A Preliminary Evaluation of the Use of Solid Residues from the Distillation of Medicinal and Aromatic Plants as Fertilizers in Mediterranean Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of the Soil and the Solid Residues fromMAP Distillation
2.2. Treatments of the Soil with the Solid Residues from MAP Distillation
2.3. Statistical Analysis
3. Results
3.1. Properties of the Original Soil and the Solid Residues from the Distillation of MAP
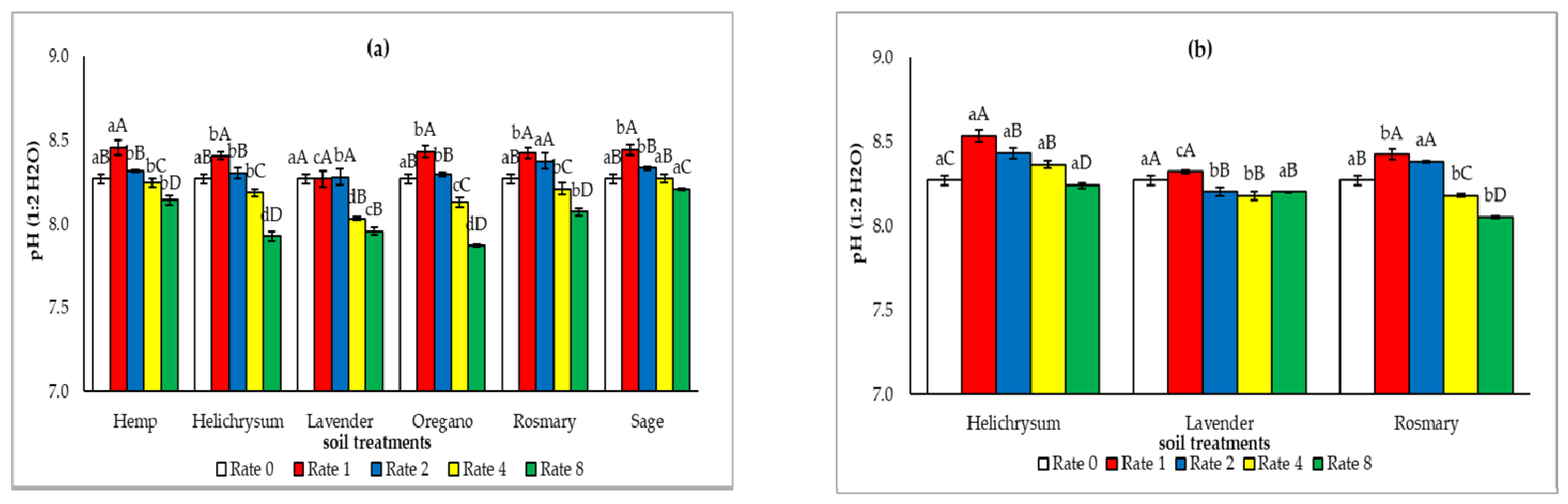
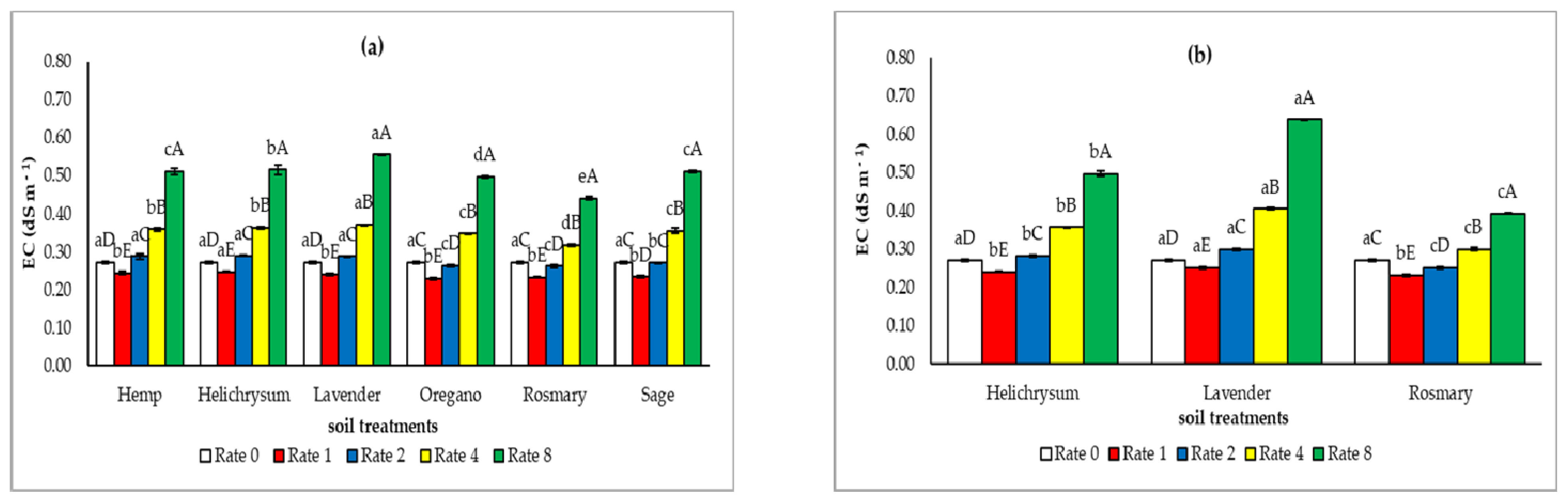
3.2. The Soil Chemical Properties After the Application of the Solid Residues from the Distillation of MAP
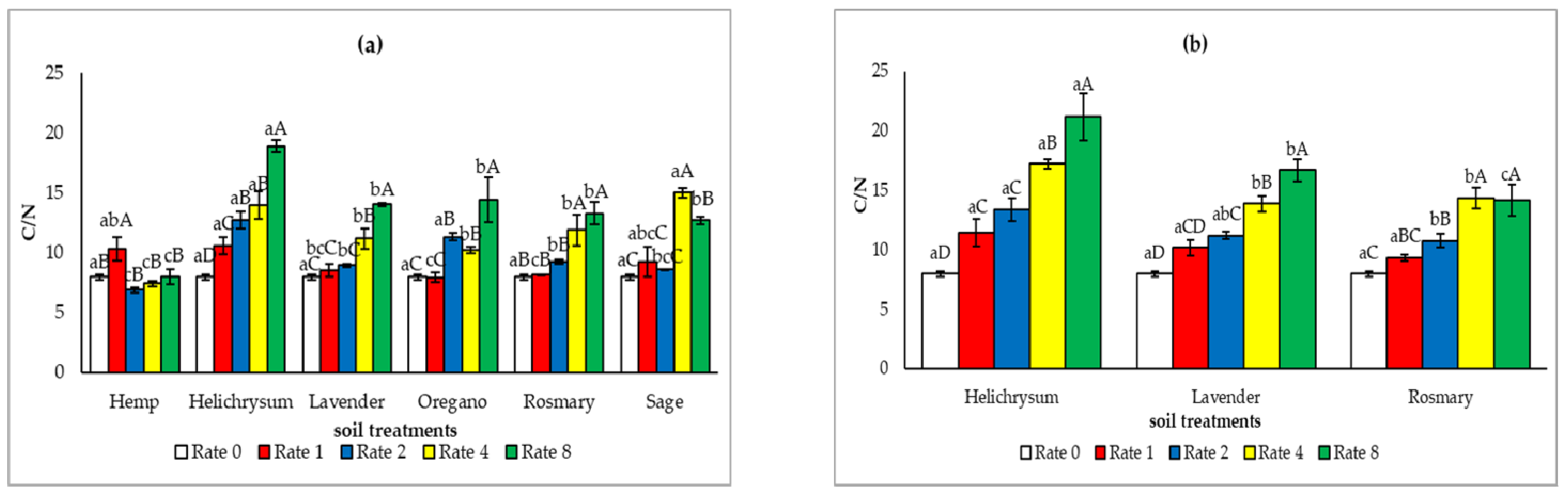
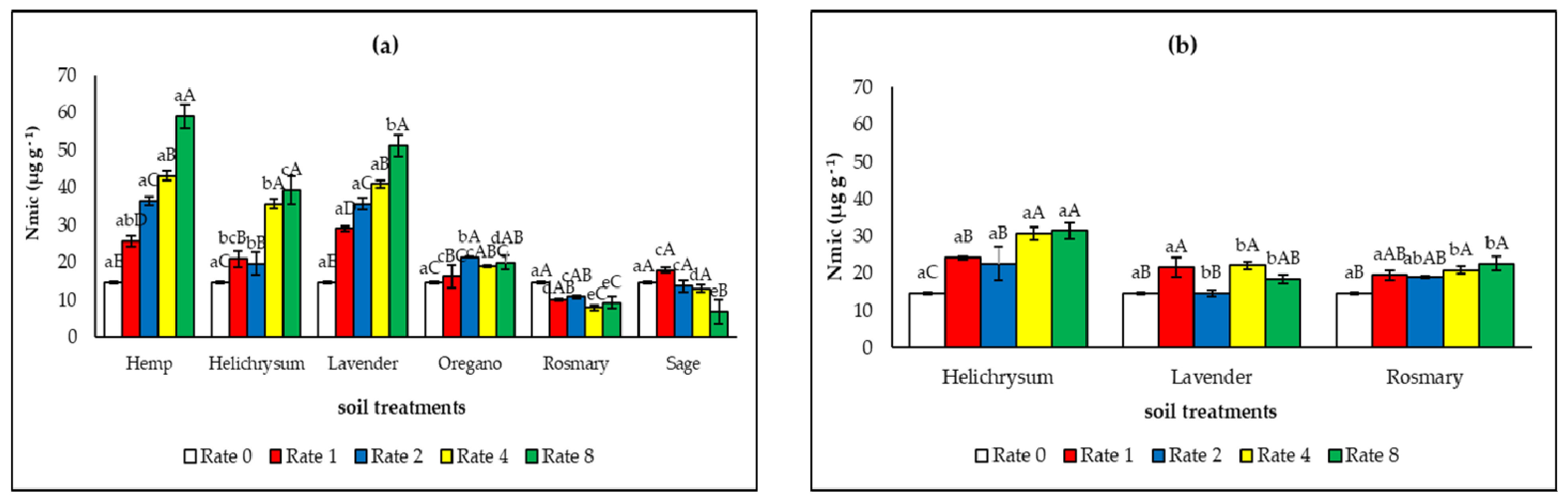
3.3. Soil Fertility and Microbial Properties After the Application of the Solid Residues from the Distillation of MAP
4. Discussion
4.1. Effect of the Solid Residues from the Distillation of MAP on the Soil Chemical Properties
4.2. Effect of the Solid Residues from the Distillation of MAP on Soil Fertility and Microbial Properties
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAP | Medicinal and Aromatic plants |
| EC | Electrical conductivity |
| CEC | Cation exchange capacity |
| MR | Microbial respiration rate |
| Nmic | Biomass microbial N |
| qCO2 | Metabolic quotient |
Appendix A
| Rate (%) | Species | |||||
|---|---|---|---|---|---|---|
| Hemp | Helichrysum | Lavender | Oregano | Rosemary | Sage | |
| ΝO3-Ν (mg kg−1) | ||||||
| 0 | 58.1 ± 1.9 aB* | 58.1 ± 1.9 aA | 58.1 ± 1.9 aA | 58.1 ± 1.9 aA | 58.1 ± 1.9 aA | 58.1 ± 1.9 aA |
| 1 | 15.6 ± 0.4 aE | 10.4 ± 0.9 bE | 9.5 ± 0.9 bD | 10.3 ± 0.6 bD | 11.6 ± 0.7 bD | 8.9 ± 0.8 bE |
| 2 | 20.2 ± 1.0 aD | 14.7 ± 0.8 bD | 11.5 ± 2.5 bD | 12.8 ± 1.3 bD | 13.4 ± 0.8 bD | 13.2 ± 0.5 bD |
| 4 | 34.3 ± 0.7 aC | 24.4 ± 1.6 cdC | 27.1 ± 4.4 bcC | 28.0 ± 0.5 bC | 21.6 ± 1.8 dC | 21.4 ± 1.4 dC |
| 8 | 63.8 ± 3.5 aA | 49.5 ± 6.2 bB | 51.1 ± 4.4 bB | 51.6 ± 2.6 bB | 39.6 ± 1.4 cB | 41.3 ± 1.2 cB |
| p F-test | ||||||
| Rate | <0.001 | |||||
| Residue | <0.001 | |||||
| Interaction | <0.001 | |||||
| ΝH4-Ν (mg kg−1) | ||||||
| 0 | 11.0 ± 2.0 aC | 11.1 ± 2.0 aA | 11.0 ± 2.0 aA | 11.0 ± 2.0 aA | 11.0 ± 2.0 aA | 11.0 ± 2.0 aA |
| 1 | 9.7 ± 0.2 aC | 9.3 ± 0.2 abB | 8.4 ± 0.3 bB | 8.2 ± 0.2 abB | 8.1 ± 0.5 bB | 8.2 ± 0.9 abB |
| 2 | 10.4 ± 0.3 aC | 8.9 ± 0.1 abB | 8.1 ± 0.4 bB | 8.2 ± 0.1 bB | 7.5 ± 0.3 bBC | 8.0 ± 0.2 bB |
| 4 | 12.9 ± 0.7 aB | 8.7 ± 0.3 bB | 8.6 ± 0.7 bB | 7.8 ± 0.5 bB | 7.3 ± 0.2 bcBC | 5.9 ± 0.4 cC |
| 8 | 16.5 ± 0.5 aA | 9.3 ± 0.5 bB | 9.0 ± 0.1 bB | 8.2 ± 0.2 bcB | 6.5 ± 0.6 dC | 6.9 ± 0.1 cdBC |
| p F-test | ||||||
| Rate | <0.001 | |||||
| Residue | <0.001 | |||||
| Interaction | <0.001 | |||||
| P (mg kg−1) | ||||||
| 0 | 9.5 ± 1.9 aE | 9.5 ± 1.9 aBC | 9.5 ± 1.9 aC | 9.5 ± 1.9 aD | 9.5 ± 1.9 aC | 9.5 ± 1.9 aC |
| 1 | 23.6 ± 0.8 aD | 9.1 ± 0.7 cC | 7.5 ± 0.4 cC | 12.6 ± 3.5 bC | 8.4 ± 0.4 cC | 8.5 ± 0.7 cC |
| 2 | 39.8 ± 2.0 aC | 11.1 ± 1.9 cBC | 7.9 ± 0.3 dC | 14.5 ± 1.0 bC | 10.7 ± 1.0 cC | 10.3 ± 0.3 cdC |
| 4 | 71.7 ± 2.5 aB | 11.9 ± 1.7 eB | 12.5 ± 1.5 deB | 26.6 ± 2.4 bB | 16.5 ± 0.8 cB | 14.8 ± 1.1 cdB |
| 8 | 122 ± 0.6 aA | 18.9 ± 0.2 dA | 18.2 ± 2.0 dA | 41.6 ± 2.6 bA | 26.1 ± 0.4 cA | 26.2 ± 1.8 cA |
| p F-test | ||||||
| Rate | <0.001 | |||||
| Residue | <0.001 | |||||
| Interaction | <0.001 | |||||
| K (mg kg−1) | ||||||
| 0 | 112 ± 10 aE | 112 ± 10 aE | 112 ± 10 aE | 112 ± 10 aE | 112 ± 10 aE | 112 ± 10 aE |
| 1 | 163 ± 3 aD | 136 ± 7 bD | 163 ± 6 aD | 175 ± 3 aD | 175 ± 3 aD | 155 ± 5 abD |
| 2 | 267 ± 15 bcC | 161 ± 3 eC | 263 ± 6 cdC | 313 ± 31 aC | 287 ± 12 bC | 243 ± 12 dC |
| 4 | 397 ± 21 bB | 243 ± 15 dB | 413 ± 6 bB | 470 ± 36 aB | 453 ± 12 aB | 337 ± 15 cB |
| 8 | 720 ± 10 cA | 373 ± 12 eA | 857 ± 12 bA | 887 ± 15 aA | 840 ± 26 bA | 557 ± 15 dA |
| p F-test | ||||||
| Rate | <0.001 | |||||
| Residue | <0.001 | |||||
| Interaction | <0.001 | |||||
| Rate (%) | Species | |||||
|---|---|---|---|---|---|---|
| Helichrysum | Lavender | Rosemary | Helichrysum | Lavender | Rosemary | |
| ΝO3-Ν (mg kg−1) | ΝH4-Ν (mg kg−1) | |||||
| 0 | 58.1 ± 1.9 aA* | 58.1 ± 1.9 aB | 58.1 ± 1.9 aA | 11.0 ± 2.0 aA | 11.0 ± 2.0 aA | 11.0 ± 2.0 aA |
| 1 | 10.1 ± 0.9 aD | 10.1 ± 2.6 aD | 10.0 ± 2.1 aC | 8.3 ± 0.2 aB | 8.7 ± 0.3 aB | 9.0 ± 0.3 aB |
| 2 | 15.7 ± 0.0 aC | 13.6 ± 0.7 aD | 9.4 ± 0.6 bC | 7.9 ± 0.5 aB | 7.7 ± 0.1 aB | 8.1 ± 0.7 aB |
| 4 | 16.0 ± 0.6 bC | 41.9 ± 5.8 aC | 12.3 ± 0.4 cC | 7.0 ± 0.1 aB | 7.4 ± 0.2 aB | 7.9 ± 0.3 aB |
| 8 | 32.1 ± 1.7 bB | 113 ± 4 aA | 17.8 ± 1.2 cB | 7.2 ± 0.7 aB | 7.4 ± 0.1 aB | 7.5 ± 0.4 aB |
| p F-test | p F-test | |||||
| Rate | <0.001 | <0.001 | ||||
| Residue | <0.001 | NS # | ||||
| Interaction | <0.001 | NS | ||||
| P (mg kg−1) | K (mg kg−1) | |||||
| 0 | 9.5 ± 1.9 aB | 9.5 ± 1.9 aD | 9.5 ± 1.9 aCD | 112 ± 10 aC | 112 ± 10 aE | 112 ± 10 aE |
| 1 | 8.9 ± 1.4 aB | 10.0 ± 1.8 aD | 8.7 ± 0.5 aD | 129 ± 7 bBC | 185 ± 4 aD | 169 ± 2 aD |
| 2 | 7.8 ± 0.1 bB | 12.3 ± 0.7 aC | 10.6 ± 0.9 aC | 140 ± 8 bBC | 303 ± 15 aC | 277 ± 6 aC |
| 4 | 8.7 ± 0.5 bB | 15.3 ± 0.8 aB | 15.1 ± 0.3 aB | 163 ± 1 cB | 523 ± 15 aB | 410 ± 20 bB |
| 8 | 14.1 ± 1.0 cA | 32.3 ± 0.3 aA | 25.6 ± 0.6 bA | 280 ± 0 cA | 1077 ± 55 aA | 707 ± 64 bA |
| p F-test | p F-test | |||||
| Rate | <0.001 | <0.001 | ||||
| Residue | <0.001 | <0.001 | ||||
| Interaction | <0.001 | <0.001 | ||||
| Rate (%) | Species | |||||
|---|---|---|---|---|---|---|
| Hemp | Helichrysum | Lavender | Oregano | Rosemary | Sage | |
| B (mg kg−1) | ||||||
| 0 | 0.55 ± 0.06 aE* | 0.55 ± 0.06 aD | 0.55 ± 0.06 aD | 0.55 ± 0.06 aD | 0.55 ± 0.06 aC | 0.55 ± 0.06 aC |
| 1 | 0.87 ± 0.04 aD | 0.75 ± 0.13 abC | 0.71 ± 0.02 bC | 0.65 ± 0.08 bcD | 0.55 ± 0.01 cC | 0.65 ± 0.02 bcC |
| 2 | 1.15 ± 0.10 aC | 0.79 ± 0.05 bcBC | 0.77 ± 0.04 cC | 0.93 ± 0.04 bC | 0.68 ± 0.05 cBC | 0.69 ± 0.06 cBC |
| 4 | 1.59 ± 0.10 aB | 0.91 ± 0.04 cB | 1.12 ± 0.14 bB | 1.15 ± 0.10 bB | 0.89 ± 0.10 cA | 0.89 ± 0.08 cA |
| 8 | 2.34 ± 0.07 aA | 1.28 ± 0.14 cA | 1.70 ± 0.26 bA | 1.34 ± 0.22 cA | 0.80 ± 0.07 dAB | 0.84 ± 0.02 dAB |
| p F-test | ||||||
| Rate | <0.001 | |||||
| Residue | <0.001 | |||||
| Interaction | <0.001 | |||||
| Cu (mg kg−1) | ||||||
| 0 | 2.06 ± 0.07 aC | 2.06 ± 0.07 aA | 2.06 ± 0.07 aD | 2.06 ± 0.07 aB | 2.06 ± 0.07 aA | 2.06 ± 0.07 aAB |
| 1 | 2.06 ± 0.04 bC | 1.93 ± 0.03 cC | 2.53 ± 0.10 aC | 2.16 ± 0.11 bB | 2.11 ± 0.06 bA | 2.11 ± 0.05 bA |
| 2 | 2.14 ± 0.04 cBC | 2.04 ± 0.05 cAB | 2.63 ± 0.06 aBC | 2.32 ± 0.32 bA | 2.06 ± 0.06 cA | 2.09 ± 0.03 cA |
| 4 | 2.21 ± 0.02 bAB | 2.03 ± 0.06 cdAB | 2.91 ± 0.01 aA | 2.10 ± 0.03 bcB | 1.93 ± 0.03 dB | 2.06 ± 0.07 cAB |
| 8 | 2.30 ± 0.06 bA | 1.99 ± 0.05 cdAB | 2.71 ± 0.06 aB | 2.08 ± 0.08 cB | 1.90 ± 0.05 dB | 1.96 ± 0.04 cdB |
| p F-test | ||||||
| Rate | <0.001 | |||||
| Residue | <0.001 | |||||
| Interaction | <0.001 | |||||
| Zn (mg kg−1) | ||||||
| 0 | 0.75 ± 0.03 aE | 0.75 ± 0.03 aC | 0.75 ± 0.03 aE | 0.75 ± 0.03 aE | 0.75 ± 0.03 aE | 0.75 ± 0.03 aE |
| 1 | 0.87 ± 0.02 bD | 0.68 ± 0.03 eD | 0.80 ± 0.04 dD | 0.82 ± 0.04 cdD | 0.86 ± 0.02 bcD | 0.93 ± 0.02 aD |
| 2 | 1.03 ± 0.03 bC | 0.77 ± 0.00 eC | 0.88 ± 0.02 dC | 0.91 ± 0.02 cdC | 0.93 ± 0.01 cC | 1.09 ± 0.02 aC |
| 4 | 1.30 ± 0.07 aB | 0.90 ± 0.01 eB | 0.97 ± 0.02 dB | 1.11 ± 0.02 bB | 1.02 ± 0.02 cB | 1.31 ± 0.02 aB |
| 8 | 1.72 ± 0.03 aA | 1.12 ± 0.04 dA | 1.07 ± 0.00 eA | 1.41 ± 0.03 bA | 1.22 ± 0.01 cA | 1.75 ± 0.03 aA |
| p F-test | ||||||
| Rate | <0.001 | |||||
| Residue | <0.001 | |||||
| Interaction | <0.001 | |||||
| Fe (mg kg−1) | ||||||
| 0 | 13.7 ± 0.6 aA | 13.7 ± 0.6 aA | 13.7 ± 0.6 aC | 13.7 ± 0.6 aAB | 13.7 ± 0.6 aB | 13.7 ± 0.6 aC |
| 1 | 10.7 ± 0.3 bB | 12.1 ± 0.9 bB | 15.2 ± 0.4 aB | 14.3 ± 1.2 aAB | 15.3 ± 1.4 aA | 15.6 ± 0.6 aAB |
| 2 | 10.7 ± 0.4 dB | 13.7 ± 1.5 cA | 15.8 ± 0.4 abB | 15.1 ± 0.8 bcA | 15.5 ± 0.5 abA | 16.7 ± 0.7 aA |
| 4 | 9.9 ± 0.8 dB | 13.7 ± 1.6 cA | 17.3 ± 0.8 aA | 9.9 ± 0.0 dC | 15.2 ± 0.9 bA | 15.0 ± 0.7 bcB |
| 8 | 10.0 ± 1.3 dB | 12.0 ± 2.4 bcB | 17.7 ± 1.0 aA | 13.1 ± 0.1 bB | 10.8 ± 1.3 cdC | 10.2 ± 0.7 dD |
| p F-test | ||||||
| Rate | <0.001 | |||||
| Residue | <0.001 | |||||
| Interaction | <0.001 | |||||
| Mn (mg kg−1) | ||||||
| 0 | 15.8 ± 1.1 aD | 15.8 ± 1.1 aD | 15.8 ± 1.1 aD | 15.8 ± 1.1 aE | 15.8 ± 1.1 aE | 15.8 ± 1.1 aD |
| 1 | 35.7 ± 2.6 cCD | 38.2 ± 2.0 cCD | 45.9 ± 3.8 cCD | 300 ± 7.3 aD | 96 ± 1.6 bD | 47 ± 5.6 cD |
| 2 | 51.4 ± 7.2 cC | 67.0 ± 5.8 cC | 60.9 ± 1.5 cC | 407 ± 1.0 aC | 207 ± 15 bC | 183 ± 15.5 bC |
| 4 | 85.0 ± 15.7 eB | 193 ± 14 dB | 209 ± 23 dB | 463 ± 33 aB | 370 ± 61 bB | 331 ± 37.2 cB |
| 8 | 154 ± 5.2 eA | 376 ± 29 dA | 413 ± 23 cA | 546 ± 40 aA | 426 ± 18 bcA | 455 ± 31.5 bA |
| p F-test | ||||||
| Rate | <0.001 | |||||
| Residue | <0.001 | |||||
| Interaction | <0.001 | |||||
| Rate (%) | Species | |||||
|---|---|---|---|---|---|---|
| Helichrysum | Lavender | Rosemary | ||||
| B (mg kg−1) | ||||||
| 0 | 0.55 ± 0.06 aD* | 0.55 ± 0.06 aD | 0.55 ± 0.06 aC | |||
| 1 | 0.67 ± 0.01 aC | 0.59 ± 0.07 abCD | 0.54 ± 0.05 bC | |||
| 2 | 0.71 ± 0.03 aC | 0.67 ± 0.04 aC | 0.61 ± 0.11 aBC | |||
| 4 | 0.90 ± 0.04 aB | 0.94 ± 0.10 aB | 0.68 ± 0.11 bB | |||
| 8 | 1.22 ± 0.04 aA | 1.09 ± 0.12 bA | 0.85 ± 0.05 cA | |||
| p F-test | ||||||
| Rate | <0.001 | |||||
| Residue | <0.001 | |||||
| Interaction | 0.004 | |||||
| Species | ||||||
| Helichrysum | Lavender | Rosemary | Helichrysum | Lavender | Rosemary | |
| Cu (mg kg−1) | Zn (mg kg−1) | |||||
| 0 | 2.06 ± 0.07 aA | 2.06 ± 0.07 aB | 2.06 ± 0.07 aB | 0.75 ± 0.03 aC | 0.75 ± 0.03 aC | 0.75 ± 0.03 aE |
| 1 | 2.05 ± 0.00 bA | 2.35 ± 0.14 aA | 2.14 ± 0.06 bAB | 0.75 ± 0.04 bC | 0.80 ± 0.03 bBC | 0.87 ± 0.06 aD |
| 2 | 2.08 ± 0.09 bA | 2.31 ± 0.25 aA | 2.16 ± 0.04 abAB | 0.77 ± 0.01 bC | 0.80 ± 0.06 bBC | 0.93 ± 0.01 aC |
| 4 | 2.08 ± 0.03 aA | 2.12 ± 0.10 aB | 2.21 ± 0.07 aAB | 0.84 ± 0.02 bB | 0.84 ± 0.03 bB | 1.06 ± 0.03 aB |
| 8 | 2.06 ± 0.06 bA | 2.13 ± 0.10 abB | 2.22 ± 0.05 aA | 0.99 ± 0.06 bA | 0.94 ± 0.00 bA | 1.29 ± 0.04 aA |
| p F-test | p F-test | |||||
| Rate | NS # | <0.001 | ||||
| Residue | 0.004 | <0.001 | ||||
| Interaction | NS | <0.001 | ||||
| Fe (mg kg−1) | Mn (mg kg−1) | |||||
| 0 | 13.7 ± 0.6 aAB | 13.7 ± 0.6 aB | 13.7 ± 0.6 aC | 15.8 ± 1.1 aD | 15.8 ± 1.1 aD | 15.8 ± 1.1 aD |
| 1 | 12.7 ± 0.8 bB | 14.0 ± 0.7 abB | 14.3 ± 0.8 aBC | 34.1 ± 0.9 aD | 31.7 ± 1.7 aCD | 25.9 ± 0.3 aD |
| 2 | 14.1 ± 1.1 bA | 16.0 ± 1.2 aA | 15.3 ± 0.7 abAB | 78.6 ± 15.3 aC | 62.0 ± 6.2 aC | 80.2 ± 1.6 aC |
| 4 | 14.4 ± 0.7 bA | 14.9 ± 0.5 abAB | 15.9 ± 0.6 aA | 175 ± 35 bB | 259 ± 36 aB | 123 ± 5 cB |
| 8 | 14.9 ± 1.6 abA | 14.1 ± 0.3 bB | 16.2 ± 0.9 aA | 340 ± 40 bA | 389 ± 35 aA | 189 ± 21 cA |
| p F-test | p F-test | |||||
| Rate | <0.001 | <0.001 | ||||
| Residue | 0.004 | <0.001 | ||||
| Interaction | NS | <0.001 | ||||
References
- Elguea-Culebras, G.O.; Bravo, E.M.; Sanchez-Vioque, R. Potential sources and methodologies for the recovery of phenolic compounds from distillation residues of Mediterranean aromatic plants. An approach to the valuation of by-products of the essential oil market—A review. Ind. Crops Prod. 2022, 175, 114261. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Choulitoudi, E.; Bimpilas, A.; Mitropoulou, G.; Kourkoutas, Y.; Oreopoulou, V. Exploitation of the biological potential of Satureja thymbra essential oil and distillation by-products. J. Appl. Res. Med. Aromat. Plants 2017, 4, 12–20. [Google Scholar] [CrossRef]
- Greff, B.; Lakatos, E.; Szigeti, J.; Varga, L. Co-composting with herbal wastes: Potential effects of essential oil residues on microbial pathogen during composting. Crit. Rev. Environ. Sci. Technol. 2020, 51, 457–511. [Google Scholar] [CrossRef]
- Saha, A.; Basak, B.B. Scope of value addition and utilization of residual biomass from medicinal and aromatic plants. Ind. Crops Prod. 2020, 145, 111979. [Google Scholar] [CrossRef]
- Zaccardelli, M.; Roscigno, G.; Pane, C.; Celano, G.; Di Matteo, M.; Mainente, M.; Vuotto, A.; Mencherine, T.; Esposito, T.; Vitti, A.; et al. Essential oil and quality composts sourced by recycling vegetable residues from aromatic plant supply chain. Ind. Crops Prod. 2021, 162, 113255. [Google Scholar] [CrossRef]
- Christaki, S.; Bouloumpasi, E.; Lalidou, E.; Chatzopoulou, P.; Irakli, M. Bioactive Profile of Distilled Solid By-Products of Rosemary, Greek Sage and Spearmint as Affected by Distillation Methods. Molecules 2022, 27, 9058. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Bou, M.; Sigoillot, J.C.; Faulds, C.B.; Lomascolo, A. Essential oils and distilled straws of lavender and lavandin: A review of current use and potential application in white biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3375–3385. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Tzortzakis, N. Residues from medicinal and aromatic plants after distillation can be used in replace some peat in the growing media for Viola X wittrockiana Production. Agronomy 2024, 14, 187. [Google Scholar] [CrossRef]
- Singh, D.; Suthar, S. Vermicomposting of herbal pharmaceutical industry solid wastes. Ecol. Eng. 2012, 39, 1–6. [Google Scholar] [CrossRef]
- United Nations; Department of Economics and Social Affairs; Population Division. Population 2030: Demographic Challenges and Opportunities for Sustainable Development Planning; ST/ESA/SER/389; United Nations: New York, NY, USA, 2015. [Google Scholar]
- European Commission. Closing the Loop—An EU Action Plan for the Circular Economy COM; European Commission: Brussels, Belgium, 2015; p. 614. [Google Scholar]
- Abdelraouf, E.A.; Nassar, I.N.; Gomma, I.; Aboukila, E.F. Valorization of Cheese Whey as a fertilizer: Effects on Maize Germination and Growth in Clay Loam and Calcareous Soil. Egypt. J. Soil Sci. 2023, 63, 489–502. [Google Scholar] [CrossRef]
- Ccacyancco-Caceres, E.; Sarmiento-Sarmiento, G.; Mena-Chacon, L. Use of processed grape pomace and whey bio ferment to improve the agronomic performance of radish (Raphanus sativus L.) in arid soils. Rev. Fac. Nac. Agron. 2024, 77, 10707–10715. [Google Scholar] [CrossRef]
- Balkrishna, A.; Srivastava, S.; Srivastava, D.; Sharma, N.; Arya, V.; Gautam, A.K. Unleashing the potential of medicinal and aromatic plant wastes with particular consideration of vermicompost: A comprehensive review of literature. J. Appl. Res. Med. Aromat. Plants 2024, 39, 100527. [Google Scholar] [CrossRef]
- Nigam, N.; Shanker, K.; Khare, P. Valorisation of residue of mentha arvensis by pyrolysis: Evaluation of agronomic and environmental benefits. Waste Biomass Valorization 2018, 9, 1909–1919. [Google Scholar] [CrossRef]
- Zhou, Y.; Selvam, A.; Wong, J.W.C. Effect of Chinese medicinal herbal residues on microbial community succession and anti-pathogenic properties during co-composting with food waste. Bioresour. Technol. 2016, 217, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Greff, B.; Szigeti, J.; Varga, A.; Lakatos, E.; Saho, A.; Varga, L. Effect of bacterial inoculation on co-composting of lavender (Lavandula angustifolia Mill.) waste and cattle manure. 3 Biotech 2021, 11, 306. [Google Scholar] [CrossRef]
- Deka, H.; Deka, S.; Baruah, C.K.; Das, J.; Hoque, S.; Sarma, H.; Sarma, N.S. Vermicomposting potentiality of Perionyx excavatus for recycling of waste biomass of java citronella—An aromatic oil yielding plant. Bioresour. Technol. 2011, 102, 11212–11217. [Google Scholar] [CrossRef]
- Haghighi, M.; Afsharikia, A.; Mozafariyan, M.; Pessarakli, M.; Bolandnazar, A. Usage of Herbal (Thyme and Chicory) Waste as an organic Substrate in Cucumber Production. Commun. Soil Sci. Plant Anal. 2014, 45, 2607–2619. [Google Scholar] [CrossRef]
- Bouloumpasi, E.; Hatzikamari, M.; Christaki, S.; Lazaridou, A.; Chatzopoulou, P.; Biliaderis, C.G.; Irakli, M. Assessment of Antioxidant and Antibacterial Potential of Phenolic Extracts from Post-Distillation Solid Residues of Oregano, Rosemary, Sage, Lemon Balm, and Spearmint. Processes 2024, 12, 14. [Google Scholar] [CrossRef]
- Chrysargyri, A.; Goumenos, C.; Tzortzakis, N. Use of medicinal and aromatic plants for partial peat substitution on growing media for Sonchus oleraceus production. Agronomy 2023, 13, 1074. [Google Scholar] [CrossRef]
- Wang, B.; Verheyen, K.; Baeten, L.; Smedt, P.D. Herb litter mediates tree litter decomposition and soil fauna composition. Soil Biol. Biochem. 2021, 152, 108063. [Google Scholar] [CrossRef]
- Hassiotis, C. Chemical compounds and essential oil release through decomposition process from Lavandula stoechas in Mediterranean region. Biochem. Syst. Ecol. 2010, 38, 493–501. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular plants of greece: An annotated checklist. Supplement. Willdenowia 2016, 46, 301–347. [Google Scholar] [CrossRef]
- Greek Payment Authority of Common Agricultural Policy (C.A.P.). Available online: https://www.opekepe.gr/ (accessed on 28 July 2025).
- Chand, S.; Anwar, M.; Patra, D.D.; Khanuja, S.P.S. Effect of mint distillation waste on soil microbial biomass in a mint-mustard cropping sequence. Commun. Soil Sci. Plant Anal. 2004, 35, 243–254. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 14th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Karagianni, A.G.; Paraschou, A.; Matsi, T. Solid residues of medicinal and aromatic plants distillation as amendments for acids soils. J. Soil Sci. Plant Nutr. 2025; under review. [Google Scholar]
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved salts. In Methods of Soil Analysis, Part 3: Chemical Methods; SSSA Book Series 5; Sparks, D.L., Ed.; Soil Science Society of America, American Society: Madison, WI, USA, 1996; pp. 417–435. [Google Scholar] [CrossRef]
- ISO 23470; Soil Quality—Determination of Effective Cation Exchange Capacity (C.E.C.) and Exchangeable Cations Using a Hexamminecobalt Trichloride Solution. International Organization for Standardization: Geneva, Switzerland, 2007.
- Walkley, A.J.; Black, I.A. Estimation of soil organic carbon by the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-Total. In Methods of Soil Analysis, Part 3: Chemical Methods; SSSA Book Series 5; Sparks, D.L., Ed.; Soil Science Society of America, America Society of Agronomy: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Mulvaney, R.L. Nitrogen—Inorganic forms. In Methods of Soil Analysis, Part 3: Chemical Methods; SSSA Book Series 5; Sparks, D.L., Ed.; Soil Scinece Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar] [CrossRef]
- Kuo, S. Phosphorus. In Methods of Soil Analysis, Part 3: Chemical Methods; SSSA Book Series 5; Sparks, D.L., Ed.; Soil Science Society of America, America Society of Agronomy: Madison, WI, USA, 1996; pp. 869–919. [Google Scholar] [CrossRef]
- Thomas, G.W. Exchangeable cations. In Agronomy Monographs; Page, A.L., Ed.; America Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 159–165. [Google Scholar] [CrossRef]
- Keren, R. Boron. In Methods of Soil Analysis, Part 3: Chemical Methods; SSSA Book Series 5; Sparks, D.L., Ed.; Soil Science Society of America, America Society of Agronomy: Madison, WI, USA, 1996; pp. 603–626. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA test for zinc, iron, manganese and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Tinsley, J.; Taylor, T.G.; Moore, J.H. The determination of carbon dioxide derived from carbonates in agricultural and biological materials. Analyst 1951, 76, 300–310. [Google Scholar] [CrossRef]
- Horwath, W.R.; Paul, E.A. Microbial Biomass. In Methods of Soil Analysis, Part 2: Microbiological and Biochemical Properties; Weaver, R.W., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 753–773. [Google Scholar] [CrossRef]
- Dahnke, W.C.; Johnson, G.V. Testing soils for available nitrogen. In Soil Testing and Plant Analysis for Available Nitrogen; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1990; pp. 128–139. [Google Scholar]
- Thomas, G.W.; Peaslee, D.E. Testing soils for phosphorus. In Soil Testing and Plant Analysis; Walsh, L.M., Beaton, J.D., Eds.; Soil Science Society of America: Madison, WI, USA, 1973; pp. 115–132. [Google Scholar]
- Haby, Y.A.; Russelle, M.P.; Skogley, E.O. Testing soils for potassium, calcium, and magnesium. In Soil Testing and Plant —Analysis; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1990; pp. 181–227. [Google Scholar]
- Sims, J.T.; Johnson, G.V. Micronutrient soil tests. In Micronutrients in Agriculture, 2nd ed.; Mortvedt, J.J., Ed.; Soil Science Society of America: Madison, WI, USA, 1991; pp. 427–476. [Google Scholar] [CrossRef]
- Furlan, V.; Bren, U. Helichrysum italicum: From extraction, distillation, and encapsulation techniques to beneficial health effects. Foods 2023, 12, 802. [Google Scholar] [CrossRef]
- Prusinowska, R.; Smigielski, K.B. Composition, biological properties and therapeutics effects of lavender (Lavandula angustifolia L.). A review. Herba Pol. 2014, 60, 0010. [Google Scholar] [CrossRef]
- Azim, K.; Soudi, B.; Boukhara, S.; Perissol, C.; Roussos, S.; Thami Alami, I. Composting parameters and compost quality: A literature review. Org. Agric. 2017, 8, 141–158. [Google Scholar] [CrossRef]
- Sloot, V.D.M.; Kleijn, D.; De Deyn, G.B.; Limpens, J. Carbon to nitrogen ratio and quantity of organic amendment interactively affect crop growth and soil mineral N retention. Crop. Environ. 2022, 1, 161–167. [Google Scholar] [CrossRef]
- Zhou, Y.; Manu, M.K.; Li, D.; Johnravindar, D.; Selvam, A.; Varjani, S.; Wong, J. Effect of Chinese medicinal herbal residues compost on tomato and Chinese cabbage plants: Assessment on phytopathogenic effect and nutrients uptake. Environ. Res. 2023, 216, 114747. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.B.; Basak, B.B.; Patel, V.J.; Senapati, N.; Ramani, V.P.; Gajbhiye, N.A.; Kalola, A.D. Enriched soil amendments influenced soil fertility, herbage yield and bioactive principle of medicinal plant (Cassia angustifolia Vahl.) grown in two different soils. Heliyon 2024, 10, e24874. [Google Scholar] [CrossRef]
- Kadoglidou, K.; Chalkos, D.; Karamanoli, K.; Eleftherohorinos, I.G.; Constantinidou, H.I.A.; Vokou, D. Aromatic plants as soil amendments: Effect of spearmint and sage on soil properties, growth and physiology of tomato seedlings. Sci. Hortic. 2014, 179, 25–35. [Google Scholar] [CrossRef]
- Chouliaras, N.; Gravanis, F.; Vasilakoglou, I.; Gougoulias, N.; Vagelas, I.; Kapotis, T.; Wogiatzi, E. The effect of basil (Ocimum basilicum L.) on soil organic matter biodegradation and other soil chemical properties. J. Sci. Food Agric. 2007, 87, 2416–2419. [Google Scholar] [CrossRef]
- Buchmann, C.; Korz, S.; Moraru, A.; Richling, E.; Sadzik, S.; Scharfenberger-Schmeer, M.; Munoz, K. From winery by-product to soil improver?- A comprehensive review of grape pomace in agriculture and its effects on soil properties and functions. Sci. Total Environ. 2025, 982, 179611. [Google Scholar] [CrossRef]
- Zhu, H.; Pan, J.; Wei, Y.; Lan, H.; Yang, S.; Li, X.; Tang, X. Manganese toxicity suppressing nitrogen-fixing bacteria growth and impairing nitrogen uptake and utilization in sugarcane. Front. Microbiol. 2025, 16, 1548896. [Google Scholar] [CrossRef]
- Saha, A.; Basak, B.B.; Banerjee, A. In-vitro antioxidant evaluation and production of biochar from distillation waste biomass of Mentha arvensis. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100428. [Google Scholar] [CrossRef]
- Santana-Meridas, O.; Polissiou, M.; Izquierdo-Melero, M.E.; Astraka, K.; Tarantilis, P.A.; Herraiz-Penalver, D.; Sanchez-Vioque, R. Polyphenol composition, antioxidant and bioplaguicide activities of the solid residue from hydrodistillation of Rosmarinus officinalis L. Ind. Crops Prod. 2014, 59, 125–134. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karagianni, A.-G.; Paraschou, A.; Matsi, T. A Preliminary Evaluation of the Use of Solid Residues from the Distillation of Medicinal and Aromatic Plants as Fertilizers in Mediterranean Soils. Agronomy 2025, 15, 1903. https://doi.org/10.3390/agronomy15081903
Karagianni A-G, Paraschou A, Matsi T. A Preliminary Evaluation of the Use of Solid Residues from the Distillation of Medicinal and Aromatic Plants as Fertilizers in Mediterranean Soils. Agronomy. 2025; 15(8):1903. https://doi.org/10.3390/agronomy15081903
Chicago/Turabian StyleKaragianni, Anastasia-Garyfallia, Anastasia Paraschou, and Theodora Matsi. 2025. "A Preliminary Evaluation of the Use of Solid Residues from the Distillation of Medicinal and Aromatic Plants as Fertilizers in Mediterranean Soils" Agronomy 15, no. 8: 1903. https://doi.org/10.3390/agronomy15081903
APA StyleKaragianni, A.-G., Paraschou, A., & Matsi, T. (2025). A Preliminary Evaluation of the Use of Solid Residues from the Distillation of Medicinal and Aromatic Plants as Fertilizers in Mediterranean Soils. Agronomy, 15(8), 1903. https://doi.org/10.3390/agronomy15081903






