Mechanisms of Resistance of Oryza sativa to Phytophagous Insects and Modulators Secreted by Nilaparvata lugens (Hemiptera, Delphacidae) When Feeding on Rice Plants
Abstract
1. Introduction
2. Genetics of N. lugens Resistance
3. Molecular Mechanisms Associated with N. lugens Resistance Genes
3.1. Classification and Structural Diversity of 17 Cloned Rice Genes Conferring Resistance to N. lugens
3.2. CNL-Type Genes
3.3. LRR-Containing Genes
3.4. Other Types of N. lugens Resistance Genes
4. N. lugens Salivary Components as Key Mediators in Rice–Insect Interactions
4.1. N. lugens Elicitors Involved in Interactions with Rice
4.2. N. lugens Effectors Involved in Interactions with Rice
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abd El-Aty, M.S.; Abo-Youssef, M.I.; Galal, A.A.; Salama, A.M.; Salama, A.A.; El-Shehawi, A.M.; Elseehy, M.M.; El-Saadony, M.T.; El-Tahan, A.M. Genetic behavior of earliness and yield traits of some rice (Oryza sativa L.) genotypes. Saudi J. Biol. Sci. 2022, 29, 2691–2697. [Google Scholar] [CrossRef] [PubMed]
- Yele, Y.; Chander, S.; Suroshe, S.S.; Nebapure, S.; Tenguri, P.; Pattathanam Sundaran, A. Ecological engineering in low land rice for brown plant hopper, Nilaparvata lugens (Stål) management. PeerJ 2023, 11, e15531. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, X.; Zhao, M.; Guo, M.; Han, K.; Dong, X.; Zhao, J.; Cai, W.; Zhang, Q.; Hua, H. Ultrabithorax is a key regulator for the dimorphism of wings, a main cause for the outbreak of planthoppers in rice. Natl. Sci. Rev. 2020, 7, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.L.; Zhuo, J.C.; Fang, G.Q.; Lu, J.B.; Ye, Y.X.; Li, D.T.; Lou, Y.H.; Zhang, X.Y.; Chen, X.; Wang, S.L.; et al. The genomic history and global migration of a windborne pest. Sci. Adv. 2024, 10, eadk3852. [Google Scholar] [CrossRef]
- Urban, J.M.; Cryan, J.R. Evolution of the planthoppers (insecta: Hemiptera: Fulgoroidea). Mol. Phylogenetics Evol. 2007, 42, 556–572. [Google Scholar] [CrossRef]
- Markevich, D.; Walczak, M.; Borodin, O.; Szwedo, J.; Brożek, J. Morphological reassessment of the movable calcar of delphacid planthoppers (hemiptera: Fulgoromorpha: Delphacidae). Sci. Rep. 2021, 11, 22294. [Google Scholar] [CrossRef]
- Gębicki, C.; Szwedo, J. The first ugyopine planthopper Serafinana perperunae gen. and sp. n. from eocene baltic amber (hemiptera, fulgoroidea: Delphacidae). Pol.J. Entomol./Pol. Pismo Entomol. 2000, 69, 389–395. [Google Scholar]
- Szwedo, J.; Bourgoin, T.; Lefèbvre, F. Fossil Planthoppers (Hemiptera Fulgoromorpha) of the World. An Annotated Catalogue with Notes on Hemiptera Classification; Studio 1: Warszawa, Poland, 2004; 199p. [Google Scholar]
- Solórzano Kraemer, M.M. Systematic, palaeoecology, and palaeobiogeography of the insect fauna from mexican amber. Palaeontogr. Abt. A 2007, 282, 1–133. [Google Scholar] [CrossRef]
- Urban, J.M.; Bartlett, C.R.; Cryan, J.R. Evolution of delphacidae (hemiptera: Fulgoroidea): Combined-evidence phylogenetics reveals importance of grass host shifts. Syst. Entomol. 2010, 35, 678–691. [Google Scholar] [CrossRef]
- Huang, Y.X.; Zheng, L.F.; Bartlett, C.R.; Qin, D.Z. Resolving phylogenetic relationships of delphacini and tropidocephalini (hemiptera: Delphacidae: Delphacinae) as inferred from four genetic loci. Sci. Rep. 2017, 7, 3319. [Google Scholar] [CrossRef]
- Wilson, S.W.; Mitter, C.; Denno, R.F.; Wilson, M.R. Evolutionary patterns of host plant use by delphacid planthoppers and their relatives. In Planthoppers: Their Ecology and Management; Denno, R.F., Perfect, T.J., Eds.; Chapman & Hall: Boca Raton, FL, USA, 1994; pp. 7–113. [Google Scholar]
- Wheeler, A. Bryophagy in the auchenorrhyncha: Seasonal history and habits of a moss specialist, javesella opaca (beamer) (fulgoroidea: Delphacidae). Proc. Entomol. Soc. Wash. 2003, 105, 599–610. [Google Scholar]
- Shi, S.; Zha, W.; Yu, X.; Wu, Y.; Li, S.; Xu, H.; Li, P.; Li, C.; Liu, K.; Chen, J.; et al. Integrated transcriptomics and metabolomics analysis provide insight into the resistance response of rice against brown planthopper. Front. Plant Sci. 2023, 14, 1213257. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, H. Studies on the influence of insecticides and bio-pesticides for the management of Brown plant hopper, Nilaparvata lugens (Stal) in the condition of western up (India). Front. Plant Sci. 2021, 41, 1441–1449. [Google Scholar] [CrossRef]
- Jones, P.; Gacesa, P.; Butlin, R. Systematics of brown planthopper and related species using nuclear. Ecol. Agric. Pests Biochem. Approaches 1996, 53, 133. [Google Scholar]
- Zhao, Y.; Huang, J.; Wang, Z.; Jing, S.; Wang, Y.; Ouyang, Y.; Cai, B.; Xin, X.-F.; Liu, X.; Zhang, C.; et al. Allelic diversity in an NLR geneBPH9enables rice to combat planthopper variation. Proc. Natl. Acad. Sci. USA 2016, 113, 12850–12855. [Google Scholar] [CrossRef]
- Yan, L.; Luo, T.; Huang, D.; Wei, M.; Ma, Z.; Liu, C.; Qin, Y.; Zhou, X.; Lu, Y.; Li, R.; et al. Recent advances in molecular mechanism and breeding utilization of brown planthopper resistance genes in rice: An integrated review. Int. J. Mol. Sci. 2023, 24, 12061. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Xiong, S.; Guan, X.; Tang, T.; Zhu, Z.; Zhu, X.; Hu, J.; Wu, J.; Zhang, S. Insight into rice resistance to the brown planthopper: Gene cloning, functional analysis, and breeding applications. Int. J. Mol. Sci. 2024, 25, 13397. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Barik, S.R.; Pandit, E.; Yadav, S.S.; Das, S.R.; Pradhan, S.K. Genetics, mechanisms anddeployment of brown planthopper resistance genes in rice. Crit. Rev. Plant Sci. 2022, 41, 91–127. [Google Scholar] [CrossRef]
- Li, C.; Xiong, Z.; Fang, C.; Liu, K. Transcriptome and metabolome analyses reveal the responses of brown planthoppers to RH resistant rice cultivar. Front. Physiol. 2022, 13, 1018470. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Tao, Z.; Ma, F.; Wu, S.; Miao, X.; Cao, L.; Shi, Z. The microRNA OsmiR393 regulates rice brown planthopper resistance by modulating the auxin-ROS signaling cross-talk. Sci. Adv. 2025, 11, eadu6722. [Google Scholar] [CrossRef]
- Khan, M.; Han, C.; Choi, N.; Kim, J. RNAseq-based carboxylesterase Nl-EST1 gene expression plasticity identification and its potential involvement in fenobucarb resistance in the brown planthopper Nilaparvata lugens. Insects 2024, 15, 743. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ge, L.; Liu, F.; Song, Q.; Stanley, D. Pesticide-induced planthopper population resurgence in rice cropping systems. Annu. Rev. Entomol. 2020, 65, 409–429. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.H.; Wang, Y.X.; Xiao, J.; Jia, Y.F.; Liu, F.; Wang, W.X.; Wei, Q.; Lai, F.X.; Fu, Q.; Wan, P.-J. Defense regulatory network associated with circRNA in rice in response to brown planthopper infestation. Plants 2024, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, X.; Ma, X.; Luo, L.; Fang, Y.; Zhao, N.; Han, Y.; Wei, Z.; Liu, F.; Qin, B.; et al. Development of new rice (Oryza. sativa L.) breeding lines through marker-assisted introgression and pyramiding of brown planthopper, blast, bacterial leaf blight resistance, and aroma genes. Agronomy 2021, 11, 2525. [Google Scholar] [CrossRef]
- Sriram, M.; Manonmani, S.; Gopalakrishnan, C.; Sheela, V.; Shanmugam, A.; Revanna Swamy, K.M.; Suresh, R. Breeding for brown plant hopper resistance in rice: Recent updates and future perspectives. Mol. Biol. Rep. 2024, 51, 1038. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, J.; Li, Z.; Liu, J.; Gao, G.; Zhang, Q.; Xiao, J.; He, Y. Evaluation and breeding application of six brown planthopper resistance genes in rice maintainer line Jin 23B. Rice 2018, 11, 22. [Google Scholar] [CrossRef]
- Balachiranjeevi, C.H.; Prahalada, G.D.; Mahender, A.; Jamaloddin, M.; Sevilla, M.A.L.; Marfori-Nazarea, C.M.; Vinarao, R.; Sushanto, U.; Baehaki, S.E.; Li, Z.K.; et al. Identification of a novel locus, BPH38(t), conferring resistance to brown planthopper (Nilaparvata lugens Stal.) using early backcross population in rice (Oryza sativa L.). Euphytica 2019, 215, 185. [Google Scholar] [CrossRef]
- Sani Haliru, B.; Rafii, M.Y.; Mazlan, N.; Ramlee, S.I.; Muhammad, I.; Silas Akos, I.; Halidu, J.; Swaray, S.; Rini Bashir, Y. Recent strategies for detection and improvement of brown planthopper resistance genes in rice: A Review. Plants 2020, 9, 1202. [Google Scholar] [CrossRef]
- Prajapati, V.K.; Vijayan, V.; Vadassery, J. Secret weapon of insects: The oral secretion cocktail and its modulation of host immunity. Plant Cell Physiol. 2024, 65, 1213–1223. [Google Scholar] [CrossRef]
- Mou, D.F.; Kundu, P.; Pingault, L.; Puri, H.; Shinde, S.; Louis, J. Monocot crop–aphid interactions: Plant resilience and aphid adaptation. Curr. Opin. Insect Sci. 2023, 57, 101038. [Google Scholar] [CrossRef]
- Huang, H.J.; Wang, Y.Z.; Li, L.L.; Lu, H.B.; Lu, J.B.; Wang, X.; Ye, Z.X.; Zhang, Z.L.; He, Y.J.; Lu, G.; et al. Planthopper salivary sheath protein LsSP1 contributes to manipulation of rice plant defenses. Nat. Commun. 2023, 14, 737. [Google Scholar] [CrossRef]
- Huang, H.J.; Zhang, C.X.; Hong, X.Y. How does saliva function in planthopper–host interactions? Arch. Insect Biochem. 2019, 100, e21537. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Wang, X.J.; Lu, J.B.; Lu, H.B.; Ye, Z.X.; Xu, Z.T.; Zhang, C.; Chen, J.P.; Li, J.M.; Zhang, C.X.; et al. Cross-kingdom RNA interference mediated by insect salivary microRNAs may suppress plant immunity. Proc. Natl. Acad. Sci. USA 2024, 121, e2318783121. [Google Scholar] [CrossRef] [PubMed]
- Kallure, G.S.; Kumari, A.; Shinde, B.A.; Giri, A.P. Characterized constituents of insect herbivore oral secretions and their influence on the regulation of plant defenses. Phytochemistry 2022, 193, 113008. [Google Scholar] [CrossRef]
- Han, W.H.; Ji, S.X.; Zhang, F.B.; Song, H.D.; Wang, J.X.; Fan, X.P.; Xie, R.; Liu, S.S.; Wang, X.W. A small RNA effector conserved in herbivore insects suppresses host plant defense by cross-kingdom gene silencing. Mol. Plant 2025, 18, 437–456. [Google Scholar] [CrossRef]
- Mahanta, D.K.; Komal, J.; Samal, I.; Bhoi, T.K.; Kumar, P.V.D.; Mohapatra, S.; Athulya, R.; Majhi, P.K.; Mastinu, A. Plant defense responses to insect herbivores through molecular signaling, secondary metabolites, and associated epigenetic regulation. Plant Environ. Interact. 2025, 6, e70035. [Google Scholar] [CrossRef]
- Ma, X.; Yin, Z.; Li, H.; Guo, J. Roles of herbivorous insects salivary proteins. Heliyon 2024, 10, e29201. [Google Scholar] [CrossRef]
- Snoeck, S.; Guayazán-Palacios, N.; Steinbrenner, A.D. Molecular tug-of-war: Plant immune recognition of herbivory. Plant Cell 2022, 34, 1497–1513. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, S.; Hua, W. Advances of herbivore-secreted elicitors and effectors in plant-insect interactions. Front. Plant Sci. 2023, 14, 1176048. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Fu, J.; Shi, Y.; Li, J.; Jing, M.; Wang, L.; Yang, S.; Tian, T.; Wang, L.; Ju, J.; et al. Vitellogenin from planthopper oral secretion acts as a novel effector to impair plant defenses. New Phytol. 2021, 232, 802–817. [Google Scholar] [CrossRef]
- Rodriguez, P.A.; Escudero-Martinez, C.; Bos, J.I.B. An aphid effector targets trafficking protein VPS52 in a host-specific manner to promote virulence. Plant Physiol. 2017, 173, 1892–1903. [Google Scholar] [CrossRef]
- Hancock, R.; Xia, A.; Dou, D.; Wu, Y.; Wu, S.; Zuo, K.; Xia, Q.; Nyawira, K.T.; Liang, D.; Zhang, M.; et al. The mirid bug apolygus lucorum deploys a glutathione peroxidase as a candidate effector to enhance plant susceptibility. J. Exp. Bot. 2020, 71, 2701–2712. [Google Scholar] [CrossRef]
- Du, B.; Zhang, W.; Liu, B.; Hu, J.; Wei, Z.; Shi, Z.; He, R.; Zhu, L.; Chen, R.; Han, B.; et al. Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc. Natl. Acad. Sci. USA 2009, 106, 22163–22168. [Google Scholar] [CrossRef]
- Shar, S.B.D.; Nguyen, C.D.; Sanada-Morimura, S.; Yasui, H.; Zheng, S.H.; Fujita, D. Development and characterization of near-isogenic lines for brown planthopper resistance genes in the genetic background of japonica rice ‘Sagabiyori’. Breed. Sci. 2023, 73, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, H.; Ogawa, T. PFLP mapping of Bph-1 (brown planthopper resistance gene) in rice. Breed. Sci. 1995, 45, 369–371. [Google Scholar]
- Kaur, P.; Neelam, K.; Sarao, P.S.; Saini, N.S.; Dhir, Y.W.; Khanna, R.; Vikal, Y.; Singh, K. Mapping of a novel recessive brown planthopper resistance gene bph46 from wild rice (Oryza nivara). Euphytica 2024, 220, 61. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, D.; Liu, D.; Hassan, M.A.; Yu, C.; Wu, X.; Huang, S.; Bian, S.; Wei, P.; Li, J. Recent advances in gene mining and hormonal mechanism for brown planthopper resistance in rice. Int. J. Mol. Sci. 2024, 25, 12965. [Google Scholar] [CrossRef]
- Ishwarya, L.V.G.; Vanisri, S.; Basavaraj, P.S.; Sreedhar, M.; Jhansi, L.V.; Muntazir, M.; Gireesh, C.; Pushpavalli, S.N.C.V.L. Harnessing advanced genomic approaches to unveil and enhance brown planthopper resistance in rice. Rice Sci. 2025, 32, 339–352. [Google Scholar] [CrossRef]
- Muduli, L.; Pradhan, S.K.; Mishra, A.; Bastia, D.N.; Samal, K.C.; Agrawal, P.K.; Dash, M. Understanding brown planthopper resistance in rice: Genetics, biochemical and molecular breeding approaches. Rice Sci. 2021, 28, 532–546. [Google Scholar] [CrossRef]
- Kamal, M.M.; Nguyen, C.D.; Sanada-Morimura, S.; Zheng, S.-H.; Fujita, D. Development of pyramided lines carrying brown planthopper resistance genes in the genetic background of Indica Group rice (Oryza sativa L.) variety ‘IR64’. Breed. Sci. 2023, 73, 450–456. [Google Scholar] [CrossRef]
- Guo, J.; Xu, C.; Wu, D.; Zhao, Y.; Qiu, Y.; Wang, X.; Ouyang, Y.; Cai, B.; Liu, X.; Jing, S.; et al. Bph6 encodes an exocyst-localized protein and confers broad resistance to planthoppers in rice. Nat. Genet. 2018, 50, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, H.; Nie, L.; Tan, D.; Zhou, C.; Zhang, Q.; Li, Y.; Du, B.; Guo, J.; Huang, J.; et al. Bph30 confers resistance to brown planthopper by fortifying sclerenchyma in rice leaf sheaths. Mol. Plant 2021, 14, 1714–1732. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Q.; Chen, Y.; Huang, J.; Guo, Q.; Li, Y.; Wang, W.; Qiu, Y.; Guan, W.; Zhang, J.; et al. Balancing selection and wild gene pool contribute to resistance in global rice germplasm against planthopper. J. Integr. Plant Biol. 2021, 63, 1695–1711. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, H.; Chen, H.; Liu, Y.; He, J.; Kang, H.; Sun, Z.; Pan, G.; Wang, Q.; Hu, J.; et al. A gene cluster encoding lectin receptor kinases confers broad-spectrum and durable insect resistance in rice. Nat. Biotechnol. 2014, 33, 301–305. [Google Scholar] [CrossRef]
- Cheng, X.; Wu, Y.; Guo, J.; Du, B.; Chen, R.; Zhu, L.; He, G. A rice lectin receptor-like kinase that is involved in innate immune responses also contributes to seed germination. Plant J. 2013, 76, 687–698. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, L.; Zhang, Y.; Cao, C.; Liu, F.; Huang, F.; Qiu, Y.; Li, R.; Lou, X. Map-based cloning and characterization of BPH29, a B3 domain-containing recessive gene conferring brown planthopper resistance in rice. J. Exp. Bot. 2015, 66, 6035–6045. [Google Scholar] [CrossRef]
- Ren, J.; Gao, F.; Wu, X.; Lu, X.; Zeng, L.; Lv, J.; Su, X.; Luo, H.; Ren, G. Bph32, a novel gene encoding an unknown SCR domain-containing protein, confers resistance against the brown planthopper in rice. Sci. Rep. 2016, 6, 37645. [Google Scholar] [CrossRef]
- Guo, J.; Wang, H.; Guan, W.; Guo, Q.; Wang, J.; Yang, J.; Peng, Y.; Shan, J.; Gao, M.; Shi, S.; et al. A tripartite rheostat controls self-regulated host plant resistance to insects. Nature 2023, 618, 799–807. [Google Scholar] [CrossRef]
- Hu, L.; Wu, Y.; Wu, D.; Rao, W.; Guo, J.; Ma, Y.; Wang, Z.; Shangguan, X.; Wang, H.; Xu, C.; et al. The coiled-coil and nucleotide binding domains of Brown Planthopper Resistance14 function in signaling and resistance against planthopper in rice. Plant Cell 2017, 29, 3157–3185. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Guo, J.; Zhang, Q.; Shi, S.; Guan, W.; Zhou, C.; Chen, R.; Du, B.; Zhu, L.; He, G. Necessity of rice resistance to planthoppers for OsEXO70H3 regulating SAMSL excretion and lignin deposition in cell walls. New Phytol. 2022, 234, 1031–1046. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhang, X.; Shi, Q.; Cheng, Z.-M. Adaptive evolution driving the young duplications in six rosaceae species. BMC Genom. 2021, 22, 112. [Google Scholar] [CrossRef]
- Huang, Z.; He, G.; Shu, L.; Li, X.; Zhang, Q. Identification and mapping of two brown planthopper resistance genes in rice. Theor. Appl. Genet. 2001, 102, 929–934. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, J.; Nie, L.; Hu, Y.; Zhang, N.; Guo, Q.; Guo, J.; Du, B.; Zhu, L.; He, G.; et al. Molecular and functional analysis of a brown planthopper resistance protein with two nucleotide-binding site domains. J. Exp. Bot. 2021, 72, 2657–2671. [Google Scholar] [CrossRef]
- Slootweg, E.J.; Spiridon, L.N.; Roosien, J.; Butterbach, P.; Pomp, R.; Westerhof, L.; Wilbers, R.; Bakker, E.; Bakker, J.; Petrescu, A.-J. Structural determinants at the interface of the ARC2 and leucine-rich repeat domains control the activation of the plant immune receptors Rx1 and Gpa2. Plant Physiol. 2013, 162, 1510–1528. [Google Scholar] [CrossRef] [PubMed]
- Takken, F.L.; Goverse, A. How to build a pathogen detector: Structural basis of NB-LRR function. Curr. Opin. Plant Biol. 2012, 15, 375–384. [Google Scholar] [CrossRef]
- Zipfel, C. Plant pattern-recognition receptors. Trends Immunol. 2014, 35, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Qing, D.; Chen, W.; Li, J.; Lu, B.; Huang, S.; Chen, L.; Zhou, W.; Pan, Y.; Huang, J.; Wu, H.; et al. TMT-based quantitative proteomics analysis of defense responses induced by the Bph3 gene following brown planthopper infection in rice. BMC Plant Biol. 2024, 24, 1092. [Google Scholar] [CrossRef]
- Li, Y.; Cheah, B.H.; Fang, Y.-F.; Kuang, Y.-H.; Lin, S.-C.; Liao, C.-T.; Huang, S.-H.; Lin, Y.-F.; Chuang, W.-P. Transcriptomics identifies key defense mechanisms in rice resistant to both leaf-feeding and phloem feeding herbivores. BMC Plant Biol. 2021, 21, 306. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Zhang, X.; Liu, J.; Tao, K.; Li, C.; Xiao, S.; Zhang, W.; Li, J.F. Plant elicitor peptide signalling confers rice resistance to piercing-sucking insect herbivores and pathogens. Plant Biotechnol. J. 2022, 20, 991–1005. [Google Scholar] [CrossRef]
- Qi, L.; Li, J.; Li, S.; Li, J.; Wang, H.; Yang, L.; Tan, X.; Zhao, Z.; Luo, G.; Jing, M.; et al. An insect salivary sheath protein triggers plant resistance to insects and pathogens as a conserved HAMP. Adv. Sci. 2025, 12, e2415474. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, X.Y.; Li, S.; Xie, Y.C.; Luo, X.M.; Yang, Y.; Pu, Z.; Zhang, L.; Lu, J.B.; Huang, H.J.; et al. Rapid intracellular acidification is a plant defense response countered by the brown planthopper. Curr. Biol. 2024, 34, 5017–5027. [Google Scholar] [CrossRef]
- Ray, S.; Gaffor, I.; Acevedo, F.E.; Helms, A.; Chuang, W.P.; Tooker, J.; Felton, G.W.; Luthe, D.S. Maize plants recognize herbivore-associated cues from caterpillar frass. J. Chem. Ecol. 2015, 41, 781–792. [Google Scholar] [CrossRef]
- Shangguan, X.; Zhang, J.; Liu, B.; Zhao, Y.; Wang, H.; Wang, Z.; Guo, J.; Rao, W.; Jing, S.; Guan, W.; et al. A mucin-like protein of planthopper is required for feeding and induces immunity response in plants. Plant Physiol. 2018, 176, 552–565. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, N.; Shan, J.; Peng, Y.; Guo, J.; Zhou, C.; Shi, S.; Zheng, X.; Wu, D.; Guan, W.; et al. Salivary protein 1 of brown planthopper is required for survival and induces immunity response in plants. Front. Plant Sci. 2020, 11, 571280. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Zheng, X.; Liu, B.; Guo, Q.; Guo, J.; Wu, Y.; Shangguan, X.; Wang, H.; Wu, D.; Wang, Z.; et al. Secretome analysis and in planta expression of salivary proteins identify candidate effectors from the brown planthopper Nilaparvata lugens. Mol. Plant Microbe In 2019, 32, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Ye, W.; Hu, W.; Jin, X.; Kuai, P.; Xiao, W.; Jian, Y.; Turlings, T.C.J.; Lou, Y. The N-terminal subunit of vitellogenin in planthopper eggs and saliva acts as a reliable elicitor that induces defenses in rice. New Phytol. 2023, 238, 1230–1244. [Google Scholar] [CrossRef]
- Gao, H.; Lin, X.; Yuan, X.; Zou, J.; Zhang, H.; Zhang, Y.; Liu, Z.; Hancock, R. The salivary chaperone protein NlDNAJB9 of Nilaparvata lugens activates plant immune responses. J. Exp. Bot. 2023, 74, 6874–6888. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zou, J.; Lin, X.; Zhang, H.; Yu, N.; Liu, Z.; Foyer, C. Nilaparvata lugens salivary protein NlG14 triggers defense response in plants. J. Exp. Bot. 2022, 73, 7477–7487. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Ma, T.; Cao, J.; Zhang, Y.; Chen, S.; Lin, S.; Liu, X.; He, G.; Wan, L. Recognition of a salivary effector by the TNL protein RCSP promotes effector-triggered immunity and systemic resistance in Nicotiana benthamiana. J. Integr. Plant Biol. 2024, 67, 150–168. [Google Scholar] [CrossRef]
- Ji, R.; Ye, W.; Chen, H.; Zeng, J.; Li, H.; Yu, H.; Li, J.; Lou, Y. A salivary endo-β-1,4-glucanase acts as an effector that enables the brown planthopper to feed on rice. Plant Physiol. 2017, 173, 1920–1932. [Google Scholar] [CrossRef]
- Ye, W.; Yu, H.; Jian, Y.; Zeng, J.; Ji, R.; Chen, H.; Lou, Y. A salivary EF-hand calcium-binding protein of the brown planthopper Nilaparvata lugens functions as an effector for defense responses in rice. Sci. Rep. 2017, 7, 40498. [Google Scholar] [CrossRef]
- Fu, J.; Shi, Y.; Wang, L.; Tian, T.; Li, J.; Gong, L.; Zheng, Z.; Jing, M.; Fang, J.; Ji, R. Planthopper-secreted salivary calmodulin acts as an effector for defense responses in rice. Front. Plant Sci. 2022, 13, 841378. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, X.; Li, H.; Ye, Y.; Li, Z.; Han, X.; Hu, Y.; Zhang, C.; Jiang, Y. Heat shock 70 kDa protein cognate 3 of brown planthopper is required for survival and suppresses immune response in plants. Insects 2022, 13, 299. [Google Scholar] [CrossRef]
- Fu, J.; Li, S.; Li, j.; Zhao, Z.; Li, J.; Tan, X.; Yu, S.; Jing, M.; Zhu-Salzman, K.; Fang, J.; et al. An insect effector mimics its host immune regulator to undermine plant immunity. Adv. Sci. 2025, 12, e2409186. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Qiu, C.-L.; Shi, J.-H.; Sun, Z.; Hu, X.-J.; Liu, L.; Wang, M.-Q. A salivary odorant-binding protein mediates Nilaparvata lugens feeding and host plant phytohormone suppression. Int. J. Mol. Sci. 2021, 22, 4988. [Google Scholar] [CrossRef]
- Filippi, A.; Petrussa, E.; Boscutti, F.; Vuerich, M.; Vrhovsek, U.; Rabiei, Z.; Braidot, E. Bioactive polyphenols modulate enzymes Involved in grapevine pathogenesis and chitinase activity at increasing complexity levels. Int. J. Mol. Sci. 2019, 20, 6357. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Y.; Liu, X.; Francis, F.; Fan, J.; Liu, H.; Wang, Q.; Sun, Y.; Zhang, Y.; Chen, J. SmCSP4 from aphid saliva stimulates salicylic acid-mediated defence responses in wheat by interacting with transcription factor TaWKRY76. Plant Biotechnol. J. 2023, 21, 2389–2407. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, Y.Z.; Lou, Y.; Zhang, C. Vitellogenin and vitellogenin-like genes in the brown planthopper. Front. Physiol. 2019, 10, 1181. [Google Scholar] [CrossRef]
- Su, Q.; Yang, F.; Hu, Y.; Peng, Z.; Huang, T.; Tong, H.; Zhang, R.; Yang, Y.; Zhou, Z.; Liang, P.; et al. Flavonoids enhance tomato plant resistance to whitefly by interfering with the expression of a salivary effector. Plant Physiol. 2025, 197, kiaf101. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Yuan, L.Y.; Li, Y.F.; Xiao, H.X.; Li, Y.F.; Zhang, Y.; Wu, W.J.; Zhang, Z.F. Salivary protein 7 of the brown planthopper functions as an effector for mediating tricin metabolism in rice plants. Sci. Rep. 2022, 12, 3205. [Google Scholar] [CrossRef] [PubMed]
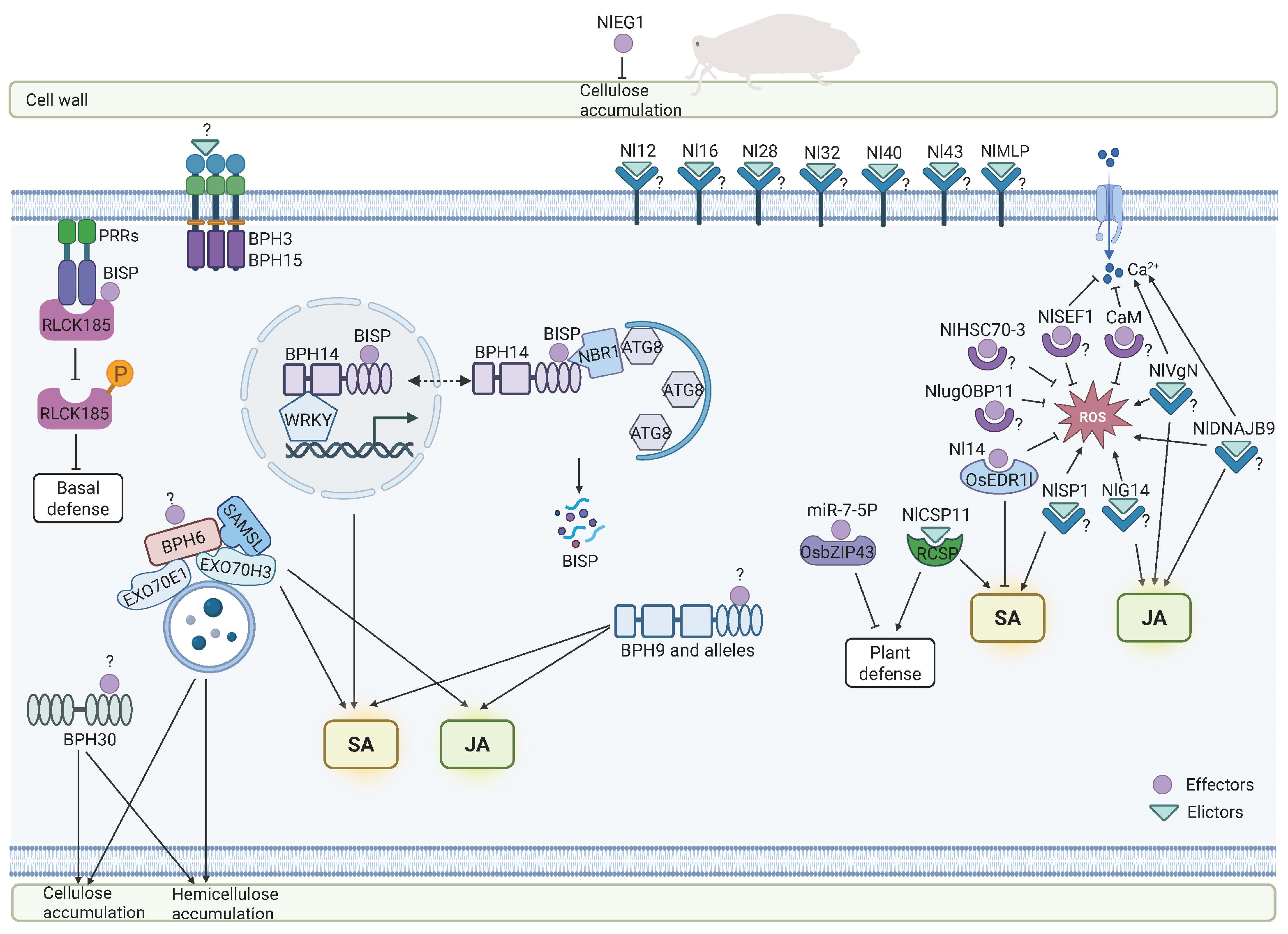
| Gene | Encoded Protein | Defense Mechanism | Ref. |
|---|---|---|---|
| Bph14 | CC-NB-LRR | Activate SA, induce callose deposition | [45] |
| Bind with BISP and activate NBR1-mediated autophagy | [60] | ||
| Interact with OsWRKY46 and OsWRKY72 and enhance their transactivation activity | [61] | ||
| Bph9 | CC-NB-NB-LRR | Activate SA | [17] |
| Bph1 | CC-NB-NB-LRR | - | [17] |
| Bph2 | CC-NB-NB-LRR | - | [17] |
| Bph7 | CC-NB-NB-LRR | - | [17] |
| Bph10 | CC-NB-NB-LRR | - | [17] |
| Bph18 | CC-NB-NB-LRR | - | [17] |
| Bph21 | CC-NB-NB-LRR | - | [17] |
| Bph26 | CC-NB-NB-LRR | - | [17] |
| Bph37 | CC-NB | - | [55] |
| Bph6 | Atypical LRR | Interact with OsEXO70E1 and promote exocytosis; reinforce plant cell wall; activate SA, JA, and CK | [53] |
| Interact with OsEXO70H3, which recruit SAMSL to enhance lignin deposition in cell wall | [62] | ||
| Bph30 | LRD | Enhance the synthesis of cellulose and hemicellulose in cell wall | [54] |
| Bph40 | LRD | Enhance cell wall | [54] |
| Bph15 | LRK | Interact with OsADF; enhance the expression of OsPR1a, OsLOX, and OsCHS | [57] |
| Bph3 | LRK | - | [56] |
| Bph29 | B3 DNA-binding | Activate SA, suppress JA/Et pathway | [58] |
| Bph32 | SCR | - | [59] |
| Name | Characterization | Function | Ref. |
|---|---|---|---|
| NlMLP | Mucin-like protein | Salivary sheath formation; induce cell death, defense-related gene expression, and callose deposition in tobacco | [75] |
| NlSP1 | Salivary protein 1 | Induce cell death, H2O2 accumulation, defense-related gene expression, and callose deposition in tobacco | [76] |
| Nl12 | Disulfide isomerase | Induce cell death, defense-related gene expression, and callose deposition in tobacco | [77] |
| Nl16 | Apolipophorin-III | Induce cell death, defense-related gene expression, and callose deposition in tobacco | [77] |
| Nl28 | Cysteine-rich protein | Induce cell death, defense-related gene expression, and callose deposition in tobacco | [77] |
| Nl32 | Chemosensory protein | Induce plant dwarfism, defense-related gene expression, and callose deposition in tobacco | [77] |
| Nl40 | N. lugens-specific salivary protein | Induce chlorosis, defense-related gene expression, and callose deposition in tobacco | [77] |
| Nl43 | Uncharacterized protein | Induce cell death, defense-related gene expression, and callose deposition in tobacco | [77] |
| NlVgN | N-terminal subunit of vitellogenin | Induce cytosolic Ca2+ and H2O2 accumulation, JA and JA-Ile production, defense-related gene expression, and volatile release in rice | [78] |
| NlDNAJB9 | DNAJ protein | Induce cell death, Ca2+ signaling, MAPK cascades, ROS accumulation, and callose deposition; activate JA pathway in tobacco | [79] |
| NlG14 | A protein specific to the salivary gland | Induce cell death, ROS accumulation, and callose deposition; activate JA pathway | [80] |
| NlCSP11 | Chemosensory protein | Induce cell death, dwarfism, and SA-dependent systemic resistance against pathogens; interact with RCSP (TNL lacking catalytic Glu) | [81] |
| NlEG1 | Endo-β-1,4-Glucanase | Enable N. lugens feeding; degrade celluloses in rice cell walls | [82] |
| BISP | BPH14-interacting salivary protein | Trigger BPH14-mediated resistance and activate NBR1-dependent selective autophagy | [60] |
| NlSEF1 | EF-hand calcium-binding protein | Suppress H2O2 and Ca2+ accumulation in rice | [83] |
| CaM | Calmodulin | Enable N. lugens feeding, bind calcium, and suppress H2O2 accumulation and callose deposition in rice | [84] |
| NIHSC70-3 | Heat shock 70 kDa protein cognate 3 | Suppress flg22-induced ROS bursts and defense-related gene expression in tobacco | [85] |
| Nl14 | 14-3-3e protein | Interact with enhanced disease resistance 1-like (OsEDR1l), suppress N. lugens-induced JA, JA-Ile and H2O2 accumulation, and facilitate N. lugens infestation | [86] |
| NlugOBP11 | Odorant-binding protein | Enable N. lugens feeding and suppress SA pathway in rice | [87] |
| miR-7-5P | microRNA | Target the immune-associated bZIP transcription factor (OsbZIP43) and suppress OsbZIP43-induced rice immunity | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Wu, W.; Huang, Y.; Xu, K.; Shangguan, X. Mechanisms of Resistance of Oryza sativa to Phytophagous Insects and Modulators Secreted by Nilaparvata lugens (Hemiptera, Delphacidae) When Feeding on Rice Plants. Agronomy 2025, 15, 1891. https://doi.org/10.3390/agronomy15081891
Zheng X, Wu W, Huang Y, Xu K, Shangguan X. Mechanisms of Resistance of Oryza sativa to Phytophagous Insects and Modulators Secreted by Nilaparvata lugens (Hemiptera, Delphacidae) When Feeding on Rice Plants. Agronomy. 2025; 15(8):1891. https://doi.org/10.3390/agronomy15081891
Chicago/Turabian StyleZheng, Xiaohong, Weiling Wu, Yuting Huang, Kedong Xu, and Xinxin Shangguan. 2025. "Mechanisms of Resistance of Oryza sativa to Phytophagous Insects and Modulators Secreted by Nilaparvata lugens (Hemiptera, Delphacidae) When Feeding on Rice Plants" Agronomy 15, no. 8: 1891. https://doi.org/10.3390/agronomy15081891
APA StyleZheng, X., Wu, W., Huang, Y., Xu, K., & Shangguan, X. (2025). Mechanisms of Resistance of Oryza sativa to Phytophagous Insects and Modulators Secreted by Nilaparvata lugens (Hemiptera, Delphacidae) When Feeding on Rice Plants. Agronomy, 15(8), 1891. https://doi.org/10.3390/agronomy15081891





