation. Laser Photonics Rev. 2024, 18, 2300827. [Google Scholar] [CrossRef]
- Underwood, W.; Koh, S.; Somerville, S.C. Visualizing cellular dynamics in plant-microbe interactions using fluorescent-tagged proteins. Methods Mol. Biol. 2011, 712, 283–291. [Google Scholar] [CrossRef]
- Naemat, A.; Sinjab, F.; McDonald, A.; Downes, A.; Elfick, A.; Elsheikha, H.M.; Notingher, I. Visualizing the interaction of Acanthamoeba castellanii with human retinal epithelial cells by spontaneous Raman and CARS imaging. J. Raman Spectrosc. 2018, 49, 412–423. [Google Scholar] [CrossRef]
- Hong, W.; Liao, C.; Zhao, H.; Younis, W.; Zhang, Y.; Seleem, M.N.; Cheng, J. In situ Detection of a Single Bacterium in Complex Environment by Hyperspectral CARS Imaging. ChemistrySelect 2016, 1, 513–517. [Google Scholar] [CrossRef]
- Petrov, G.I.; Yakovlev, V.V.; Sokolov, A.V.; Scully, M.O. Detection of Bacillus subtilis spores in water by means of broadband coherent anti-Stokes Raman spectroscopy. Opt. Express 2005, 13, 9537–9542. [Google Scholar] [CrossRef] [PubMed]
- Strycker, B.D.; Han, Z.; Commer, B.; Shaw, B.D.; Sokolov, A.V.; Scully, M.O. CARS spectroscopy of Aspergillus nidulans spores. Sci. Rep. 2019, 9, 1789. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Wang, X.; Wang, Y.; Xie, Z.; Tian, Y.; Lu, Y. Culture-Free Detection of Crop Pathogens at the Single-Cell Level by Micro-Raman Spectroscopy. Adv. Sci. 2017, 4, 1700127. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, M.; Zhao, H.; Ren, X.; Lin, T.; Zhang, P.; Zheng, D. Rapid detection and identification of fungi in grain crops using colloidal Au nanoparticles based on surface-enhanced Raman scattering and multivariate statistical analysis. World J. Microbiol. Biotechnol. 2022, 39, 26. [Google Scholar] [CrossRef]
- Wang, X.; Ai, S.; Xiong, A.; Zhou, W.; He, L.; Teng, J.; Geng, X.; Wu, R. SERS combined with QuEChERS using NBC and Fe3O4 MNPs as cleanup agents to rapidly and reliably detect chlorpyrifos pesticide in citrus. Anal. Methods 2023, 15, 6266–6274. [Google Scholar] [CrossRef]
- Lian, S.; Li, X.; Lv, X. A Novel SERS Label-Free Sensing Strategy for DON and NIV: A DFT Study on the Interaction between DON/NIV and Ag/Au. Langmuir 2024, 40, 20954–20965. [Google Scholar] [CrossRef]
- Rodriguez, R.S.; Szlag, V.M.; Reineke, T.M.; Haynes, C.L. Multiplex surface-enhanced Raman scattering detection of deoxynivalenol and ochratoxin A with a linear polymer affinity agent. Mater. Adv. 2020, 1, 3256–3266. [Google Scholar] [CrossRef]
- Alieva, R.; Sokolova, S.; Zhemchuzhina, N.; Pankin, D.; Povolotckaia, A.; Novikov, V.; Kuznetsov, S.; Gulyaev, A.; Moskovskiy, M.; Zavyalova, E. A Surface-Enhanced Raman Spectroscopy-Based Aptasensor for the Detection of Deoxynivalenol and T-2 Mycotoxins. Int. J. Mol. Sci. 2024, 25, 9534. [Google Scholar] [CrossRef]
- Lin, X.; Yu, W.; Tong, X.; Li, C.; Duan, N.; Wang, Z.; Wu, S. Application of Nanomaterials for Coping with Mycotoxin Contamination in Food Safety: From Detection to Control. Crit. Rev. Anal. Chem. 2022, 54, 355–388. [Google Scholar] [CrossRef]
- Ma, C.-H.; Zhang, J.; Hong, Y.-C.; Wang, Y.-R.; Chen, X. Determination of carbendazim in tea using surface enhanced Raman spectroscopy. Chin. Chem. Lett. 2015, 26, 1455–1459. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.; Tian, S.; Hong, T. Novel Surface-Enhanced Raman Spectroscopy Techniques for DNA, Protein and Drug Detection. Sensors 2019, 19, 1712. [Google Scholar] [CrossRef]
- Chen, X.; Lin, M.; Sun, L.; Xu, T.; Lai, K.; Huang, M.; Lin, H. Detection and quantification of carbendazim in Oolong tea by surface-enhanced Raman spectroscopy and gold nanoparticle substrates. Food Chem. 2019, 293, 271–277. [Google Scholar] [CrossRef]
- Kulakovich, O.S.; Matsukovich, A.S.; Trotsiuk, L.L. Challenges in surface-enhanced Raman scattering detection of pesticide carbendazim and ways to overcome. J. Nanophotonics 2022, 16, 046002. [Google Scholar] [CrossRef]
- Yan, D.; Ma, C.; Jing, X.; Zhang, J.; Qian, R.; Chen, L. Effects of aggregating agents on the surface-enhanced Raman spectroscopic detection of carbendazim. In Proceedings of the Fifteenth International Conference on Information Optics and Photonics (CIOP 2024), Xi’an, China, 11–15 August 2024. [Google Scholar] [CrossRef]
- Oliveira, M.J.d.S.; Ruiz, G.C.M.; Rubira, R.J.G.; Sanchez-Cortes, S.; Constantino, C.J.L.; Furini, L.N. Consequences of Surface Composition and Aggregation Conditions of Ag Nanoparticles on Surface-Enhanced Raman Scattering (SERS) of Pesticides. Chemosensors 2025, 13, 13. [Google Scholar] [CrossRef]
- Song, J.; Chen, Z.-P.; Jin, J.-W.; Chen, Y.; Yu, R.-Q. Quantitative surface-enhanced Raman spectroscopy based on the combination of magnetic nanoparticles with an advanced chemometric model. Chemom. Intell. Lab. Syst. 2014, 135, 31–36. [Google Scholar] [CrossRef]
- Sivaraj, S.; Ramasamy, P.; Sathe, V.; Mahalingam, U. Synergistic Effects of plasmonic and semiconductor nanoparticles on graphene oxide for thiodicarb pesticide detection and Detoxification. Appl. Surf. Sci. 2025, 689, 162469. [Google Scholar] [CrossRef]
- Li, J.; Cheng, J.; Du, J.; Xiao, M.; Wang, M.; Wang, J.; She, Y.; El-Aty, A.A.; Cao, X. Detection of myclobutanil and tebuconazole in apple using magnetic molecularly imprinted polymer surface-enhanced Raman spectroscopy. J. Food Compos. Anal. 2024, 133, 106380. [Google Scholar] [CrossRef]
- Chen, Z.; Dong, X.; Liu, C.; Wang, S.; Dong, S.; Huang, Q. Rapid detection of residual chlorpyrifos and pyrimethanil on fruit surface by surface-enhanced Raman spectroscopy integrated with deep learning approach. Sci. Rep. 2023, 13, 19855. [Google Scholar] [CrossRef]
- Chen, Z.; Tan, R.; Zeng, M.; Yuan, X.; Zhuang, K.; Feng, C.; He, Y.; Luo, X. SERS detection of triazole pesticide residues on vegetables and fruits using Au decahedral nanoparticles. Food Chem. 2023, 439, 138110. [Google Scholar] [CrossRef]
- Ouyang, L.; Ren, W.; Zhu, L.; Irudayaraj, J. Prosperity to challenges: Recent approaches in SERS substrate fabrication. Rev. Anal. Chem. 2017, 36, 20160027. [Google Scholar] [CrossRef]
- Lin, T.; Song, Y.-L.; Liao, J.; Liu, F.; Zeng, T.-T. Applications of surface-enhanced Raman spectroscopy in detection fields. Nanomedicine 2020, 15, 2971–2989. [Google Scholar] [CrossRef]
- Kim, M.; Kang, J.; Park, S.; Lee, M. Surface-enhanced Raman spectroscopy of quinomethionate adsorbed on silver colloids. Bull. Korean Chem. Soc. 2003, 24, 633–637. [Google Scholar] [CrossRef][Green Version]
- Sun, Y.; Zheng, X.; Wang, H.; Yan, M.; Chen, Z.; Yang, Q.; Shao, Y. Research advances of SERS analysis method based on silent region molecules for food safety detection. Microchim. Acta 2023, 190, 387. [Google Scholar] [CrossRef] [PubMed]
- Camden, J.P.; Dieringer, J.A.; Wang, Y.; Masiello, D.J.; Marks, L.D.; Schatz, G.C.; Van Duyne, R.P. Probing the structure of Single-molecule surface-enhanced Raman scattering hot spots. J. Am. Chem. Soc. 2008, 130, 12616–12617. [Google Scholar] [CrossRef]
- Weng, S.; Yuan, H.; Zhang, X.; Li, P.; Zheng, L.; Zhao, J.; Huang, L. Deep learning networks for the recognition and quantitation of surface-enhanced Raman spectroscopy. Analyst 2020, 145, 4827–4835. [Google Scholar] [CrossRef]
- Zhang, D.; Liang, P.; Chen, W.; Tang, Z.; Li, C.; Xiao, K.; Jin, S.; Ni, D.; Yu, Z. Rapid field trace detection of pesticide residue in food based on surface-enhanced Raman spectroscopy. Microchim. Acta 2021, 188, 370. [Google Scholar] [CrossRef]
- Weng, S.; Yu, S.; Dong, R.; Zhao, J.; Liang, D. Detection of Pirimiphos-Methyl in Wheat Using Surface-Enhanced Raman Spectroscopy and Chemometric Methods. Molecules 2019, 24, 1691. [Google Scholar] [CrossRef]
- Han, M.; Lu, H.; Zhang, Z. Fast and Low-Cost Surface-Enhanced Raman Scattering (SERS) Method for On-Site Detection of Flumetsulam in Wheat. Molecules 2020, 25, 4662. [Google Scholar] [CrossRef]
- Lukošiūtė-Stasiukonienė, A.; Almogdad, M.; Semaškienė, R.; Mačiulytė, V. Crop Density and Sowing Timing Effect on Tan Spot Occurrence in Spring Wheat. Agriculture 2024, 14, 1284. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, N.; Liu, J.; Liu, W.; Wang, G.-L. The role of effectors and host immunity in plant–necrotrophic fungal interactions. Virulence 2014, 5, 722–732. [Google Scholar] [CrossRef]
- Shao, D.; Smith, D.L.; Kabbage, M.; Roth, M.G. Effectors of Plant Necrotrophic Fungi. Front. Plant Sci. 2021, 12, 687713. [Google Scholar] [CrossRef]
- Derbyshire, M.C.; Raffaele, S. Till death do us pair: Co-evolution of plant–necrotroph interactions. Curr. Opin. Plant Biol. 2023, 76, 102457. [Google Scholar] [CrossRef] [PubMed]
- Mironenko, N.V.; Baсильeвнa, M.H.; Orina, A.S.; Cтaнислaвoвнa, O.A.; Kovalenko, N.M.; Mихaйлoвнa, K.H. Expression of the ToxA and PtrPf2 genes of the phytopathogenic fungus Pyrenophora tritici-repentis at the beginning of the infection process. Ecol. Genet. 2019, 18, 149–155. [Google Scholar] [CrossRef]
- Mironenko, N.V.; Orina, A.S.; Kovalenko, N.M. Novel ToxA Insertion Element in Pyrenophora tritici-repentis. Russ. J. Genet. 2024, 60, 1161–1167. [Google Scholar] [CrossRef]
- Tran, V.A.; Aboukhaddour, R.; Strelkov, I.S.; Bouras, N.; Spaner, D.; Strelkov, S.E. The sensitivity of Canadian wheat genotypes to the necrotrophic effectors produced by Pyrenophora tritici-repentis. Can. J. Plant Pathol. 2017, 39, 149–162. [Google Scholar] [CrossRef]
- Faris, J.D.; Friesen, T.L. Plant genes hijacked by necrotrophic fungal pathogens. Curr. Opin. Plant Biol. 2020, 56, 74–80. [Google Scholar] [CrossRef]
- Tan, K.-C.; Oliver, R.P.; Solomon, P.S.; Moffat, C.S. Proteinaceous necrotrophic effectors in fungal virulence. Funct. Plant Biol. 2010, 37, 907–912. [Google Scholar] [CrossRef]
- Liao, C.-J.; Hailemariam, S.; Sharon, A.; Mengiste, T. Pathogenic strategies and immune mechanisms to necrotrophs: Differences and similarities to biotrophs and hemibiotrophs. Curr. Opin. Plant Biol. 2022, 69, 102291. [Google Scholar] [CrossRef]
- Sun, J.; Gong, L.; Wang, W.; Gong, Z.; Wang, D.; Fan, M. Surface-enhanced Raman spectroscopy for on-site analysis: A review of recent developments. Luminescence 2020, 35, 808–820. [Google Scholar] [CrossRef]
- Zhu, A.; Ali, S.; Jiao, T.; Wang, Z.; Ouyang, Q.; Chen, Q. Advances in surface-enhanced Raman spectroscopy technology for detection of foodborne pathogens. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1466–1494. [Google Scholar] [CrossRef]
- Afroozeh, A. A Review of Developed Surface-Enhanced Raman Spectroscopy (SERS)-Based Sensors for the Detection of Common Hazardous Substances in the Agricultural Industry. Plasmonics 2024, 20, 63–81. [Google Scholar] [CrossRef]
- Atanasov, P.A.; Nedyalkov, N.N.; Fukata, N.; Jevasuwan, W.; Subramani, T.; Terakawa, M.; Nakajima, Y. Surface-Enhanced Raman Spectroscopy (SERS) of Mancozeb and Thiamethoxam Assisted by Gold and Silver Nanostructures Produced by Laser Techniques on Paper. Appl. Spectrosc. 2018, 73, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Sharma, A.K.; Agarwal, A.; Prajesh, R. Label-free detection of Thiram pesticide on flexible SERS-active substrate. Mater. Chem. Phys. 2022, 295, 127088. [Google Scholar] [CrossRef]
- Feng, Z.; Yang, X.; Guo, X.; Yu, J.; Li, F.; Shao, J.; Liang, H.; Jiang, H. Sensitive Determination of Carbendazim in Tobacco Leaves by Surface Enhanced Raman Spectroscopy (SERS) Using a Silica–Silver Nanoparticle Substrate and QuEChERs Pretreatment. Anal. Lett. 2024, 57, 2644–2653. [Google Scholar] [CrossRef]
- Cialla-May, D.; Bonifacio, A.; Bocklitz, T.; Markin, A.; Markina, N.; Fornasaro, S.; Dwivedi, A.; Dib, T.; Farnesi, E.; Liu, C.; et al. Biomedical SERS—The current state and future trends. Chem. Soc. Rev. 2024, 53, 8957–8979. [Google Scholar] [CrossRef]
- Lau, H.Y.; Wang, Y.; Wee, E.J.H.; Botella, J.R.; Trau, M. Field Demonstration of a Multiplexed Point-of-Care Diagnostic Platform for Plant Pathogens. Anal. Chem. 2016, 88, 8074–8081. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagelas, I. Raman Spectroscopy as a Tool for Early Identification of Tan Spot Disease and Assessment of Fungicide Response in Wheat. Agronomy 2025, 15, 1952. https://doi.org/10.3390/agronomy15081952
Vagelas I. Raman Spectroscopy as a Tool for Early Identification of Tan Spot Disease and Assessment of Fungicide Response in Wheat. Agronomy. 2025; 15(8):1952. https://doi.org/10.3390/agronomy15081952
Chicago/Turabian StyleVagelas, Ioannis. 2025. "Raman Spectroscopy as a Tool for Early Identification of Tan Spot Disease and Assessment of Fungicide Response in Wheat" Agronomy 15, no. 8: 1952. https://doi.org/10.3390/agronomy15081952
APA StyleVagelas, I. (2025). Raman Spectroscopy as a Tool for Early Identification of Tan Spot Disease and Assessment of Fungicide Response in Wheat. Agronomy, 15(8), 1952. https://doi.org/10.3390/agronomy15081952





