Engineering Crops for Enhanced Drought Stress Tolerance: A Strategy for Sustainable Agriculture
Abstract
1. Introduction
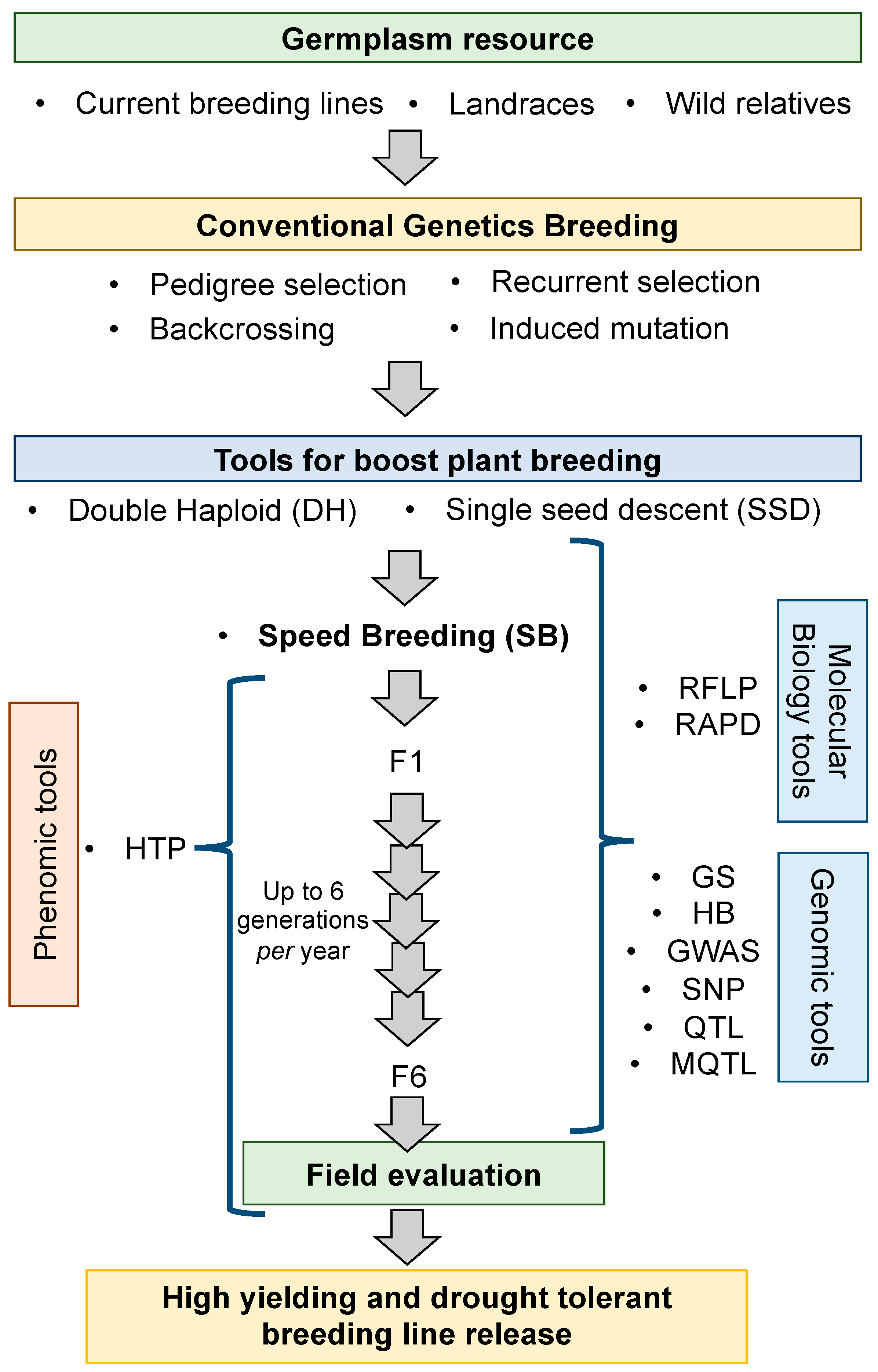
2. Development of Resistant Plants with a Drought-Tolerant Phenotype
2.1. Screening for Natural Resistance
2.2. Genetic Improvement by Traditional Genetics
2.3. Novel Tools to Speed up Plant Breeding
2.4. Early Molecular Tools
2.5. High-Throughput Genotyping to Boost Plant Breeding
2.6. High Throughput Phenotyping to Boost Plant Breeding
3. Recombinant DNA Technology
3.1. Agrobacterium Infection
3.2. Gene Edition by Clustered Regularly Interspaced Short Palindromic Repeats with Cas 9 Associated Endonuclease (CRISPR/Cas 9)
3.3. RNAi Technology
3.4. Other Novel Genetic Engineering Tools
4. Limitations and Future Directions
5. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Biswas, A.; Sarkar, S.; Das, S.; Dutta, S.; Choudhury, M.R.; Giri, A.; Bera, B.; Bag, K.; Mukherjee, B.; Banerjee, K.; et al. Water scarcity: A global hindrance to sustainable development and agricultural production—A critical review of the impacts and adaptation strategies. Camb. Prism. Water 2025, 3, e4. [Google Scholar] [CrossRef]
- Terán-Samaniego, K.; Robles-Parra, J.M.; Vargas-Arispuro, I.; Martínez-Téllez, M.Á.; Garza-Lagler, M.C.; Félix-Gurrlola, D.; Maycotte-de la Peña, M.L.; Tafolla-Arellano, J.C.; García-Figueroa, J.A.; Espinoza-López, P.C. Agroecology and sustainable agriculture: Conceptual challenges and opportunities—A systematic literature review. Sustainability 2025, 17, 1805. [Google Scholar] [CrossRef]
- López-López, C.C.; Exebio-García, A.A.; Flores-Velázquez, J.; Bolaños-González, M.A.; Rubiños-Panta, J.E. Evaluación Asociativa del Impacto Económico del Estrés Hídrico en la Producción Agrícola del Noreste de México Mediante Indicadores. Rev. TERRA Latinoam. 2025, 43, e2077. [Google Scholar]
- Ahmar, S.; Gill, R.A.; Jung, K.-H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent advances and future outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef]
- Raza, A.; Mubarik, M.S.; Sharif, R.; Habib, M.; Jabeen, W.; Zhang, C.; Chen, H.; Chen, Z.H.; Siddique, K.H.; Zhuang, W. Developing drought-smart, ready-to-grow future crops. Plant Genome 2023, 16, e20279. [Google Scholar] [CrossRef]
- Bakala, H.S.; Devi, J.; Singh, G.; Singh, I. Drought and heat stress: Insights into tolerance mechanisms and breeding strategies for pigeonpea improvement. Planta 2024, 259, 123. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.K.; Barmukh, R.; Roorkiwal, M.; Qi, Y.; Kholova, J.; Tuberosa, R.; Reynolds, M.P.; Tardieu, F.; Siddique, K.H. Breeding custom-designed crops for improved drought adaptation. Adv. Genet. 2021, 2, e202100017. [Google Scholar] [CrossRef] [PubMed]
- Oladosu, Y.; Rafii, M.Y.; Samuel, C.; Fatai, A.; Magaji, U.; Kareem, I.; Kamarudin, Z.S.; Muhammad, I.i.; Kolapo, K. Drought resistance in rice from conventional to molecular breeding: A review. Int. J. Mol. Sci. 2019, 20, 3519. [Google Scholar] [CrossRef] [PubMed]
- Haroon, M.; Wang, X.; Afzal, R.; Zafar, M.M.; Idrees, F.; Batool, M.; Khan, A.S.; Imran, M. Novel plant breeding techniques shake hands with cereals to increase production. Plants 2022, 11, 1052. [Google Scholar] [CrossRef]
- Rosero, A.; Granda, L.; Berdugo-Cely, J.A.; Šamajová, O.; Šamaj, J.; Cerkal, R. A dual strategy of breeding for drought tolerance and introducing drought-tolerant, underutilized crops into production systems to enhance their resilience to water deficiency. Plants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Yadav, O.; Kaliamoorthy, S.; Gupta, S.; Serba, D.D.; Choudhary, S.; Govindaraj, M.; Kholová, J.; Murugesan, T.; Satyavathi, C.T. Breeding drought-tolerant pearl millet using conventional and genomic approaches: Achievements and prospects. Front. Plant Sci. 2022, 13, 781524. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, K.B.; Singh, N.; Kaur, P.; Rani, S.; Babu, H.P.; Singh, K. Deployment of wild relatives for genetic improvement in rice (Oryza sativa). Plant Breed. 2021, 140, 23–52. [Google Scholar] [CrossRef]
- Anilkumar, C.; Sah, R.P.; Beena, R.; Azharudheen TP, M.; Kumar, A.; Behera, S.; Sunitha, N.; Pradhan, S.; Reshmi Raj, K.; Marndi, B. Conventional and contemporary approaches for drought tolerance rice breeding: Progress and prospects. Plant Breed 2023, 142, 418–438. [Google Scholar] [CrossRef]
- Patil, K.S.; Gupta, S.K.; Marathi, B.; Danam, S.; Thatikunta, R.; Rathore, A.; Das, R.R.; Dangi, K.S.; Yadav, O.P. African and Asian origin pearl millet populations: Genetic diversity pattern and its association with yield heterosis. Crop Sci. 2020, 60, 3035–3048. [Google Scholar] [CrossRef]
- Acquaah, G. Conventional plant breeding principles and techniques. In Advances in Plant Breeding Strategies: Breeding, Biotechnology and Molecular Tools; Al-Khayri, J., Jain, S., Johnson, D., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 115–158. [Google Scholar]
- Yahaya, M.A.; Shimelis, H. Drought stress in sorghum: Mitigation strategies, breeding methods and technologies—A review. J. Agron. Crop Sci. 2022, 208, 127–142. [Google Scholar] [CrossRef]
- Mondal, S.; Dutta, S.; Crespo-Herrera, L.; Huerta-Espino, J.; Braun, H.J.; Singh, R.P. Fifty years of semi-dwarf spring wheat breeding at CIMMYT: Grain yield progress in optimum, drought and heat stress environments. Field Crops Res. 2020, 250, 107757. [Google Scholar] [CrossRef]
- Soriano, J.M.; Alvaro, F. Discovering consensus genomic regions in wheat for root-related traits by QTL meta-analysis. Sci. Rep. 2019, 9, 10537. [Google Scholar] [CrossRef]
- Bucksch, A.; Burridge, J.; York, L.M.; Das, A.; Nord, E.; Weitz, J.S.; Lynch, J.P. Image-based high-throughput field phenotyping of crop roots. Plant Physiol. 2014, 166, 470–486. [Google Scholar] [CrossRef]
- Purushothaman, R.; Krishnamurthy, L.; Upadhyaya, H.D.; Vadez, V.; Varshney, R.K. Genotypic variation in soil water use and root distribution and their implications for drought tolerance in chickpea. Funct. Plant Biol. 2016, 44, 235–252. [Google Scholar] [CrossRef]
- Kivuva, B.M.; Githiri, S.M.; Yencho, G.C.; Sibiya, J. Screening sweetpotato genotypes for tolerance to drought stress. Field Crops Res. 2015, 171, 11–22. [Google Scholar] [CrossRef]
- Geetika, G.; Van Oosterom, E.J.; George-Jaeggli, B.; Mortlock, M.Y.; Deifel, K.S.; McLean, G.; Hammer, G.L. Genotypic variation in whole-plant transpiration efficiency in sorghum only partly aligns with variation in stomatal conductance. Funct. Plant Biol. 2019, 46, 1072–1089. [Google Scholar] [CrossRef]
- Goche, T.; Shargie, N.G.; Cummins, I.; Brown, A.P.; Chivasa, S.; Ngara, R. Comparative physiological and root proteome analyses of two sorghum varieties responding to water limitation. Sci. Rep. 2020, 10, 11835. [Google Scholar] [CrossRef]
- Awika, H.; Hays, D.; Mullet, J.; Rooney, W.; Weers, B. QTL mapping and loci dissection for leaf epicuticular wax load and canopy temperature depression and their association with QTL for staygreen in Sorghum bicolor under stress. Euphytica 2017, 213, 1–22. [Google Scholar] [CrossRef]
- Fadoul, H.E.; El Siddig, M.A.; Abdalla, A.W.H.; El Hussein, A.A. Physiological and proteomic analysis of two contrasting Sorghum bicolor genotypes in response to drought stress. Aust. J. Crop Sci. 2018, 12, 1543–1551. [Google Scholar] [CrossRef]
- Chun, S.C.; Paramasivan, M.; Chandrasekaran, M. Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front. Microbiol. 2018, 9, 2525. [Google Scholar] [CrossRef] [PubMed]
- Beena, R.; Kirubakaran, S.; Nithya, N.; Manickavelu, A.; Sah, R.P.; Abida, P.S.; Sreekumar, J.; Jaslam, P.M.; Rejeth, R.; Jayalekshmy, V.G. Association mapping of drought tolerance and agronomic traits in rice (Oryza sativa L.) landraces. BMC Plant Biol. 2021, 21, 484. [Google Scholar] [CrossRef]
- Pandit, E.; Panda, R.K.; Sahoo, A.; Pani, D.R.; Pradhan, S.K. Genetic relationship and structure analysis of root growth angle for improvement of drought avoidance in early and mid-early maturing rice genotypes. Rice Sci. 2020, 27, 124–132. [Google Scholar] [CrossRef]
- Duc, G.; Agrama, H.; Bao, S.; Berger, J.; Bourion, V.; de Ron, A.M.; Gowda, C.L.; Mikic, A.; Millot, D.; Singh, K.B. Breeding annual grain legumes for sustainable agriculture: New methods to approach complex traits and target new cultivar ideotypes. Crit. Rev. Plant Sci. 2015, 34, 381–411. [Google Scholar] [CrossRef]
- Pang, Y.; Chen, K.; Wang, X.; Xu, J.; Ali, J.; Li, Z. Recurrent selection breeding by dominant male sterility for multiple abiotic stresses tolerant rice cultivars. Euphytica 2017, 213, 268. [Google Scholar] [CrossRef]
- Efendi, B.; Sabaruddin, Z.; Lukman, H. Mutation with gamma raysirradiation to assemble green super rice tolerant to drought stress and high yield rice (Oryza sativa L.). Int. J. Adv. Sci. Eng. Technol. 2017, 5, 1–5. [Google Scholar]
- Soe, H.; Myat, M.; Khaing, Z.; Nyo, N.; Phyu, P. Development of drought tolerant mutant from rice var. Manawthukha through mutation breeding technique using 60Co gamma source. Int. J. Innov. Res. Sci. Eng. Technol. 2016, 4, 11205–11212. [Google Scholar]
- Hallajian, M.; Ebadi, A.; Mohammadi, M.; Muminjanov, H.; Jamali, S.; Aghamirzaei, M. Integration of mutation and conventional breeding approaches to develop new superior drought-tolerant plants in rice (Oryza sativa). Annu. Res. Rev. Biol. 2014, 4, 1173. [Google Scholar] [CrossRef]
- Fikre, A.; Degefu, T.; Geleta, T.; Thudi, M.; Gaur, P.; Ojiewo, C.; Hickey, L.; Varshney, R.K. Rapid Generation Advance in Chickpea for Accelerated Breeding Gain in Ethiopia: What Speed Breeding Imply? Ethiop. J. Agric. Sci. 2021, 31, 1–10. [Google Scholar]
- Voss-Fels, K.P.; Stahl, A.; Hickey, L.T. Q.&.A.: Modern crop breeding for future food security. BMC Biol. 2019, 17, 18. [Google Scholar]
- Christopher, J.; Richard, C.; Chenu, K.; Christopher, M.; Borrell, A.; Hickey, L. Integrating rapid phenotyping and speed breeding to improve stay-green and root adaptation of wheat in changing, water-limited, Australian environments. Procedia Environ. Sci. 2015, 29, 175–176. [Google Scholar] [CrossRef]
- Ghosh, S.; Watson, A.; Gonzalez-Navarro, O.E.; Ramirez-Gonzalez, R.H.; Yanes, L.; Mendoza-Suárez, M.; Simmonds, J.; Wells, R.; Rayner, T.; Green, P. Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nat. Protoc. 2018, 13, 2944–2963. [Google Scholar] [CrossRef] [PubMed]
- Hickey, L.T.; Germán, S.E.; Pereyra, S.A.; Diaz, J.E.; Ziems, L.A.; Fowler, R.A.; Platz, G.J.; Franckowiak, J.D.; Dieters, M.J. Speed breeding for multiple disease resistance in barley. Euphytica 2017, 213, 64. [Google Scholar] [CrossRef]
- Rizal, G.; Karki, S.; Alcasid, M.; Montecillo, F.; Acebron, K.; Larazo, N.; Garcia, R.; Slamet-Loedin, I.H.; Quick, W.P. Shortening the breeding cycle of sorghum, a model crop for research. Crop Sci. 2014, 54, 520–529. [Google Scholar] [CrossRef]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.-D.; Asyraf Md Hatta, M.; Hinchliffe, A.; Steed, A.; Reynolds, D. Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef]
- Chaikam, V.; Molenaar, W.; Melchinger, A.E.; Boddupalli, P.M. Doubled haploid technology for line development in maize: Technical advances and prospects. Theor. Appl. Genet. 2019, 132, 3227–3243. [Google Scholar] [CrossRef]
- Cooper, M.; Messina, C.D.; Podlich, D.; Totir, L.R.; Baumgarten, A.; Hausmann, N.J.; Wright, D.; Graham, G. Predicting the future of plant breeding: Complementing empirical evaluation with genetic prediction. Crop Pasture Sci. 2014, 65, 311–336. [Google Scholar] [CrossRef]
- Gaffney, J.; Schussler, J.; Löffler, C.; Cai, W.; Paszkiewicz, S.; Messina, C.; Groeteke, J.; Keaschall, J.; Cooper, M. Industry-scale evaluation of maize hybrids selected for increased yield in drought-stress conditions of the US Corn Belt. Crop Sci. 2015, 55, 1608–1618. [Google Scholar] [CrossRef]
- Sandhu, N.; Dixit, S.; Swamy, B.; Raman, A.; Kumar, S.; Singh, S.; Yadaw, R.; Singh, O.; Reddy, J.; Anandan, A. Marker assisted breeding to develop multiple stress tolerant varieties for flood and drought prone areas. Rice 2019, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.; Sandhu, N.; Bartholome, J. Genome-Wide Association Study for yield and yield related traits under reproductive stage drought in a diverse indica-aus rice panel. Rice 2020, 13, 53. [Google Scholar] [CrossRef]
- Pantaliao, G.F.; Narciso, M.; Guimaraes, C.; Castro, A.; Colombari, J.M.; Breseghello, F.; Rodrigues, L.; Vianello, R.P.; Borba, T.O.; Brondani, C. Genome wide association study (GWAS) for grain yield in rice cultivated under water deficit. Genetica 2016, 144, 651–664. [Google Scholar] [CrossRef]
- Acuña-Galindo, M.A.; Mason, R.E.; Subramanian, N.K.; Hays, D.B. Meta-analysis of wheat QTL regions associated with adaptation to drought and heat stress. Crop Sci. 2015, 55, 477–492. [Google Scholar] [CrossRef]
- Selamat, N.; Nadarajah, K.K. Meta-analysis of quantitative traits loci (QTL) identified in drought response in rice (Oryza sativa L.). Plants 2021, 10, 716. [Google Scholar] [CrossRef]
- Zhang, X.; Shabala, S.; Koutoulis, A.; Shabala, L.; Zhou, M. Meta-analysis of major QTL for abiotic stress tolerance in barley and implications for barley breeding. Planta 2017, 245, 283–295. [Google Scholar] [CrossRef]
- Khahani, B.; Tavakol, E.; Shariati, V.; Rossini, L. Meta-QTL and ortho-MQTL analyses identified genomic regions controlling rice yield, yield-related traits and root architecture under water deficit conditions. Sci. Rep. 2021, 11, 6942. [Google Scholar] [CrossRef]
- Sinha, P.; Singh, V.K.; Saxena, R.K.; Khan, A.W.; Abbai, R.; Chitikineni, A.; Desai, A.; Molla, J.; Upadhyaya, H.D.; Kumar, A. Superior haplotypes for haplotype-based breeding for drought tolerance in pigeonpea (Cajanus cajan L.). Plant Biotechnol. J. 2020, 18, 2482–2490. [Google Scholar] [CrossRef]
- Vanhaeren, H.; Gonzalez, N.; Inzé, D. A journey through a leaf: Phenomics analysis of leaf growth in Arabidopsis thaliana. Arab. Book/Am. Soc. Plant Biol. 2015, 13, e0181. [Google Scholar] [CrossRef]
- Young, S.N.; Kayacan, E.; Peschel, J.M. Design and field evaluation of a ground robot for high-throughput phenotyping of energy sorghum. Precis. Agric. 2019, 20, 697–722. [Google Scholar] [CrossRef]
- Condorelli, G.E.; Maccaferri, M.; Newcomb, M.; Andrade-Sanchez, P.; White, J.W.; French, A.N.; Sciara, G.; Ward, R.; Tuberosa, R. Comparative aerial and ground based high throughput phenotyping for the genetic dissection of NDVI as a proxy for drought adaptive traits in durum wheat. Front. Plant Sci. 2018, 9, 349736. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S. Recombinant DNA Technology for Sustainable Plant Growth and Production. In Harsh Environment and Plant Resilience: Molecular and Functional Aspects; Husen, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Tiwari, R.; Rajam, M.V. RNA- and miRNA-interference to enhance abiotic stress tolerance in plants. J. Plant Biochem. Biotechnol. 2022, 31, 689–704. [Google Scholar] [CrossRef]
- Rai, G.K.; Khanday, D.M.; Kumar, P.; Magotra, I.; Choudhary, S.M.; Kosser, R.; Kalunke, R.; Giordano, M.; Corrado, G.; Rouphael, Y.; et al. Enhancing Crop Resilience to Drought Stress through CRISPR-Cas9 Genome Editing. Plants 2023, 12, 2306. [Google Scholar] [CrossRef] [PubMed]
- Gerashchenkov, G.; Rozhnova, N.; Kuluev, B.; Kiryanova, O.Y.; Gumerova, G.; Knyazev, A.; Vershinina, Z.; Mikhailova, E.; Chemeris, D.; Matniyazov, R. Design of guide RNA for CRISPR/Cas plant genome editing. Mol. Biol. 2020, 54, 24–42. [Google Scholar] [CrossRef]
- Alamillo, J.M.; López, C.M.; Martínez Rivas, F.J.; Torralbo, F.; Bulut, M.; Alseekh, S. Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein and hairy roots: A perfect match for gene functional analysis and crop improvement. Curr. Opin. Biotechnol. 2023, 79, 102876. [Google Scholar] [CrossRef]
- Zurbriggen, M.D.; Hajirezaei, M.R.; Carrillo, N. Engineering the future. Development of transgenic plants with enhanced tolerance to adverse environments. Biotechnol. Genet. Eng. Rev. 2010, 27, 33–55. [Google Scholar] [CrossRef]
- Hu, H.H.; Xiong, L.Z. Genetic Engineering and Breeding of Drought-Resistant Crops. Annu. Rev. Plant Biol. 2014, 65, 715–741. [Google Scholar] [CrossRef]
- Kantar, M.; Lucas, S.J.; Budaki, H. Drought Stress: Molecular Genetics and Genomics Approaches. In Advances in Botanical Research; Turkan, I., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 57, pp. 445–493. [Google Scholar]
- Guo, W.F.; Li, G.Q.; Wang, N.; Yang, C.F.; Zhao, Y.N.; Peng, H.K.; Liu, D.H.; Chen, S.F. A Na+/H+ antiporter, K2-NhaD, improves salt and drought tolerance in cotton (Gossypium hirsutum L.). Plant Mol. Biol. 2020, 102, 553–567. [Google Scholar] [CrossRef]
- Hassan, S.; Qadir, I.; Aslam, A.; Rashid, B.; Sarwar, M.B.; Husnain, T. Cloning, Genetic Transformation and Cellular Localization of Abiotic Stress Responsive Universal Stress Protein Gene (GUSP1) in Gossypium hirsutum. Iran. J. Biotechnol. 2020, 18, 37–46. [Google Scholar] [CrossRef]
- Qi, X.H.; Tang, X.; Liu, W.G.; Fu, X.; Luo, H.Y.; Ghimire, S.; Zhang, N.; Si, H.J. A potato RING-finger protein gene StRFP2 is involved in drought tolerance. Plant Physiol. Biochem. 2020, 146, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Sahid, S.; Roy, C.; Paul, S.; Datta, R. Rice lectin protein r40c1 imparts drought tolerance by modulating S-adenosylmethionine synthase 2, stress-associated protein 8 and chromatin-associated proteins. J. Exp. Bot. 2020, 71, 7331–7346. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cheon, K.S.; Kim, S.Y.; Kim, J.H.; Kwon, O.H.; Lee, H.J.; Kim, W.H. Expression of SOD2 enhances tolerance to drought stress in roses. Hortic. Environ. Biotechnol. 2020, 61, 569–576. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, A.Y.; Lim, S.D.; Jang, C.S. Mutation of a RING E3 ligase, OsDIRH2, enhances drought tolerance in rice with low stomata density. Physiol. Plant. 2024, 176, e14565. [Google Scholar] [CrossRef]
- Kim, M.S.; Ko, S.R.; Jung, Y.J.; Kang, K.K.; Lee, Y.J.; Cho, Y.G. Knockout Mutants of OsPUB7 Generated Using CRISPR/Cas9 Revealed Abiotic Stress Tolerance in Rice. Int. J. Mol. Sci. 2023, 24, 5338. [Google Scholar] [CrossRef] [PubMed]
- Li, G.M.; Ma, Y.X.; Wang, X.P.; Cheng, N.N.; Meng, D.Y.; Chen, S.Y.; Wang, W.; Wang, X.T.; Hu, X.J.; Yan, L.; et al. CRISPR/Cas9 Gene Editing of NtAITRs, a Family of Transcription Repressor Genes, Leads to Enhanced Drought Tolerance in Tobacco. Int. J. Mol. Sci. 2022, 23, 15268. [Google Scholar] [CrossRef]
- Li, H.; Ma, W.Y.; Wang, X.; Hu, H.L.; Cao, L.A.; Ma, H.; Lin, J.W.; Zhong, M. A WUSCHEL-related homeobox transcription factor, SlWOX4, negatively regulates drought tolerance in tomato. Plant Cell Rep. 2024, 43, 253. [Google Scholar] [CrossRef]
- Linghu, B.; Song, M.; Mu, J.X.; Huang, S.H.; An, R.; Chen, N.A.; Xie, C.G.; Zhu, Y.T.; Guan, Z.B.; Zhang, Y.F. Comprehensive analysis of U-box E3 ubiquitin ligases gene family revealed BnPUB18 and BnPUB19 negatively regulated drought tolerance in Brassica napus. Ind. Crops Prod. 2023, 200, 116875. [Google Scholar] [CrossRef]
- Liu, S.H.; Zhang, Y.C.; Pan, X.H.; Li, B.; Yang, Q.; Yang, C.Q.; Zhang, J.H.; Wu, F.Y.; Yang, A.G.; Li, Y.T. PIF1, a phytochrome-interacting factor negatively regulates drought tolerance and carotenoids biosynthesis in tobacco. Int. J. Biol. Macromol. 2023, 247, 125693. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.Q.; Zhang, C.; Guo, J.; Liu, Q.; Yin, Y.J.; Hu, Y.; Xia, H.C.; Li, B.Y.; Sun, X.P.; et al. Gene editing of ZmGA20ox3 improves plant architecture and drought tolerance in maize. Plant Cell Rep. 2024, 43, 18. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.M.; Wang, X.W.; Dong, X.N.; Gao, M.; Dong, D.H.; Li, C.H.; Jing, S.R.; Guo, Y.D.; Zhang, N. CRISPR/Cas9 edited SlGT30 improved both drought resistance and fruit yield through endoreduplication. Plant Cell Environ. 2024, 48, 2581–2595. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.L.; Li, X.X.; Jin, X.K.; Sun, Y.; Wu, Y.Y.; Wang, W.M.; Huang, J.L. Rice OsPUB16 modulates the 'SAPK9-OsMADS23-OsAOC' pathway to reduce plant water-deficit tolerance by repressing ABA and JA biosynthesis. PLoS Genet. 2022, 18, e1010520. [Google Scholar] [CrossRef] [PubMed]
- Parmar, H.; Goel, A.; Achary, V.M.M.; Sonti, R.; Reddy, M.K. Plasticity of OsERF109 mitigates drought stress by modulating the antioxidant defense system and morphophysiological traits in rice. Plant Stress 2025, 15, 100701. [Google Scholar] [CrossRef]
- Rashid, A.; Achary, V.M.M.; Abdin, M.Z.; Karippadakam, S.; Parmar, H.; Panditi, V.; Prakash, G.; Bhatnagar-Mathur, P.; Reddy, M.K. Cytokinin oxidase2-deficient mutants improve panicle and grain architecture through cytokinin accumulation and enhance drought tolerance in indica rice. Plant Cell Rep. 2024, 43, 207. [Google Scholar] [CrossRef]
- Aydin, A.; Yerlikaya, B.A.; Yerlikaya, S.; Yilmaz, N.N.; Kavas, M. CRISPR-mediated mutation of cytokinin signaling genes (SlHP2 and SlHP3) in tomato: Morphological, physiological, and molecular characterization. Plant Genome 2025, 18, e20542. [Google Scholar] [CrossRef]
- Saikia, B.; Remya, S.; Debbarma, J.; Maharana, J.; Sastry, G.N.; Chikkaputtaiah, C. CRISPR/Cas9-based genome editing and functional analysis of SlHyPRP1 and SlDEA1 genes of Solanum lycopersicum L. in imparting genetic tolerance to multiple stress factors. Front. Plant Sci. 2024, 15, 1304381. [Google Scholar] [CrossRef]
- Shen, H.; Zhou, Y.; Liao, C.G.; Xie, Q.L.; Chen, G.P.; Hu, Z.L.; Wu, T. The AlkB Homolog SlALKBH10B Negatively Affects Drought and Salt Tolerance in Solanum lycopersicum. Int. J. Mol. Sci. 2024, 25, 173. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.H.; Yu, W.C.; Wang, L.P.; Lan, Q.K.; Wang, Y.; Chen, C.B.; Zhang, Y. Knocking Out the Transcription Factor OsNAC092 Promoted Rice Drought Tolerance. Biology 2022, 11, 1830. [Google Scholar] [CrossRef]
- D'Inca, R.; Mattioli, R.; Tomasella, M.; Tavazza, R.; Macone, A.; Incocciati, A.; Martignago, D.; Polticelli, F.; Fraudentali, I.; Cona, A.; et al. A Solanum lycopersicum polyamine oxidase contributes to the control of plant growth, xylem differentiation, and drought stress tolerance. Plant J. 2024, 119, 960–981. [Google Scholar] [CrossRef]
- Dong, A.Y.; Wang, N.; Zenda, T.; Zhai, X.Z.; Zhong, Y.; Yang, Q.; Xing, Y.; Duan, H.J.; Yan, X.C. ZmDnaJ-ZmNCED6 module positively regulates drought tolerance via modulating stomatal closure in maize. Plant Physiol. Biochem. 2025, 218, 109286. [Google Scholar] [CrossRef]
- Wang, C.L.; Zhou, Y.Y.; Wang, Y.M.; Jiao, P.; Liu, S.Y.; Guan, S.Y.; Ma, Y.Y. CRISPR-Cas9-mediated editing of ZmPL1 gene improves tolerance to drought stress in maize. GM Crop. Food. 2025, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Luo, Q.; Yang, W.C.; Ahammed, G.J.; Ding, S.T.; Chen, X.Y.; Wang, J.; Xia, X.J.; Shi, K. A Novel Role of Pipecolic Acid Biosynthetic Pathway in Drought Tolerance through the Antioxidant System in Tomato. Antioxidants 2021, 10, 1923. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.C.; Liu, T.; Deng, Z.C.; Zhang, Z.L.; Wang, Q.; Wang, W.F.; Li, W.; Guo, Y.F. Characterization of NAC transcription factor NtNAC028 as a regulator of leaf senescence and stress responses. Front. Plant Sci. 2022, 13, 941026. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.T.; Karikari, B.; Wang, L.; Chang, F.G.; Zhao, T.J. Structure characterization and potential role of soybean phospholipases A multigene family in response to multiple abiotic stress uncovered by CRISPR/Cas9 technology. Environ. Exp. Bot. 2021, 188, 104521. [Google Scholar] [CrossRef]
- Xu, L.; Gao, Q.; Feng, J.Y.; Xu, Y.; Jiang, J.R.; Deng, L.L.; Lu, Y.F.; Zeng, W.L.; Xing, J.X.; Xiang, H.Y.; et al. Physiological and phosphoproteomic analyses revealed that the NtPOD63 L knockout mutant enhances drought tolerance in tobacco. Ind. Crops Prod. 2023, 193, 116218. [Google Scholar] [CrossRef]
- Yang, Y.; Li, A.Q.; Liu, Y.Q.; Shu, J.G.; Wang, J.R.; Guo, Y.X.; Li, Q.Z.; Wang, J.H.; Zhou, A.; Wu, C.Y.; et al. ZmASR1 negatively regulates drought stress tolerance in maize. Plant Physiol. Biochem. 2024, 211, 108684. [Google Scholar] [CrossRef]
- Zhong, X.B.; Hong, W.; Shu, Y.; Li, J.F.; Liu, L.L.; Chen, X.Y.; Islam, F.; Zhou, W.J.; Tang, G.X. CRISPR/Cas9 mediated gene-editing of GmHdz4 transcription factor enhances drought tolerance in soybean (Glycine max L. Merr.). Front. Plant Sci. 2022, 13, 988505. [Google Scholar] [CrossRef]
- Matzke, M.A.; Primig, M.; Trnovsky, J.; Matzke, A.J. Reversible methylation and inactivation of marker genes in sequentially transformed tobacco plants. EMBO J. 1989, 8, 643–649. [Google Scholar] [CrossRef]
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in trans. Plant Cell 1990, 2, 279–289. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, C.; Xu, Y.; Jiang, R.; Han, Y.; Xu, Z.; Chong, K. A practical vector for efficient knockdown of gene expression in rice (Oryza sativa L.). Plant Mol. Biol. Report. 2004, 22, 409–417. [Google Scholar] [CrossRef]
- Hirai, S.; Kodama, H. RNAi vectors for manipulation of gene expression in higher plants. Open Plant Sci. J. 2008, 2, 21–30. [Google Scholar] [CrossRef]
- Yan, P.; Shen, W.; Gao, X.; Li, X.; Zhou, P.; Duan, J. High-throughput construction of intron-containing hairpin RNA vectors for RNAi in plants. PLoS ONE 2012, 7, e38186. [Google Scholar] [CrossRef]
- Mujtaba, M.; Wang, D.; Carvalho, L.B.; Oliveira, J.L.; Espirito Santo Pereira, A.d.; Sharif, R.; Jogaiah, S.; Paidi, M.K.; Wang, L.; Ali, Q. Nanocarrier-mediated delivery of miRNA, RNAi, and CRISPR-Cas for plant protection: Current trends and future directions. ACS Agric. Sci. Technol. 2021, 1, 417–435. [Google Scholar] [CrossRef]
- Zhao, J.-H.; Liu, Q.-Y.; Xie, Z.-M.; Guo, H.-S. Exploring the challenges of RNAi-based strategies for crop protection. Adv. Biotechnol. 2024, 2, 23. [Google Scholar] [CrossRef]
- Akbar, S.; Wei, Y.; Zhang, M.-Q. RNA Interference: Promising Approach to Combat Plant Viruses. Int. J. Mol. Sci. 2022, 23, 5312. [Google Scholar] [CrossRef]
- Cai, X.; Tang, L.Y.; Wang, H.T.; Zhang, S.J.; Li, X.H.; Liu, C.J.; Zhang, X.Y.; Zhang, J.H. Identification of the cysteine-rich transmembrane module CYSTM family in upland cotton and functional analysis of GhCYSTM5_A in cold and drought stresses. Int. J. Biol. Macromol. 2025, 292, 139058. [Google Scholar] [CrossRef]
- Cui, X.Y.; Zhang, P.Y.; Chen, C.C.; Zhang, J.X. VyUSPA3, a universal stress protein from the Chinese wild grape Vitis yeshanensis, confers drought tolerance to transgenic V. vinifera. Plant Cell Rep. 2023, 42, 181–196. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, K.; Zhu, L.; Zhang, N.; Si, H. StMAPKK5 positively regulates response to drought and salt stress in potato. Int. J. Mol. Sci. 2024, 25, 3662. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, P.; Song, H.; Li, Q. NtNCED3 regulates responses to phosphate deficiency and drought stress in Nicotiana tabacum. Gene 2023, 872, 147458. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, X.; Tang, Y.; Yu, T.; Zheng, L.; Chen, J.; Zhou, Y.; Liu, Y.; Chen, M.; Xu, Z.-S. GmSAP5, a soybean A20/AN1 domain-containing stress-associated protein gene activated by GmAREB3, increases drought stress resistance in soybean by mediating ABA signaling. Crop J. 2022, 10, 1601–1610. [Google Scholar] [CrossRef]
- Lee, S.-U.; Mun, B.-G.; Bae, E.-K.; Kim, J.-Y.; Kim, H.-H.; Shahid, M.; Choi, Y.-I.; Hussain, A.; Yun, B.-W. Drought stress-mediated transcriptome profile reveals NCED as a key player modulating drought tolerance in Populus davidiana. Front. Plant Sci. 2021, 12, 755539. [Google Scholar] [CrossRef] [PubMed]
- Huan, X.; Wang, X.; Zou, S.; Zhao, K.; Han, Y.; Wang, S. Transcription factor ERF194 modulates the stress-related physiology to enhance drought tolerance of poplar. Int. J. Mol. Sci. 2023, 24, 788. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, N.; Zou, J.; Ran, J.; Chen, X. Rice MYB transcription factor OsMYB1R1 negatively regulates drought resistance. Plant Growth Regul. 2023, 99, 515–525. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, N.; Deng, Y.; Liu, S.; Yang, L.; Wang, X.; Wen, R.; Si, H. Functional analysis of the StERF79 gene in response to drought stress in potato (Solanum tuberosum L.). BMC Plant Biol. 2025, 25, 387. [Google Scholar] [CrossRef]
- Pu, Z.; Qin, T.; Wang, Y.; Wang, X.; Shi, N.; Yao, P.; Liu, Y.; Bai, J.; Bi, Z.; Sun, C. Genome-Wide Analysis of the JAZ Gene Family in Potato and Functional Verification of StJAZ23 Under Drought Stress. Int. J. Mol. Sci. 2025, 26, 2360. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, X.; Zhao, B.; Huang, J.; Zhou, H.; Li, M.; Ma, F. The ubiquitin-binding protein MdRAD23D1 affects WUE under long-term moderate drought stress in transgenic apple (Malus domestica). Sci. Hortic. 2023, 319, 112164. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, X.; Liu, J.; Wu, Y.; Zhou, Q.; Fang, L.; Liu, Z. DROUGHT-INDUCED UNKNOWN PROTEIN 1 positively modulates drought tolerance in cultivated alfalfa (Medicago sativa L.). Crop J. 2023, 11, 57–70. [Google Scholar] [CrossRef]
- Zhao, H.; Abulaizi, A.; Wang, C.; Lan, H. Overexpression of CgbHLH001, a positive regulator to adversity, enhances the photosynthetic capacity of maize seedlings under drought stress. Agronomy 2022, 12, 1149. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, K.; Shan, D.; Wang, C.; Yan, T.; Hu, Z.; Zheng, X.; Zhang, T.; Song, H.; Li, R. The WRKY17-WRKY50 complex modulates anthocyanin biosynthesis to improve drought tolerance in apple. Plant Sci. 2024, 340, 111965. [Google Scholar] [CrossRef]
- Cao, G.; Gu, H.; Jiang, W.; Tian, Z.; Shi, G.; Chen, W.; Tian, B.; Wei, X.; Zhang, L.; Wei, F. BrDHC1, a novel putative DEAD-box helicase gene, confers drought tolerance in transgenic Brassica rapa. Horticulturae 2022, 8, 707. [Google Scholar] [CrossRef]
- Lv, K.; Wei, H.; Liu, G. A R2R3-MYB transcription factor gene, BpMYB123, regulates BpLEA14 to improve drought tolerance in Betula platyphylla. Front. Plant Sci. 2021, 12, 791390. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Wei, C.; Liu, X.; Jiang, L.; Gao, J. Multifunctional transcription factor YABBY6 regulates morphogenesis, drought and cold stress responses in rice. Rice 2024, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Q.-Y.; Xu, P.; Wang, X.-H.; Dai, S.-J.; Liu, Z.-N.; Xu, M.; Cao, X.; Cui, X.-Y. GmTRAB1, a Basic Leucine Zipper Transcription Factor, Positively Regulates Drought Tolerance in Soybean (Glycine max. L). Plants 2024, 13, 3104. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, X.; Wu, J.; Shou, H.; Wang, W. The F-Box Protein TaFBA1 Positively Regulates Drought Resistance and Yield Traits in Wheat. Plants 2024, 13, 2588. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Liang, Y.; Xiao, D.; Fu, T.; Yang, X.; Liu, J.; Wang, S.; Wang, Y. PagTPS1 and PagTPS10, the trehalose-6-phosphate synthase genes, increase trehalose content and enhance drought tolerance. Int. J. Biol. Macromol. 2024, 279, 135518. [Google Scholar] [CrossRef]
- Lv, A.; Su, L.; Fan, N.; Wen, W.; Wang, Z.; Zhou, P.; An, Y. Chloroplast-targeted late embryogenesis abundant 1 increases alfalfa tolerance to drought and aluminum. Plant Physiol. 2023, 193, 2750–2767. [Google Scholar] [CrossRef]
- Wang, C.; Hou, Z.-H.; Kong, Y.-N.; Jin, L.-G.; Zheng, L.; Chen, J.; Zhou, Y.-B.; Chen, M.; Ma, Y.-Z.; Fang, Z.-W. GmCIPK29 positively regulates drought tolerance through involvement in the reactive oxygen species scavenging and abscisic acid signaling pathway. Environ. Exp. Bot. 2023, 210, 105340. [Google Scholar] [CrossRef]
- Geng, L.; Ren, J.; Ji, X.; Yan, S.; Song, X.S. Over-expression of DREB46 enhances drought tolerance in Populus trichocarpa. J. Plant Physiol. 2023, 281, 153923. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, C.; Lan, H. A bHLH transcription factor from Chenopodium glaucum confers drought tolerance to transgenic maize by positive regulation of morphological and physiological performances and stress-responsive genes' expressions. Mol. Breed. 2021, 41, 74. [Google Scholar] [CrossRef]
- Iannelli, M.A.; Nicolodi, C.; Coraggio, I.; Fabriani, M.; Baldoni, E.; Frugis, G. A Novel Role of Medicago truncatula KNAT3/4/5-like Class 2 KNOX Transcription Factors in Drought Stress Tolerance. Int. J. Mol. Sci. 2023, 24, 12668. [Google Scholar] [CrossRef]
- Zhang, Y.; Iaffaldano, B.; Qi, Y. CRISPR ribonucleoprotein-mediated genetic engineering in plants. Plant Commun. 2021, 2, 100168. [Google Scholar] [CrossRef]
- Joshi, R.K.; Bharat, S.S.; Mishra, R. Engineering drought tolerance in plants through CRISPR/Cas genome editing. 3 Biotech 2020, 10, 400. [Google Scholar] [CrossRef]
- Augustine, S.M.; Cherian, A.V.; Seiling, K.; Di Fiore, S.; Raven, N.; Commandeur, U.; Schillberg, S. Targeted mutagenesis in Nicotiana tabacum ADF gene using shockwave-mediated ribonucleoprotein delivery increases osmotic stress tolerance. Physiol. Plant. 2021, 173, 993–1007. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rosas, E.; Hernández-Oñate, M.Á.; Fernandez-Valverde, S.-L.; Tiznado-Hernández, M.E. Plant long non-coding RNAs: Identification and analysis to unveil their physiological functions. Front. Plant Sci. 2023, 14, 1275399. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.V.; Chekanova, J.A. Chapter 5: Long noncoding RNAs in plants. In Long Non Coding RNA Biology. Advances in Experimental Medicine and Biology; Rao, M., Ed.; Springer: Singapore, 2017; pp. 133–154. [Google Scholar]
- Jarroux, J.; Morillon, A.; Pinskaya, M. Chapter 1: History, discovery, and classification of lncRNAs. In Long Non Coding RNA biology. Advances in Experimental Medicine and Biology; Rao, M., Ed.; Springer: Singapore, 2017; pp. 1–46. [Google Scholar]
- Rai, M.I.; Alam, M.; Lightfoot, D.A.; Gurha, P.; Afzal, A.J. Classification and experimental identification of plant long non-coding RNAs. Genomics 2019, 111, 997–1005. [Google Scholar] [CrossRef]
- Gelaw, T.A.; Sanan-Mishra, N. Non-coding RNAs in response to drought stress. Int. J. Mol. Sci. 2021, 22, 12519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Fan, Y.; Sun, X.; Chen, L.; Terzaghi, W.; Bucher, E.; Li, L.; Dai, M. A large-scale circular RNA profiling reveals universal molecular mechanisms responsive to drought stress in maize and Arabidopsis. Plant J. 2019, 98, 697–713. [Google Scholar] [CrossRef]
- Xu, W.-B.; Guo, Q.-H.; Liu, P.; Dai, S.; Wu, C.-A.; Yang, G.-D.; Huang, J.-G.; Zhang, S.-Z.; Song, J.-M.; Zheng, C.-C.; et al. A long non-coding RNA functions as a competitive endogenous RNA to modulate TaNAC018 by acting as a decoy for tae-miR6206. Plant Mol. Biol. 2024, 114, 36. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, Y.; Qi, X. LncRNA TCONS_00021861 is functionally associated with drought tolerance in rice (Oryza sativa L.) via competing endogenous RNA regulation. BMC Plant Biol. 2021, 21, 410. [Google Scholar] [CrossRef]
- Luo, L.; Xia, H.; Lu, B.-R. Editorial: Crop Breeding for Drought Resistance. Front. Plant Sci. 2019, 10, 314. [Google Scholar] [CrossRef]
- Das, T.; Anand, U.; Pal, T.; Mandal, S.; Kumar, M.; Radha; Gopalakrishnan, A.V.; de la Lastra, J.M.P.; Dey, A. Exploring the potential of CRISPR/Cas genome editing for vegetable crop improvement: An overview of challenges and approaches. Biotechnol. Bioeng. 2023, 120, 1215–1228. [Google Scholar] [CrossRef]
| Physical or Agronomic Traits | Physiological Traits | Biochemical Traits |
|---|---|---|
| Germination rate | Flowering time | Abscisic acid biosynthesis |
| Seedling vigor | Osmotic adjustment | Auxin biosynthesis |
| Plant height | Internal water status | Osmolytes biosynthesis |
| Harvest index | Cell-membrane stability | Proline biosynthesis |
| Seed yield | Cuticle deposition | Heat shock protein biosynthesis |
| Leaf area | Photosynthetic capacity | Dehydrins biosynthesis |
| Root architecture | Leaf water content | |
| Root mass | Chlorophyll stability | |
| Root volume | Stomatal conductance | |
| Water-use efficiency | ||
| Canopy temperature | ||
| Cuticular wax leaf content | ||
| Stomatal density |
| Species | Gene Studied | Phenotypic Effect | Ref. | |
|---|---|---|---|---|
| Increase | Decrease | |||
| Brassica napus | BnPUB18 and BnPUB19 | Antioxidant activity and survival percentage. | Water loss, MDA content, and electrolyte leakage percentage. | [72] |
| Glycine max | GmpPLA-IIe and GmPLA-IIz | Survival rate, plant height, plant weight, shoot length, and root length. | [88] | |
| GmHdz4 | Root dry weight, number of root tips, proline and soluble sugar content, and enzymatic activity of catalase, SOD, and peroxidase. | Hydrogen peroxide, superoxide, and malondialdehyde content. | [91] | |
| Nicotiana tabacum | NtAITR | Sensitivity to ABA, expression of an ABA receptor gene, and survival percentage. | [70] | |
| NtPIF | Survival rate, RWC, proline content, photosynthetic rate, expression of catalase, peroxidase, and SOD genes. | MDA and hydrogen peroxide content. Degree of wilted leaves. | [73] | |
| NtNAC028 | Survival rate, expression of the senescence-related cysteine proteinase 1 genes and the stress-responsive genes RD29A, RD26, DREB1B, and NCED3-2. Activity of SOD, catalase, and peroxidase. | MDA content and velocity of leaf senescence. | [87] | |
| NtPOD63l9 | Activity of peroxidase and SOD. Proline and lignin content. Expression of the stress-related genes RD29A and DREB2A. Recovery and survival rate. Number of interfascicular fibers in stems. | Water loss rate and stomatal opening. | [89] | |
| Oriza sativa | OsDIRH2 | Chlorophyll content, shoot fresh weight, and survival rate. | Stomatal density. | [68] |
| OsPUB7 | Proline content and recovery rate. | Percentage of ion leakage and damage level. | [69] | |
| OsPUB16 | Stomatal density. Sensitive to methyl jasmonic acid and ABA. | Water loss in leaves, the size of stomata, and plant growth. | [76] | |
| OsERF109 | Expression of SOD, catalase, and ascorbate peroxidase genes. RWC in leaves, photosynthesis, concentration of intercellular CO2, proline content, shoot length, root length, and survival rate. | Canopy temperature, velocity of leaf senescence, water loss in leaves, stomatal conductance, and transpiration. MDA, superoxide, and hydrogen peroxide content. | [77] | |
| OsCKX2 | RWC, photosynthesis rate, proline content, and survival rate. Expression of catalase, SOD, and ascorbate peroxidase genes. | Water loss, transpiration, stomatal conductance, and internal CO2 concentration. MDA and endonucleases associated with programmed cell death content. | [78] | |
| OsNACO92 | RWC, chlorophyll content, and survival rate. Expression of SOD, peroxidase, and catalase genes. | Wilting. MDA, superoxide, and hydrogen peroxide content. | [82] | |
| Solanum lycopersicum | SlGT30 | Photosynthetic CO2 assimilation rate, fruit weight, and fruit size. Size and number of fruit pericarp cells. | Water loss, number of stomata, electrolyte leakage, wilting symptoms, and leaf rolling. Superoxide and hydrogen peroxide content. | [75] |
| SlWOX4 | Plant growth, survival percentage, proline content, and root length. | Area stomatal open, water loss, electrolyte leakage, and MDA content. | [71] | |
| SlHyPRP1 and SlDEA1 | Survival rate, chlorophyll content, and proline content. | [80] | ||
| SlALKBH10B | RWC, chlorophyll, proline, soluble sugars, and starch content. Expression of ascorbate peroxidases, catalases, SOD, and proline synthase genes. | Leaves wilting, water loss, MDA, and hydrogen peroxide content. | [81] | |
| Slald1 | Activity of SOD, peroxidase, catalase, ascorbate peroxidase, dehydroascorbate reductase, and glutathione reductase. Stomatal conductance and transpiration rate. | Electrolyte leakage, leaf rolling, and wilting. MDA, hydrogen peroxide, and superoxide ion content. | [86] | |
| Zea mays | ZmGA20ox3 | Jasmonic and ABA content. Plant height. Expression of transcription factor genes related to drought stress | Gibberellin GA1 content. | [74] |
| ZmPL1 | Survival percentage, RWC, root length, root branches, and proline content. Expression of SOD, peroxide, and catalase genes. Expression of stress-related genes ZmLTP3, ZmRD22, and ZmCBF4. | Degree of wilted leaves. Superoxide ion, hydrogen peroxide, and MDA content. | [85] | |
| ZmASR1 | Survival rate, RWC, chlorophyll content, and net photosynthetic rate. Activity of peroxidase, catalase, and SOD. | Stomatal openness. | [90] | |
| Species | Gene Targeted | Method | Phenotypic Effect | Ref. | |
|---|---|---|---|---|---|
| Increase | Decrease | ||||
| Arabidopsis thaliana | GmSAP5 | RNAi hairpin | Degree of leaf wilting and malondialdehyde content. | Survival rate, leaf RWC, proline, and ABA content. | [105] |
| Betula platyphylla | BpMYB123 | RNAi hairpin | Superoxide, hydrogen peroxide, and MDA content. Electrolyte leakage and degree of wilting in leaves. | Proline content. Activity of peroxidase and SOD. | [116] |
| Brassica rapa | BrDHC1 | RNAi intron-containing hairpin | MDA content. Leaf water loss. Yellow color and curled leaves. | RWC, roots length, seedling fresh weight, and stomatal aperture. Proline, soluble sugars, and chlorophyll content. Activity of SOD, catalase, and peroxidase. Expression of the stress-related genes CAO, CHLG, POD, P5CR, MYB44, and ABRE2. | [115] |
| Glycine max | GmTRAB1 | Antisense RNAi intron-containing hairpin | Leaf wilting. MDA and hydrogen peroxide content. | Survival rate and root fresh weight. Proline content. Activity of catalase and peroxidase. Expression of ABA-responsive, antioxidant-related, and drought-induced genes. | [118] |
| GmCIPK29 | RNAi hairpin | Leaf wilting. MDA, superoxide, and hydrogen peroxide content. | RWC and survival rate. Activity of catalase and peroxidase. Expression of stress-related genes. | [122] | |
| Gossypium hirsutum | GhCYSTM5_A | Antisense RNAi hairpin | Leaf wilting. MDA content. | Proline content. Activity of SOD. | [101] |
| Malus domestica | MdRAD23D1 | RNAi intron-containing hairpin | MDA, superoxide, and hydrogen peroxide content. | RWC, water utilization efficiency, plant height, trunk diameter, biomass accumulation, and photosynthetic activity. Root weight and root hydraulic conductivity. Activity of SOD, peroxidase, and catalase | [111] |
| MdWRKY50 | RNAi hairpin | Leaf wilting. Reactive oxygen species and MDA content. | . Photosynthetic rate, fresh weight, and leaf area. Expression of the anthocyanin biosynthetic genes MdCHS, MdCHI, MdF3H, MdF3’H, and MdDFR. | [114] | |
| Medicago sativa | MsDIUP1 | RNAi hairpin | MDA content. | Proline, soluble sugar, and chlorophyll content. | [112] |
| MsLEA1 | Antisense RNAi intron-containing hairpin | Wilting and yellowish color in leaves. Stomatal aperture and stomatal conductance. Electrolyte leakage and MDA content. | Fresh weight, water use efficiency, photosynthetic rate, transpiration rate, rubisco activity, and hydrogen peroxide content. | [121] | |
| Medicago truncatula | MtKNOX3-like | RNAi intron-containing hairpin | Senescence symptoms, water loss, and ion leakage in leaves. | Chlorophyll content in leaves. Proline content and expression of the proline dehydrogenase gene. | [125] |
| Nicotiana tabacum | NtNCED3 | RNAi * | Photosynthesis. Shoot and root development. | [104] | |
| Oriza sativa | MYB (Os04g0583900) | RNAi intron-containing hairpin | Shoot height, root length, and proline content. | MDA content and relative electric conductivity. | [108] |
| OsYABBY6 | Antisense RNAi | Water loss and survival rate. Soluble sugar and proline content. Expression of a receptor-like kinase that activates the antioxidant system. | MDA, superoxide ion, and hydrogen peroxide content. Expression of three NADPH oxidase genes. | [117] | |
| Populus alba x P. glandulosa | PagTPS1 and PagTPS10 | RNAi hairpin | Leaf wilting. | Trehalose content and expression of the genes TPS and trehalose-6-phosphate phosphatase. Expression of drought-responsive genes. | [120] |
| Populous tremolo × P. Alba | ERF194 | RNAi * | MDA content. | RWC, number of leaves, and root length. Activity of catalase, peroxidase, and SOD. Expression of stress-related genes. | [107] |
| Populus trichocarpa | DREB46 | RNAi hairpin | Plant height and leaf wilting. | RWC and survival rate. Activity of SOD, catalase, and peroxidase. Chlorophyll, proline, and lignin content. | [123] |
| PdNCED3 | RNAi intron-containing hairpin | Stomatal aperture. Curling and rolling in leaf. Hydrogen peroxide content. Expression of the Abscisic acid-insensitive 5-like protein 2 gene. | ABA content and expression of ABA signaling transcription factor genes. | [106] | |
| Solanum tuberosum | StMAPKK5 | RNAi by amiRNA | Leaf wilting and MDA content. | Leaf RWC, plant growth velocity, and proline content. Activity of catalase, SOD, and peroxidase. | [103] |
| StERF79 | RNAi by amiRNA | Water loss, leaf wilting, and MDA content. | RWC and proline content. Activity of SOD, peroxidase, and catalase. Expression of the dehydrin gene StDHN-2. | [109] | |
| StJAZ23 | RNAi miRNA | Height, number of leaves, root area, root length, and root tips. Jasmonic acid and ABA content. Activity of SOD, peroxidase, and catalase. | [110] | ||
| Triticum aestivum | TaFBA1 | Antisense RNAi hairpin | Water loss, leaf wilting, and electrolyte leakage. Proline, MDA, superoxide, and hydrogen peroxide content. Activity of the stress-inducible delta 1-pyrroline-5-carboylate synthetase. | RWC, plant height, weight of seeds, net photosynthetic rate, transpiration, stomatal conductance, chlorophyll content, soluble sugars content, and aquaporin activity. Activity of SOD, catalase, ascorbate peroxidase, peroxidases, glutathione peroxidase, monodehydroascorbate reductase, and dehydroascorbate reductase. Expression of antioxidant-related and stress-induced genes. | [119] |
| Vitis vinifera | VyUSPA3 | RNAi intron-containing hairpin | Leaf wilting and the number of dead leaves. MDA, hydrogen peroxide, and peroxide ion content. | Activity of SOD, catalase, and peroxidase. Expression of the stress-related genes RD22, RD29B, DREB2A, and NCED1. | [102] |
| Zea mays | bHLH | RNAi * | Starch content, activity of NAD-malic enzyme, and RUBISCO. | [113] | |
| CgbHLH00 | Antisense RNAi hairpin | Leaf wilting. Hydrogen peroxide, superoxide, and MDA content. | Proline and soluble sugar content. Activity of catalase, peroxidase, and SOD. Expression of drought-inducible genes. | [124] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Coronado, H.; Ojeda-Contreras, A.-J.; Berumen-Varela, G.; Robles-Parra, J.-M.; Handa, A.K.; Tiznado-Hernández, M.-E. Engineering Crops for Enhanced Drought Stress Tolerance: A Strategy for Sustainable Agriculture. Agronomy 2025, 15, 1912. https://doi.org/10.3390/agronomy15081912
García-Coronado H, Ojeda-Contreras A-J, Berumen-Varela G, Robles-Parra J-M, Handa AK, Tiznado-Hernández M-E. Engineering Crops for Enhanced Drought Stress Tolerance: A Strategy for Sustainable Agriculture. Agronomy. 2025; 15(8):1912. https://doi.org/10.3390/agronomy15081912
Chicago/Turabian StyleGarcía-Coronado, Heriberto, Angel-Javier Ojeda-Contreras, Guillermo Berumen-Varela, Jesús-Martín Robles-Parra, Avtar K. Handa, and Martín-Ernesto Tiznado-Hernández. 2025. "Engineering Crops for Enhanced Drought Stress Tolerance: A Strategy for Sustainable Agriculture" Agronomy 15, no. 8: 1912. https://doi.org/10.3390/agronomy15081912
APA StyleGarcía-Coronado, H., Ojeda-Contreras, A.-J., Berumen-Varela, G., Robles-Parra, J.-M., Handa, A. K., & Tiznado-Hernández, M.-E. (2025). Engineering Crops for Enhanced Drought Stress Tolerance: A Strategy for Sustainable Agriculture. Agronomy, 15(8), 1912. https://doi.org/10.3390/agronomy15081912









