Suspension Fertilizers Based on Waste Organic Matter from Peanut Oil Extraction By-Products
Abstract
1. Introduction
- (1)
- Grinding of Raw Materials
- (2)
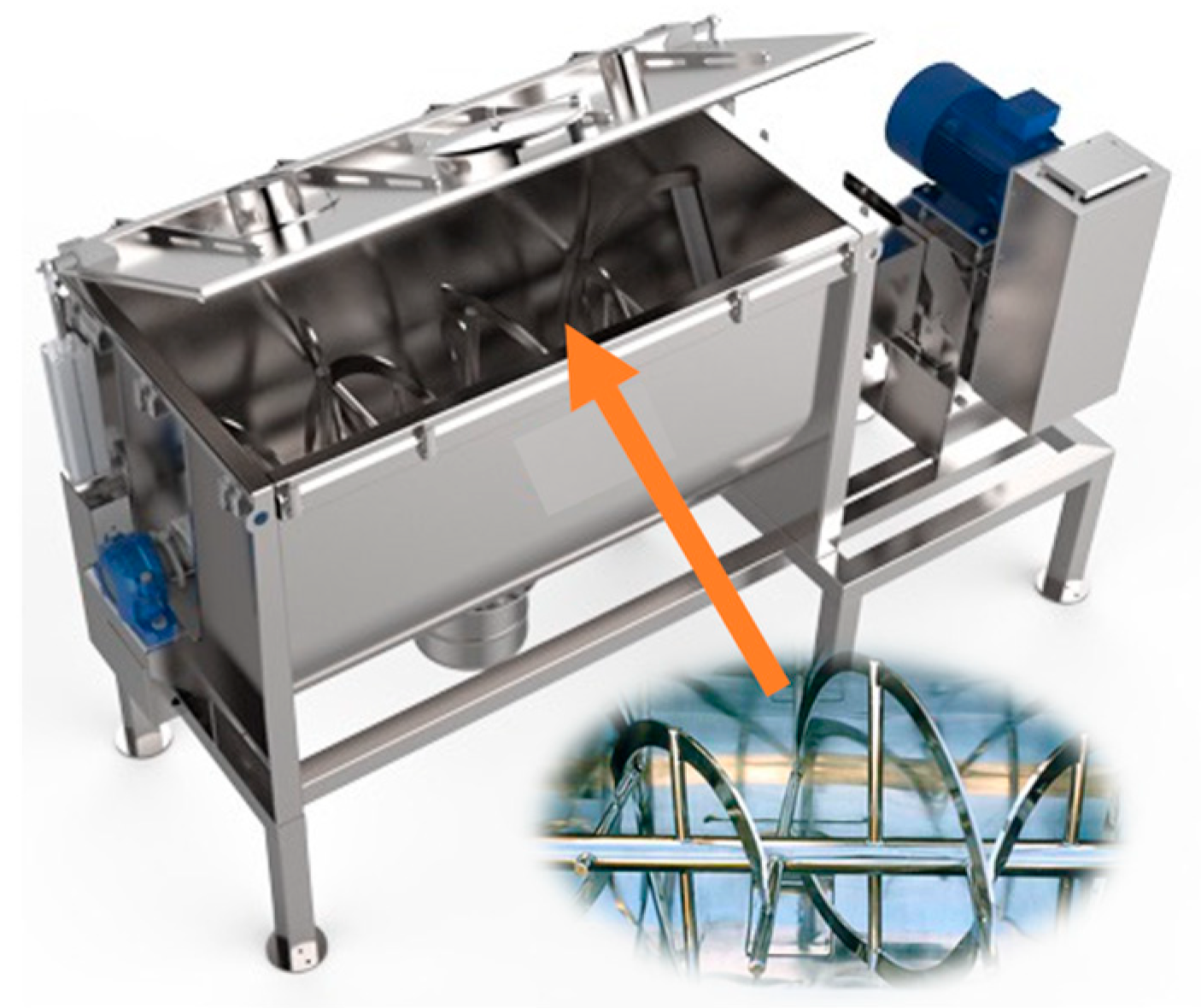
- Manufacturing of Suspension System
- (3)
- Mixing and Dispersing
2. Materials and Methods
2.1. Fertilizer Raw Materials
2.2. Auxiliary Agent
2.2.1. Stabilizing Agent
2.2.2. Emulsifier and Suspending Agent
2.3. Research Methodology
2.3.1. Sample Preparation
- (1)
- Temperature treatments:
- (2)
- Formulation trials:
- ①
- Single-factor screening: bran 16–30% (w/w); clay minerals 0–1%.
- ②
- Orthogonal optimization: L16(44) matrix examining four levels each of monoglyceride, sucrose ester, κ-carrageenan and xanthan gum, using 7-day sedimentation ratio as the response.
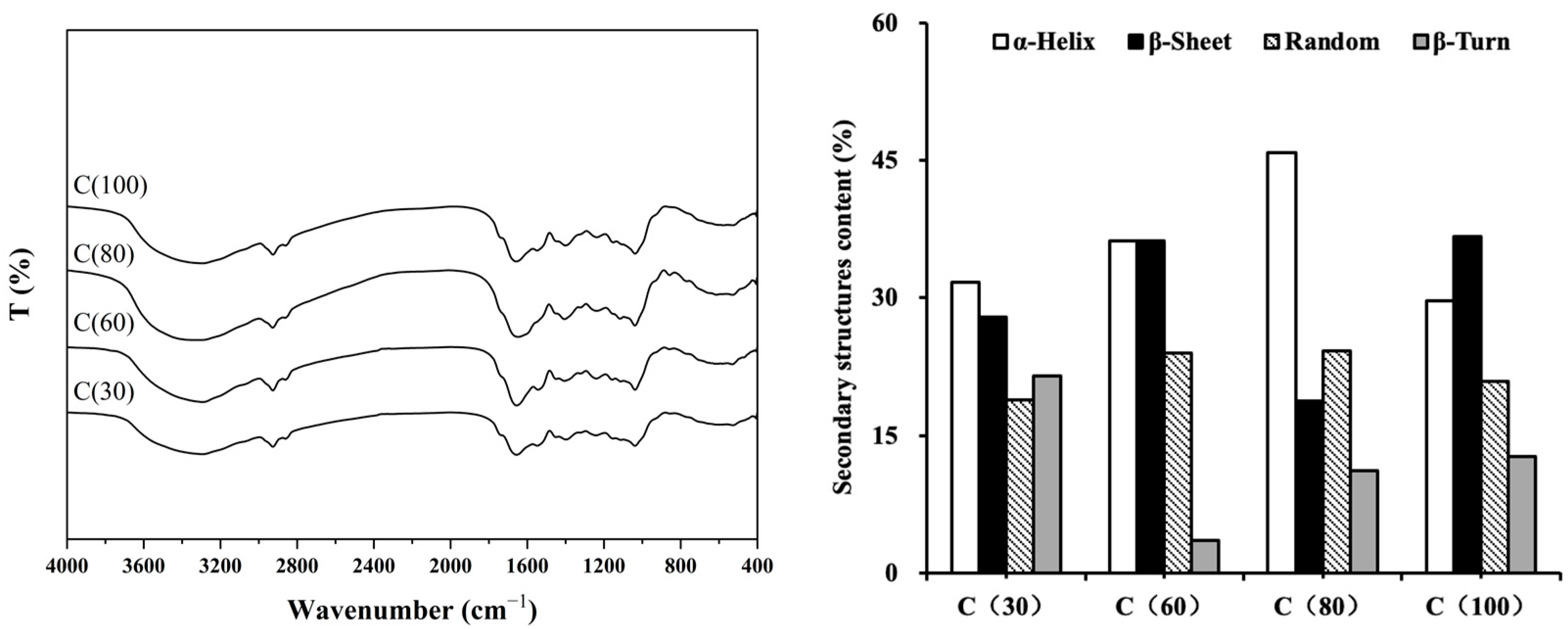
2.3.2. FT-IR Analysis and Spectrum Processing
2.3.3. Stability Tests
2.3.4. Castability Test
2.4. Statistical Analysis
3. Results and Discussion
3.1. Heating Temperature for Stable Suspension
- (1)
- Effect of Different Temperatures on Pure Peanut Bran Dispersions
- (2)
- Effect of Different Temperatures on Suspension Fertilizers
3.2. Suspension Fertilizer Formulation
- (1)
- Peanut Bran Usage
- (2)
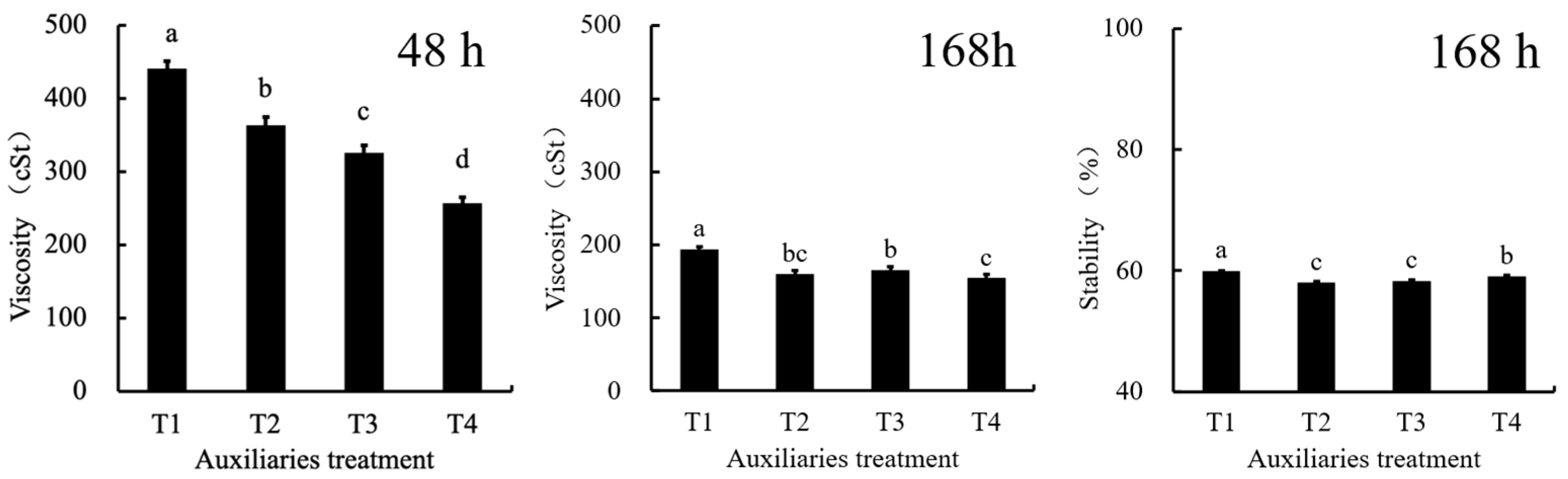
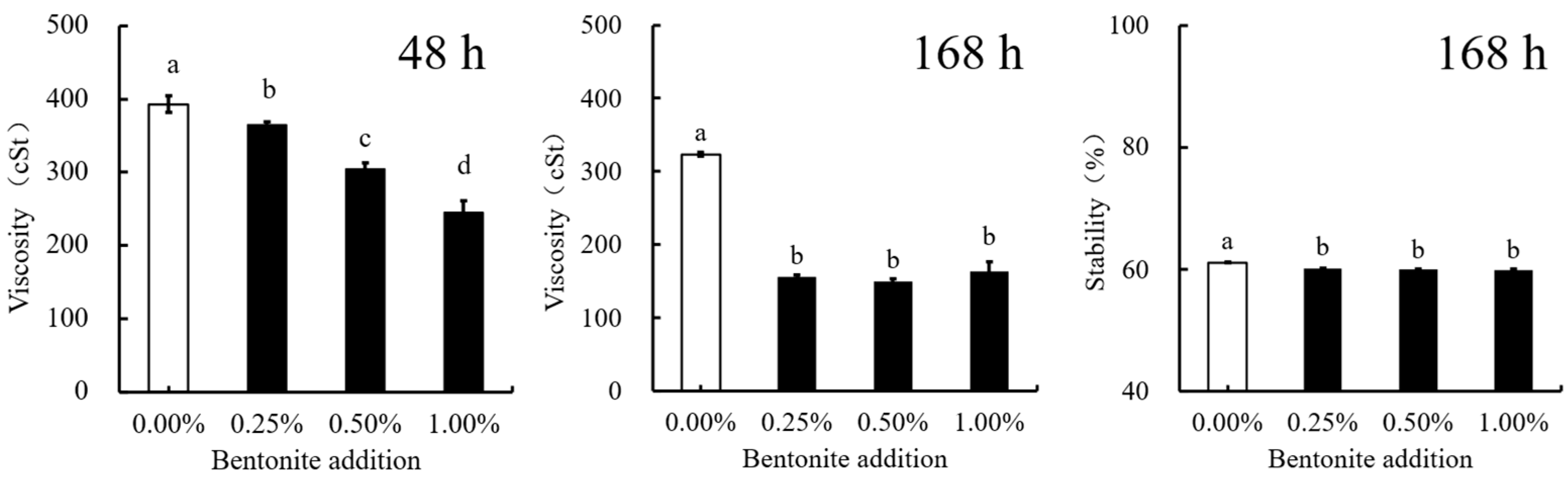
- Stabilizing Agent Selection
- (3)
- Emulsifier and Suspending Agent Selection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Yang, Y.; Xie, Y.; Zeng, Y.; Li, K.; Hu, L. Improving soil carbon sequestration stability in Siraitia grosvenorii farmland through co-application of rice straw and its biochar. Front. Plant Sci. 2024, 15, 1470486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tian, Y. Research on the spatio-temporal coupling relationship between agricultural green development efficiency and food security system in China. Heliyon 2024, 10, e31893. [Google Scholar] [CrossRef]
- Shen, G.; Chen, Y.; Zhang, J.; Wu, Y.; Yi, Y.; Li, S.; Yin, S. Quantitative analysis of index factors in agricultural compost by infrared spectroscopy. Heliyon 2023, 9, e14010. [Google Scholar] [CrossRef]
- Zheng, P.; Li, Y.; Qi, Y. Exploring the drivers of agricultural wages growth in China: A comprehensive framework utilizing input-output and structural decomposition methods. PLoS ONE 2024, 19, e0299067. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, Y.; Xu, D.; Yang, J.; Wang, X.; Luo, T.; Zhang, Z. Hydrolysis Mechanism of Water-Soluble Ammonium Polyphosphate Affected by Zinc Ions. ACS Omega 2023, 8, 17573–17582. [Google Scholar] [CrossRef]
- Mikła, D.; Hoffmann, K.; Hoffmann, J. Production of suspension fertilizers as a potential way of managing industrial waste. Pol. J. Chem. Technol. 2007, 9, 9–11. [Google Scholar] [CrossRef]
- Hinsui, J.; Krasae, K.; Jantaratch, N.; Mahae, N. Protein and water recovery from tuna defrosting wastewater. Heliyon 2024, 10, e26669. [Google Scholar] [CrossRef]
- Bogusz, P.; Rusek, P.; Brodowska, M.S. Suspension Fertilizers Based on Waste Phosphates from the Production of Polyols. Molecules 2022, 27, 7916. [Google Scholar] [CrossRef]
- Bogusz, P.; Rusek, P.; Brodowska, M.S. Suspension Fertilizers: How to Reconcile Sustainable Fertilization and Environmental Protection. Agriculture 2021, 11, 1008. [Google Scholar] [CrossRef]
- Florowska, A.; Hilal, A.; Florowski, T.; Wroniak, M. Addition of Selected Plant-Derived Proteins as Modifiers of Inulin Hydrogels Properties. Foods 2020, 9, 845. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, M.; Glenn-Davi, K.; Mohammadi, N.; Fitzgerald, R.J. Key Factors Influencing Gelation in Plant vs. Animal Proteins: A Comparative Mini-Review. Gels 2024, 10, 575. [Google Scholar] [CrossRef]
- Sebesta, M.; Nemcek, L.; Urik, M.; Kolencik, M.; Bujdos, M.; Vavra, I.; Dobrocka, E.; Matus, P. Partitioning and stability of ionic, nano- and microsized zinc in natural soil suspensions. Sci. Total Environ. 2020, 700, 134445. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lv, X.; Qin, Y.; Zhang, D.; Zhang, C.; Song, Z.; Liu, D.; Jiang, L.; Huang, B.; Wang, J. Effects of different botanical oil meal mixed with cow manure organic fertilizers on soil microbial community and function and tobacco yield and quality. Front. Microbiol. 2023, 14, 1191059. [Google Scholar] [CrossRef]
- Yang, T.; Wang, P.; Zhou, Q.; Wang, X.; Cai, J.; Huang, M.; Jiang, D. Investigation on the Molecular and Physicochemical Changes of Protein and Starch of Wheat Flour during Heating. Foods 2021, 10, 1419. [Google Scholar] [CrossRef]
- Charan, H.; Glebe, U.; Anand, D.; Kinzel, J.; Zhu, L.; Bocola, M.; Garakani, T.M.; Schwaneberg, U.; Boker, A. Nano-thin walled micro-compartments from transmembrane protein-polymer conjugates. Soft Matter 2017, 13, 2866–2875. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.B. Structural and functional properties of self-assembled peanut protein nanoparticles prepared by ultrasonic treatment: Effects of ultrasound intensity and protein concentration. Food Chem. 2023, 413, 135626. [Google Scholar] [CrossRef]
- Fadimu, G.J.; Le, T.T.; Gill, H.; Farahnaky, A.; Olatunde, O.O.; Truong, T. Enhancing the Biological Activities of Food Protein-Derived Peptides Using Non-Thermal Technologies: A Review. Foods 2022, 11, 1823. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Tao, Y.; Chen, H.; Zhang, D.; Wang, C. Hydrothermal-induced changes in the gel properties of Mung bean proteins and their effect on the cooking quality of developed compound noodles. Front. Nutr. 2022, 9, 957487. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Roos, Y.H.; Miao, S. Plant Protein versus Dairy Proteins: A pH-Dependency Investigation on Their Structure and Functional Properties. Foods 2023, 12, 368. [Google Scholar] [CrossRef]
- Matthias, B. Denaturation of proteins: Electrostatic effects vs. hydration. RSC Adv. 2022, 12, 10105–10113. [Google Scholar]
- Guo, Y.; Ma, C.; Xu, Y.; Du, L.; Yang, X. Food Gels Based on Polysaccharide and Protein: Preparation, Formation Mechanisms, and Delivery of Bioactive Substances. Gels 2024, 10, 735. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Sun, L.; Guan, C.; Ren, T.; Zhang, Q.; Pan, S.; Zhang, Q.; Chen, H. Preparation and Properties of Salecan-Soy Protein Isolate Composite Hydrogel Induced by Thermal Treatment and Transglutaminase. Int. J. Mol. Sci. 2022, 23, 9383. [Google Scholar] [CrossRef]
- Pang, M.; Liu, Z.; Li, H.; Liang, L.; Li, L. Effect of Fatty Acids on Vegetable-Oil-Derived Sustainable Polyurethane Coatings for Controlled-Release Fertilizer. Coatings 2024, 14, 1183. [Google Scholar] [CrossRef]
- Bhakay, A.; Rahman, M.; Dave, R.N.; Bilgili, E. Bioavailability Enhancement of Poorly Water-Soluble Drugs via Nanocomposites: Formulation-Processing Aspects and Challenges. Pharmaceutic 2018, 10, 86. [Google Scholar] [CrossRef]
- Cholakova, D.; Tcholakova, S. Sucrose ester surfactants: Current understanding and emerging perspectives. Curr. Opin. Colloid. Interface Sci. 2024, 73, 101832. [Google Scholar] [CrossRef]
- Zhang, S.; Bao, Z.; Wu, Y.; Wang, Y.; Liu, R.; Gao, Y.; Zhao, X.; Zhang, C.; Du, F. Enhancing the Stability and Effectivity of Multiple Pesticide Formulation Mixtures by Adding an Eco-Friendly Adjuvant. ACS Sustain. Chem. Eng. 2023, 11, 15385–15396. [Google Scholar] [CrossRef]
- Hejrani, T.; Sheikholeslami, Z.; Mortazavi, A.; Davoodi, M.G. The properties of part baked frozen bread with guar and xanthan gums. Food Hydrocoll. 2016, 71, 252–257. [Google Scholar] [CrossRef]
- Sutariya, S.G.; Salunke, P. Effect of Hyaluronic Acid and Kappa-Carrageenan on Milk Properties: Rheology, Protein Stability, Foaming, Water-Holding, and Emulsification Properties. Foods 2023, 12, 913. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.K.; Greis, M.; Lu, J.; Nolden, A.A.; McClements, D.J.; Kinchla, A.J. Functional Performance of Plant Proteins. Foods 2022, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Roos, Y.H.; Miao, S. Structure, gelation mechanism of plant proteins versus dairy proteins and evolving modification strategies. Trends Food Sci. Technol. 2024, 147, 104464. [Google Scholar] [CrossRef]
- Wang, H.; Li, W.; Luo, S.; Hu, X.; Liu, C. Gelling ability and gel structure of soy protein isolate influenced by heating in the presence of various acids. Food Chem. 2025, 464, 141745. [Google Scholar] [CrossRef]
- Spöttel, J.; Brockelt, J.; Falke, S.; Rohn, S. Characterization of Conjugates between α-Lactalbumin and Benzyl Isothiocyanate-Effects on Molecular Structure and Proteolytic Stability. Molecules 2021, 26, 6247. [Google Scholar] [CrossRef]
- Pan, T.; Xu, J.L.; Sun, Q. Editorial: The future food analysis. Front. Nutr. 2022, 9, 1014322. [Google Scholar] [CrossRef]
- Wei, L.; Ren, Y.; Huang, L.; Ye, X.; Li, H.; Li, J.; Cao, J.; Liu, X. Quality, Thermo-Rheology, and Microstructure Characteristics of Cubic Fat Substituted Pork Patties with Composite Emulsion Gel Composed of Konjac Glucomannan and Soy Protein Isolate. Gels 2024, 10, 111. [Google Scholar] [CrossRef]
- Macdougall, L.J.; Pérez-Madrigal, M.M.; Arno, M.C.; Dove, A.P. Nonswelling Thiol-Yne Cross-Linked Hydrogel Materials as Cytocompatible Soft Tissue Scaffolds. Biomacromolecules 2018, 19, 1378–1388. [Google Scholar] [CrossRef]
- Wang, K.; Ren, X.; Yang, F.; Huang, Y.; Zhang, K.; Liu, C.; Huang, C. Study on the Gel Properties of Soy and Pea Protein Isolate Composites Induced by Heat. China Condiment 2022, 47, 7–11. (In Chinese) [Google Scholar]
- Tao, Y.; Hasan, A.; Deeb, G.; Hu, C.; Han, H. Rheological and Mechanical Behavior of Silk Fibroin Reinforced Waterborne Polyurethane. Polymers 2016, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Totea, A.M.; Sabin, J.; Dorin, I.; Hemming, K.; Laity, P.R.; Conway, B.R.; Waters, L.; Asare-Addo, K. Thermodynamics of clay-drug complex dispersions: Isothermal titration calorimetry and high-performance liquid chromatography. J. Pharm. Anal. 2020, 10, 78–85. [Google Scholar] [CrossRef]
- Cui, Y.; Tan, Z. Experimental Study of High Performance Synchronous Grouting Materials Prepared with Clay. Materials 2021, 14, 1362. [Google Scholar] [CrossRef]
- Yao, X.; Xu, J.; Xun, Y.; Du, T.; Huang, M.; Guo, J. High gelatinous salted duck egg white protein powder gel: Physicochemical, microstructure and techno-functional properties. Front. Nutr. 2023, 10, 1110786. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Binks, B.; Cui, Z. Edible oil-in-water emulsions stabilized by hydrophile–lipophile balanced sucrose ester. J. Am. Oil Chem. Soc. 2023, 100, 711–721. [Google Scholar] [CrossRef]






| Order | Patentee/Applicant | City | Country | Applications |
|---|---|---|---|---|
| 1 | Guangzhou Yixiang Agricultural Technology Co., Ltd. | Guangzhou | China | 9 |
| 2 | South China Agriculture University | Guangzhou | China | 6 |
| 3 | Shenzhen Batian Ecotypic Engineering Co., Ltd. | Shenzhen | China | 4 |
| 4 | Dongguan Desheng Fertilizer Technology Co., Ltd. | Dongguan | China | 3 |
| 5 | Guangdong Fengkang Biotechnology Co., Ltd. | Guangzhou | China | 3 |
| 6 | Kingenta Ecological Engineering Group Co., Ltd. | Linyi | China | 2 |
| 7 | Woda Agricultural Technology Co., Ltd. | Beijing | China | 2 |
| 8 | South Subtropical Crops Research Institute CATAS | Zhanjiang | China | 2 |
| 9 | Environment and Soil Fertilizer Research Institute FJAAS | Fuzhou | China | 2 |
| Parameter | Content (%) | Fertilizing Ingredients | Value (%) |
|---|---|---|---|
| Protein | 51.40 | N | 6.45 |
| Oil | 0.40 | P2O5 | 1.17 |
| Ash | 5.61 | K2O | 1.42 |
| Starch | 17.30 | Ca | 0.84 |
| Soluble Saccharide | 10.87 | Mg | 0.19 |
| Sample | Factors | HLB | Stability (%) | |||
|---|---|---|---|---|---|---|
| A Monoglyceride | B Sucrose Ester | C Carrageenan | D Xanthan Gum | |||
| 1 | 0.00 | 0.30 | 0.00 | 0.10 | 13.0 | 37.81 |
| 2 | 0.00 | 0.10 | 0.06 | 0.20 | 13.0 | 44.44 |
| 3 | 0.10 | 0.00 | 0.04 | 0.30 | 3.5 | 59.99 |
| 4 | 0.30 | 0.10 | 0.04 | 0.00 | 5.9 | 45.91 |
| 5 | 0.30 | 0.20 | 0.00 | 0.10 | 7.3 | 43.09 |
| 6 | 0.10 | 0.30 | 0.00 | 0.20 | 10.6 | 48.37 |
| 7 | 0.20 | 0.10 | 0.00 | 0.30 | 6.7 | 59.36 |
| 8 | 0.30 | 0.30 | 0.06 | 0.30 | 8.3 | 61.50 |
| 9 | 0.10 | 0.20 | 0.06 | 0.00 | 9.8 | 50.22 |
| 10 | 0.20 | 0.00 | 0.06 | 0.10 | 3.5 | 39.25 |
| 11 | 0.30 | 0.00 | 0.02 | 0.20 | 3.5 | 48.00 |
| 12 | 0.00 | 0.20 | 0.02 | 0.30 | 13.0 | 59.95 |
| 13 | 0.10 | 0.10 | 0.02 | 0.10 | 8.3 | 44.26 |
| 14 | 0.20 | 0.30 | 0.02 | 0.00 | 9.2 | 48.15 |
| 15 | 0.00 | 0.00 | 0.00 | 0.00 | - | 48.72 |
| 16 | 0.20 | 0.20 | 0.04 | 0.20 | 8.3 | 49.39 |
| K1 | 190.91 | 195.95 | 199.54 | 192.98 | ||
| K2 | 202.83 | 193.96 | 200.36 | 164.41 | ||
| K3 | 196.14 | 202.64 | 193.08 | 190.19 | ||
| K4 | 198.49 | 195.82 | 195.40 | 240.80 | ||
| R | 11.92 | 8.68 | 7.27 | 76.39 | ||
| Excellent level | A2 | B3 | C2 | D4 | ||
| Priority order | D > A > B > C | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, S.; Li, B.; Lyu, Y. Suspension Fertilizers Based on Waste Organic Matter from Peanut Oil Extraction By-Products. Agronomy 2025, 15, 1885. https://doi.org/10.3390/agronomy15081885
Xiang S, Li B, Lyu Y. Suspension Fertilizers Based on Waste Organic Matter from Peanut Oil Extraction By-Products. Agronomy. 2025; 15(8):1885. https://doi.org/10.3390/agronomy15081885
Chicago/Turabian StyleXiang, Sainan, Baoshen Li, and Yang Lyu. 2025. "Suspension Fertilizers Based on Waste Organic Matter from Peanut Oil Extraction By-Products" Agronomy 15, no. 8: 1885. https://doi.org/10.3390/agronomy15081885
APA StyleXiang, S., Li, B., & Lyu, Y. (2025). Suspension Fertilizers Based on Waste Organic Matter from Peanut Oil Extraction By-Products. Agronomy, 15(8), 1885. https://doi.org/10.3390/agronomy15081885






