Supplementation of Calcium Through Seed Enrichment Technique Enhances Germinability and Early Growth of Timothy (Phleum pratense L.) Under Salinity Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Calcium Application Through Seed Enrichment
2.3. Germination Tests
2.4. Measurement of the Catalase Activity and Water-Soluble Sugar Content of the Seeds
2.5. Plant Growth Test Under Salt Stress Conditions
2.6. Statistical Analysis
3. Results
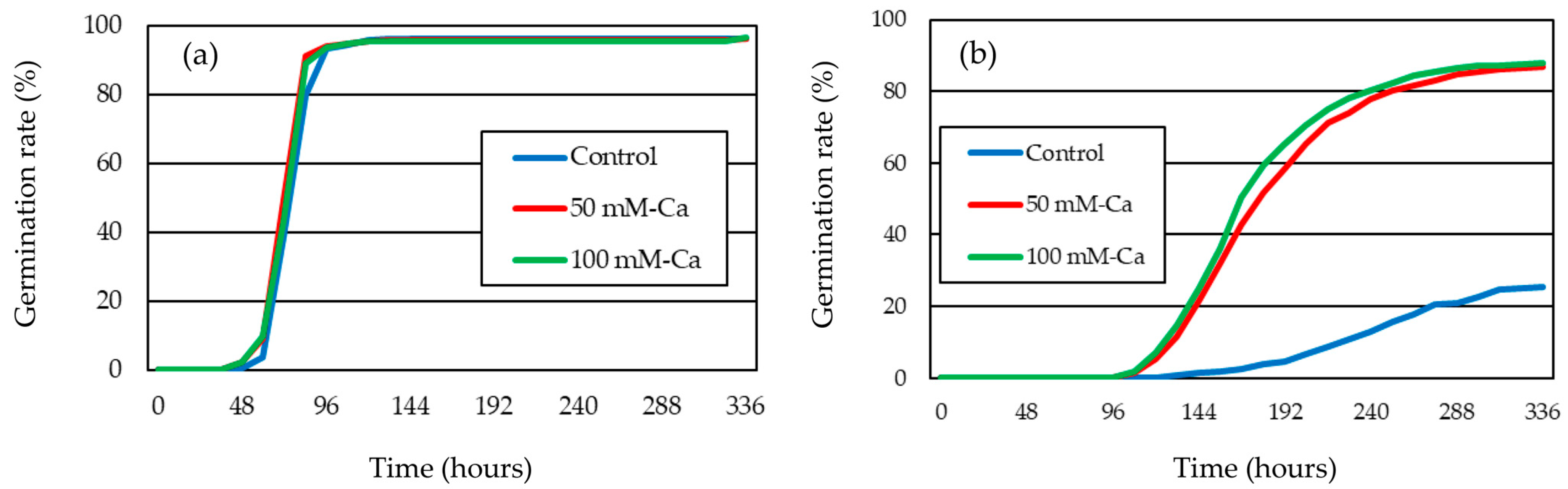
3.1. Germinability of Timothy Under Salt Stress
3.2. CAT Activity and Soluble Sugar Content of the Seeds
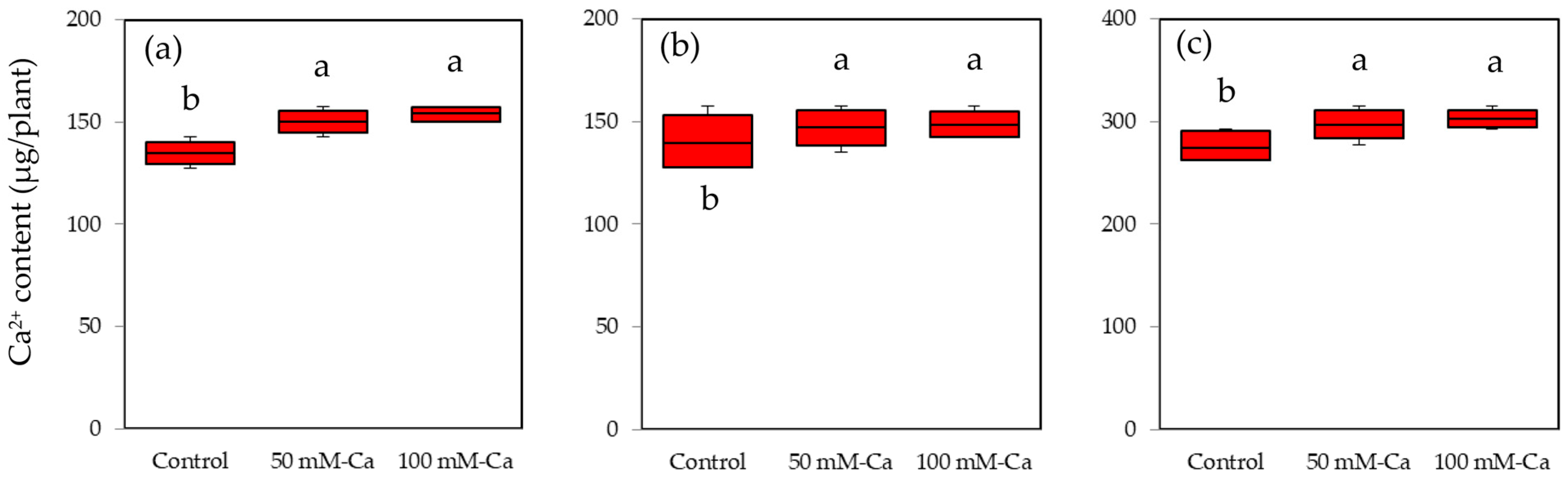
3.3. Calcium Content of the Plants
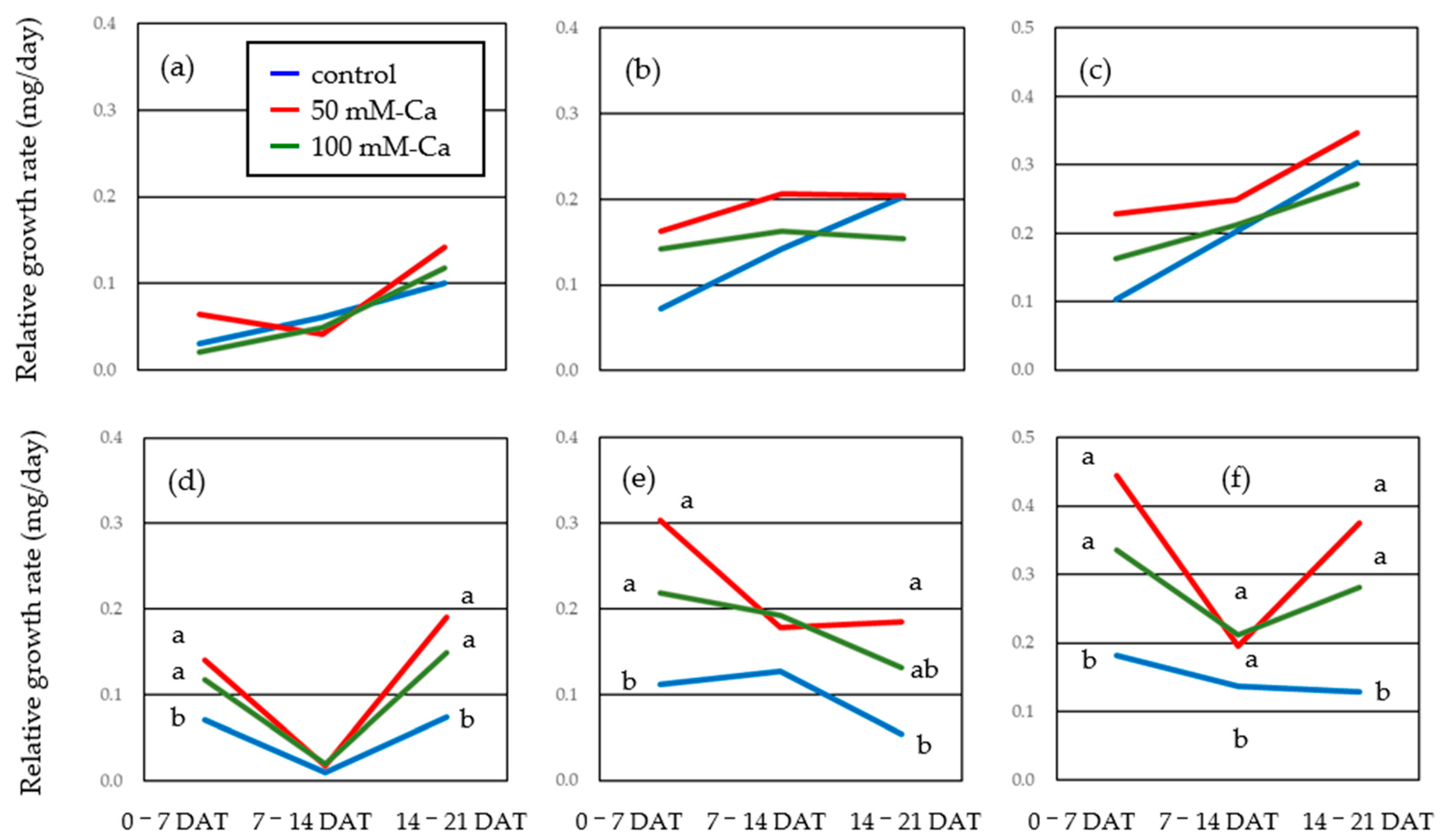
3.4. Plant Growth Under Salt Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moser, L.E.; Hoveland, C.S. Cool-season grass overview. In Cool-Season Forage Grasses; Wiley: Hoboken, HJ, USA, 2015; pp. 1–14. [Google Scholar]
- Schrauf, G.E.; Alonso Nogara, F.; Rush, P.; Roa, P.P.; Musacchio, E.; Ghio, S.; Couso, L.; Ramos, E.; Schrauf, M.F.; Voda, L.; et al. Genetic improvement of perennial forage plants for salt tolerance. In Saline and Alkaline Soils in Latin America: Natural Resources, Management and Productive Alternatives; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 399–414. [Google Scholar]
- Saito, T.; Matsukura, C.; Ban, Y.; Shoji, K.; Sugiyama, M.; Fukuda, N.; Nishimura, S. Salinity Stress Affects Assimilate Metabolism at the Gene-expression Level during Fruit Development and Improves Fruit Quality in Tomato (Solanum lycopersicum L.). J. Jpn. Soc. Hortic. Sci. 2008, 77, 61–68. [Google Scholar] [CrossRef]
- Chairawiwut, W.; McMartin, D.W.; Azam, S. Salts removal from synthetic solution-potash brine by non-planted constructed wetlands. Water 2016, 8, 113. [Google Scholar] [CrossRef]
- Hou, M.; Zhu, L.; Jin, Q. Surface drainage and mulching drip-irrigated tomatoes reduces soil salinity and improves fruit yield. PLoS ONE 2016, 11, e0154799. [Google Scholar] [CrossRef]
- Santos, E.S.; Salazar, M.; Mendes, S.; Lopes, M.; Pacheco, J.; Marques, D. Rehabilitation of abandoned areas from a Mediterranean nature reserve by Salicornia crop: Influence of the salinity and shading. Arid. Land. Res. Manag. 2017, 31, 29–45. [Google Scholar] [CrossRef]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H.M. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sust. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Roy, S.J.; Negrão, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biot. 2014, 26, 115–124. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, P.; Sun, P.; Wei, L.; Chen, G.; Wu, D. Interactive salt—Alkali stress and exogenous Ca2+ effects on growth and osmotic adjustment of Lolium multiflorum in a coastal estuary. Flora Morphol. Distrib. Func. Ecol. Plants 2017, 229, 92–99. [Google Scholar] [CrossRef]
- Javadi, A.; Khomari, S.; Sofalian, O. Seed vigor and boron and calcium nutrition influence oilseed rape germinability and seedling growth under salt stress. J. Plant Nutr. 2016, 39, 1688–1696. [Google Scholar] [CrossRef]
- Joshi, S.V.; Patel, N.T.; Pandey, I.B.; Pandey, A.N. Effect of supplemental Ca 2+ on NaCl− stressed castor plants (Ricinus communis L.). Acta Bota. Croa. 2012, 71, 13–29. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, X.; Zhang, Y.; Xuan, Y.; Yan, Y. Effects of salt stress and exogenous Ca2+ on Na+ compartmentalization, ion pump activities of tonoplast and plasma membrane in Nitraria tangutorum Bobr. leaves. Acta Phys. Plant. 2014, 36, 2183–2193. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Rahman, A.; Alam, M.; Mahmud, J.A.; Suzuki, T.; Fujita, M. Polyamines confer salt tolerance in mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and Methylglyoxal detoxification systems. Front. Plant Sci. 2016, 7, 1104. [Google Scholar] [CrossRef]
- Paul, S.; Roychoudhury, A. Effect of seed priming with spermine/spermidine on transcriptional regulation of stress-responsive genes in salt-stressed seedlings of an aromatic rice cultivar. Plant Gene 2017, 11, 133–142. [Google Scholar] [CrossRef]
- Seckin, B.; Sekmen, A.H.; Türkan, I. An enhancing effect of exogenous mannitol on the antioxidant enzyme activities in roots of wheat under salt stress. J. Plant Growth Regu. 2009, 28, 12–20. [Google Scholar] [CrossRef]
- Maeda, Y.; Nakazawa, R. Effects of the timing of calcium application on the alleviation of salt stress in the maize, tall fescue, and reed canarygrass seedlings. Biol. Plant. 2008, 52, 153–156. [Google Scholar] [CrossRef]
- Salahshoor, F.; Kazemi, F. Effect of calcium on reducing salt stress in seed germination and early growth stage of Festuca ovina L. Plant Soil Environ. 2016, 62, 460–466. [Google Scholar] [CrossRef]
- Wilkins, K.A.; Matthus, E.; Swarbreck, S.M.; Davies, J.M. Calcium-mediated abiotic stress signaling in roots. Front. Plant Sci. 2016, 7, 1296. [Google Scholar] [CrossRef]
- Yang, S.; Hou, L.L.; Guo, F.; Zhang, J.L.; Geng, Y.; Meng, J.J.; Li, X.G.; Wan, S.B. Effects of exogenous Ca2+ on growth and development, physiology and yield of peanut under salt stress. Chin. J. Appl. Ecol. 2017, 28, 894–900. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, R.; Guo, Q.; Gu, Z. NaCl stress and supplemental CaCl2 regulating GABA metabolism pathways in germinating soybean. Euro. Food Res. Technol. 2014, 238, 781–788. [Google Scholar] [CrossRef]
- Farooq, M.; Usman, M.; Nadeem, F.; Rehman, H.U.; Wahid, A.; Basra, S.M.A.; Siddique, K.H.M. Seed priming in field crops: Potential benefits, adoption and challenges. Crop Past. Sci. 2019, 70, 731–771. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Siddique, K.H.M. Micronutrient application through seed treatments—A review. J. Soil. Sci. Plant Nutr. 2012, 12, 125–142. [Google Scholar] [CrossRef]
- Johnson, S.E.; Lauren, J.G.; Welch, R.M.; Duxbury, J.M. A comparison of the effects of micronutrient seed priming and soil fertilization on the mineral nutrition of chickpea (Cicer arietinum), lentil (Lens culinaris), rice (Oryza sativa) and wheat (Triticum aestivum) in Nepal. Expe. Agri. 2005, 41, 427–448. [Google Scholar] [CrossRef]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A. Selenium (Se) seed priming induced growth and biochemical changes in wheat under water deficit conditions. Biol. Trace Elem. Res. 2013, 151, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Rai-Kalal, P.; Jajoo, A. Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Phys. Bioch. 2021, 160, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, N.; Yano, K. Seed P-enrichment as an effective P supply to wheat. Plant Soil. 2010, 327, 347–354. [Google Scholar] [CrossRef]
- Shah, A.R.; Ara, N.; Shafi, G. Seed priming with phosphorus increased germination and yield of okra. Afr. J. Agric. Res. 2011, 6, 3859–3876. [Google Scholar]
- Teixeira, W.T.; Fagan, E.B.; Soares, L.H.; Umburanas, R.C.; Reichardt, K.; Neto, D.D. Foliar and seed application of amino acids affects the antioxidant metabolism of the soybean crop. Front. Plant Sci. 2017, 8, 327. [Google Scholar] [CrossRef]
- Afzal, I.; Basra, S.M.A.; Ahmad, N.; Cheema, M.A.; Haq, M.A.; Kazmi, M.H.; Irfan, S. Enhancement of antioxidant defense system induced by hormonal priming in wheat. Cereal Res. Commun. 2011, 39, 334–342. [Google Scholar] [CrossRef]
- Aymen, E.M. Seed priming with plant growth regulators to improve crop abiotic stress tolerance. In Advances in Seed Priming; Springer: Berlin/Heidelberg, Germany, 2018; pp. 95–106. [Google Scholar]
- Farooq, M.; Aziz, T.; Basra, S.M.A.; Cheema, M.A.; Rehman, H. Chilling tolerance in hybrid maize induced by seed priming with salicylic acid. J. Agron. Crop Sci. 2008, 194, 161–168. [Google Scholar] [CrossRef]
- Korkmaz, A. Inclusion of acetyl salicylic acid and methyl jasmonate into the priming solution improves low-temperature germination and emergence of sweet pepper. HortScience 2005, 40, 197–200. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Daly, P.; Sharma, A.; Shaghaleh, H.; Hamoud, Y.A.; El-Esawi, M.A.; Pan, R.; Wan, Q.; et al. Seed priming and foliar application with jasmonic acid enhance salinity stress tolerance of soybean (Glycine max L.) seedlings. J. Sci. Food Agri. 2021, 101, 2027–2041. [Google Scholar] [CrossRef]
- Yaqoob, H.; Akram, N.A.; Iftikhar, S.; Ashraf, M.; Khalid, N.; Sadiq, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Seed pretreatment and foliar application of proline regulate morphological, physio-biochemical processes and activity of antioxidant enzymes in plants of two cultivars of quinoa (Chenopodium quinoa willd.). Plants 2019, 8, 588. [Google Scholar] [CrossRef]
- Sghayar, S.; Debez, A.; Lucchini, G.; Abruzzese, A.; Zorrig, W.; Negrini, N.; Morgutti, S.; Abdelly, C.; Sacchi, G.A.; Pecchioni, N. Seed priming mitigates high salinity impact on germination of bread wheat (Triticum aestivum L.) by improving carbohydrate and protein mobilization. Plant Direct 2023, 7, e497. [Google Scholar] [CrossRef]
- Youssef, R.B.; Jelali, N.; Boukari, N.; Albacete, A.; Martinez, C.; Alfocea, F.P.; Abdelly, C. The efficiency of different priming agents for improving germination and early seedling growth of local Tunisian barley under salinity stress. Plants 2021, 10, 2264. [Google Scholar] [CrossRef] [PubMed]
- Maaroufi-Dguimi, H.; Mohammed, S.G.; Abdalgadir, H.; Omari Al Zahrani, F. Effects of chemical seed priming on germination performance and seedling growth of Lycopersicon esculentum (Mill.) under salt stress. Agron. Res. 2024, 22, 672–684. [Google Scholar] [CrossRef]
- Harrison, J.; Huhtanen, P.; Collins, M. Perennial grasses. In Silage Science and Technology; Wiley: Hoboken, HJ, USA, 2015; pp. 665–747. [Google Scholar]
- Berg, C.C.; McElroy, A.R.; Kunelius, H.T. Timothy. In Cool-Season Forage Grasses; Wiley: Hoboken, HJ, USA, 2015; pp. 643–664. [Google Scholar]
- Israelsen, K.R.; Ransom, C.V.; Waldron, B.L. Salinity tolerance of foxtail barley (Hordeum jubatum) and desirable pasture grasses. Weed Sci. 2011, 59, 500–505. [Google Scholar] [CrossRef]
- Soliman, W.S.; Sugiyama, S.I.; Abbas, A.M. Contribution of avoidance and tolerance strategies towards salinity stress resistance in eight C3 turfgrass species. Hortic. Environ. Biotechnol. 2018, 59, 29–36. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, P.; Chen, G.; Pan, L.; Li, J.; Dong, L. Environmental factors on seed germination and seedling emergence of Phleum paniculatum Huds. Chile. J. Agri. Res. 2018, 78, 370–377. [Google Scholar] [CrossRef]
- Maeda, Y.; Ukai, H.; Takenaga, H. Salt tolerance of five temperate grass at germination and different growth stages. J. Jap. Soc. Reveg. Technol. 2001, 27, 499–506. [Google Scholar] [CrossRef][Green Version]
- Dodd, J.L.; Lauenroth, W.K. Analysis of the response of grassland ecosystem to stress. In Perspectives in Grassland Ecology; French, N., Ed.; Springer: New York, NY, USA, 1979; pp. 43–58. [Google Scholar]
- Kader, M.A. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. R. Soc. New South Wales 2005, 138, 65–75. [Google Scholar] [CrossRef]
- Ranal, M.A.; De Santana, D.G. How and why to measure the germination process? Rev. Bras. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Debez, A.; Ben Slimen, I.D.; Bousselmi, S.; Atia, A.; Farhat, N.; El Kahoui, S.; Abdelly, C. Comparative analysis of salt impact on sea barley from semi-arid habitats in Tunisia and cultivated barley with special emphasis on reserve mobilization and stress recovery aptitude. Plant Biosyst. 2020, 154, 544–552. [Google Scholar] [CrossRef]
- Liu, L.; Xia, W.; Li, H.; Zeng, H.; Wei, B.; Han, S.; Yin, C. Salinity inhibits rice seed germination by reducing α-amylase activity via decreased bioactive gibberellin content. Front. Plant Sci. 2018, 9, 275. [Google Scholar] [CrossRef]
- Farooq, M.; Aziz, T.; Basra, S.M.A.; Wahid, A.; Khaliq, A.; Cheema, M.A. Exploring the role of calcium to improve chilling tolerance in hybrid maize. J. Agron. Crop Sci. 2008, 194, 350–359. [Google Scholar] [CrossRef]
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front. Plant Sci. 2016, 7, 609. [Google Scholar] [CrossRef] [PubMed]
- Ben Youssef, R.; Boukari, N.; Abdelly, C.; Jelali, N. Mitigation of salt stress and stimulation of growth by salicylic acid and calcium chloride seed priming in two barley species. Plant Biosyst. 2023, 157, 758–768. [Google Scholar] [CrossRef]
- Tsialtas, J.T.; Shabala, S.; Matsi, T. A prominent role for leaf calcium as a yield and quality determinant in upland cotton (Gossypium hirsutum L.) varieties grown under irrigated Mediterranean conditions. J. Agron. Crop Sci. 2016, 202, 161–173. [Google Scholar] [CrossRef]
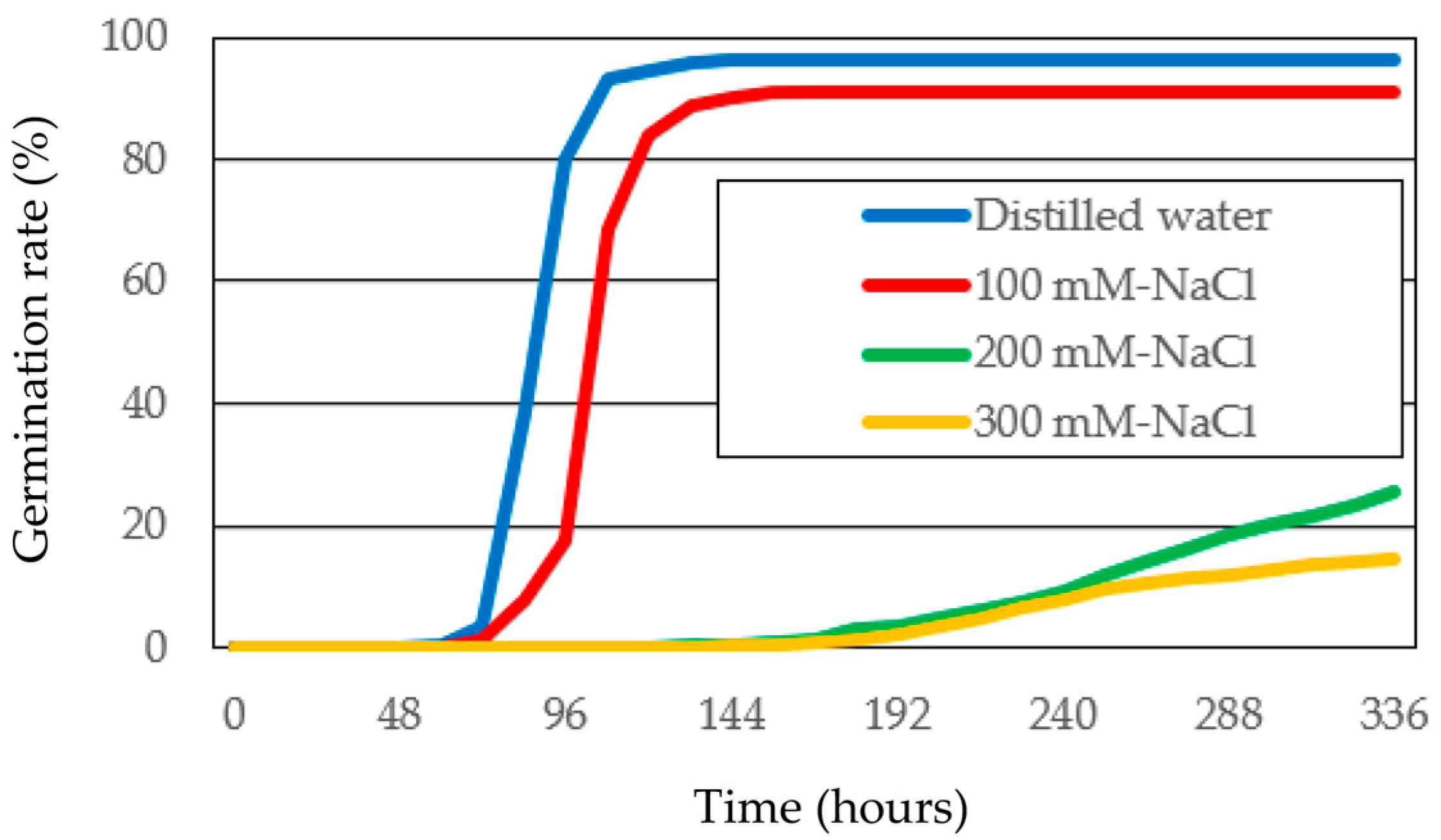



| Germination Media | GR (%) | MGT (Hours) | CUG | GI | ||||
|---|---|---|---|---|---|---|---|---|
| Distilled Water | 96.2 ± 1.0 | a | 93.3 ± 1.6 | d | 4.79 ± 0.38 | a | 0.73 ± 0.01 | a |
| 100 mM NaCl | 91.1 ± 0.5 | b | 108.8 ± 2.0 | c | 3.65 ± 0.63 | b | 0.68 ± 0.01 | b |
| 200 mM NaCl | 25.6 ± 4.7 | c | 239.7 ± 1.9 | b | 0.27 ± 0.03 | c | 0.27 ± 0.03 | c |
| 300 mM NaCl | 15.7 ± 0.3 | d | 280.3 ± 3.5 | a | 0.34 ± 0.04 | c | 0.06 ± 0.01 | d |
| GR (%) | MGT (Hours) | CUG | GI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Distilled Water | |||||||||
| Control | 96.2 ± 1.0 | a | 93.3 ± 1.6 | c | 4.79 ± 0.38 | b | 0.73 ± 0.01 | a | |
| 50 mM-Ca | 95.6 ± 1.3 | a | 94.1 ± 3.1 | c | 5.70 ± 0.80 | a | 0.74 ± 0.01 | a | |
| 100 mM-Ca | 95.6 ± 0.6 | a | 93.1 ± 1.1 | c | 5.05 ± 0.47 | b | 0.73 ± 0.02 | a | |
| 200 mM-NaCl | |||||||||
| Control | 25.6 ± 4.7 | c | 239.7 ± 1.9 | a | 0.27 ± 0.03 | d | 0.27 ± 0.03 | c | |
| 50 mM-Ca | 86.8 ± 1.7 | b | 183.5 ± 11.0 | b | 0.31 ± 0.02 | c | 0.42 ± 0.02 | b | |
| 100 mM-Ca | 87.9 ± 2.7 | b | 177.0 ± 9.3 | b | 0.34 ± 0.01 | c | 0.45 ± 0.02 | b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akimoto, M.; Ma, L. Supplementation of Calcium Through Seed Enrichment Technique Enhances Germinability and Early Growth of Timothy (Phleum pratense L.) Under Salinity Conditions. Agronomy 2025, 15, 1905. https://doi.org/10.3390/agronomy15081905
Akimoto M, Ma L. Supplementation of Calcium Through Seed Enrichment Technique Enhances Germinability and Early Growth of Timothy (Phleum pratense L.) Under Salinity Conditions. Agronomy. 2025; 15(8):1905. https://doi.org/10.3390/agronomy15081905
Chicago/Turabian StyleAkimoto, Masahiro, and Li Ma. 2025. "Supplementation of Calcium Through Seed Enrichment Technique Enhances Germinability and Early Growth of Timothy (Phleum pratense L.) Under Salinity Conditions" Agronomy 15, no. 8: 1905. https://doi.org/10.3390/agronomy15081905
APA StyleAkimoto, M., & Ma, L. (2025). Supplementation of Calcium Through Seed Enrichment Technique Enhances Germinability and Early Growth of Timothy (Phleum pratense L.) Under Salinity Conditions. Agronomy, 15(8), 1905. https://doi.org/10.3390/agronomy15081905






