Sustainable Cotton Production in Sicily: Yield Optimization Through Varietal Selection, Mycorrhizae, and Efficient Water Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design and Crop Management
2.3. Irrigation Management
2.4. Microbial Biostimulants Management
2.5. Variety Selection
2.6. Agronomic Management
2.7. Weather Data
2.8. Data Collection
2.8.1. Growth Stage Data
2.8.2. Morphological Data
2.8.3. Yield Data
2.9. Statistical Analysis
3. Results
3.1. Irrigation Data
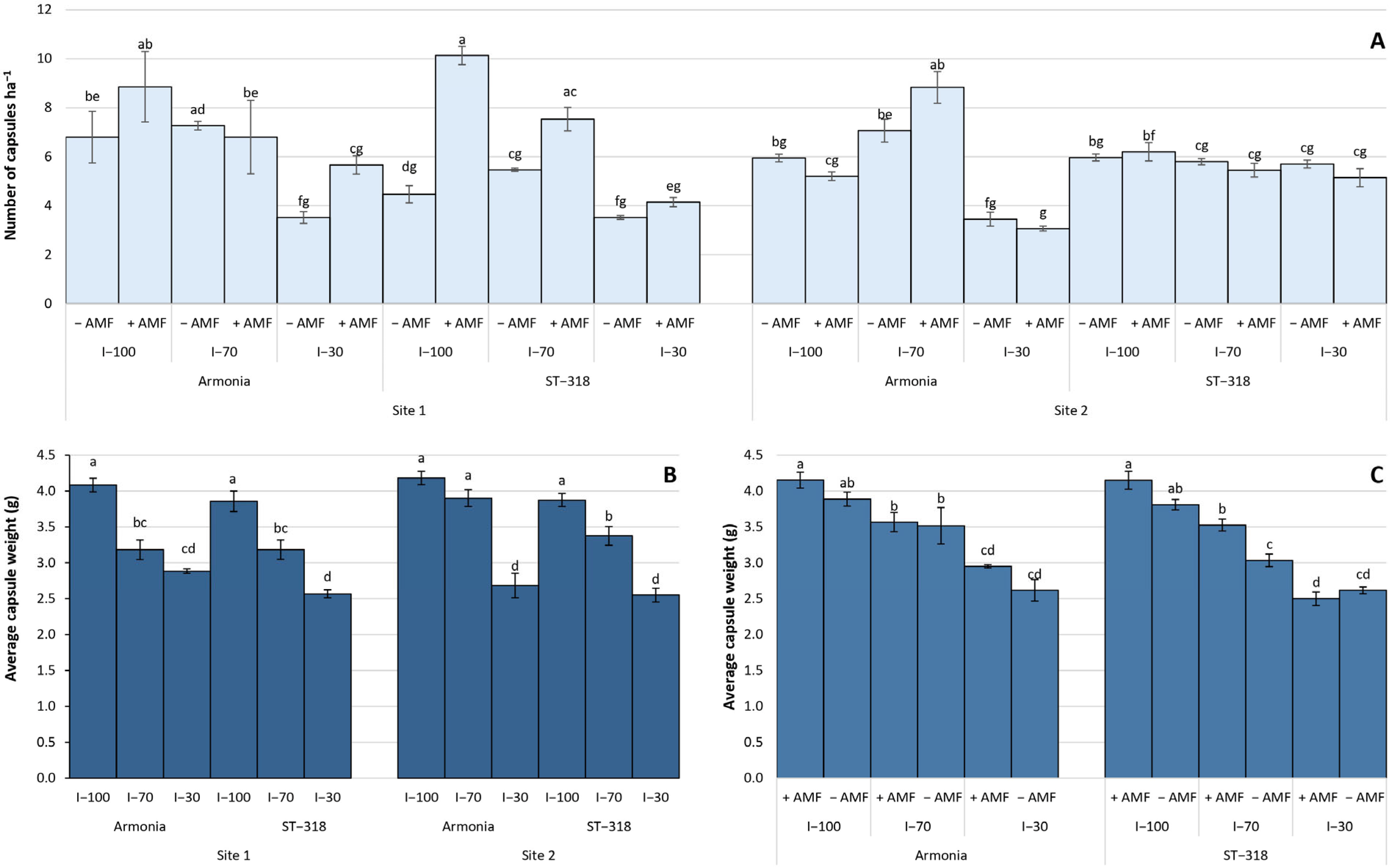
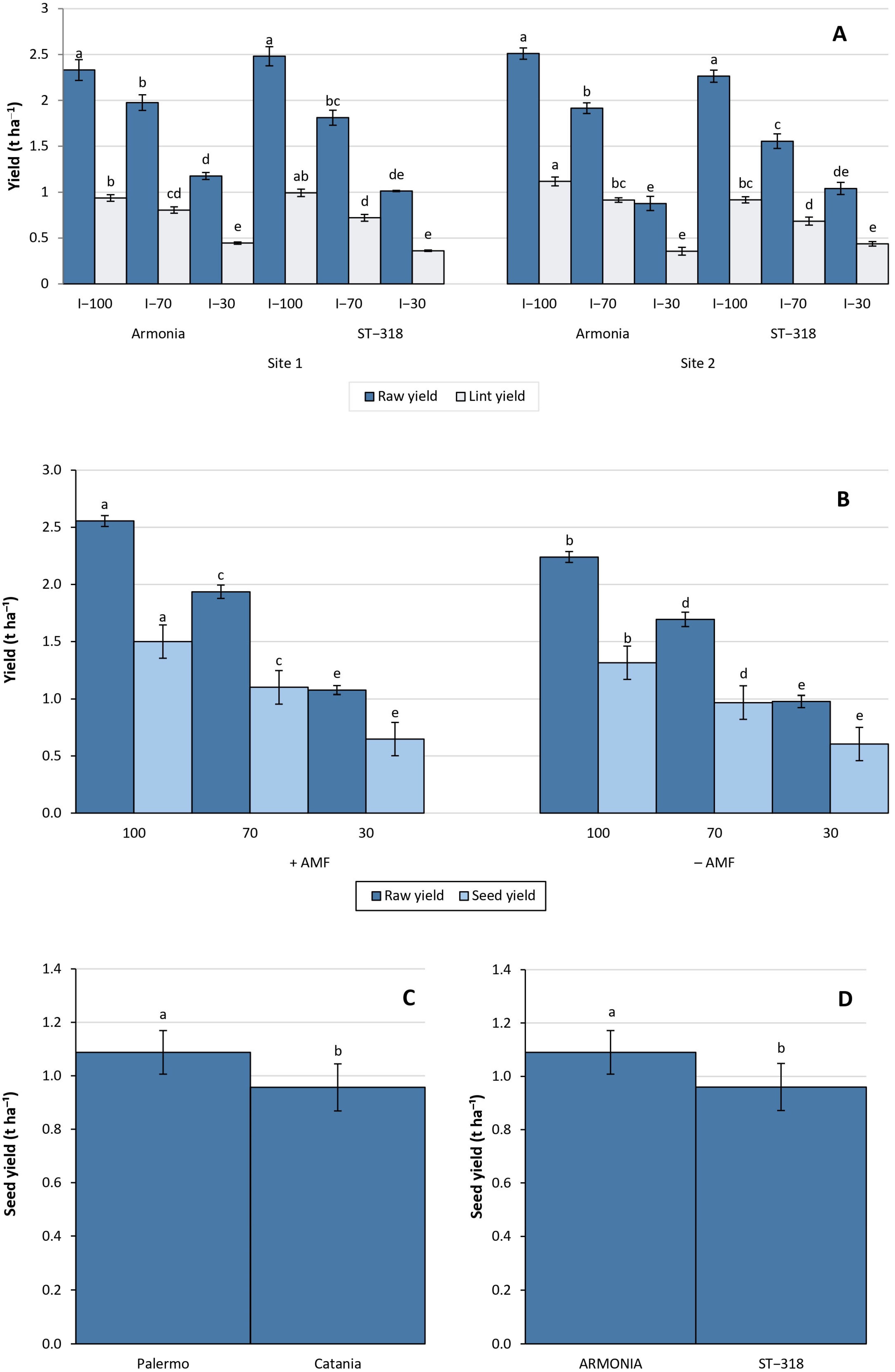
3.2. Agronomic and Yield Data
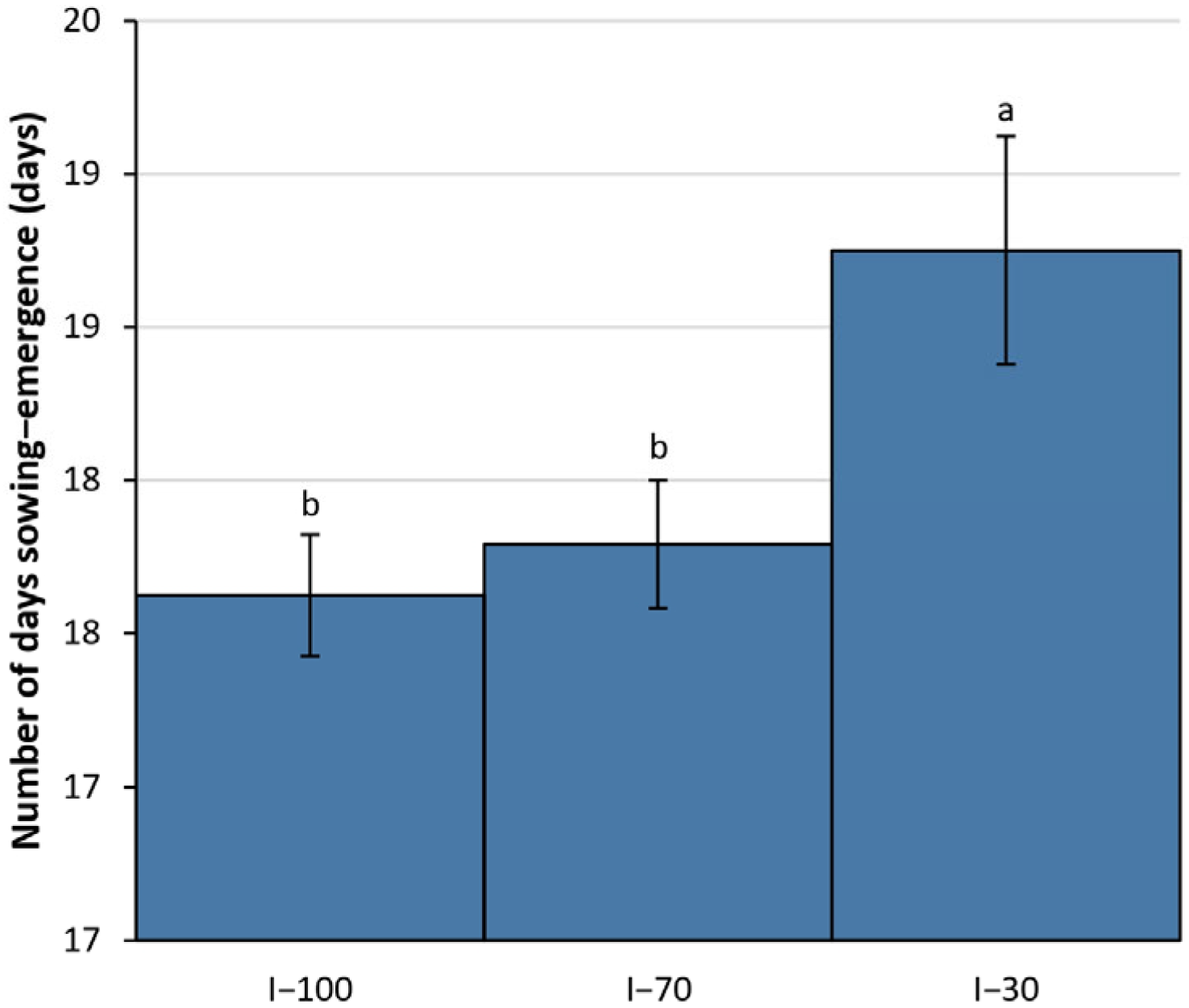
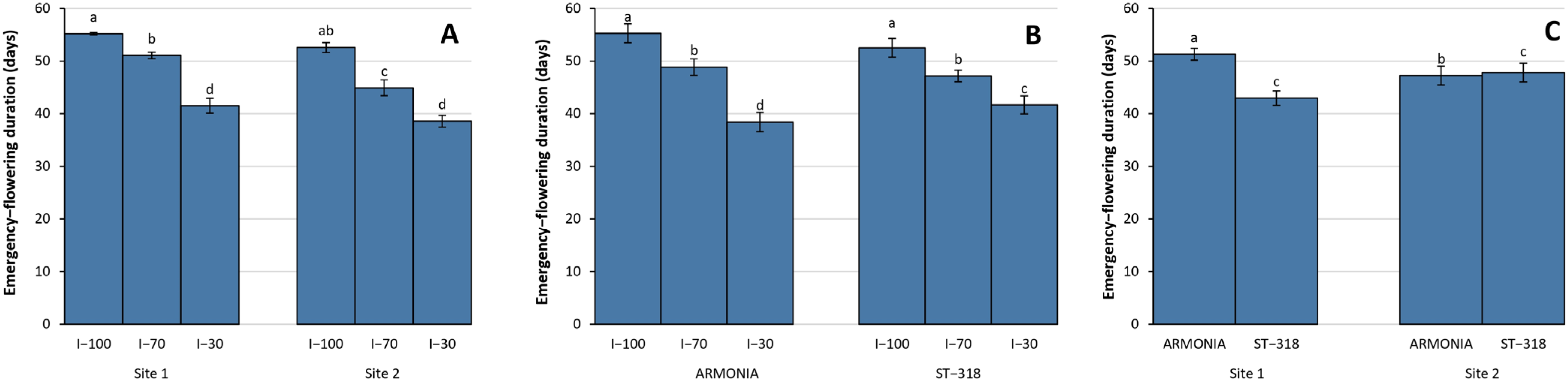
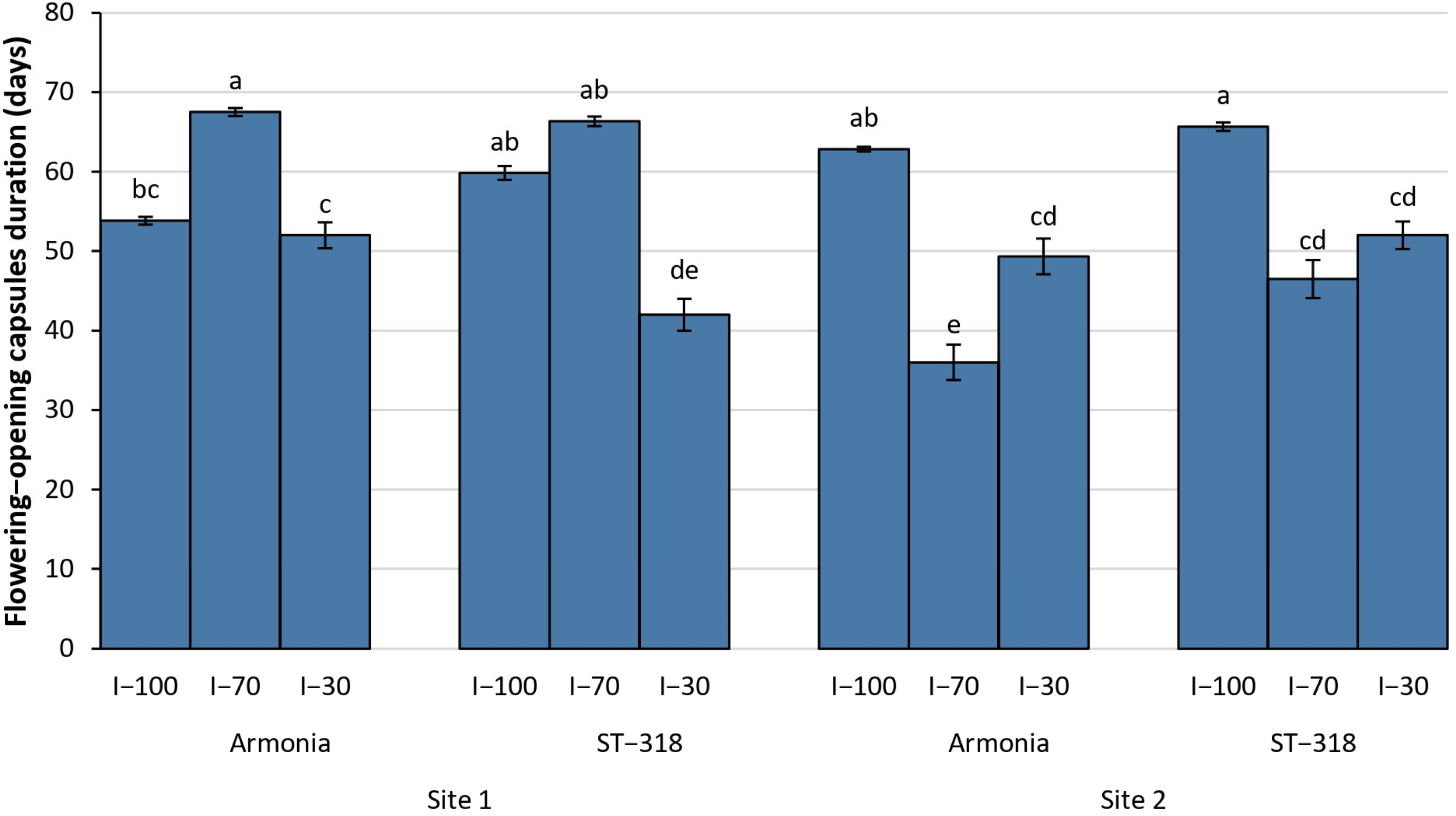
3.3. Phenological Data
3.4. Crop Water Productivity
4. Discussion
4.1. Irrigation and Water Productivity
4.2. Agronomic and Yield Performance
4.3. Phenological Performance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMF | Arbuscular Mycorrhizal Fungi |
| FC | Field Capacity |
| ETc | reference crop evapotranspiration |
| GDDs | growth degree days |
| I-100 | 100% ETc |
| I-70 | 70% ETc |
| I-30 | 30% ETc |
| ET0 | daily reference evapotranspiration |
| S | site |
| G | genotype |
| I | irrigation |
| M | mycorrhization |
| +AMF | mycorrhized plants |
| −AMF | non-mycorrhized plants |
| Kc | crop coefficients |
References
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 2 July 2025).
- Hunsaker, D.J.; French, A.N.; Waller, P.M.; Bautista, E.; Thorp, K.R.; Bronson, K.F.; Andrade-Sanchez, P. Comparison of Traditional and ET-Based Irrigation Scheduling of Surface-Irrigated Cotton in the Arid Southwestern USA. Agric. Water Manag. 2015, 159, 209–224. [Google Scholar] [CrossRef]
- Koudahe, K.; Sheshukov, A.Y.; Aguilar, J.; Djaman, K. Irrigation-Water Management and Productivity of Cotton: A Review. Sustainability 2021, 13, 10070. [Google Scholar] [CrossRef]
- Bordovsky, J.P.; Mustian, J.T.; Ritchie, G.L.; Lewis, K.L. Cotton Irrigation Timing with Variable Seasonal Irrigation Capacities in the Texas South Plains. Appl. Eng. Agric. 2015, 31, 883–897. [Google Scholar] [CrossRef]
- Chapagain, A.K.; Hoekstra, A.Y.; Savenije, H.H.G.; Gautam, R. The Water Footprint of Cotton Consumption: An Assessment of the Impact of Worldwide Consumption of Cotton Products on the Water Resources in the Cotton Producing Countries. Ecol. Econ. 2006, 60, 186–203. [Google Scholar] [CrossRef]
- Wu, B.; Tian, F.; Zhang, M.; Piao, S.; Zeng, H.; Zhu, W.; Liu, J.; Elnashar, A.; Lu, Y. Quantifying Global Agricultural Water Appropriation with Data Derived from Earth Observations. J. Clean. Prod. 2022, 358, 131891. [Google Scholar] [CrossRef]
- Engonopoulos, V.; Papastylianou, P.; Kakabouki, I.; Bilalis, D. Agro-Meteorological Indices in Relation to Cotton Yield in a Mediterranean Environment. Not. Sci. Biol. 2025, 17, 12330. [Google Scholar] [CrossRef]
- Di Nunno, F.; Granata, F. Future Trends of Reference Evapotranspiration in Sicily Based on CORDEX Data and Machine Learning Algorithms. Agric. Water Manag. 2023, 280, 108232. [Google Scholar] [CrossRef]
- Barnes, E.M.; Campbell, B.T.; Vellidis, G.; Porter, W.M.; Payero, J.O.; Leib, B.G.; Sui, R.; Fisher, D.K.; Anapalli, S.; Colaizzi, P.D.; et al. Forty Years of Increasing Cotton’s Water Productivity and Why the Trend Will Continue. Appl. Eng. Agric. 2020, 36, 457–478. [Google Scholar] [CrossRef]
- Mai, W.X.; Xue, X.R.; Azeem, A. Growth of cotton crop (Gossypium hirsutum L.) higher under drip irrigation because of better phosphorus uptake. Appl. Ecol. Environ. Res. 2022, 20, 4865–4878. [Google Scholar] [CrossRef]
- Vitale, G.S.; Scavo, A.; Zingale, S.; Tuttolomondo, T.; Santonoceto, C.; Pandino, G.; Lombardo, S.; Anastasi, U.; Guarnaccia, P. Agronomic Strategies for Sustainable Cotton Production: A Systematic Literature Review. Agriculture 2024, 14, 1597. [Google Scholar] [CrossRef]
- Eyupoglu, S. Organic Cotton and Environmental Impacts; Springer: Singapore, 2019; pp. 157–176. [Google Scholar] [CrossRef]
- Bevilacqua, M.; Ciarapica, F.E.; Mazzuto, G.; Paciarotti, C. Environmental Analysis of a Cotton Yarn Supply Chain. J. Clean. Prod. 2014, 82, 154–165. [Google Scholar] [CrossRef]
- La Rosa, A.D.; Grammatikos, S.A. Comparative Life Cycle Assessment of Cotton and Other Natural Fibers for Textile Applications. Fibers 2019, 7, 101. [Google Scholar] [CrossRef]
- USDA-National Agricultural Statistics Service-Surveys-Agricultural Chemical Use Program. Available online: https://www.nass.usda.gov/Surveys/Guide_to_NASS_Surveys/Chemical_Use/ (accessed on 7 July 2025).
- Vulchi, R.; Bagavathiannan, M.; Nolte, S.A. History of Herbicide-Resistant Traits in Cotton in the U.S. and the Importance of Integrated Weed Management for Technology Stewardship. Plants 2022, 11, 1189. [Google Scholar] [CrossRef] [PubMed]
- Huff, J.A.; Reynolds, D.B.; Dodds, D.M.; Irby, J.T. Glyphosate Tolerance in Enhanced Glyphosate-Resistant Cotton (Gossypium hirsutum). Weed Technol. 2010, 24, 289–294. [Google Scholar] [CrossRef]
- Langarica-Fuentes, A.; Straub, D.; Wimmer, B.; Thompson, K.; Nahnsen, S.; Huhn, C.; Kleindienst, S. Subtle Microbial Community Changes despite Rapid Glyphosate Degradation in Microcosms with Four German Agricultural Soils. Appl. Soil Ecol. 2024, 198, 105381. [Google Scholar] [CrossRef]
- Q&A on Glyphosate; World Health Organization: Geneva, Switzerland, 2016.
- Vasseur, C.; Serra, L.; El Balkhi, S.; Lefort, G.; Ramé, C.; Froment, P.; Dupont, J. Glyphosate Presence in Human Sperm: First Report and Positive Correlation with Oxidative Stress in an Infertile French Population. Ecotoxicol. Environ. Saf. 2024, 278, 116410. [Google Scholar] [CrossRef]
- Ballester, C.; Hornbuckle, J.; Brinkhoff, J.; Quayle, W.C. Effects of Three Frequencies of Irrigation and Nitrogen Rates on Lint Yield, Nitrogen Use Efficiency and Fibre Quality of Cotton under Furrow Irrigation. Agric. Water Manag. 2021, 248, 106783. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, H.; Li, W.; Zhao, Q.; Dai, J.; Tian, L.; Dong, H. Effects of Reduced Nitrogen Rate on Cotton Yield and Nitrogen Use Efficiency as Mediated by Application Mode or Plant Density. Field Crops Res. 2018, 218, 150–157. [Google Scholar] [CrossRef]
- Salehin, S.M.U.; Rajan, N.; Mowrer, J.; Casey, K.D.; Tomlinson, P.; Somenahally, A.; Bagavathiannan, M. Cover Crops in Organic Cotton Influence Greenhouse Gas Emissions and Soil Microclimate. Agron. J. 2025, 117, e21735. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.O.; White, S. The Story of Phosphorus: Global Food Security and Food for Thought. Glob. Environ. Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Mycorrhizal Fungi Can Dominate Phosphate Supply to Plants Irrespective of Growth Responses. Plant Physiol. 2003, 133, 16–20. [Google Scholar] [CrossRef]
- Costantini, E.A.C.; Lorenzetti, R. Soil Degradation Processes in the Italian Agricultural and Forest Ecosystems. Ital. J. Agron. 2013, 8, e28. [Google Scholar] [CrossRef]
- Acar, M.; Celik, I.; Budak, M.; Acir, N. Evaluating Soil Quality: A Comparative Study of Contrasting Soils Using Geospatial and Analytical Methods in Çukurova Region, Turkey. Eurasian Soil Sci. 2025, 58, 95. [Google Scholar] [CrossRef]
- Ortega, E.; Lozano, F.J.; Martínez, F.J.; Bienes, R.; Gallardo, J.F.; Asensio, C. Soils of the Mediterranean Areas. In The Soils of Spain; Springer: Cham, Switzerland, 2016; pp. 163–187. [Google Scholar] [CrossRef]
- Thompson, J.P.; Seymour, N.P.; Clewett, T.G. Stunted Cotton (Gossypium hirsutum L.) Fully Recovers Biomass and Yield of Seed Cotton after Delayed Root Inoculation with Spores of an Arbuscular Mycorrhizal Fungus (Glomus mosseae). Australas. Plant Pathol. 2012, 41, 431–437. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Update on Arbuscular Mycorrhizas and Phosphorus Nutrition Roles of Arbuscular Mycorrhizas in Plant Phosphorus Nutrition: Interactions between Pathways of Phosphorus Uptake in Arbuscular Mycorrhizal Roots Have Important Implications for Understanding and Manipulating Plant Phosphorus Acquisition 1. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Mai, W.; Xue, X.; Feng, G.; Tian, C. Simultaneously Maximizing Root/Mycorrhizal Growth and Phosphorus Uptake by Cotton Plants by Optimizing Water and Phosphorus Management. BMC Plant Biol. 2018, 18, 334. [Google Scholar] [CrossRef]
- Ortas, I.; Iqbal, M.T. Mycorrhizal Inoculation Enhances Growth and Nutrition of Cotton Plant. J. Plant Nutr. 2019, 42, 2043–2056. [Google Scholar] [CrossRef]
- Zhou, J.; Chai, X.; Zhang, L.; George, T.S.; Wang, F.; Feng, G. Different Arbuscular Mycorrhizal Fungi Cocolonizing on a Single Plant Root System Recruit Distinct Microbiomes. mSystems 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Wang, J.; Du, G.; Tian, J.; Zhang, Y.; Jiang, C.; Zhang, W. Effect of Irrigation Methods on Root Growth, Root-Shoot Ratio and Yield Components of Cotton by Regulating the Growth Redundancy of Root and Shoot. Agric. Water Manag. 2020, 234, 106120. [Google Scholar] [CrossRef]
- Li, X. The Role of Funneliformis Mosseae Symbiosis on Cotton Plants under Lead Toxicity: Molecular and Physiological Aspects. Russ. J. Plant Physiol. 2024, 71, 83. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Monaco, A.L.; Ruta, C.; Mauromicale, G. Mycorrhizal Inoculation Improves Plant Growth and Yield of Micropropagated Early Globe Artichoke under Field Conditions. Agriculture 2022, 12, 114. [Google Scholar] [CrossRef]
- IstatData. Available online: https://esploradati.istat.it/databrowser/#/it (accessed on 7 July 2025).
- Tuttolomondo, T.; Virga, G.; Rossini, F.; Anastasi, U.; Licata, M.; Gresta, F.; La Bella, S.; Santonoceto, C. Effects of Environment and Sowing Time on Growth and Yield of Upland Cotton (Gossypium hirsutum L.) Cultivars in Sicily (Italy). Plants 2020, 9, 1209. [Google Scholar] [CrossRef]
- Lotti, G. Official Methods of Analysis, 22nd Edition (2023)—AOAC INTERNATIONAL. Available online: https://www.aoac.org/official-methods-of-analysis/ (accessed on 23 July 2025).
- Kjeldahl, J. Neue Methode Zur Bestimmung Des Stickstoffs in Organischen Körpern. Zeitschrift für Analytische Chemie 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists; Volume 1, Agricultural Chemicals; Contaminants; Drugs; AOAC International: Rockville, MD, USA, 1990. [Google Scholar]
- Dane, J.H.; Topp, G.C.; Campbell, G.S.; Horton, R.; Jury, W.A.; Nielsen, D.R.; van Es, H.M.; Wierenga, P.J.; Al-Amoodi, L.; Dick, W.A. Methods of Soil Analysis, Part 4: Physical Methods; Soil Science Society of America: Madison, WI, USA, 2018; pp. 1–1692. [Google Scholar] [CrossRef]
- Sumner, M.E.; Miller, W.P. Cation Exchange Capacity and Exchange Coefficients. In Methods of Soil Analysis, Part 3: Chemical Methods; Soil Science Society of America: Madison, WI, USA, 2018; pp. 1201–1229. [Google Scholar] [CrossRef]
- Blake, G.R.; Hartge, K.H. Bulk Density. In Methods of Soil Analysis, Part 1: Physical and Mineralogical Methods; Soil Science Society of America: Madison, WI, USA, 2018; pp. 363–375. [Google Scholar] [CrossRef]
- Klute, A. Water Retention: Laboratory Methods. In Methods of Soil Analysis, Part 1: Physical and Mineralogical Methods; Soil Science Society of America: Madison, WI, USA, 2018; pp. 635–662. [Google Scholar] [CrossRef]
- Flint, A.L.; Flint, L.E. 2.2 Particle Density. In Methods of Soil Analysis. Part 4, Physical Methods; Soil Science Society of America: Madison, WI, USA, 2018. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper 56; Fao: Rome, Italy, 1998. [Google Scholar]
- Zhangjin; Soothar, R.K.; Shar, S.U.; Alharthi, B.; Shaikh, I.A.; Laghari, M.; Das Suthar, J.; Samoon, A.; Jamali, N.A.; Fiaz, S.; et al. Responses of Cotton Yield and Water Productivity to Irrigation Management: Assessment of Economic Costs, Interactive Effects of Deficit Irrigation Water and Soil Types. Discover Life 2025, 55, 1–18. [Google Scholar] [CrossRef]
- Lai, Z.; Fan, J.; Yang, R.; Xu, X.; Liu, L.; Li, S.; Zhang, F.; Li, Z. Interactive Effects of Plant Density and Nitrogen Rate on Grain Yield, Economic Benefit, Water Productivity and Nitrogen Use Efficiency of Drip-Fertigated Maize in Northwest China. Agric Water Manag 2022, 263, 107453. [Google Scholar] [CrossRef]
- Filintas, A.; Nteskou, A.; Kourgialas, N.; Gougoulias, N.; Hatzichristou, E. A Comparison between Variable Deficit Irrigation and Farmers’ Irrigation Practices under Three Fertilization Levels in Cotton Yield (Gossypium hirsutum L.) Using Precision Agriculture, Remote Sensing, Soil Analyses, and Crop Growth Modeling. Water 2022, 14, 2654. [Google Scholar] [CrossRef]
- Moussouraki, M.A.; Tani, E.; Velliou, A.; Goufa, M.; Psychogiou, M.; Papadakis, I.E.; Abraham, E.M. Growth, Physiological and Biochemical Responses of Two Greek Cotton Cultivars to Salt Stress and Their Impact as Selection Indices for Salt Tolerance. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 706–715. [Google Scholar] [CrossRef]
- Lancashire, P.D.; Bleiholder, H.; Boom, T.V.D.; Langelüddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A Uniform Decimal Code for Growth Stages of Crops and Weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Gowda, P.H.; Baumhardt, R.L.; Esparza, A.M.; Marek, T.H.; Howell, T.A. Suitability of Cotton as an Alternative Crop in the Ogallala Aquifer Region. Agron. J. 2007, 99, 1397–1403. [Google Scholar] [CrossRef]
- Beegum, S.; Reddy, K.R.; Ambinakudige, S.; Reddy, V. Planting for Perfection: How to Maximize Cotton Fiber Quality with the Right Planting Dates in the Face of Climate Change. Field Crops Res. 2024, 315, 109483. [Google Scholar] [CrossRef]
- Shah, M.A.; Farooq, M.; Shahzad, M.; Khan, M.B.; Hussain, M. Yield and Phenological Responses of Bt Cotton to Different Sowing Dates in Semi-Arid Climate. J. Agric. Sci. 2017, 54, 233–239. [Google Scholar] [CrossRef]
- Zhao, Y.; Bian, Q.; Dong, Z.; Rao, X.; Wang, Z.; Fu, Y.; Chen, B. The Input of Organic Fertilizer Can Improve Soil Physicochemical Properties and Increase Cotton Yield in Southern Xinjiang. Front. Plant Sci. 2025, 15, 1520272. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-Z.; Chu, K.-Y.; Tang, H.-Y.; Nie, Y.-C.; Zhang, X.-L. Fertilizer 15 N Accumulation, Recovery and Distribution in Cotton Plant as Affected by N Rate and Split. J. Integr. Agric. 2013, 12, 999–1007. [Google Scholar] [CrossRef]
- Wang, H.; Li, G.; Huang, W.; Li, Z.; Wang, X.; Gao, Y. Compensation of Cotton Yield by Nitrogen Fertilizer in Non-Mulched Fields with Deficit Drip Irrigation. Agric. Water Manag. 2024, 298, 108850. [Google Scholar] [CrossRef]
- St Aime, R.; Rhodes, G.; Jones, M.; Campbell, B.T.; Narayanan, S. Evaluation of Root Traits and Water Use Efficiency of Different Cotton Genotypes in the Presence or Absence of a Soil-Hardpan. Crop J. 2021, 9, 945–953. [Google Scholar] [CrossRef]
- Bryant, C.J.; Locke, M.A.; Krutz, L.J.; Reynolds, D.B.; Golden, B.R.; Irby, T.; Steinriede, R.W.; Spencer, G.D. Furrow-Irrigation Application Efficiency in Mid-Southern U.S. Conservation Tillage Systems. Agron. J. 2021, 113, 397–406. [Google Scholar] [CrossRef]
- Chauhdary, J.N.; Li, H.; Jiang, Y.; Pan, X.; Hussain, Z.; Javaid, M.; Rizwan, M. Advances in Sprinkler Irrigation: A Review in the Context of Precision Irrigation for Crop Production. Agronomy 2023, 14, 47. [Google Scholar] [CrossRef]
- Bai, Z.; Li, Z.; Li, L.; Li, P.; Gong, P.; Wang, T.; Fan, J.; Liu, H. Deep Vertical Rotary Tillage Reduced Soil Salinity and Improved Seed Cotton Yield and Water Productivity under Limited Irrigation in Saline-Alkaline Fields. Ind. Crops Prod. 2024, 218, 118943. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, N.; Wang, L.; Lin, T.; Zheng, Z.; Cui, J.; Tian, L. Subsoiling Depth Affects the Morphological and Physiological Traits of Roots in Film-Mulched and Drip-Irrigated Cotton. Soil Tillage Res. 2023, 234, 105826. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Z.; Zhang, J.; Zhou, B.; Lv, T.; Li, W. Effects of Soil Texture on Soil Leaching and Cotton (Gossypium hirsutum L.) Growth under Combined Irrigation and Drainage. Water 2021, 13, 3614. [Google Scholar] [CrossRef]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of Salinity Stress in Plants by Arbuscular Mycorrhizal Symbiosis: Current Understanding and New Challenges. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Jajoo, A.; Mathur, S. Role of Arbuscular Mycorrhizal Fungi as an Underground Saviuor for Protecting Plants from Abiotic Stresses. Physiol. Mol. Biol. Plants 2021, 27, 2589–2603. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, M.; Sharma, L.; Kaushik, P.; Singh, A.; Sharma, M.M. Mycorrhiza: An Ecofriendly Bio-Tool for Better Survival of Plants in Nature. Sustainability 2022, 14, 10220. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, M.; Ma, X.; Guo, C.; Li, M.; Zhao, W.; Liu, Y.; Ma, F. Variation of Nitrogen, Phosphorus, and Potassium Contents in Drip-Irrigated Cotton at Different Yield Levels under Combined Effects of Nitrogen, Phosphorus and Potassium. Agronomy 2024, 14, 503. [Google Scholar] [CrossRef]
- Melillo, M.; Brunetti, M.T.; Peruccacci, S.; Gariano, S.L.; Guzzetti, F. Rainfall Thresholds for the Possible Landslide Occurrence in Sicily (Southern Italy) Based on the Automatic Reconstruction of Rainfall Events. Landslides 2016, 13, 165–172. [Google Scholar] [CrossRef]
- Istipliler, D.; Ekizoğlu, M.; Çakaloğulları, U.; Tatar, Ö. The Impact of Environmental Variability on Cotton Fiber Quality: A Comparative Analysis of Primary Cotton-Producing Regions in Türkiye. Agronomy 2024, 14, 1276. [Google Scholar] [CrossRef]
- Gao, X.; Guo, H.; Zhang, Q.; Guo, H.; Zhang, L.; Zhang, C.; Gou, Z.; Liu, Y.; Wei, J.; Chen, A.; et al. Arbuscular Mycorrhizal Fungi (AMF) Enhanced the Growth, Yield, Fiber Quality and Phosphorus Regulation in Upland Cotton (Gossypium hirsutum L.). Sci. Rep. 2020, 10, 2084. [Google Scholar] [CrossRef]
- Gao, H.; Li, N.; Li, J.; Khan, A.; Ahmad, I.; Wang, Y.; Wang, F.; Luo, H. Improving Boll Capsule Wall, Subtending Leaves Anatomy and Photosynthetic Capacity Can Increase Seed Cotton Yield under Limited Drip Irrigation Systems. Ind. Crops Prod. 2021, 161, 113214. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, D.; Sun, Y.; Li, P.; Xiang, D.; Zhang, Y.; Yang, M.; Gou, L.; Tian, J.; Zhang, W. Cotton Harvest Aids Promote the Translocation of Bur-Stored Photoassimilates to Enhance Single Boll Weight. Ind. Crops Prod. 2023, 195, 116375. [Google Scholar] [CrossRef]
- Xu, W.; Chen, P.; Zhan, Y.; Chen, S.; Zhang, L.; Lan, Y. Cotton Yield Estimation Model Based on Machine Learning Using Time Series UAV Remote Sensing Data. Int. J. Appl. Earth Obs. Geoinf. 2021, 104, 102511. [Google Scholar] [CrossRef]
- Yang, L.; Duan, J.; Liu, Y.; Hu, W.; Liu, X.; Wang, Y.; Zhou, Z.; Zhao, W. Changes in Carbohydrate Distribution in Cotton Photosynthetic Organs and Increase in Boll Weight Reduce Yield Loss under High Temperature. J. Exp. Bot. 2024, 75, 3483–3499. [Google Scholar] [CrossRef]
- Hou, X.; Li, H.; Ding, R.; Du, T. Hydraulic, Morphological, and Anatomical Changes over the Development of Cotton Bolls and Pedicels Leading to Boll Opening under Well-Watered and Water Deficit Conditions. Environ. Exp. Bot. 2024, 228, 105996. [Google Scholar] [CrossRef]
- Herritt, M.T.; Thompson, A.; Thorp, K. Irrigation Management Impacts on Cotton Reproductive Development and Boll Distribution. Crop Sci. 2022, 62, 1559–1572. [Google Scholar] [CrossRef]
- Ferrol, N.; Pozo, M.J.; Antelo, M.; Azcón-Aguilar, C. Arbuscular Mycorrhizal Symbiosis Regulates Plasma Membrane H+-ATPase Gene Expression in Tomato Plants. J. Exp. Bot. 2002, 53, 13–1687. [Google Scholar] [CrossRef]
- Salmeron-santiago, I.A.; Martínez-trujillo, M.; Valdez-alarcón, J.J.; Pedraza-santos, M.E.; Santoyo, G.; Pozo, M.J.; Chávez-bárcenas, A.T. An Updated Review on the Modulation of Carbon Partitioning and Allocation in Arbuscular Mycorrhizal Plants. Microorganisms 2021, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Madouh, T.A.; Quoreshi, A.M. The Function of Arbuscular Mycorrhizal Fungi Associated with Drought Stress Resistance in Native Plants of Arid Desert Ecosystems: A Review. Diversity 2023, 15, 391. [Google Scholar] [CrossRef]
- Pathan, S.I.; Ganugi, P.; Arfaioli, P.; Masoni, A.; Pietramellara, G. Resilience of Root and Soil Bacteria to Drought Stress Depends on Host Plant’s Colonization Affinity towards Arbuscular Mycorrhiza Fungi. Eur. J. Soil Biol. 2023, 118, 103540. [Google Scholar] [CrossRef]
- Ma, J.; Ding, Y.; Zhang, J.; Bai, Y.; Cui, B.; Hao, X.; Zheng, M.; Ding, B.; Yang, S. Impact of “Dry Sowing and Wet Emergence” Water Regulation on Physiological Growth Characteristics and Water Productivity of Cotton Fields in Southern Xinjiang Province. Agronomy 2024, 14, 734. [Google Scholar] [CrossRef]
- Vories, E.D.; Hogan, R.; Tacker, P.L.; Glover, R.E.; Lancaster, S.W. Estimating the Impact of Delaying Irrigation for Midsouth Cotton on Clay Soil. Trans. ASABE 2007, 50, 929–937. [Google Scholar] [CrossRef]
- Mauget, S.A.; Himanshu, S.K.; Goebel, T.S.; Ale, S.; Lascano, R.J.; Gitz, D.C. Soil and Soil Organic Carbon Effects on Simulated Southern High Plains Dryland Cotton Production. Soil Tillage Res. 2021, 212, 105040. [Google Scholar] [CrossRef]
- Raphael, J.P.A.; Gazola, B.; Nunes, J.G.S.; Macedo, G.C.; Rosolem, C.A. Cotton Germination and Emergence under High Diurnal Temperatures. Crop Sci. 2017, 57, 2761–2769. [Google Scholar] [CrossRef]
- Bange, M.P.; Long, R.L.; Caton, S.J.; Finger, N. Prediction of Upland Cotton Micronaire Accounting for the Effects of Environment and Crop Demand from Fruit Growth. Crop Sci. 2022, 62, 397–409. [Google Scholar] [CrossRef]
- Luo, Q.; Bange, M.; Clancy, L. Cotton Crop Phenology in a New Temperature Regime. Ecol. Model. 2014, 285, 22–29. [Google Scholar] [CrossRef]
- Tian, Y.; Shi, F.; Shi, X.; Zhang, H.; Li, Z.; Lin, H.; Luo, H.; Chenu, K. Improving Cotton Productivity and Nitrogen Use Efficiency through Late Nitrogen Fertilization: Evidence from a Three-Year Field Experiment in the Xinjiang. Field Crops Res. 2024, 313, 109433. [Google Scholar] [CrossRef]




| Soil Characteristics | Unit | Value | Method | |
|---|---|---|---|---|
| Site 1 | Site 2 | |||
| Sand | % | 59 | 16.6 | [39] |
| Loam | % | 13 | 27.8 | [39] |
| Clay | % | 28 | 55.6 | [39] |
| N total | g kg−1 | 1.3 | 1 | Kjeldahl [40] |
| P | mg kg−1 | 9.16 | 2.18 | Ferrari [41] |
| K | mg kg−1 | 112.9 | 203.3 | Dirks and Scheffer [41] |
| Organic matter | % | 1.46 | 1.1 | Walkley and Black [41] |
| Electrical Conductivity | mS/cm | 0.8643 | 0.15 | [42] |
| Cation Exchange Capacity (CEC) | meq/% | 27.05 | 14.8 | [43] |
| pH | 7.4 | 7.6 | In water solution | |
| Bulk Density | t m3 | 1.16 | 1.2 | [44] |
| Field Capacity at −0.03 MPa | % | 27.5 | 27 | [45] |
| Wilting Point at −1.5 MPa | % | 16.7 | 11 | [46] |
| Phase | Description | Kc | Depth of Soil Explored by Roots (cm) |
|---|---|---|---|
| Initial | Germination: from dry seed (00) to emergence of hypocotyl with cotyledons (09) | 0.4–0.5 | 30 |
| Development | Leaf development: from cotyledons completely unfolded (10) to canopy closure (39) | 0.7–0.8 | 50 |
| Mid-season | Inflorescence emergence: from first detectable bud (51) to about 90% of capsules having reached their final size (79) | 1.05–1.25 | 50 |
| End-season | Senescence: from about 10% of discolored or abscessed leaves (91) to above-ground parts of dead plants | 0.65–0.70 | 50 |
| Irrigation Level | Phenological Phase | Site 1 | Site 2 | ||
|---|---|---|---|---|---|
| Rainfall (m3 ha−1) | Irrigation (m3 ha−1) | Rainfall (m3 ha−1) | Irrigation (m3 ha−1) | ||
| I-30 | Initial (BBCH 00–09) | 163.4 | 58.1 | 589 | 95 |
| I-30 | Development (BBCH 10–50) | 92.3 | 0 | 17 | 0 |
| I-30 | Mid-season (BBCH 51–79) | 365.3 | 0 | 335 | 0 |
| I-30 | End-season (BBCH 80–89) | 62.4 | 0 | 49 | 0 |
| Total water supplied (m3 ha−1) | 741.5 | 1085 | |||
| I-70 | Initial (BBCH 00–09) | 163.4 | 58.1 | 589 | 95 |
| I-70 | Development (BBCH 10–50) | 92.3 | 92.2 | 17 | 108.9 |
| I-70 | Mid-season (BBCH 51–79) | 365.3 | 90.7 | 335 | 115 |
| I-70 | End-season (BBCH 80–89) | 62.4 | 0 | 49 | 0 |
| Total water supplied (m3 ha−1) | 924.4 | 1308.9 | |||
| I-100 | Initial (BBCH 00–09) | 163.4 | 58.1 | 589 | 83.2 |
| I-100 | Development (BBCH 10–50) | 92.3 | 127.3 | 17 | 154.6 |
| I-100 | Mid-season (BBCH 51–79) | 365.3 | 141.7 | 335 | 250 |
| I-100 | End-season (BBCH 80–89) | 62.4 | 0 | 49 | 0 |
| Total water supplied (m3 ha−1) | 1010.5 | 1477.8 | |||
| Source of Variation | Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| df | Plant Height | First Fruiting Branch Height | Number of Capsules ha−1 × 10−6 | Average Capsule Weight | Raw Yield | Lint Yield | Seed Yield | |
| G | 1 | 0.01 ns | 12.17 *** | 1.16 ns | 10.77 ** | 22.77 *** | 41.18 *** | 6.75 * |
| M | 1 | 54.91 *** | 2.3 ns | 27.15 *** | 18 *** | 46.35 *** | 42.9 *** | 29.98 *** |
| I | 2 | 591.55 *** | 74.6 *** | 52.4 *** | 205.68 *** | 612.07 *** | 533.75 *** | 418.65 *** |
| S | 1 | 5.81 * | 425.62 *** | 5.44 * | 6.34 * | 10.53 ** | 3.25 ns | 35.48 *** |
| G × M | 1 | 0.06 ns | 12.92 *** | 2.05 ns | 0.04 ns | 0.04 ns | 0.24 ns | 0 ns |
| G × I | 2 | 5.91 ** | 3.49 * | 7.66 *** | 1.63 ns | 6.01 ** | 10.39 *** | 1.95 ns |
| G × S | 1 | 0.05 ns | 8.78 ** | 2.56 ns | 7.42 ** | 0.03 ns | 1.91 ns | 1.46 ns |
| M × I | 2 | 0.42 ns | 2.57 ns | 1.24 ns | 1.26 ns | 3.94 * | 2.21 ns | 3.56 * |
| M × S | 1 | 25.97 *** | 5.42 * | 13.57 *** | 0.07 ns | 0.02 ns | 1.86 ns | 1.22 ns |
| I × S | 2 | 28.16 *** | 10.24 *** | 7.09 ** | 9.71 *** | 1.8 ns | 1.37 ns | 2.64 ns |
| G × M × I | 2 | 15.6 *** | 3.88 * | 3.06 ns | 5.82 ** | 1.02 ns | 2.34 ns | 0.39 ns |
| G × M× S | 1 | 8.93 ** | 12.04 *** | 3.89 ns | 0.07 ns | 0.42 ns | 0.48 ns | 0.22 ns |
| G × I × S | 2 | 3.29 * | 9.45 *** | 9.16 *** | 4.92 * | 6.27 ** | 11.86 *** | 1.98 ns |
| M × I × S | 2 | 2.15 ns | 1.46 ns | 9.3 *** | 2.41 ns | 1.8 ns | 0.32 ns | 3.09 ns |
| G × M × I × S | 2 | 4.89 * | 5.74 ** | 3.74 * | 2.49 ns | 0.16 ns | 1 ns | 0.34 ns |
| Source of Variation | Parameters | ||||
|---|---|---|---|---|---|
| df | S–Em | Em–F | F–Oc | Oc–H | |
| G | 1 | 0.27 n.s. | 0.39 n.s. | 3.88 n.s. | 8.57 ** |
| M | 1 | 0.12 n.s. | 0.96 n.s. | 1.86 n.s. | 14.56 *** |
| I | 2 | 4.83 * | 164.94 *** | 54.57 *** | 237.74 *** |
| S | 1 | 0.76 n.s. | 38.81 *** | 28.12 *** | 11.98 ** |
| G × M | 1 | 0.76 n.s. | 0.07 n.s. | 1.12 n.s. | 0.07 n.s. |
| G × I | 2 | 0.48 n.s. | 8.75 ** | 8.92 ** | 31.25 *** |
| G × S | 1 | 0 n.s. | 51 *** | 14.8 *** | 1.77 n.s. |
| M × I | 2 | 1.64 n.s. | 0.49 n.s. | 0.28 n.s. | 1.28 n.s. |
| M × S | 1 | 0.27 n.s. | 1.14 n.s. | 0.4 n.s. | 0.95 n.s. |
| I × S | 2 | 0.73 n.s. | 3.35 * | 130.18 *** | 51.05 *** |
| G × M × I | 2 | 0.33 n.s. | 0.29 n.s. | 0.71 n.s. | 0.5 n.s. |
| G × M × S | 1 | 3.03 n.s. | 0.03 n.s. | 0.452 n.s. | 4.17 * |
| G × I × S | 2 | 1.3 n.s. | 1.31 n.s. | 7.8 ** | 72.31 *** |
| M × I × S | 2 | 2.07 n.s. | 1.43 n.s. | 0.02 n.s. | 0.29 n.s. |
| G × M × I × S | 2 | 0.14 n.s. | 0.436 n.s. | 0.29 n.s. | 1.43 n.s. |
| Water Supplied (m3 ha−1) | IWP (kg m−3) | |||
|---|---|---|---|---|
| Site | Irrigation level | Seed Yield | Lint Yield | |
| Site 1 | ||||
| I-30 | 741.5 | 0.93 | 0.54 | |
| I-70 | 924.4 | 1.22 | 0.83 | |
| I-100 | 1010.5 | 1.43 | 0.95 | |
| Site 2 | ||||
| I-30 | 1203.9 | 0.52 | 0.37 | |
| I-70 | 1308.9 | 0.72 | 0.61 | |
| I-100 | 1477.8 | 0.93 | 0.69 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, G.S.; Iacuzzi, N.; Tortorici, N.; Indovino, G.; Franco, L.; Mosca, C.; Giovino, A.; Scavo, A.; Lombardo, S.; Tuttolomondo, T.; et al. Sustainable Cotton Production in Sicily: Yield Optimization Through Varietal Selection, Mycorrhizae, and Efficient Water Management. Agronomy 2025, 15, 1892. https://doi.org/10.3390/agronomy15081892
Vitale GS, Iacuzzi N, Tortorici N, Indovino G, Franco L, Mosca C, Giovino A, Scavo A, Lombardo S, Tuttolomondo T, et al. Sustainable Cotton Production in Sicily: Yield Optimization Through Varietal Selection, Mycorrhizae, and Efficient Water Management. Agronomy. 2025; 15(8):1892. https://doi.org/10.3390/agronomy15081892
Chicago/Turabian StyleVitale, Giuseppe Salvatore, Nicolò Iacuzzi, Noemi Tortorici, Giuseppe Indovino, Loris Franco, Carmelo Mosca, Antonio Giovino, Aurelio Scavo, Sara Lombardo, Teresa Tuttolomondo, and et al. 2025. "Sustainable Cotton Production in Sicily: Yield Optimization Through Varietal Selection, Mycorrhizae, and Efficient Water Management" Agronomy 15, no. 8: 1892. https://doi.org/10.3390/agronomy15081892
APA StyleVitale, G. S., Iacuzzi, N., Tortorici, N., Indovino, G., Franco, L., Mosca, C., Giovino, A., Scavo, A., Lombardo, S., Tuttolomondo, T., & Guarnaccia, P. (2025). Sustainable Cotton Production in Sicily: Yield Optimization Through Varietal Selection, Mycorrhizae, and Efficient Water Management. Agronomy, 15(8), 1892. https://doi.org/10.3390/agronomy15081892











