Monitoring Rose Black Spot Disease Using Electrical Impedance Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Treatment Methods
2.3. Parameters Measurement
2.4. Statistical Analysis
3. Results
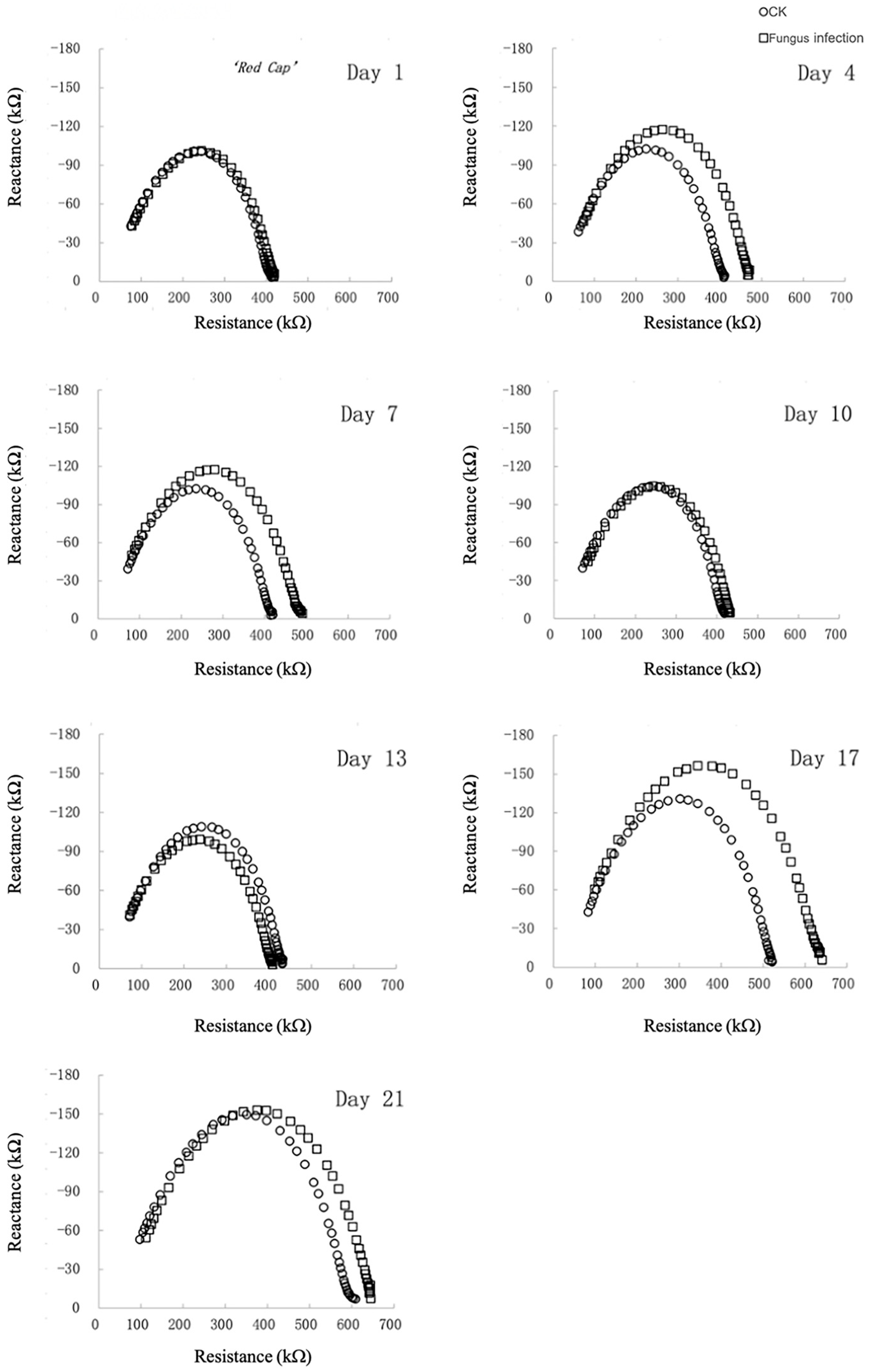
3.1. Changes in Electrical Impedance Spectroscopy and Parameters
3.1.1. Electrical Impedance Spectroscopy
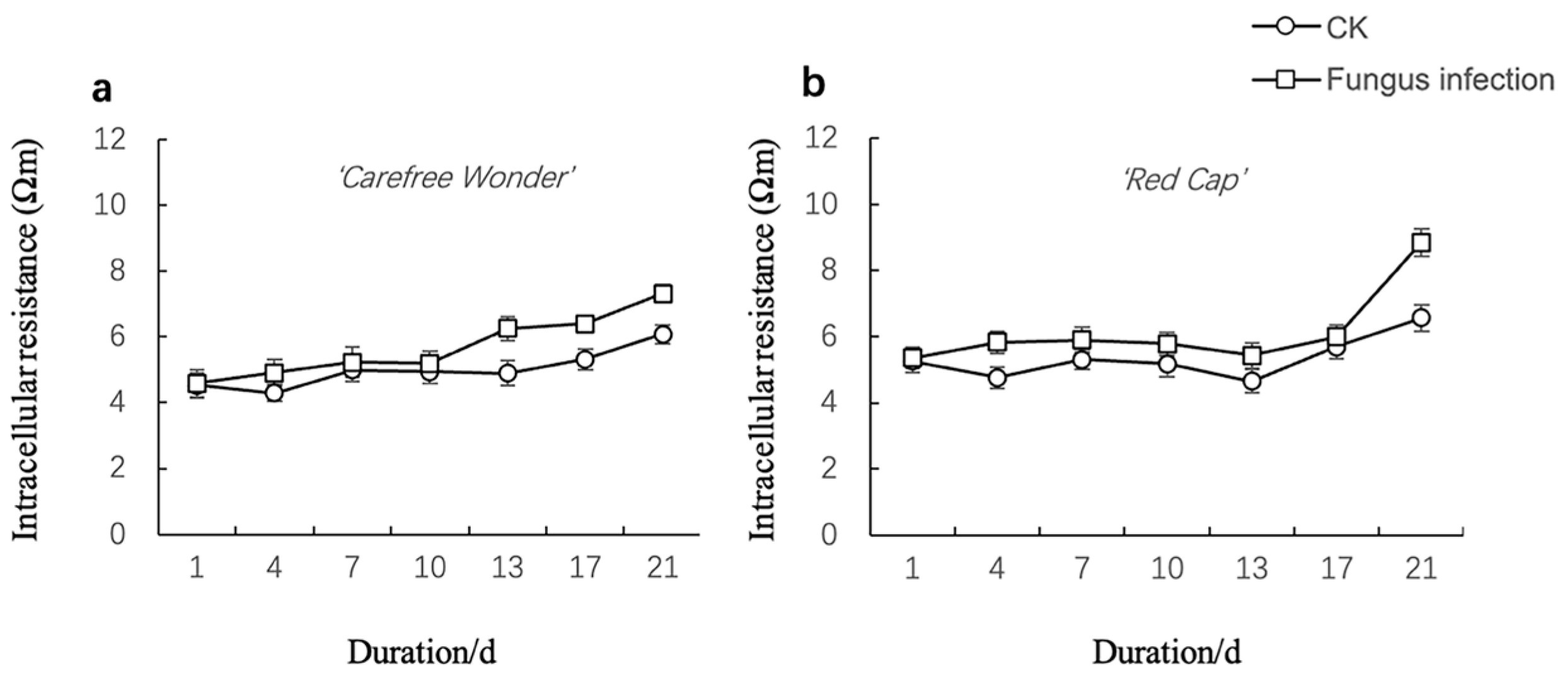
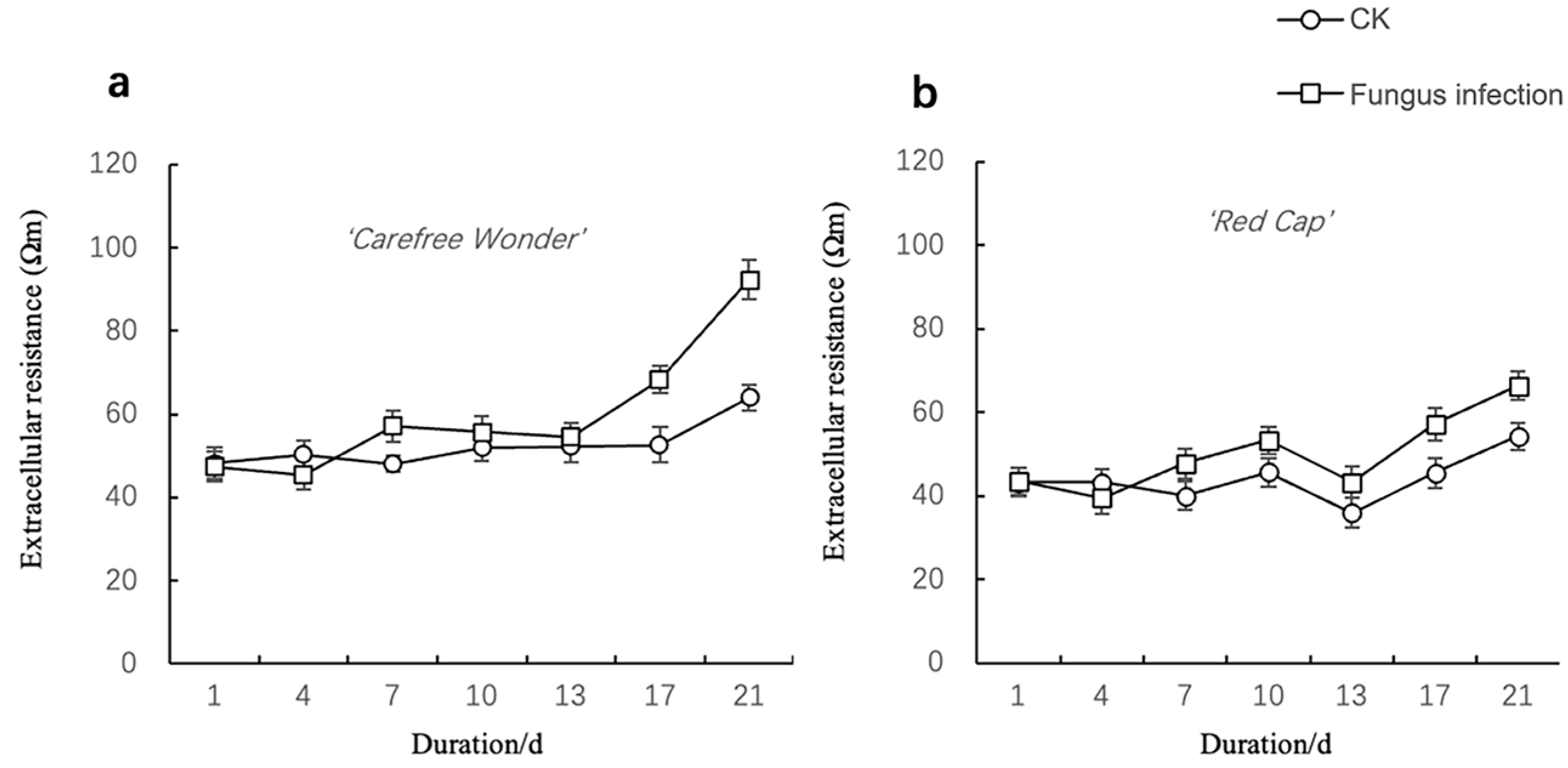
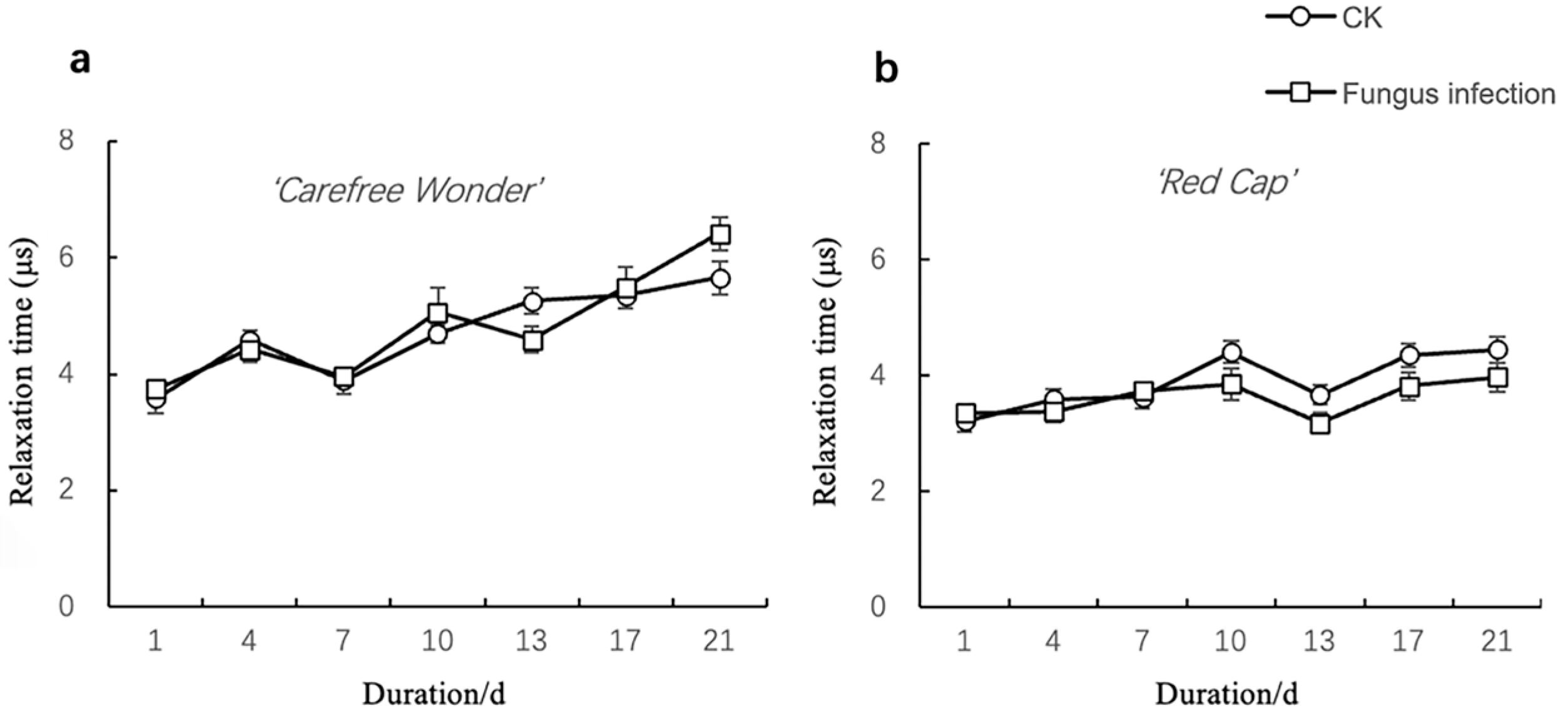
3.1.2. Changes in Electrical Impedance Parameters
Intracellular Resistivity (ri)
Extracellular Resistivity (re)
Relaxation Time (τ)
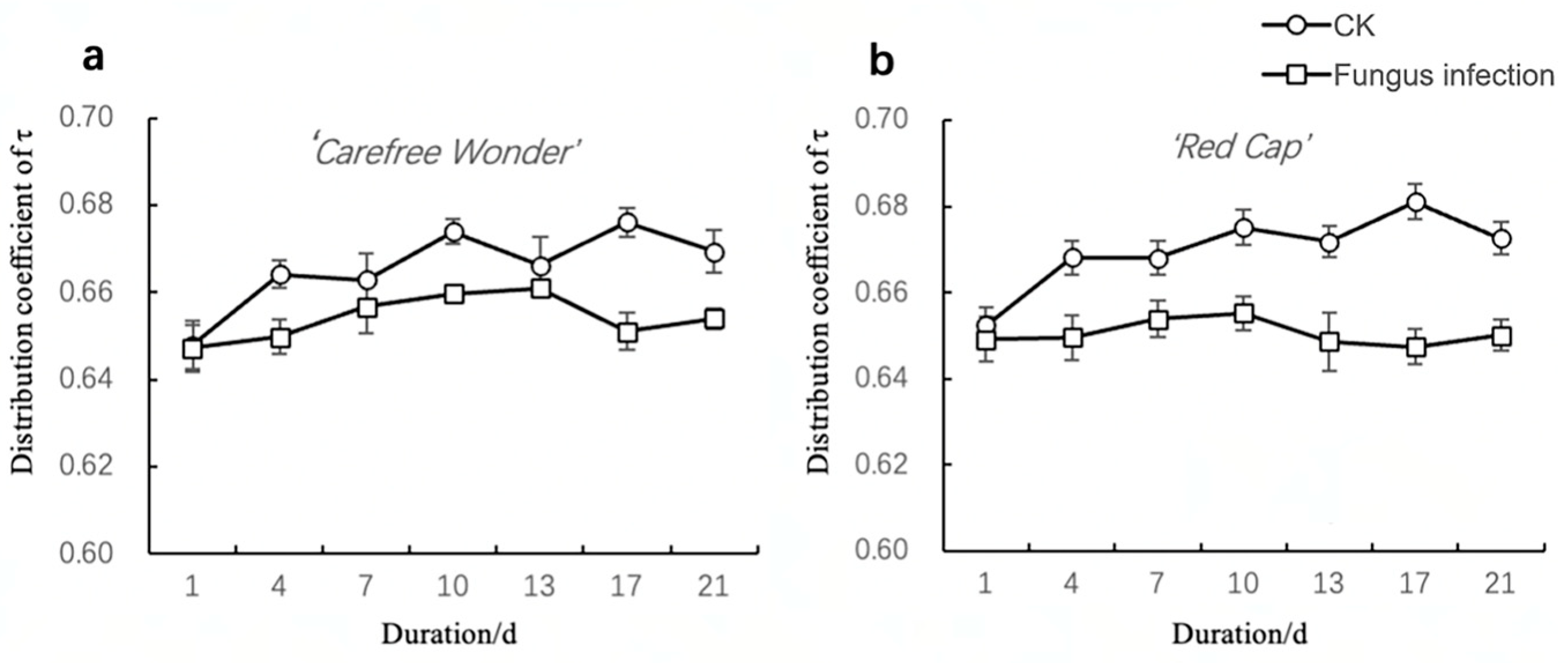
Dispersion Coefficient of Relaxation Time (ψ)
3.2. Changes in Osmotic Adjustment Substance Content
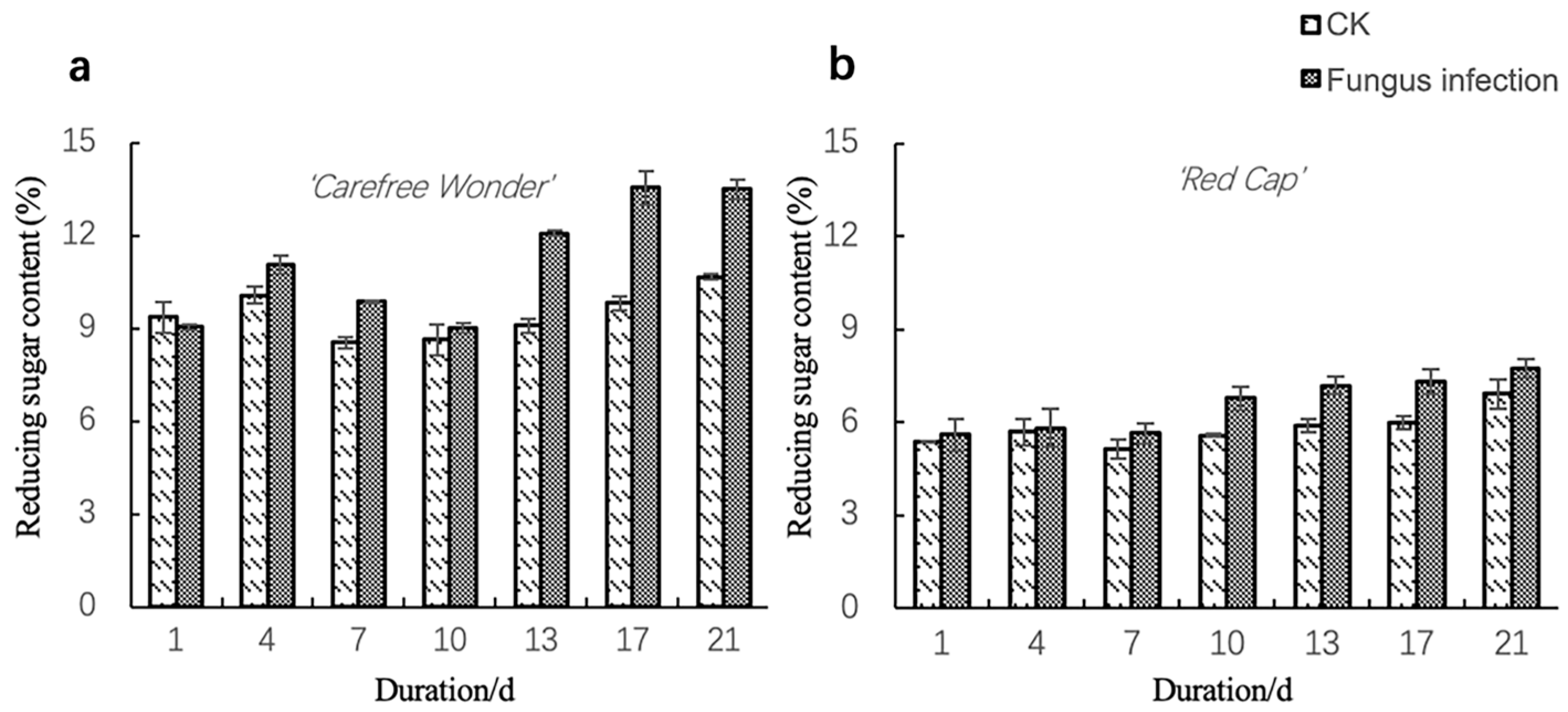
3.2.1. Reducing Sugar
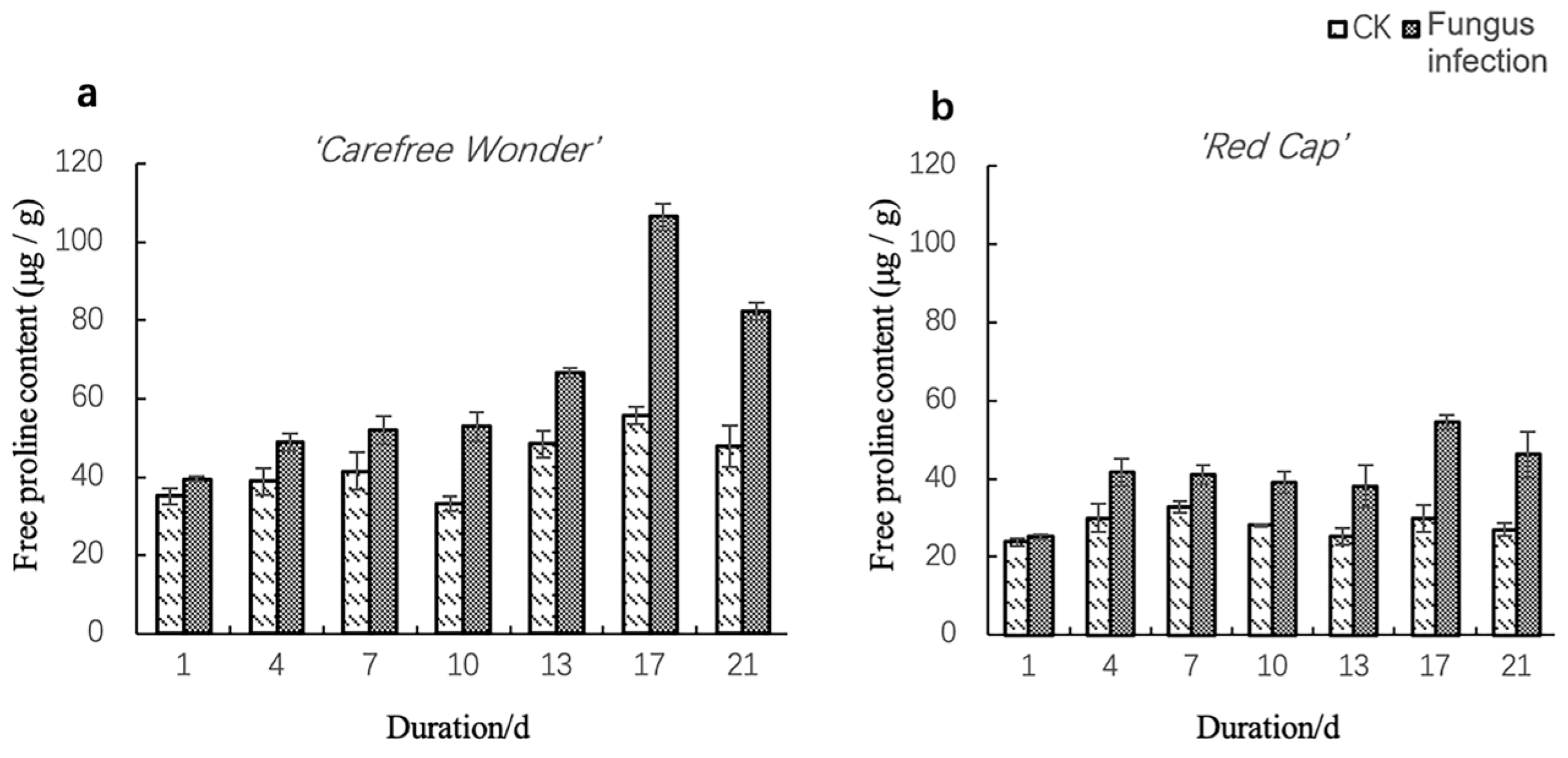
3.2.2. Free Proline Content
3.3. Changes in the Activity of Pathogenesis-Related Proteases
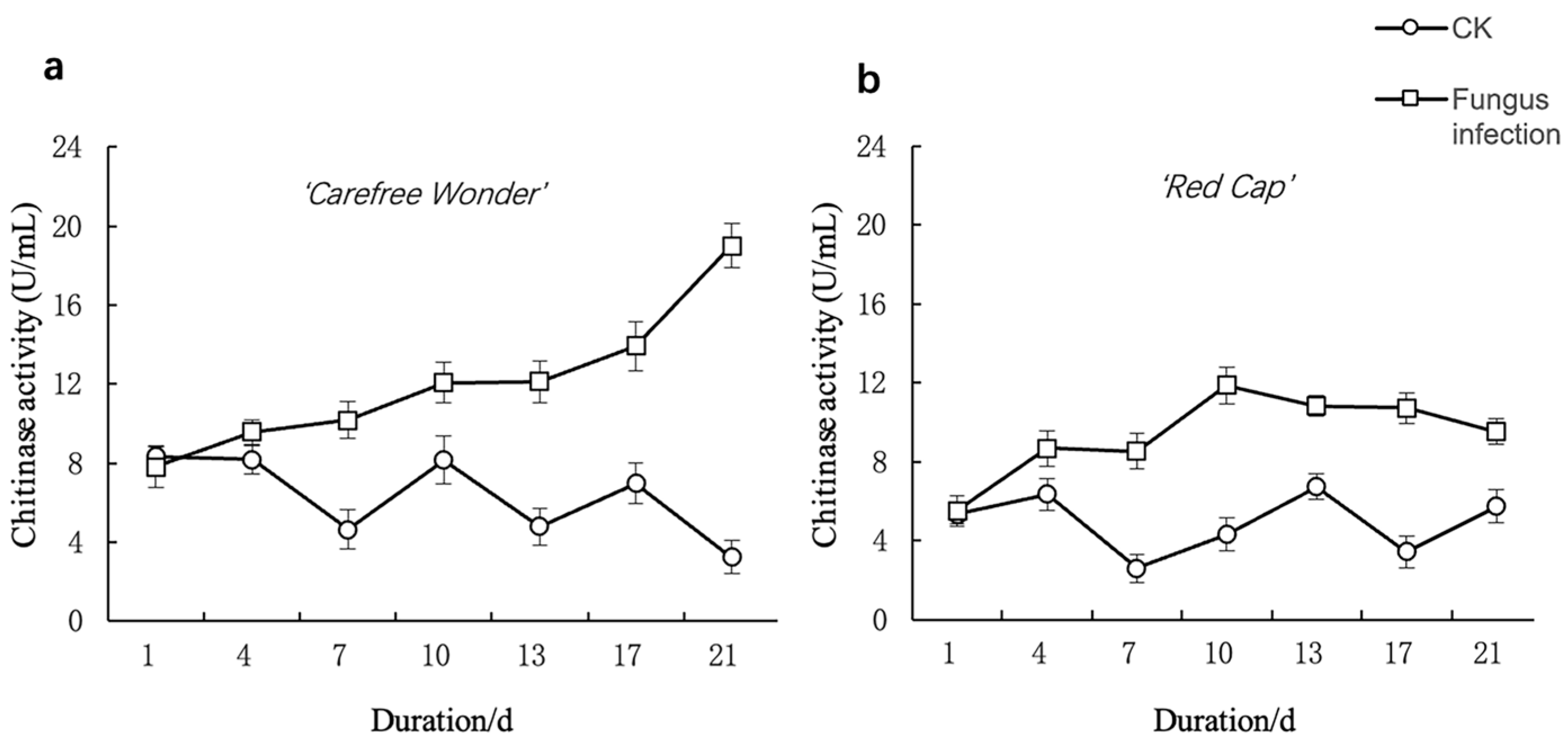
3.3.1. Chitinase Asctivity
3.3.2. β-1,3-Glucanase Activity
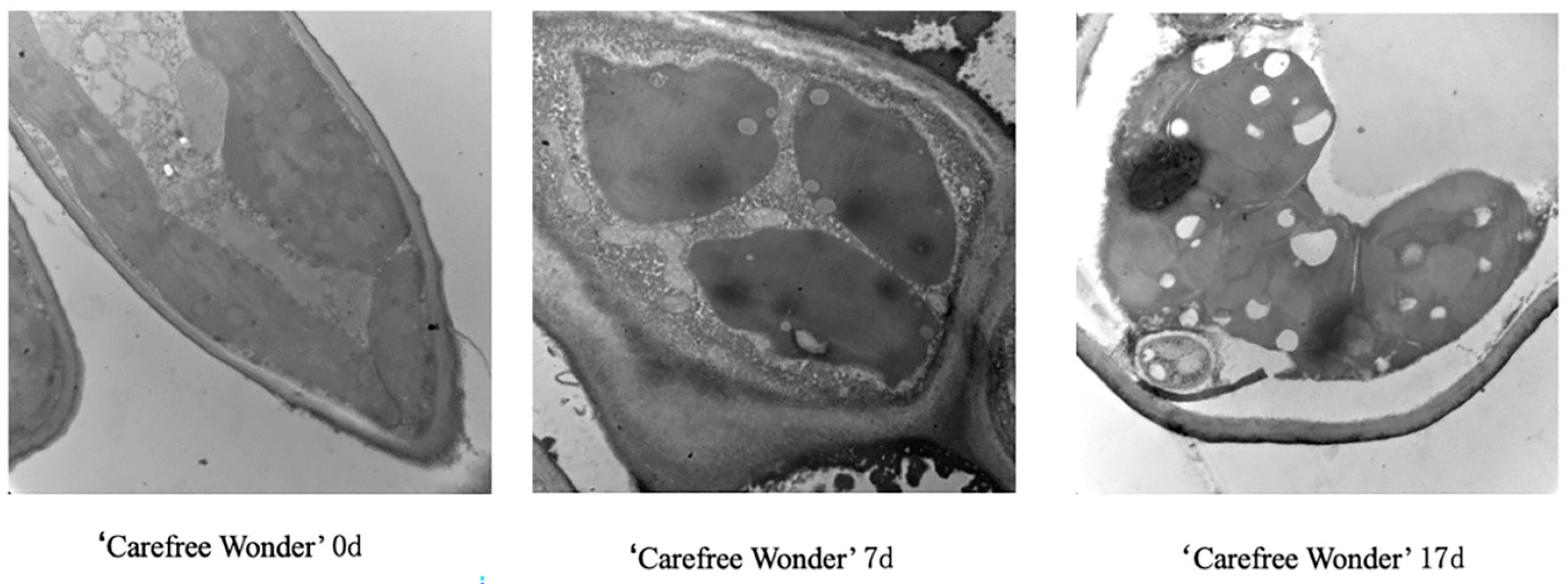
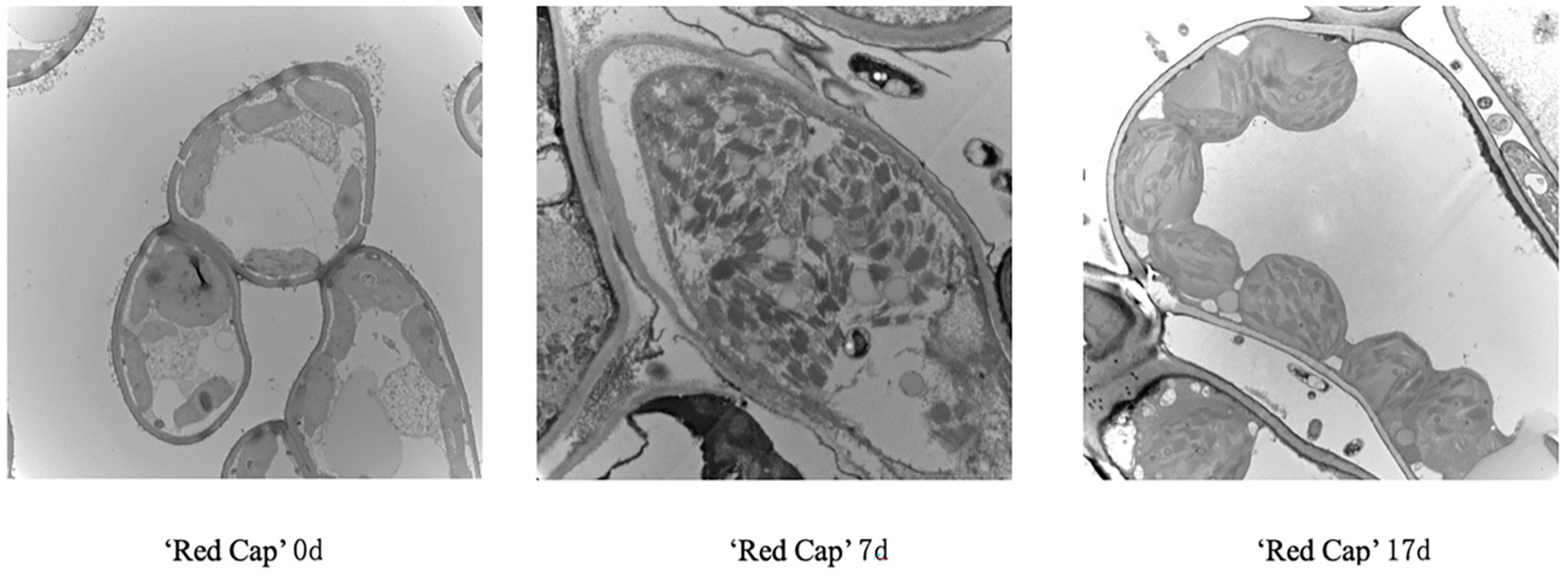
3.4. Ultrastructural Changes in Leaf Tissues
3.5. Correlation Analysis Between EIS Parameters and Physiological Indicators
4. Discussion
4.1. Changes in Plant Physiological Characteristics in Relation to Rose Black Spot Disease
4.2. Changes in Electrical Impedance Spectroscopy (EIS) and Rose Black Spot Disease
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bai, J.R.; Zhang, Q.X.; Luo, L.; Pan, H.T.; Yu, C. Pollen morphology of some Chinese traditional roses. Bull. Bot. Res. 2011, 31, 15. [Google Scholar]
- Li, C.; Li, Y.; Chu, P.; Hao-Hao, Z.; Wei, Z.; Cheng, Y.; Liu, X.; Zhao, F.; Li, Y.J.; Zhang, Z.; et al. Effects of salt stress on sucrose metabolism and growth in Chinese rose (Rosa chinensis). Biotechnol. Biotechnol. Equip. 2022, 36, 706–716. [Google Scholar] [CrossRef]
- Debener, T.; Byrne, D.H. Disease resistance breeding in rose: Current status and potential of biotechnological tools. Plant Sci. 2014, 228, 107–117. [Google Scholar]
- Drewes-Alvarez, R. Black Spot. In Encyclopedia of RoseScience; Roberts, A., Debener, T., Gudin, S., Eds.; Elsevier Science: Oxford, UK, 2003; pp. 148–153. [Google Scholar]
- Horst, R.K. Compendium of Rose Diseases; APS Press: St Paul, MN, USA, 2007. [Google Scholar]
- Dovas, C.I.; Katis, N.I. A spot nested RT-PCR methods for the simultaneous detection of members of the Vitivirus and F-oveavirus genera in grapevine. J. Virol. Methods 2003, 170, 99–106. [Google Scholar] [CrossRef]
- Shen, S.; Xu, W.; Lu, J.; Wang, S.; Huang, Y.; Zeng, X.; Xiao, W.; Yin, J. Recent progress on fluorescent probes for viruses. Chin. Chem. Lett. 2024, 35, 108360. [Google Scholar] [CrossRef]
- Chaerle, L.; Caeneghem, W.V.; Messens, E.; Lambers, H.; Van Montagu, M.; Van Der Straeten, D. Presymptomatic visualization of plant–virus interactions by thermography. Nat. Biotechnol. 1999, 17, 813–816. [Google Scholar] [CrossRef]
- Staskawicz, B.J. Genetics of plant-pathogen interactions specifying plant disease resistance. Plant Physiol. 2001, 125, 73–76. [Google Scholar] [CrossRef]
- Wang, L.M.; Zhang, Q.X. A Review of the Research on the Genetic Engineering of Rose Black-spot (Diplocarpon rosae Wolf) Fungus Disease. J. Cent. South Univ. For. Technol. 2003, 5, 92–95. [Google Scholar]
- Santi, S.; De Marco, F.; Polizzotto, R.; Grisan, S.; Musetti, R. Recovery from stolbur disease in grapevine involves changes in sugar transport and metabolism. Front. Plant Sci. 2013, 4, 171. [Google Scholar] [CrossRef]
- Molano, J.; Polacheck, I.; Duran, A.; Cabib, E. An endochitinase from wheat germ. Activity on nascent and preformed chitin. J. Biol. Chem. 1979, 254, 4901–4907. [Google Scholar]
- Chandra, S.; Dutta, A.K.; Chandrashekara, K.N.; Acharya, K. In silico Characterization, Homology Modeling of Camellia sinensis Chitinase and its Evolutionary Analyses with Other Plant Chitinases. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 685–695. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Vashisht, D.; Cletus, J.; Sakthivel, N. Plant β-1,3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 2012, 34, 1983–1990. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.M.; Qian, J.; Di, B.; Zhang, G.; Ren, Z.H. Electrical impedance spectroscopy (EIS) in plant roots research: Are view. Plant Methods 2021, 17, 118. [Google Scholar] [CrossRef]
- Di, B.; Luoranen, J.; Lehto, T.; Himanen, K.; Silvennoinen, M.; Silvennoinen, R.; Repo, T. Biophysical changes in the roots of Scots pine seedlings during cold acclimation and after frost damage. For. Ecol. Manag. 2019, 431, 63–72. [Google Scholar] [CrossRef]
- Astashev, M.E.; Konchekov, E.M.; Kolik, L.V.; Gudkov, S.V. Electric impedance spectroscopy in trees condition analysis: Theory and experiment. Sensors 2022, 22, 8310. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.F.; Di, B.; Repo, T.; Roitto, M.; Zhang, G. Responses of parameters for electrical impedance spectroscopy and pressure-volume curves to drought stress in Pinus bungeana seedlings. Forests 2020, 11, 359. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.-Q.; Dong, S.-H. Assessing frost hardiness of Pinus bungeana shoots and needles by electrical impedance spectroscopy with and without freezing tests. J. Plant Ecol. 2010, 3, 285–293. [Google Scholar] [CrossRef]
- Repo, T.; Korhonen, A.; Lehto, T.; Silvennoinen, R. Assessment of frost damage in mycorrhizal and non-mycorrhizal roots of Scots pine seedlings using classification analysis of their electrical impedance spectra. Trees 2016, 30, 483–495. [Google Scholar] [CrossRef]
- Xiang, D.; Zhang, G.; Gong, R.; Di, B.; Tian, Y. Effect of Cadmium Stress on Growth and Electrical Impedance Spectroscopy Parameters of Cotinus coggygria Roots. Water Air Soil Pollut. 2018, 229, 279. [Google Scholar] [CrossRef]
- Ryyppö, A.; Repo, T.; Vapaavuori, E. Development of freezing tolerance in roots and shoots of Scots pine seedlings at nonfreezing temperatures. Can. J. For. Res. 1998, 28, 557–565. [Google Scholar] [CrossRef]
- Väinölä, A.; Repo, T. Impedance spectroscopy in frost hardiness evaluation of Rhododendron leaves. Ann. Bot. 2000, 86, 799–805. [Google Scholar] [CrossRef]
- Sharma, S.R.; Kolte, S.J. Effect of soil-applied NPK fertilizers on severity of black spot disease (Alternaria brassicae) and yield of oilseed rape. Plant Soil 1994, 167, 313–320. [Google Scholar] [CrossRef]
- Allum, J.F.; Bringloe, D.H.; Roberts, A.V. Interac tions of four pathotypes of Diplocarpon rosae with species and hybrids of Rosa. Plant Pathol. 2010, 59, 516. [Google Scholar] [CrossRef]
- Zhang, G.; Ryyppö, A.; Repo, T. The electrical impedance spectroscopy of Scots pine needles during cold acclimation. Physiol. Plant. 2002, 115, 385–392. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Troll, W.; Lindsley, J. A photometric method for the determination of proline. J. Biol. Chem. 1955, 215, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; Mauch, F. Colorimetric Assay for Chitinase. Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1988; pp. 430–435. [Google Scholar]
- Ramada, M.H.S.; Lopes, F.Á.C.; Ulhoa, C.J.; do Nascimento Silva, R. Optimized microplate β-1,3-glucanase assay system for Trichoderma spp. screening. J. Microbiol. Methods 2010, 81, 6–10. [Google Scholar] [CrossRef]
- Gaveliene, V.; Pakalniskyte, L.; Novickiene, L. Regulation of proline and ethylene levels in rape seedlings for freezing tolerance. Cent. Eur. J. Biol. 2014, 9, 1099–1107. [Google Scholar] [CrossRef]
- Xu, M. Effect of eose pigment and amino acids by rose black spot. Acta Hortic. Sin. 1985, 1, 61–64. [Google Scholar]
- Xu, D.; Meng, Z. Researchonthe Resistant Mechanism of the Rose Black Spot. J. Anhui Agri. Sci. 2007, 27, 8532–8533. [Google Scholar]
- Albrecht, U.; Bowman, K.D. Gene expression in Citrus sinensis (L.) Osbeck following infection with the bacterial pathogen Candidatus liberibacter asiaticus causing Huanglongbing in Florida. Plant Sci. 2008, 175, 291–306. [Google Scholar] [CrossRef]
- Martinelli, F.; Uratsu, S.L.; Albrecht, U.; Reagan, R.L.; Phu, M.L.; Britton, M.; Buffalo, V.; Fass, J.; Leicht, E.; Zhao, W.; et al. Transcriptome profiling of citrus fruit response to huanglongbing disease. PloS ONE 2012, 7, e38039. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Path-Physiology Research of Jujube Fruit Shrink Disease; Hebei Agricultural University: Baoding, China, 2011. [Google Scholar]
- Song, F.; Zheng, Z.; Ge, X. Changes in contents of sugars and invertase activity in cotton seedings after infection by Fusarium oxysporum. Acta Agric. Zhejiangensis 1996, 2, 29–33. [Google Scholar]
- Fang, W.; Sanz, A.B.; Bartual, S.G.; Wang, B.; Ferenbach, A.T.; Farkaš, V.; Hurtado-Guerrero, R.; Arroyo, J.; Van Aalten, D.M. Mechanisms of redundancy and specificity of the Aspergillus fumigatus Crh transglycosylases. Nat. Commun. 2019, 10, 1669. [Google Scholar] [CrossRef]
- Toufiq, N.; Tabassum, B.; Bhatti, M.U.; Khan, A.; Tariq, M.; Shahid, N.; Nasir, I.A.; Husnain, T. Improved antifungal activity of barley derived chitinase I gene that overexpress a 32 kDa recombinant chitinase in Escherichia coli host. Braz. J. Microbiol. 2018, 49, 414–421. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep. 2007, 26, 2027–2038. [Google Scholar] [CrossRef] [PubMed]
- Mauch, F.; Mauch-Mani, B.; Boller, T. Antifungal Hydrolases in Pea Tissue 1: II. Inhibition of Fungal Growth by Combinations of Chitinase and β-1,3-Glucanase. Plant Physiol. 1988, 88, 936–942. [Google Scholar] [CrossRef]
- Brogue, K.; Chet, I.; Holliday, M.; Cressman, R.; Biddle, P.; Knowlton, S.; Mauvais, C.J.; Broglie, R. Transgenic Plants with Enhanced Resistance to the Fungal Pathogen Rhizoctonia solani. Science 1991, 254, 1194–1197. [Google Scholar] [CrossRef]
- Grover, A. Plant Chitinases: Genetic Diversity and Physiological Roles. Crit. Rev. Plant Sci. 2012, 31, 57–73. [Google Scholar] [CrossRef]
- van Loon, L.C. Pathogenesis-related proteins. Plant Mol. Biol. 1985, 4, 111–116. [Google Scholar] [CrossRef]
- Li, S.; Du, C. Effects of lipopeptide on chitinase and glucanase of Magnaporthe grisea. Acta Phytopathol. Sin. 2021, 51, 789–795. [Google Scholar]
- Song, Q.; Pratishka, S.; Rajesh, K.S.; Wang, L.; Nong, Y.; Yang, L.; Li, Y. Changes of related enzyme activity and endogenous hormone content in sugarcane response to smut fungus infection. J. South. Agric. 2018, 49, 650–655. [Google Scholar]


| EIS Parameters | Reducing Sugar | Free Proline | Chitinase | β-1,3-Glucanase |
|---|---|---|---|---|
| re | 0.678 ** | 0.627 ** | 0.631 ** | 0.513 * |
| τ | 0.774 ** | 0.607 ** | 0.605 ** | 0.710 ** |
| EIS Parameter | Physiological Parameters | Regression Model | R2 | Significance |
|---|---|---|---|---|
| re | Reducing sugar Free proline | y = −1.819 + 0.259x − 0.001x2 y = −16.541 + 1.476x − 0.005x2 | 0.479 0.399 | 0.003 ** 0.009 ** |
| Chitinase | y = 3.934 + 0.095x | 0.399 | 0.009 ** | |
| β-1,3-glucanase | y = −6.214 + 0.381x − 0.002x2 | 0.401 | 0.009 ** | |
| τ | Reducing sugar Free proline | y = −8.539 + 5.888x − 0.42x2 y = 11.458 + 6.359x + 0.468x2 | 0.637 0.369 | 0.000 ** 0.016 * |
| Chitinase β-1,3-glucanase | Y = 17.749 − 4.895x − 0.686x2 y = −16.05 + 8.895x − 0.78x2 | 0.419 0.725 | 0.007 ** 0.000 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Tan, D.; Wang, R.; Li, T.; Wang, Y.; Shan, G.; Qian, J.; Di, B. Monitoring Rose Black Spot Disease Using Electrical Impedance Spectroscopy. Agronomy 2025, 15, 1800. https://doi.org/10.3390/agronomy15081800
Ma T, Tan D, Wang R, Li T, Wang Y, Shan G, Qian J, Di B. Monitoring Rose Black Spot Disease Using Electrical Impedance Spectroscopy. Agronomy. 2025; 15(8):1800. https://doi.org/10.3390/agronomy15081800
Chicago/Turabian StyleMa, Tianyi, Dongyu Tan, Rui Wang, Tianyi Li, Yiying Wang, Guilin Shan, Ji Qian, and Bao Di. 2025. "Monitoring Rose Black Spot Disease Using Electrical Impedance Spectroscopy" Agronomy 15, no. 8: 1800. https://doi.org/10.3390/agronomy15081800
APA StyleMa, T., Tan, D., Wang, R., Li, T., Wang, Y., Shan, G., Qian, J., & Di, B. (2025). Monitoring Rose Black Spot Disease Using Electrical Impedance Spectroscopy. Agronomy, 15(8), 1800. https://doi.org/10.3390/agronomy15081800






