Multistrain Microbial Inoculant Enhances Yield and Medicinal Quality of Glycyrrhiza uralensis in Arid Saline–Alkali Soil and Modulate Root Nutrients and Microbial Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiment Site and Design
2.2. Sample Preparation and Analysis
2.3. High-Throughput Sequencing of Rhizospheric Microbiota and Data Processing
2.4. Data Analysis
3. Results
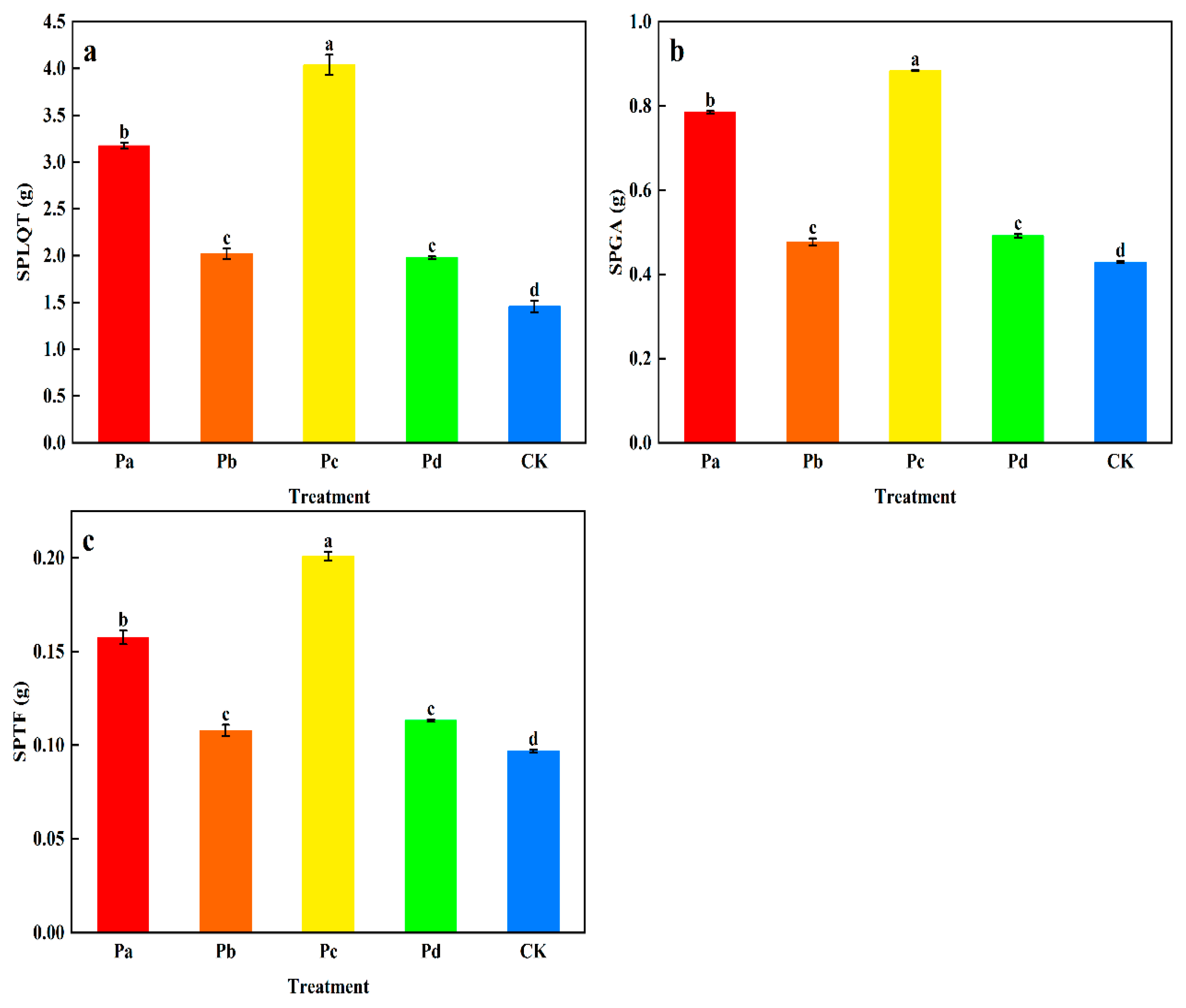
3.1. Effects of Different Microbial Inoculants on Growth Indices, Yield, and Component Content of G. uralensis
3.2. Effect of Inoculant Pc Application on Rhizospheric Soil Physicochemical Properties of G. uralensis in Saline–Alkali Soil
3.3. Effect of Inoculant Pc Application on Rhizospheric Soil Microbial Diversity of G. Uralensis in Saline–Alkali Soil
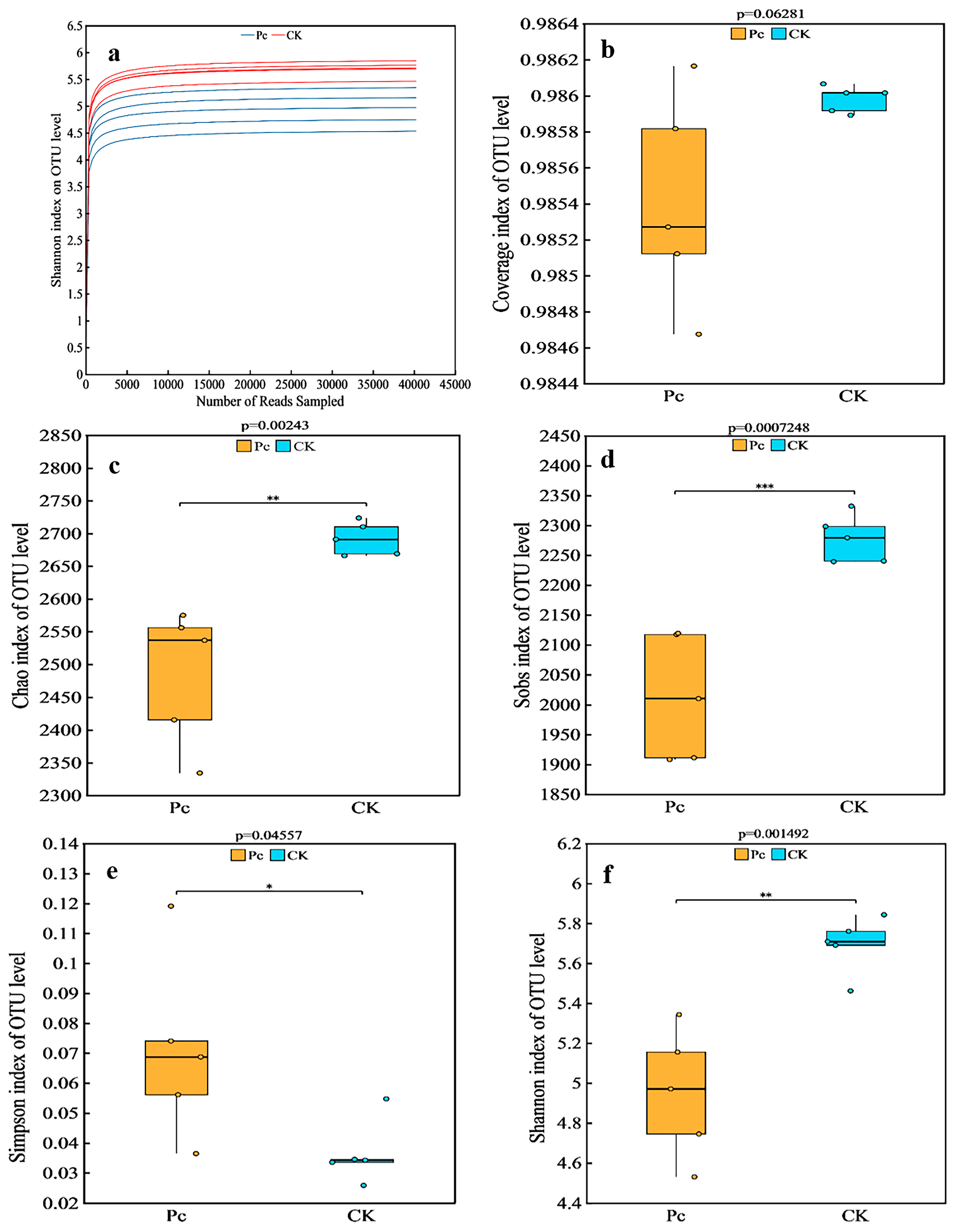
3.3.1. Sequencing Depth and α Diversity of the Sampled Soils
3.3.2. β Diversity of Microbial Communities in the Sampled Soils
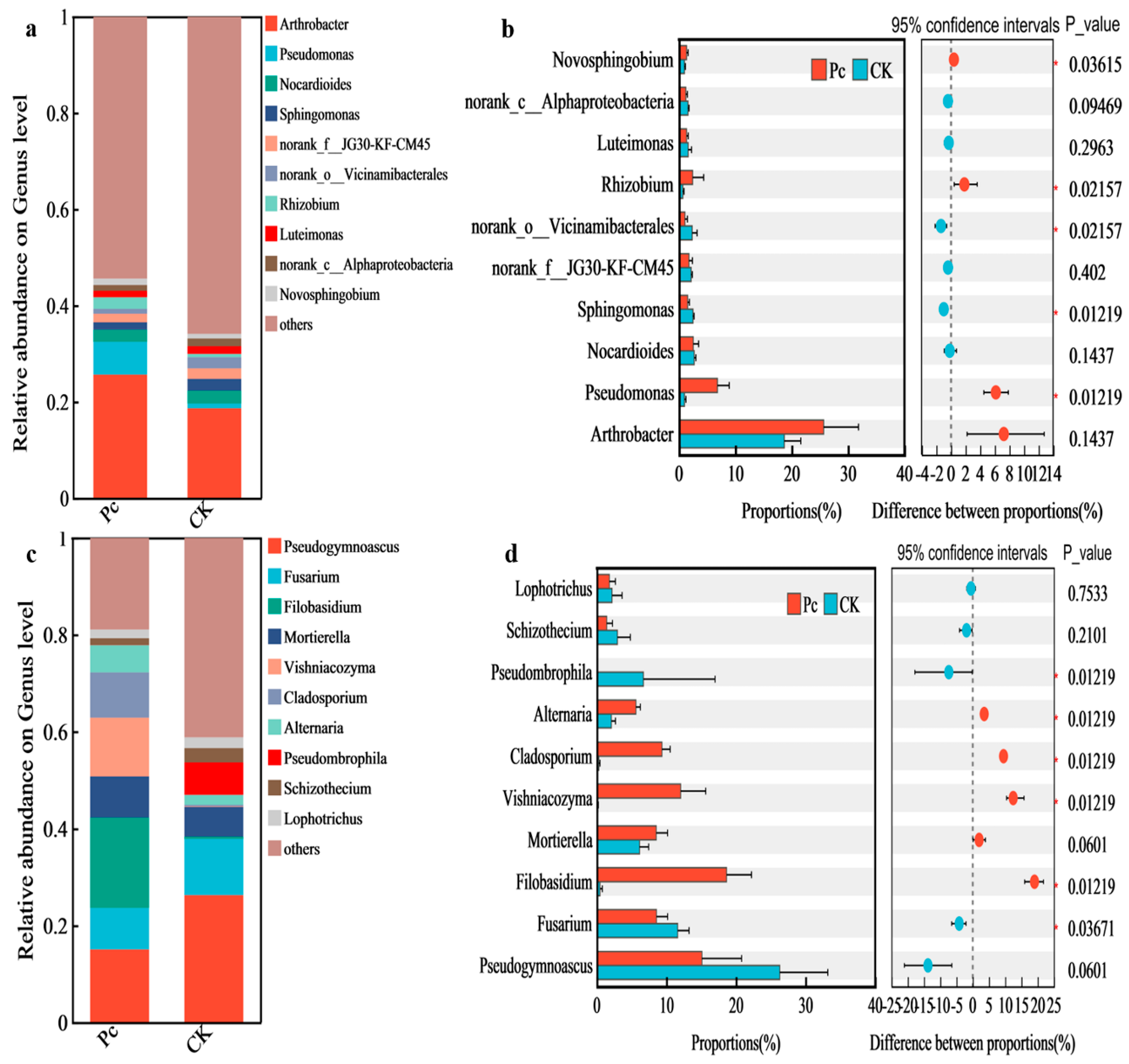
3.3.3. Relative Abundance and Differential Analysis of Dominant Rhizospheric Genera of Microbial Communities in the Sampled Soils
3.4. Correlations Between Dominant Rhizospheric Microbes in G. Uralensis and Soil Nutrients, Plant Growth, and Bioactive Compounds
4. Discussion
4.1. Microbial Inoculant-Mediated Enhancement of G. uralensis Performance
4.2. Impact on Rhizospheric Soil Properties
4.3. Microbial Community Restructuring by Pc Inoculation
4.4. Mechanistic Links Between Microbes, Nutrients, and Plant Traits
4.5. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRL | Main root length |
| MRD | Main root diameter |
| LRN | Number of lateral roots |
| SPDW | Single-plant dry weight |
| SPLQT | Single-plant liquiritin |
| SPGA | Single-plant glycyrrhizic acid |
| SPTF | Single-plant total flavonoids |
| SSS | Soil soluble salt |
| SOM | Soil organic matter |
| TN | Total nitrogen |
| AP | Available phosphorus |
| AK | Available potassium |
| NO3−-N | Nitrate nitrogen |
References
- FAO. Global Map of Salt Affected Soils Version 1.0. Available online: https://www.fao.org/soils-portal/data-hub/soil-maps-and-databases/global-map-of-salt-affected-soils/zh/ (accessed on 1 March 2025).
- Hopmans, J.W.; Qureshi, A.S.; Kisekka, I.; Munns, R.; Grattan, S.R.; Rengasamy, P.; Ben-Gal, A.; Assouline, S.; Javaux, M.; Minhas, P.S.; et al. Chapter One-Critical Knowledge Gaps and Research Priorities in Global Soil Salinity. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, UK, 2021; Volume 169, pp. 1–191. [Google Scholar]
- Wang, L.; Zhao, Z.Y.; Zhang, K.; Tian, C.Y. Reclamation and utilization of saline soils in arid northwestern China: A promising halophyte drip-irrigation system. Environ. Sci. Technol. 2013, 47, 5518–5519. [Google Scholar] [CrossRef] [PubMed]
- Gansu Provincial Soil Survey Office. Gansu Soil; China Agricultural Press: Beijing, China, 1993.
- Nan, L.; Guo, Q.; Cao, S.; Zhan, Z. Diversity of bacterium communities in saline-alkali soil in arid regions of Northwest China. BMC Microbiol. 2022, 22, 11. [Google Scholar] [CrossRef]
- Kerbab, S.; Silini, A.; Chenari Bouket, A.; Cherif-Silini, H.; Eshelli, M.; El Houda Rabhi, N.; Belbahri, L. Mitigation of NaCl Stress in Wheat by Rhizosphere Engineering Using Salt Habitat Adapted PGPR Halotolerant Bacteria. Appl. Sci. 2021, 11, 1034. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Shaygan, M.; Baumgartl, T. Reclamation of Salt-Affected Land: A Review. Soil Syst. 2022, 6, 61. [Google Scholar] [CrossRef]
- Singh, K.; Pandey, V.C.; Singh, B.; Singh, R.R. Ecological restoration of degraded sodic lands through afforestation and cropping. Ecol. Eng. 2012, 43, 70–80. [Google Scholar] [CrossRef]
- Oster, J.D.; Jayawardane, N.S. Agricultural Management of Sodic Soils. In Sodic Soil: Distribution, Management and Environmental Consequences; Oxford University Press: Oxford, UK, 1998; pp. 126–147. [Google Scholar]
- Keyes, K.L.; Mott, J.B.; Barnes, S.S.; Jensen, D.A. Remediation of brine contaminated soil using Atriplex spp. (Chenopodiaceae). In Proceedings of the Internationl Oil Spill Conference, Seattle, WA, USA, 7–12 March, 1999; pp. 757–764. [Google Scholar]
- You, Q.G.; Xue, X.; Huang, C.H. Preliminary Study on the Effects of Saline Water Irrigation on Soil Salinization in Deep Groundwater Area:A case study of Minqin oasis. J. Desert Res. 2011, 31, 302–308. [Google Scholar]
- Birru, G.A.; Clay, D.E.; DeSutter, T.M.; Reese, C.L.; Kennedy, A.C.; Clay, S.A.; Bruggeman, S.A.; Owen, R.K.; Malo, D.D. Chemical Amendments of Dryland Saline–Sodic Soils Did Not Enhance Productivity and Soil Health in Fields without Effective Drainage. Agron. J. 2019, 111, 496–508. [Google Scholar] [CrossRef]
- Hasnain, M.; Abideen, Z.; Ali, F.; Hasanuzzaman, M.; El-Keblawy, A. Potential of Halophytes as Sustainable Fodder Production by Using Saline Resources: A Review of Current Knowledge and Future Directions. Plants 2023, 12, 2150. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Zhao, Z.; Zhang, K.; Tian, C.; Mai, W. Progress of Euhalophyte Adaptation to Arid Areas to Remediate Salinized Soil. Agriculture 2023, 13, 704. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, D.; Cao, D.; Chen, J.; Wei, X. Exploring the potentials of Sesuvium portulacastrum L. for edibility and bioremediation of saline soils. Front. Plant Sci. 2024, 15, 1387102. [Google Scholar] [CrossRef]
- Zhang, Z.; He, K.; Zhang, T.; Tang, D.; Li, R.; Jia, S. Physiological responses of Goji berry (Lycium barbarum L.) to saline-alkaline soil from Qinghai region, China. Sci. Rep. 2019, 9, 12057. [Google Scholar] [CrossRef]
- Omer, E.; Ahl, H.S.-A.; El Gendy, A.G. Yield and Essential Oil of Ajwain (Trachyspermum ammi) Plant Cultivated in Saline Soil of North Sinai in Egypt. J. Essent. Oil Bear. Plants 2014, 17, 469–477. [Google Scholar] [CrossRef]
- Muneeb, A.; Ahmad, I.; Ahmad, M.S.A.; Fatima, S.; Hameed, M.; Ahmad, F.; Asghar, A.; Basharat, S.; Shah, S.M.R.; Shafqat, J.; et al. Ethnobotanical and economic uses of some medicinal plants from native saline areas. Int. J. Appl. Exp. Biol. 2022, 2, 147–154. [Google Scholar] [CrossRef]
- Agrawal, S.; Singh, A.; Gawande, V.; Bhanuvally, M.; Mubeen; Gourkhede, P.H.; Kumar, A. Salt Stress Tolerance in Medicinal and Aromatic Plants: A Review. Int. J. Environ. Clim. Change 2023, 13, 3382–3391. [Google Scholar] [CrossRef]
- Ventura, Y.; Sagi, M. Halophyte crop cultivation: The case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Garcia-Caparros, P.; Al-Azzawi, M.J.; Flowers, T.J. Economic Uses of Salt-Tolerant Plants. Plants 2023, 12, 2669. [Google Scholar] [CrossRef]
- Alhaddad, F.A.; Abu-Dieyeh, M.H.; ElAzazi, E.M.; Ahmed, T.A. Salt tolerance of selected halophytes at the two initial growth stages for future management options. Sci. Rep. 2021, 11, 10194. [Google Scholar] [CrossRef]
- He, Q.; Silliman, B.R.; Cui, B. Incorporating thresholds into understanding salinity tolerance: A study using salt-tolerant plants in salt marshes. Ecol. Evol. 2017, 7, 6326–6333. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Cao, D.; Zhang, W.; Yang, N.; Li, Z.; Zhang, C.; Wang, D.; Ye, G.; Chen, J.; Wei, X. Proteomic and metabolomic analyses uncover integrative mechanisms in Sesuvium portulacastrum tolerance to salt stress. Front. Plant Sci. 2023, 14, 1277762. [Google Scholar] [CrossRef]
- Liu, A.; Xiao, Z.; Li, M.-W.; Wong, F.-L.; Yung, W.-S.; Ku, Y.-S.; Wang, Q.; Wang, X.; Xie, M.; Yim, A.K.-Y.; et al. Transcriptomic reprogramming in soybean seedlings under salt stress. Plant Cell Environ. 2019, 42, 98–114. [Google Scholar] [CrossRef]
- Li, T.; Ren, G.; Zhou, N.; Qiao, Z.; Li, M.; Yin, Y.; Jiang, D.; Liu, C. A new simplified synthetic endophyte community regulates the synthesis of active ingredients in Glycyrrhiza uralensis Fisch. Ind. Crops Prod. 2025, 227, 120781. [Google Scholar] [CrossRef]
- Zhong, R.; Wen, C.; Qiu, Y.; Shen, X.; Sun, Z.; Peng, L.; Liu, T.; Huang, S.; Peng, X. Anti-Inflammatory and Immunomodulatory Effects of Glycyrrhiza uralensis Fisch. on Ulcerative Colitis in Rats: Role of Nucleotide-binding oligomerization domain 2/Receptor-interacting protein 2/Nuclear factor-kappa B Signaling Pathway. J. Ethnopharmacol. 2025, 344, 119457. [Google Scholar] [CrossRef]
- Fang, Y.; Lin, Z.; Lv, Y.; Ma, C.; Wang, Z.; Dang, J.; Li, G. Isolation and preparation of flavonoids from Glycyrrhiza uralensis fisch. Using multi-dimensional chromatography for treating non-alcoholic fatty liver disease. J. Mol. Struct. 2025, 1340, 142516. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Wang, W.; Zhang, Y.; Yang, Z.; Jin, Y.; Ge, H.M.; Li, E.; Yang, G. Glycyrrhizic acid as the antiviral component of Glycyrrhiza uralensis Fisch. against coxsackievirus A16 and enterovirus 71 of hand foot and mouth disease. J. Ethnopharmacol. 2013, 147, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ma, Z.; Liao, X.-L.; Liu, J.; Fu, Q.; Ma, S. Immunoregulatory effects of Glycyrrhizic acid exerts anti-asthmatic effects via modulation of Th1/Th2 cytokines and enhancement of CD4+CD25+Foxp3+ regulatory T cells in ovalbumin-sensitized mice. J. Ethnopharmacol. 2013, 148, 755–762. [Google Scholar] [CrossRef]
- He, R.; Ma, T.-T.; Gong, M.-X.; Xie, K.-L.; Wang, Z.-M.; Li, J. The correlation between pharmacological activity and contents of eight constituents of Glycyrrhiza uralensis Fisch. Heliyon 2023, 9, e14570. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, J.; Li, Y.-J.; Zheng, Y.-F.; Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013, 141, 1063–1071. [Google Scholar] [CrossRef]
- Xiao, J.; Xiao, J.; Gao, P.; Zhang, Y.; Yan, B.; Wu, H.; Zhang, Y. Enhanced salt tolerance in Glycyrrhiza uralensis Fisch. via Bacillus subtilis inoculation alters microbial community. Microbiol. Spectr. 2024, 12, e03812–e03823. [Google Scholar] [CrossRef] [PubMed]
- Behdad, A.; Mohsenzadeh, S.; Azizi, M.; Moshtaghi, N. Salinity effects on physiological and phytochemical characteristics and gene expression of two Glycyrrhiza glabra L. populations. Phytochemistry 2020, 171, 112236. [Google Scholar] [CrossRef]
- Gu, J.; Yao, S.; Ma, M. Maternal Effects of Habitats Induce Stronger Salt Tolerance in Early-Stage Offspring of Glycyrrhiza uralensis from Salinized Habitats Compared with Those from Non-Salinized Habitats. Biology 2024, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Han, Y.N.; Gao, R.; Ma, M.; Zhao, H.Y. The Respondence of Morphology and Structure of Glycyrrhiza uralensis Seedling Under Salt Stress. Seed 2015, 34, 25–31+34. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, Q.; Ma, X.; Lang, D.; Guo, Z.; Zhang, X. Physiological Biochemistry-Combined Transcriptomic Analysis Reveals Mechanism of Bacillus cereus G2 Improved Salt-Stress Tolerance of Glycyrrhiza uralensis Fisch. Seedlings by Balancing Carbohydrate Metabolism. Front. Plant Sci. 2022, 12, 712363. [Google Scholar] [CrossRef]
- Makhanova, U.; Ibraeva, M. Phytoremediation of saline soils using Glycyrrhiza glabra for enhanced soil fertility in arid regions of South Kazakhstan. Eurasian J. Soil Sci. 2025, 14, 22–37. [Google Scholar] [CrossRef]
- Ha-Tran, D.M.; Nguyen, T.T.M.; Hung, S.-H.; Huang, E.; Huang, C.-C. Roles of Plant Growth-Promoting Rhizobacteria (PGPR) in Stimulating Salinity Stress Defense in Plants: A Review. Int. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially Mediated Plant Salt Tolerance and Microbiome-based Solutions for Saline Agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef]
- Chialva, M.; Lanfranco, L.; Bonfante, P. The plant microbiota: Composition, functions, and engineering. Curr. Opin. Biotechnol. 2022, 73, 135–142. [Google Scholar] [CrossRef]
- Chen, L.J.; Feng, Q.; Cheng, A.F. Spatial distribution of soil water and salt contents and reasons of saline soils’ development in the Minqin Oasis. J. Arid Land Resour. 2013, 27, 99–105. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China, Part 1 ed.; China Medical Science Press: Beijing, China, 2020; pp. 88–89.
- Liu, F.Z.; Yang, J. Effect of exogenous sucrose on growth and active ingredient content of licorice seedlings under salt stress conditions. China J. Chin. Mater. Med. 2015, 40, 4384–4388. [Google Scholar]
- Matić, P.; Sabljić, M.; Jakobek, L. Validation of Spectrophotometric Methods for the Determination of Total Polyphenol and Total Flavonoid Content. J. AOAC Int. 2019, 100, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000; pp. 25–114. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Q.; Yang, H.; Li, X.; Pei, P.Y.; Li, Z.G.; Zhao, J.; Wang, P.Y. Isolation and identification of Arthrobacter sp.GCG3 from glycyrrhiza rhizosphere and its antifungal activity and growth-promoting property. Jiangsu Agric. Sci 2024, 52, 247–255. [Google Scholar] [CrossRef]
- Csorba, C.; Rodić, N.; Zhao, Y.; Antonielli, L.; Brader, G.; Vlachou, A.; Tsiokanos, E.; Lalaymia, I.; Declerck, S.; Papageorgiou, V.P.; et al. Metabolite Production in Alkanna tinctoria Links Plant Development with the Recruitment of Individual Members of Microbiome Thriving at the Root-Soil Interface. mSystems 2022, 7, e00451-22. [Google Scholar] [CrossRef] [PubMed]
- Chamkhi, I.; Benali, T.; Aanniz, T.; El Menyiy, N.; Guaouguaou, F.-E.; El Omari, N.; El-Shazly, M.; Zengin, G.; Bouyahya, A. Plant-microbial interaction: The mechanism and the application of microbial elicitor induced secondary metabolites biosynthesis in medicinal plants. Plant Physiol. Biochem. 2021, 167, 269–295. [Google Scholar] [CrossRef]
- Tshikhudo, P.P.; Ntushelo, K.; Mudau, F.N. Sustainable Applications of Endophytic Bacteria and Their Physiological/Biochemical Roles on Medicinal and Herbal Plants: Review. Microorganisms 2023, 11, 453. [Google Scholar] [CrossRef]
- Ng, C.W.W.; Yan, W.H.; Xia, Y.T.; Tsim, K.W.K.; To, J.C.T. Plant growth-promoting rhizobacteria enhance active ingredient accumulation in medicinal plants at elevated CO2 and are associated with indigenous microbiome. Front. Microbiol. 2024, 15, 1426893. [Google Scholar] [CrossRef]
- Zamioudis, C.; Mastranesti, P.; Dhonukshe, P.; Blilou, I.; Pieterse, C.M.J. Unraveling Root Developmental Programs Initiated by Beneficial Pseudomonas spp. Bacteria. Plant Physiol. 2013, 162, 304–318. [Google Scholar] [CrossRef]
- Li, Q.; Li, H.; Yang, Z.; Cheng, X.; Zhao, Y.; Qin, L.; Bisseling, T.; Cao, Q.; Willemsen, V. Plant growth-promoting rhizobacterium Pseudomonas sp. CM11 specifically induces lateral roots. New Phytol 2022, 235, 1575–1588. [Google Scholar] [CrossRef]
- Chu, T.N.; Bui, L.V.; Hoang, M.T.T. Pseudomonas PS01 Isolated from Maize Rhizosphere Alters Root System Architecture and Promotes Plant Growth. Microorganisms 2020, 8, 471. [Google Scholar] [CrossRef]
- Chhetri, G.; Kim, I.; Kang, M.; So, Y.; Kim, J.; Seo, T. An Isolated Arthrobacter sp. Enhances Rice (Oryza sativa L.) Plant Growth. Microorganisms 2022, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, T.; Wu, P.; Wang, C.; Zheng, C. Recent advances in the nitrogen cycle involving actinomycetes: Current situation, prospect and challenge. Bioresour. Technol. 2025, 419, 132100. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yao, Q.; Gao, X.; Jiang, C.; Harberd, N.P.; Fu, X. Shoot-to-Root Mobile Transcription Factor HY5 Coordinates Plant Carbon and Nitrogen Acquisition. Curr. Biol. 2016, 26, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, I.; Nakao, T.; Maita, H.; Sato, S.; Chijiiwa, R.; Yamada, E.; Arima, S.; Kojoma, M.; Ishimaru, K.; Akashi, R.; et al. Mesorhizobium sp. J8 can establish symbiosis with Glycyrrhiza uralensis, increasing glycyrrhizin production:Original Papers. Plant Biotechnol. 2021, 38, 57–66. [Google Scholar] [CrossRef]
- Li, L.; Sinkko, H.; Montonen, L.; Wei, G.; Lindström, K.; Räsänen, L.A. Biogeography of symbiotic and other endophytic bacteria isolated from medicinal G lycyrrhiza species in C hina. FEMS Microbiol. Ecol. 2012, 79, 46–68. [Google Scholar] [CrossRef]
- Wen, Y.; Wu, R.; Qi, D.; Xu, T.; Chang, W.; Li, K.; Fang, X.; Song, F. The effect of AMF combined with biochar on plant growth and soil quality under saline-alkali stress: Insights from microbial community analysis. Ecotoxicol. Environ. Saf. 2024, 281, 116592. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil microbial inoculants for sustainable agriculture: Limitations and opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
- Basu, S.; Kumari, S.; Subhadarshini, P.; Rishu, A.K.; Shekhar, S.; Kumar, G. Plant growth promoting rhizobacterium Bacillus sp. BSE01 alleviates salt toxicity in chickpea (Cicer arietinum L.) by conserving ionic, osmotic, redox and hormonal homeostasis. Physiol. Plant. 2023, 175, e14076. [Google Scholar] [CrossRef]
- Peng, M.; Jiang, Z.; Zhou, F.; Wang, Z. From salty to thriving: Plant growth promoting bacteria as nature’s allies in overcoming salinity stress in plants. Front Microbiol 2023, 14, 1169809. [Google Scholar] [CrossRef]
- AbuQamar, S.F.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.-S.M.; Elrys, A.S.; El-Mageed, T.A.A.; Semida, W.M.; Abdelkhalik, A.; Mosa, W.F.A.; Al Kafaas, S.S.; et al. Halotolerant plant growth-promoting rhizobacteria improve soil fertility and plant salinity tolerance for sustainable agriculture—A review. Plant Stress 2024, 12, 100482. [Google Scholar] [CrossRef]
- Latif, A.; Ahmad, R.; Ahmed, J.; Mueen, H.; Khan, S.A.; Bibi, G.; Mahmood, T.; Hassan, A. Novel halotolerant PGPR strains alleviate salt stress by enhancing antioxidant activities and expression of selected genes leading to improved growth of Solanum lycopersicum. Sci. Hortic. 2024, 338, 113625. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, H.; Zhang, Z.; Sun, Z.; Liu, S.; Ma, C.; Wang, X.; Liu, Z. Effects of microbial communities during the cultivation of three salt-tolerant plants in saline-alkali land improvement. Front. Microbiol. 2024, 15, 1470081. [Google Scholar] [CrossRef]
- Tedeschi, A.; Schillaci, M.; Balestrini, R. Mitigating the impact of soil salinity: Recent developments and future strategies. Ital. J. Agron. 2023, 18, 2173. [Google Scholar] [CrossRef]
- Paz, A.M.; Amezketa, E.; Canfora, L.; Castanheira, N.; Falsone, G.; Goncalves, M.C.; Gould, I.; Hristov, B.; Mastrorilli, M.; Ramos, T.; et al. Salt-affected soils: Field-scale strategies for prevention, mitigation, and adaptation to salt accumulation. Ital. J. Agron. 2023, 18, 2166. [Google Scholar] [CrossRef]
- Thepbandit, W.; Athinuwat, D. Rhizosphere microorganisms supply availability of soil nutrients and Induce plant defense. Microorganisms 2024, 12, 558. [Google Scholar] [CrossRef] [PubMed]
- Askegaard, M.; Hansen, H.C.B.; Schjoerring, J.K. A cation exchange resin method for measuring long-term potassium release rates from soil. Plant Soil 2005, 271, 63–74. [Google Scholar] [CrossRef]
- Masood, S.; Bano, A. Mechanism of potassium solubilization in the agricultural soils by the help of soil microorganisms. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer India: New Delhi, India, 2016; pp. 137–147. [Google Scholar]
- Sharma, R.; Sindhu, S.S.; Glick, B.R. Potassium Solubilizing Microorganisms as Potential Biofertilizer: A Sustainable Climate-Resilient Approach to Improve Soil Fertility and Crop Production in Agriculture. J. Plant Growth Regul. 2024, 43, 2503–2535. [Google Scholar] [CrossRef]
- Nawaz, A.; Qamar, Z.U.; Marghoob, M.U.; Imtiaz, M.; Imran, A.; Mubeen, F. Contribution of potassium solubilizing bacteria in improved potassium assimilation and cytosolic K+/Na+ ratio in rice (Oryza sativa L.) under saline-sodic conditions. Front. Microbiol. 2023, 14, 1196024. [Google Scholar] [CrossRef]
- Prabhu, N.; Borkar, S.; Garg, S. Chapter 11-Phosphate solubilization by microorganisms: Overview, mechanisms, applications and advances. In Advances in Biological Science Research; Meena, S.N., Naik, M.M., Eds.; Academic Press: Cambridge, UK, 2019; pp. 161–176. [Google Scholar]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Illmer, P.; Schinner, F. Solubilization of inorganic calcium phosphates—Solubilization mechanisms. Soil Biol. Biochem. 1995, 27, 257–263. [Google Scholar] [CrossRef]
- He, D.; Wan, W. Distribution of Culturable Phosphate-Solubilizing Bacteria in Soil Aggregates and Their Potential for Phosphorus Acquisition. Microbiol. Spectr. 2022, 10, e0029022. [Google Scholar] [CrossRef]
- Bhagat, N.; Raghav, M.; Dubey, S.; Bedi, N. Bacterial Exopolysaccharides: Insight into Their Role in Plant Abiotic Stress Tolerance. J. Microbiol. Biotechnol. 2021, 31, 1045–1059. [Google Scholar] [CrossRef]
- Wu, N.; Pan, H.-X.; Qiu, D.; Zhang, Y.-M. Feasibility of EPS-producing bacterial inoculation to speed up the sand aggregation in the Gurbantunggut Desert, Northwestern China. J. Basic Microbiol. 2014, 54, 1378–1386. [Google Scholar] [CrossRef]
- Kümmerli, R. Iron acquisition strategies in pseudomonads: Mechanisms, ecology, and evolution. Biometals 2023, 36, 777–797. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Gu, S.; Zhang, X.; Xue, J.; Yan, T.; Guo, S.; Pommier, T.; Jousset, A.; Yang, T.; Xu, Y.; et al. Siderophore interactions drive the ability of Pseudomonas spp. consortia to protect tomato against Ralstonia solanacearum. Hortic. Res. 2024, 11, uhae186. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Soni, S.K.; Kalra, A. Synergy between Glomus fasciculatum and a beneficial Pseudomonas in reducing root diseases and improving yield and forskolin content in Coleus forskohlii Briq. under organic field conditions. Mycorrhiza 2013, 23, 35–44. [Google Scholar] [CrossRef]
- Molina, L.; Udaondo, Z.; Montero-Curiel, M.; Wittich, R.-M.; García-Puente, A.; Segura, A. Clover Root Exudates Favor Novosphingobium sp. HR1a Establishment in the Rhizosphere and Promote Phenanthrene Rhizoremediation. mSphere 2021, 6, e0041221. [Google Scholar] [CrossRef]
- Sajjad, A.; Muhammad, N.; Latif, K.A.; Ahmed, A.-H. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef]
- Li, S.; Zhao, X.T.; Wang, G.Q. Research Progress of Cladosporium Fungi in Plant Pest Control. Agric. Sci. Technol. Equip. 2023, 6, 31–35. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.M.; Imran, M.; Sallam, N.M.A.; Abdel-Aal, A.M.K.; Assiri, M.E.; Abdel-Rahim, I.R. Sustainable biocontrol of purple blotch disease in Allium cepa L. by biocontrol yeasts, Pichia kluyveri and Filobasidium wieringae. Egypt. J. Biol. Pest Control 2024, 34, 776. [Google Scholar] [CrossRef]
- Nian, L.; Xie, Y.; Zhang, H.; Wang, M.; Yuan, B.; Cheng, S.; Cao, C. Vishniacozyma victoriae: An endophytic antagonist yeast of kiwifruit with biocontrol effect to Botrytis cinerea. Food Chem. 2023, 411, 135442. [Google Scholar] [CrossRef] [PubMed]
- Gorordo, M.F.; Lucca, M.E.; Sangorrín, M.P. Statistical media optimization using cheese whey powder for production of Vishniacozyma victoriae postharvest biocontrol yeast in pears. Biol. Control 2023, 180, 105203. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the Rhizosphere Microbiome for Disease-Suppressive Bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Albornoz, F.E.; Prober, S.M.; Ryan, M.H.; Standish, R.J. Ecological interactions among microbial functional guilds in the plant-soil system and implications for ecosystem function. Plant Soil 2022, 476, 301–313. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Liu, H.; Macdonald, C.A.; Singh, B.K. Microbial inoculants with higher capacity to colonize soils improved wheat drought tolerance. Microb. Biotechnol. 2023, 16, 2131–2144. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, S.; Wang, Y.; Li, Y.; Li, P.; Chen, L.; Jie, X.; Hu, D.; Feng, B.; Yue, K.; et al. Rare fungus, Mortierella capitata, promotes crop growth by stimulating primary metabolisms related genes and reshaping rhizosphere bacterial community. Soil Biol. Biochem. 2020, 151, 108017. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-Induced Phenylpropanoid Metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Functions and Biotechnology of Plant Secondary Metabolites, 2nd ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2010; pp. 1–410. [Google Scholar]
- Li, Y.; Chen, C.; Xie, Z.; Xu, J.; Wu, B.; Wang, W. Integrated Analysis of mRNA and microRNA Elucidates the Regulation of Glycyrrhizic Acid Biosynthesis in Glycyrrhiza uralensis Fisch. Int. J. Mol. Sci. 2020, 21, 3101. [Google Scholar] [CrossRef]
- Huo, Y.; Feng, P.; Bi, H.; Wang, K.; Zhang, Y.; Fang, Y.; Wang, M.; Tan, T. Synergistic acetyl-CoA augmentation strategy (SATS) for improved terpenoid biosynthesis in Saccharomyces cerevisiae. Biochem. Eng. J. 2025, 213, 109572. [Google Scholar] [CrossRef]




| Strains | Source |
|---|---|
| Bacillus subtilis | Tamarix chinensis |
| Paenibacillus peoriae | Lycium ruthenicum |
| Pseudomonas silesiensis | Elaeagnus angustifolia |
| Arthrobacter globiformis | Glycyrrhiza uralensis |
| Sinorhizobium meliloti | Medicago sativa |
| Arthrobacter sp. GCG3 | Glycyrrhiza uralensis |
| Rhizobium sp. DG1 | Nitraria tangutorum |
| Strains | Nitrogenase Activity (IU·L−1) | IAA Increment (μg·mL−1) | Organic Phosphorus Increment (μg·mL−1) | Inorganic Phosphorus Increment (μg·mL−1) | ESP Increment (mg·L−1) | Siderophore Halo Diameter (mm) | Antifungal Rate (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bc | Fs | Fo | Rs | Ss | |||||||
| Bacillus subtilis | 197.77 ± 1.69 a | 6.10 ± 0.78 c | 0.00 ± 0.07 c | 1.44 ± 0.43 b | 234.38 ± 16.44 a | 2.67 ± 1.20 c | - | - | - | - | - |
| Paenibacillus peoriae | 186.49 ± 1.95 b | 4.44 ± 0.70 d | 7.27 ± 1.56 a | 0.00 ± 0.56 c | 195.49 ± 9.63 bc | 5.50 ± 0.29 b | - | - | - | - | - |
| Pseudomonas silesiensis | 170.88 ± 0.99 c | 8.28 ± 1.33 b | 4.41 ± 1.77 b | 3.38 ± 0.44 a | 171.45 ± 2.59 c | 8.17 ± 0.44 a | - | - | - | - | - |
| Arthrobacter globiformis | 168.44 ± 2.28 c | 8.82 ± 0.69 b | 0.00 ± 0.05 c | 0.00 ± 0.06 c | 177.14 ± 4.86 c | 4.33 ± 0.72 bc | 39.09 ± 0.89 b | 15.69 ± 0.91 b | 15.89 ± 0.54 b | 0.00 ± 0.11 c | 8.25 ± 0.23 b |
| Sinorhizobium meliloti | 184.46 ± 2.21 b | 15.94 ± 1.35 a | 0.00 ± 0.17 c | 0.00 ± 0.04 c | 171.82 ± 5.04 c | 5.83 ± 0.44 b | - | - | - | - | - |
| Arthrobacter sp. GCG3 | 169.64 ± 2.00 c | 17.70 ± 2.89 a | 0.00 ± 0.04 c | 0.00 ± 0.30 c | 200.26 ± 3.88 bc | 0.00 ± 0.00 d | 76.36 ± 1.05 a | 53.92 ± 0.76 a | 52.34 ± 0.57 a | 63.27 ± 1.65 a | 80.00 ± 0.88 a |
| Rhizobium sp. DG1 | 154.58 ± 1.42 d | 8.82 ± 0.29 b | 4.74 ± 0.89 b | 0.00 ± 0.02 c | 211.08 ± 5.28 ab | 6.17 ± 0.44 ab | 73.82 ± 0.56 a | 50.98 ± 1.38 a | 49.72 ± 2.06 a | 21.43 ± 0.06 b | 76.67 ± 2.33 a |
| Treatments | pH | SSS (g kg−1) | TN (mg kg−1) | AK (mg kg−1) | AP (mg·kg−1) | NO3−-N (μg·g−1) | SOM (mg·g−1) |
|---|---|---|---|---|---|---|---|
| Pc | 8.05 ± 0.08 a | 7.38 ± 0.02 a | 291.65 ± 1.61 b | 269.37 ± 0.40 a | 45.91 ± 0.39 a | 13.56 ± 0.54 c | 4.30 ± 0.07 c |
| CK | 7.91 ± 0.05 a | 7.75 ± 0.18 a | 356.64 ± 2.57 a | 97.42 ± 2.20 c | 36.64 ± 0.60 c | 15.14 ± 0.34 b | 6.48 ± 0.08 b |
| Basic soil data. | 7.80 ± 0.13 ab | 7.38 ± 0.13 a | 216.11 ± 1.97 c | 227.22 ± 0.52 b | 41.80 ± 0.68 b | 48.02 ± 0.48 a | 6.84 ± 0.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, X.; Pei, P.; Wang, P.; Guo, Q.; Yang, H.; Xue, X. Multistrain Microbial Inoculant Enhances Yield and Medicinal Quality of Glycyrrhiza uralensis in Arid Saline–Alkali Soil and Modulate Root Nutrients and Microbial Diversity. Agronomy 2025, 15, 1879. https://doi.org/10.3390/agronomy15081879
Zhang J, Li X, Pei P, Wang P, Guo Q, Yang H, Xue X. Multistrain Microbial Inoculant Enhances Yield and Medicinal Quality of Glycyrrhiza uralensis in Arid Saline–Alkali Soil and Modulate Root Nutrients and Microbial Diversity. Agronomy. 2025; 15(8):1879. https://doi.org/10.3390/agronomy15081879
Chicago/Turabian StyleZhang, Jun, Xin Li, Peiyao Pei, Peiya Wang, Qi Guo, Hui Yang, and Xian Xue. 2025. "Multistrain Microbial Inoculant Enhances Yield and Medicinal Quality of Glycyrrhiza uralensis in Arid Saline–Alkali Soil and Modulate Root Nutrients and Microbial Diversity" Agronomy 15, no. 8: 1879. https://doi.org/10.3390/agronomy15081879
APA StyleZhang, J., Li, X., Pei, P., Wang, P., Guo, Q., Yang, H., & Xue, X. (2025). Multistrain Microbial Inoculant Enhances Yield and Medicinal Quality of Glycyrrhiza uralensis in Arid Saline–Alkali Soil and Modulate Root Nutrients and Microbial Diversity. Agronomy, 15(8), 1879. https://doi.org/10.3390/agronomy15081879







