Characteristics of Lodging Resistance of Wheat Cultivars from Different Breeding Decades as Affected by the Application of Paclobutrazol Under Shading Stress
Abstract
1. Introduction
2. Materials and Methods
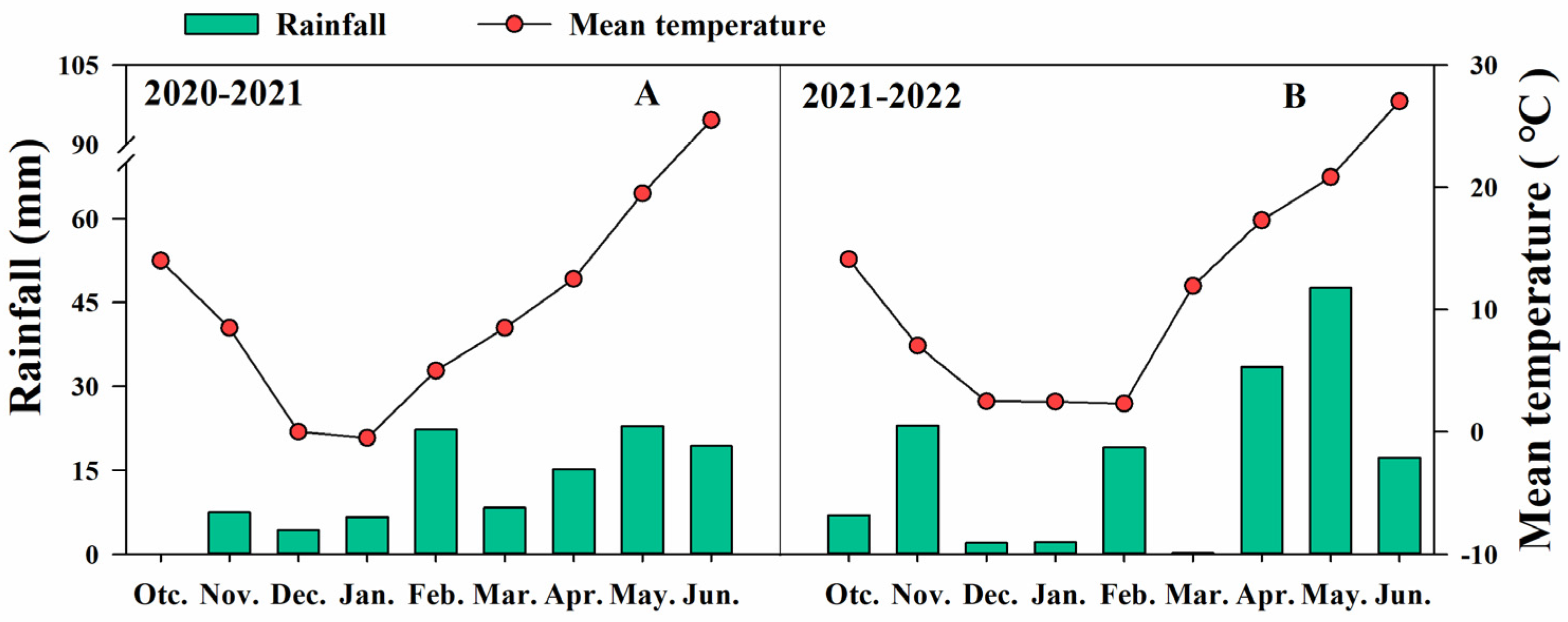
2.1. Plant Material and Experimental Design
2.2. Sampling, Lodging, and Yield Investigation
2.3. Measurements of Mechanical Traits, Lignin Synthesis, and Hormones in Culms
2.3.1. Determination of Mechanical Traits
2.3.2. Determination of Lignin Content
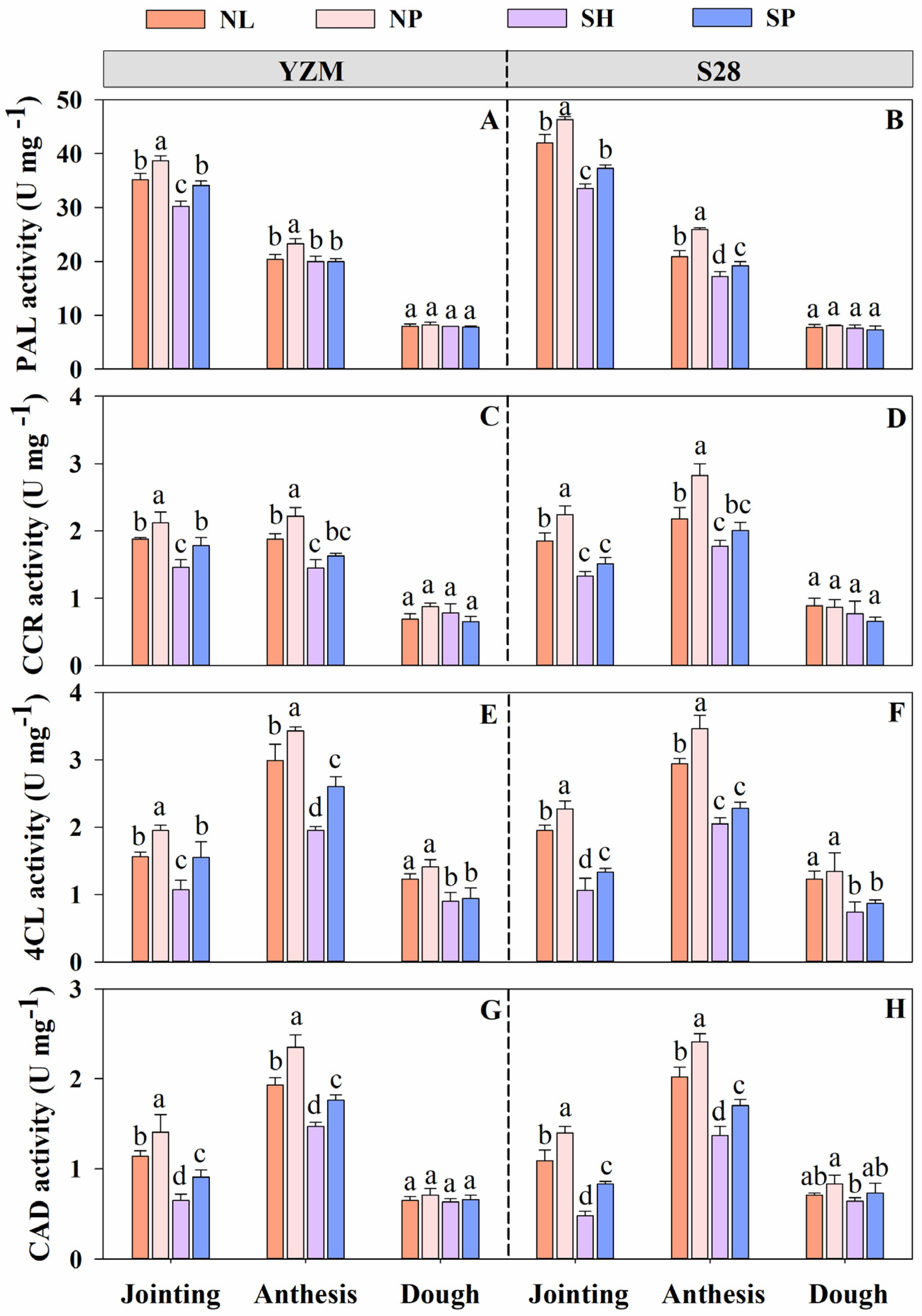
2.3.3. Determination of Activities of Enzymes Involved in Lignin Biosynthesis
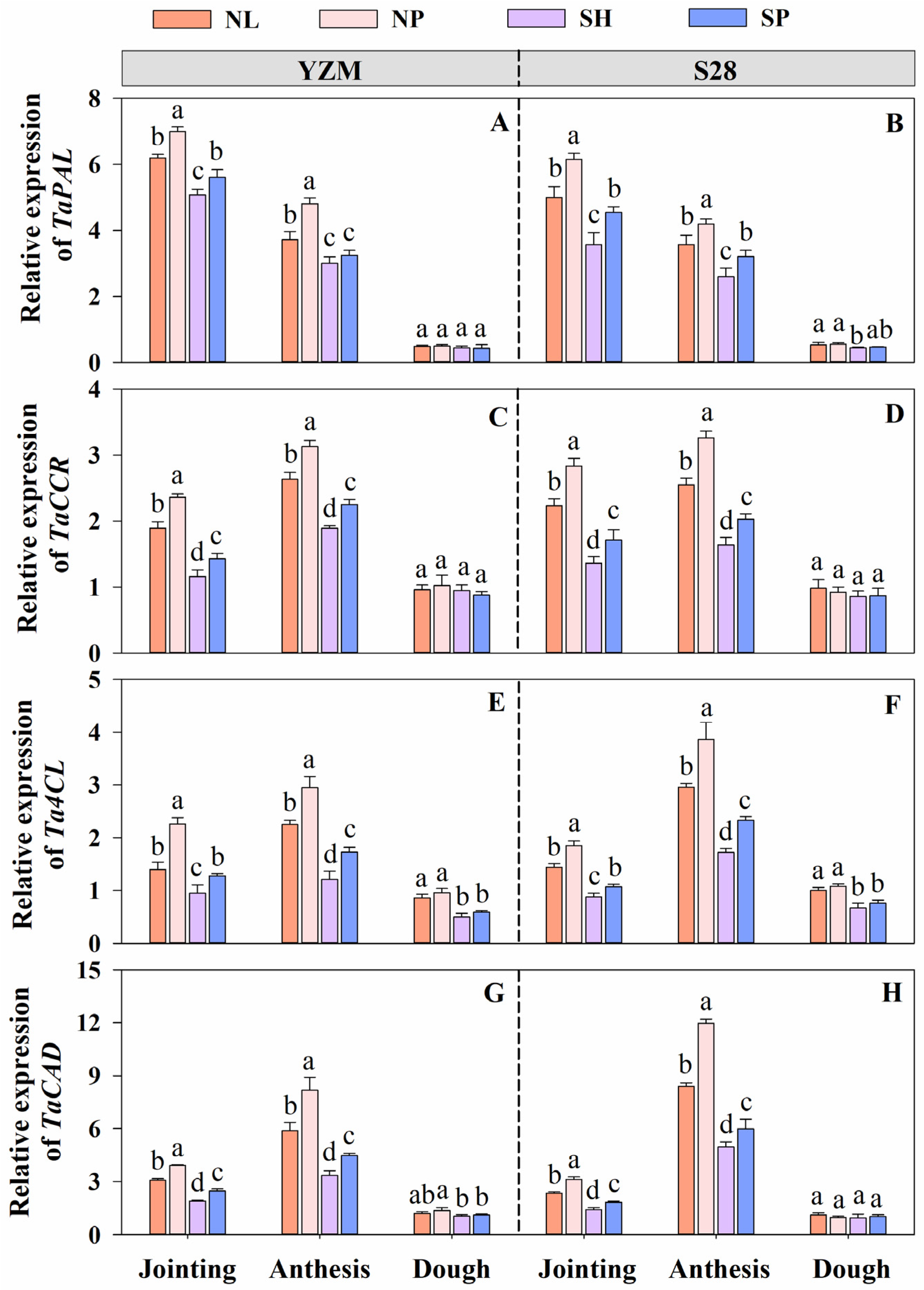
2.3.4. Determination of Expression of Genes Encoding Key Enzymes
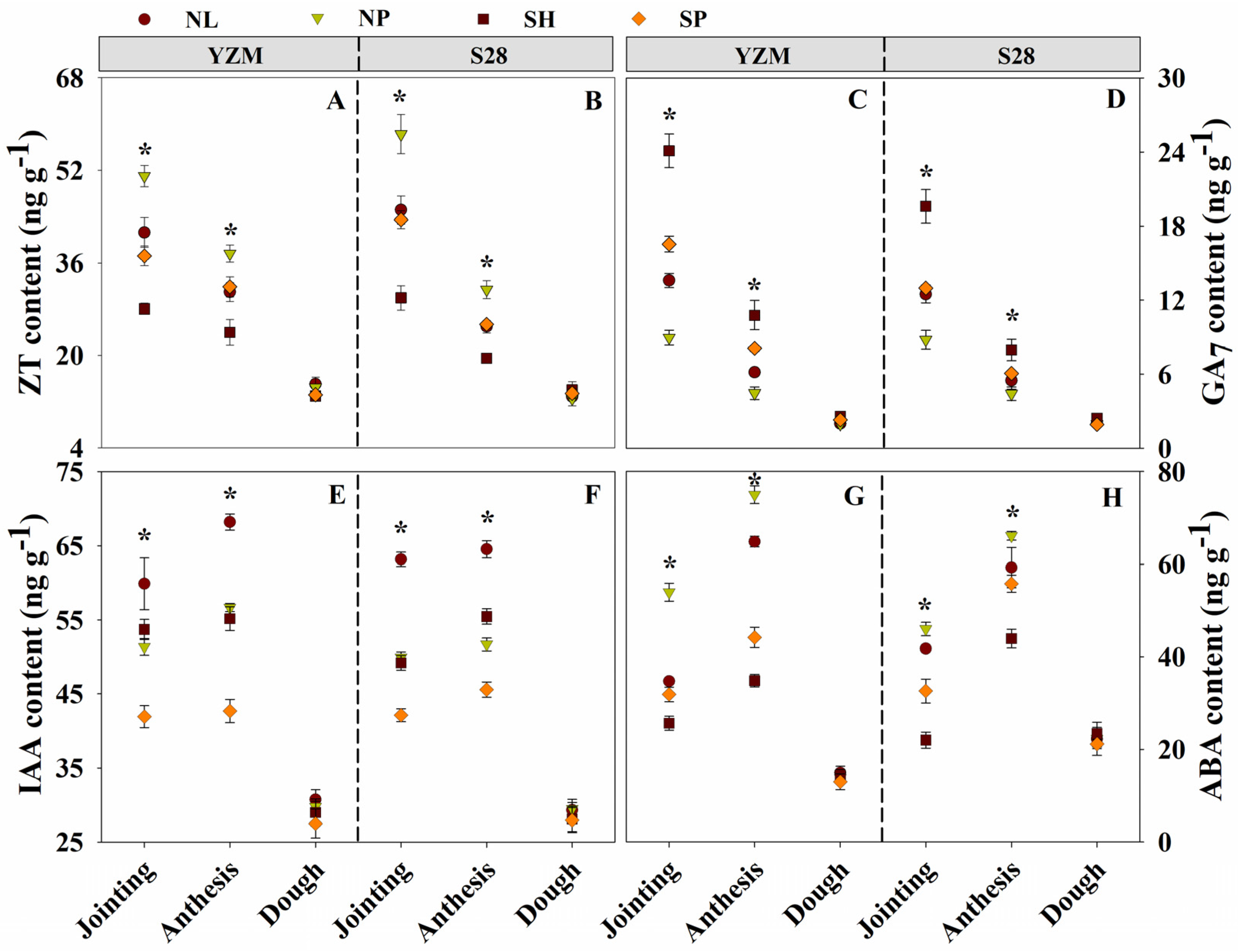
2.3.5. Determination of Endogenous Hormones of Culms
2.4. Statistical Analyses
3. Results
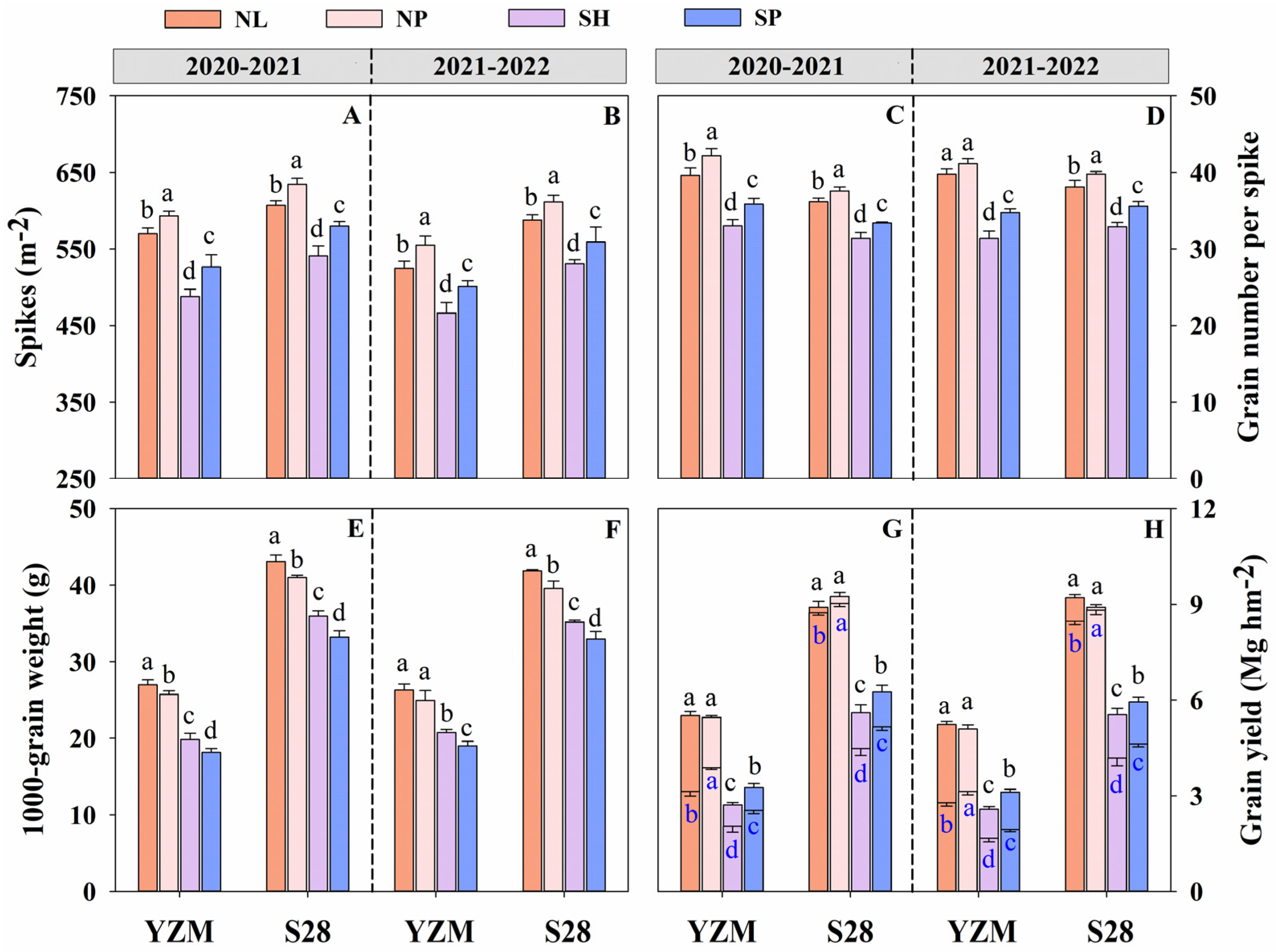
3.1. Grain Yield and Yield Components
3.2. Incidence of Lodging in the Field
3.3. Culm Morphological Characteristics
3.4. Filling Degree and Section Modulus
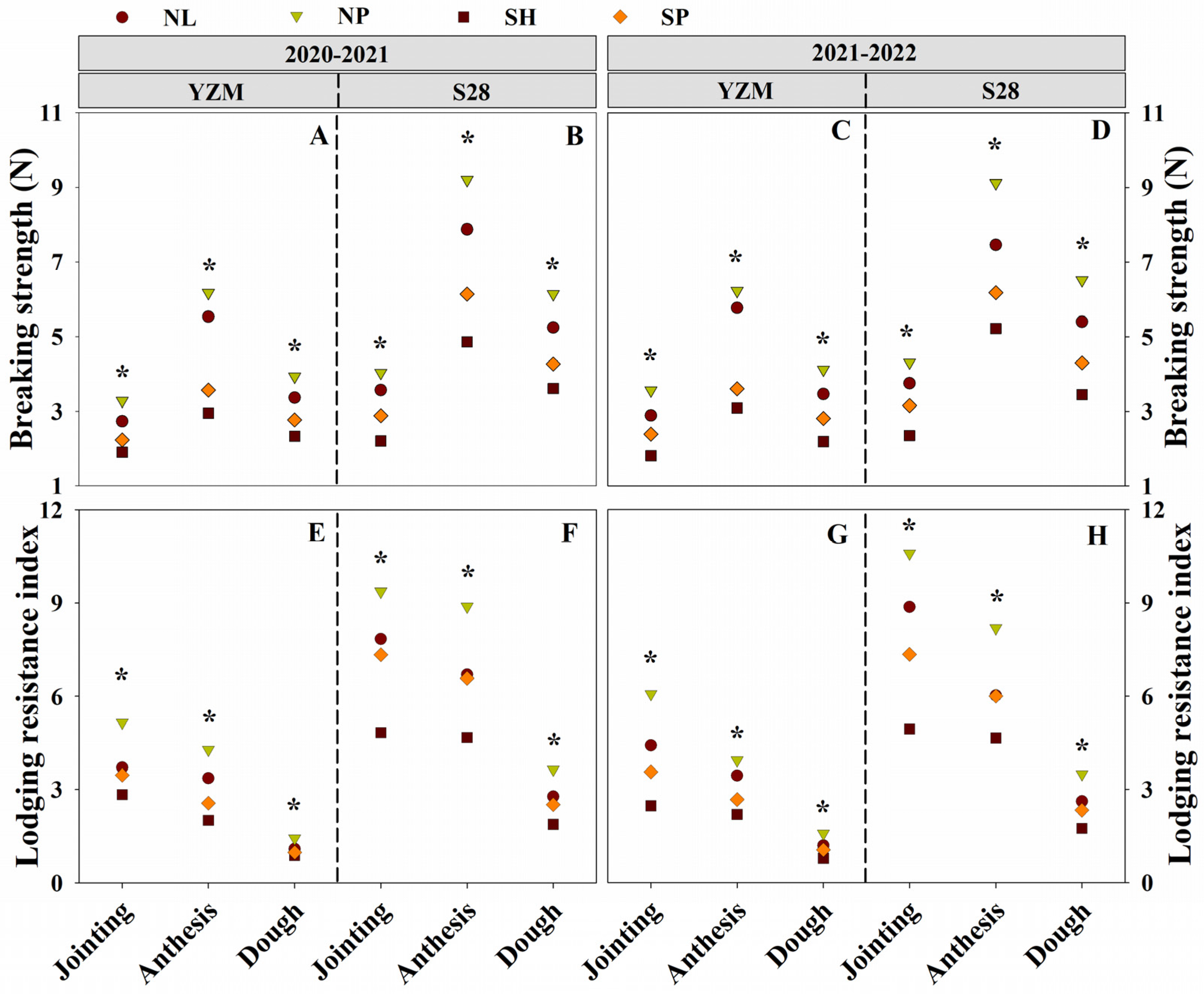
3.5. Breaking Strength and Lodging Resistance Index
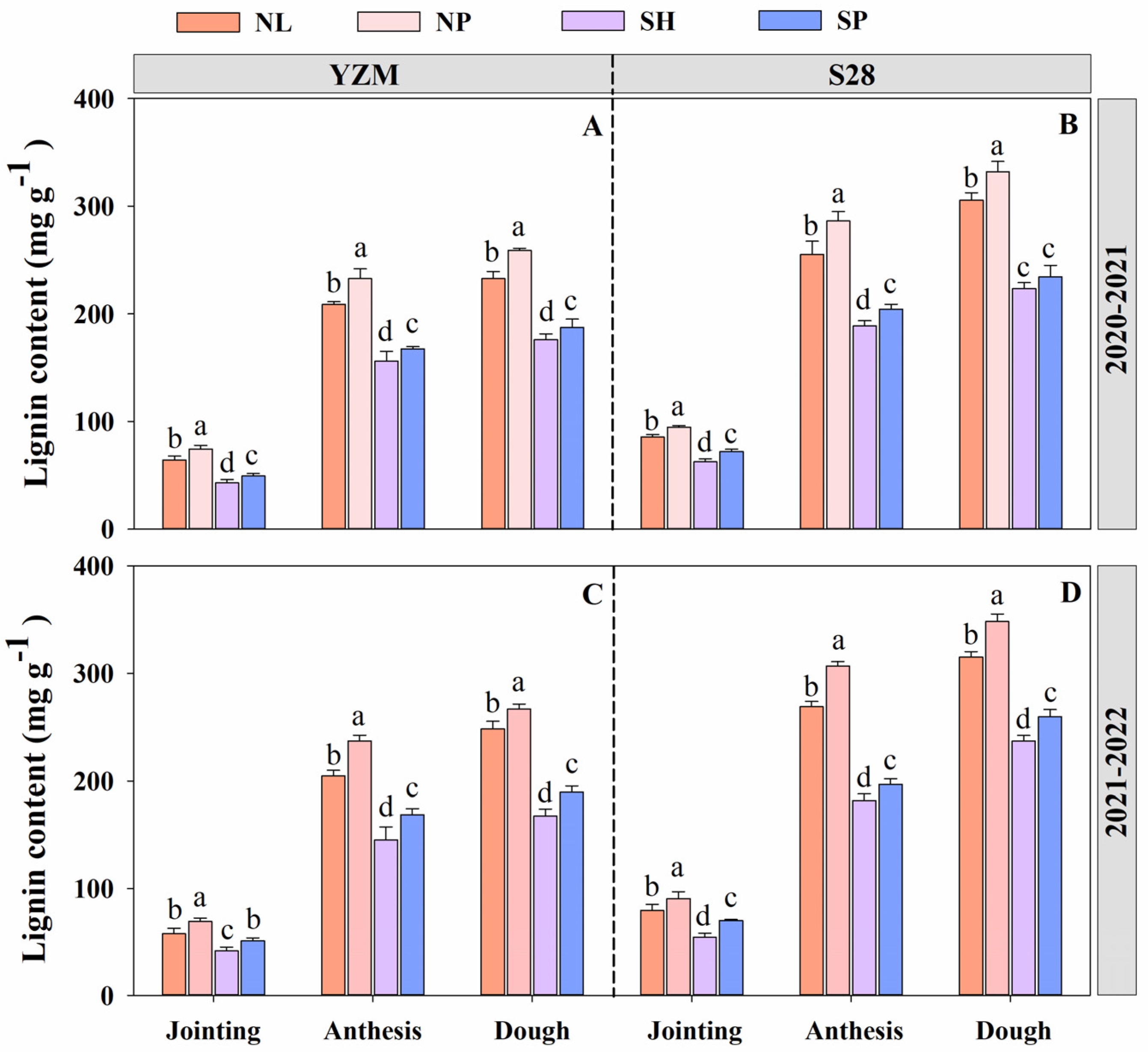
3.6. The Accumulation of Lignin
3.7. Activities of Enzymes Involved in Lignin Biosynthesis
3.8. Expression of Genes Encoding Key Enzymes in Lignin Biosynthesis
3.9. Endogenous Hormones of Basal Internode
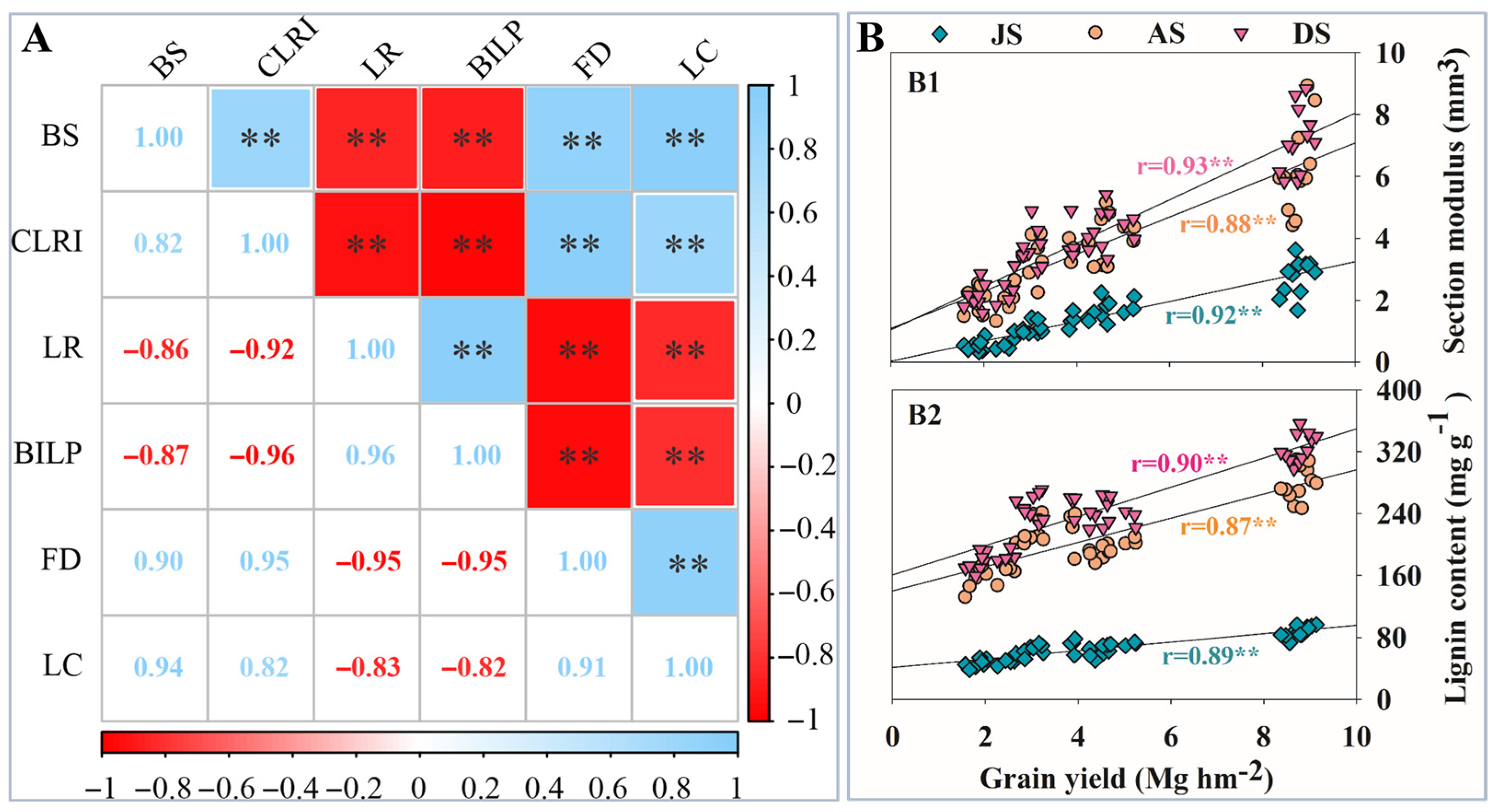
3.10. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Weibel, R.O.; Pendleton, J.W. Effect of artificial lodging on winter wheat grain yield and quality. Agron. J. 1964, 56, 487–488. [Google Scholar] [CrossRef]
- Acreche, M.M.; Slafer, G.A. Lodging yield penalties as affected by breeding in Mediterranean wheats. Field Crops Res. 2011, 122, 40–48. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, J.W.; Wang, X.Q.; Hu, Y.B.; Ren, X.L.; Jia, Z.K.; Liu, T.N.; Wang, Z.L.; Cai, T. Non-uniform wheat population distribution enhances wheat yield and lodging resistance synchronously. Eur. J. Agron. 2024, 152, 127033. [Google Scholar] [CrossRef]
- Flintham, J.E.; Borner, A.; Worland, A.J.; Gale, M.D. Optimizing wheat grain yield: Effects of Rht (gibberellin-insensitive) dwarfing genes. J. Agric. Sci. 1997, 128, 11–25. [Google Scholar] [CrossRef]
- Miralles, D.J.; Slafer, G.A. Yield, biomass and yield components in dwarf, semidwarf and tall isogenic lines of spring wheat under recommended and late sowing dates. Plant Breed. 1995, 114, 392–396. [Google Scholar] [CrossRef]
- Feng, F.; Han, Y.L.; Wang, S.G.; Yin, S.J.; Peng, Z.Y.; Zhou, M.; Gao, W.Q.; Wen, X.X.; Qin, X.L.; Kadambot, H.M. The Effect of grain position on genetic improvement of grain, number and thousand grain weight in winter wheat in north China. Front. Plant Sci. 2018, 9, 129. [Google Scholar] [CrossRef]
- Bastos, L.M.; Carciochi, W.; Lollato, R.P.; Jaenisch, B.R.; Rezende, C.R.; Schwalbert, R.; Prasad, P.; Zhang, G.R.; Fritz, A.K.; Foster, C.; et al. Winter wheat yield response to plant density as a function of yield environment and tillering potential: A review and field studies. Front. Plant Sci. 2020, 11, 54. [Google Scholar] [CrossRef]
- Qin, F.; Zhang, J.W.; Cheng, L.; Guo, X.J.; Su, L.H.; Zhao, W.J.; Jia, Z.K.; Ren, X.L.; Zhang, P.; Liu, T.N.; et al. Mechanism responsible for restricted synthesis and accumulation of lignin in wheat stems under low light conditions. Field Crops Res. 2025, 328, 109952. [Google Scholar] [CrossRef]
- Li, C.H.; Luo, Y.L.; Jin, M.; Sun, S.F.; Wang, Z.L.; Li, Y. Response of lignin metabolism to light quality in wheat population. Front. Plant Sci. 2021, 12, 729647. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Y.P.; Li, D.X.; Liu, L.T.; Qiao, Y.Z.; Sun, H.Y.; Liu, W.W.; Qiao, W.J.; Ma, Y.Z.; Liu, M.Y.; et al. Wheat escapes low light stress by altering pollination types. Front. Plant Sci. 2022, 13, 924565. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Jiang, D. The effect of climate change on winter wheat production in China. J. Agro-Environ. Sci. 2011, 30, 1726–1733. [Google Scholar] [CrossRef]
- Pantazopoulou, C.K.; Bongers, F.J.; Pierik, R. Reducing shade avoidance can improve arabidopsis canopy performance against competitors. Plant Cell Environ. 2021, 44, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.H. Functional analysis of a cinnamyl alcohol dehydrogenase involved in lignin biosynthesis in wheat. J. Exp. Bot. 2010, 61, 2735–2744. [Google Scholar] [CrossRef]
- Welton, F.A. Lodging in oats and wheat. Bot. Gaz. 1928, 85, 121–151. [Google Scholar] [CrossRef]
- Jones, L.; Ennos, A.R.; Turner, S.R. Cloning and characterization of irregular xylem4 (irx4): A severely lignin-deficient mutant of Arabidopsis. Plant J. 2001, 26, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Liu, T.; Iqbal, N.; Brestic, M.; Pang, T.; Mumtaz, M.; Shafiq, L.; Li, S.; Wang, L.; Gao, Y.; et al. Effects of lignin, cellulose, hemicellulose, sucrose and monosaccharaides carbohydrates on soybean physical stem strength and yield in intercropping. Photochem. Photobiol. Sci. 2020, 19, 462–472. [Google Scholar] [CrossRef]
- Li, B.; Gao, F.; Ren, B.Z.; Dong, S.T.; Liu, P.; Zhao, B.; Zhang, J.W. Lignin metabolism regulates lodging resistance of maize hybrids under varying planting density. J. Integr. Agric. 2021, 20, 2077–2089. [Google Scholar] [CrossRef]
- Bonawitz, N.D.; Chapple, C. The genetics of lignin biosynthesis: Connecting genotype to phenotype. Annu. Rev. Genet. 2010, 44, 337–363. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Oliveira, D.M.; Andreotti, G.C.; Souza, L.A.; Dos Santos, W.D.; Bonato, C.M.; Antunes, W.C. Increased gibberellins and light levels promotes cell wall thickness and enhance lignin deposition in xylem fibers. Front. Plant Sci. 2018, 9, 1391. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Ke, S.; Luan, X.; Liang, J.; Hung, Y.H.; Hsieh, T.F.; Zhang, X.Q. Rice OsPEX1, an extensin-like protein, affects lignin biosynthesis and plant growth. Plant Mol. Biol. 2019, 100, 151–161. [Google Scholar] [CrossRef]
- Xu, C.L.; Gao, Y.B.; Tian, B.J.; Ren, J.H.; Meng, Q.F.; Wang, P. Effects of EDAH, a novel plant growth regulator, on mechanical strength, stalk vascular bundles and grain yield of summer maize at high densities. Field Crops Res. 2017, 200, 71–79. [Google Scholar] [CrossRef]
- Crook, M.J.; Ennos, A.R. The effects of nitrogen and growth regulators on stem and root characteristics associated with lodging in two cultivars of winter wheat. J. Exp. Bot. 1995, 46, 931–938. [Google Scholar] [CrossRef]
- Zhen, G.; Liang, X.; Zhang, L.; Lin, S.; Zhao, X.; Zhou, L.; Shen, S.; Zhou, S. Spraying exogenous 6-benzyladenine and brassinolide at tasseling increases maize yield by enhancing source and sink capacity. Field Crops Res. 2017, 211, 1–9. [Google Scholar] [CrossRef]
- Schluttenhofer, C.M.; Massa, G.D.; Mitchell, C.A. Use of uniconazole to control plant height for an industrial/pharmaceutical maize platform. Ind. Crops Prod. 2011, 33, 720–726. [Google Scholar] [CrossRef]
- Zhang, W.J.; Duan, X.J.; Li, M.Y.; Luo, X.; Liu, Q.M.; Tang, Y.Q.; Li, J.Y.; Yao, X. Effects of foliar application uniconazole on culm morphological characters, anatomical traits and stem lodging resistance of hybrid indica rice under low light stress. Sci. Agric. Sin. 2024, 57, 2946–2963. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Shi, Y.; Li, Y.; Yin, Y.; Yang, D.; Luo, Y.; Pang, D.; Xu, X.; Li, W.; et al. Manipulation of lignin metabolism by plant densities and its relationship with lodging resistance in wheat. Sci. Rep. 2017, 7, 41805. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Ma, B.; Fan, J.; Sun, M.; Yi, Y.; Guo, W.; Voldeng, H.D. Management of nitrogen fertilization to balance reducing lodging risk and increasing yield and protein content in spring wheat. Field Crops Res. 2019, 241, 107584. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Yi, Y.; Ding, J.; Zhu, M.; Li, C.; Guo, W.; Feng, C.; Zhu, X. Effect of nitrogen levels and nitrogen ratios on lodging resistance and yield potential of winter wheat (Triticum aestivum L.). PLoS ONE. 2017, 12, e0187543. [Google Scholar] [CrossRef]
- Liu, X.B.; Wu, D.Q.; Wang, C.; Hu, D.; Yang, H.; She, H.Z.; Ruan, R.W.; Yuan, X.H.; Yi, Z.L. Effects of spraying uniconazole on lodging resistance of culm and yield in common buckwheat. Sci. Agric. Sin. 2015, 48, 4903–4915. [Google Scholar] [CrossRef]
- Pan, S.G.; Rasul, F.; Li, W.; Tian, H.; Mo, Z.W.; Duan, M.Y.; Tang, X.R. Roles of plant growth regulators on yield, grain qualities and antioxidant enzyme activities in super hybrid rice (Oryza sativa L.). Rice 2013, 6, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Xing, P.P.; Duan, M.Y.; Liu, Y.H.; Mo, Z.W.; Lai, R.F.; Tang, X.R. Enhancement of yield, grain quality characters, 2-acetyl-1-pyrroline content, and photosynthesis of fragrant rice cultivars by foliar application of paclobutrazol. J. Plant Growth Regul. 2023, 42, 748–758. [Google Scholar] [CrossRef]
- Peng, D.L.; Chen, X.G.; Yin, Y.P.; Lu, K.L.; Yang, W.B.; Tang, Y.H.; Wang, Z.L. Lodging resistance of winter wheat (Triticum aestivum L.): Lignin accumulation and its related enzymes activities due to the application of paclobutrazol or gibberellin acid. Field Crops Res. 2014, 157, 1–7. [Google Scholar] [CrossRef]
- Kamran, M.; Ahmad, S.; Ahmad, I.; Hussain, I.; Meng, X.P.; Zhang, X.D.; Javed, T.; Ullah, M.; Ding, R.X.; Xu, P.Z.; et al. Paclobutrazol application favors yield improvement of maize under semiarid regions by delaying leaf senescence and regulating photosynthetic capacity and antioxidant system during grain-filling stage. Agronomy 2020, 10, 187. [Google Scholar] [CrossRef]
- Liu, W.; Gai, D.; Liang, J.; Cui, J.; Geng, Y.; Zhang, Q.; Guo, L.; Shao, X. Paclobutrazol enhances lodging resistance and yield of direct-seeded rice by optimizing plant type and canopy light transmittance. Field Crops Res. 2025, 331, 109882. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Yu, Z.W. National Wheat High Yield and Efficient Cultivation Technique Specification; Shandong Science and Technology Press: Jinan, China, 2015; pp. 96–105. [Google Scholar]
- Piñera-Chávez, F.J.; Berry, P.M.; Foulkes, M.J.; Molero, G.; Reynolds, M.P. Optimizing phenotyping methods to evaluate lodging risk for wheat. Field Crops Res. 2020, 258, 107933. [Google Scholar] [CrossRef]
- Wang, C.; Hu, D.; Liu, X.; She, H.; Ruan, R.; Yang, H.; Yi, Z.; Wu, D. Effects of uniconazole on the lignin metabolism and lodging resistance of culm in common buckwheat (Fagopyrum esculentum M.). Field Crops Res. 2015, 180, 46–53. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Grzesiak, S. Contents of total phenolics and ferulic acid, and PAL activity during water potential changes in leaves of maize single-cross hybrids of different drought tolerance. J. Agron. Crop Sci. 2008, 194, 104–112. [Google Scholar] [CrossRef]
- Kamran, M.; Cui, W.; Ahmad, I.; Meng, X.; Zhang, X.; Su, W.; Chen, J.; Ahmad, S.; Fahad, S.; Han, Q.; et al. Effect of paclobutrazol, a potential growth regulator on stalk mechanical strength, lignin accumulation and its relation with lodging resistance of maize. Plant Growth Regul. 2018, 84, 317–332. [Google Scholar] [CrossRef]
- Morrison, T.A.; Kessler, J.R.; Hatfield, R.D.; Buxton, D.R. Activity of two lignin biosynthesis enzymes during development of a maize internode. J. Sci. Food Agric. 1994, 65, 133–139. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 2, 402–408. [Google Scholar] [CrossRef]
- Yang, T.X.; Wei, A.Z.; Zheng, Y.; Yang, H.; Yang, X.N.; Zhang, R. Simultaneous determination of 8 endogenous hormones in apricot floral bud by high performance liquid chromatography. J. Anal. Chem. 2007, 35, 1359–1361. [Google Scholar] [CrossRef]
- Cai, T.; Xu, H.C.; Peng, D.L.; Yin, Y.P.; Yang, W.B.; Ni, Y.L.; Chen, X.G.; Xu, C.L.; Yang, D.Q.; Cui, Z.Y.; et al. Exogenous hormonal application improves grain yield of wheat by optimizing tiller. Field Crops Res. 2014, 155, 172–183. [Google Scholar] [CrossRef]
- Donmez, E.; Sears, R.G.; Shroyer, J.P.; Paulsen, G.M. Genetic gain in yield attributes of winter wheat in the great plains. Crop Sci. 2001, 41, 1412–1419. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.G.; Wang, H.W.; Dong, H.B.; Qi, X.L.; Zhao, M.Z.; Fang, Y.; Gao, C.; Hu, L. Progress in genetic improvement of grain yield and related physiological traits of Chinese wheat in Henan Province. Field Crops Res. 2016, 199, 117–128. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, H.Z.; Cai, S.B.; He, Z.H.; Zhang, X.K.; Xia, X.C.; Zhang, G.S. Genetic improvement of grain yield and associated traits in the southern China winter wheat region: 1949 to 2000. Euphytica 2007, 157, 465–473. [Google Scholar] [CrossRef]
- Xiao, Y.G.; Qian, Z.G.; Wu, K.; Liu, J.J.; Xia, X.C.; Ji, W.Q.; He, Z.H. Genetic gains in grain yield and physiological traits of winter wheat in Shandong Province, China, from 1969 to 2006. Crop Sci. 2012, 52, 44–56. [Google Scholar] [CrossRef]
- Beed, F.; Paveley, N.; Sylvester-Bradley, R. Predictability of wheat growth and yield in light-limited conditions. J. Agric. Sci. 2007, 145, 63–79. [Google Scholar] [CrossRef]
- Liu, W.; Tollenaar, M. Response of yield heterosis to increasing plant density in maize. Crop Sci. 2009, 49, 1807–1816. [Google Scholar] [CrossRef]
- Mo, Z.; Li, W.; Pan, S.; Fitzgerald, T.L.; Xiao, F.; Tang, Y.; Wang, Y.; Duan, M.; Tian, H.; Tang, X. Shading during the grain filling period increases 2-acetyl-1-pyrroline content in fragrant rice. Rice 2015, 8, 9–19. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, W.; Ding, Y.; Zhang, J.; Cambula, E.D.; Weng, F.; Liu, Z.; Ding, C.; Tang, S.; Chen, L.; et al. Shading contributes to the reduction of stem mechanical strength by decreasing cell wall synthesis in Japonica Rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 881. [Google Scholar] [CrossRef]
- Zhang, G.P.; Chen, J.X.; Bull, D.A. The effects of timing of N application and plant growth regulators on morphogenesis and yield formation in wheat. Plant Growth Regul. 2001, 35, 239–245. [Google Scholar] [CrossRef]
- Rajala, A.; Peltonen-Sainio, P.; Onnela, M.; Jackson, M. Effects of applying stem shortening plant growth regulators to leaves on root elongation by seedlings of wheat, oat and barley: Mediation by ethylene. Plant Growth Regul. 2002, 38, 51–59. [Google Scholar] [CrossRef]
- Berry, P.M.; Sylvester-Bradley, R.; Berry, S. Ideotype design for lodging resistant wheat. Euphytica 2007, 154, 165–179. [Google Scholar] [CrossRef]
- Zhang, W.J.; Duan, X.J.; Yao, X.; Liu, Q.M.; Xiao, R.P.; Zhang, X.W.; Tang, Y.; Wen, M.; Li, J.Y. Effects of shading on stem morphological characteristics and lodging resistance of heavy panicle hybrid rice. Chin. Rice 2020, 26, 9–13. [Google Scholar] [CrossRef]
- Luo, Y.L.; Chang, Y.; Li, C.; Wang, Y.; Cui, H.; Jin, M.; Wang, Z.L.; Li, Y. Shading decreases lodging resistance of wheat under different planting densities by altering lignin monomer composition of stems. Front. Plant Sci. 2022, 13, 1056193. [Google Scholar] [CrossRef]
- Liu, W.; Ren, M.; Liu, T.; Du, Y.; Zhou, T.; Liu, X.; Liu, J.; Hussain, S.; Yang, W. Effect of shade stress on lignin biosynthesis in soybean stems. J. Integr. Agric. 2018, 17, 1594–1604. [Google Scholar] [CrossRef]
- Chang, Y.; Cui, H.; Wang, Y.; Li, C.; Wang, J.; Jin, M.; Luo, Y.; Li, Y.; Wang, Z. Silicon spraying enhances wheat stem resistance to lodging under light stress. Agronomy 2023, 13, 2565. [Google Scholar] [CrossRef]
- O’Dogherty, M.J.; Huber, J.A.; Dyson, J.; Marshall, C.J. A study of the physical and mechanical properties of wheat straw. J. Agric. Eng. Res. 1995, 62, 133–142. [Google Scholar] [CrossRef]
- Yuan, Z.H.; Zhang, X.L.; Xu, G.Z.; Zhao, X.X. The influences of the stem structure and elastic modulus on wheat lodging. Adv. Mater. Res. 2012, 524, 2330–2333. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, X.; Zhang, B.; Yuan, X.; Xing, Y.; Liu, H.; Luo, L.; Chen, G.; Xiong, L. Genetic analyses of lodging resistance and yield provide insights into post-GreenRevolution breeding in rice. Plant Biotechnol. J. 2021, 19, 814–829. [Google Scholar] [CrossRef]
- Zhang, R.; Jia, Z.; Ma, X.; Ma, H.; Zhao, Y. Characterising the morphological characters and carbohydrate metabolism of oat culms and their association with lodging resistance. Plant Biol. 2020, 22, 267–276. [Google Scholar] [CrossRef]
- Molina-Contreras, M.J.; Paulisic, S.; Then, C.; Moreno-Romero, J.; Pastor-Andreu, P.; Morelli, L.; Roig-Villanova, I.; Jenkins, H.; Hallab, A.; Gan, X. Photoreceptor activity contributes to contrasting responses to shade in cardamine and arabidopsis seedlings. Plant Cell 2019, 31, 2649–2663. [Google Scholar] [CrossRef]
- Lorrain, S.; Allen, T.; Duek, P.D.; Whitelam, G.C.; Fankhauser, C. Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 2008, 53, 312–323. [Google Scholar] [CrossRef]
- Kamran, M.; Ahmad, I.; Wu, X.; Liu, T.; Ding, R.; Han, Q. Application of paclobutrazol: A strategy for inducing lodging resistance of wheat through mediation of plant height, stem physical strength, and lignin biosynthesis. Environ. Sci. Pollut. Res. 2018, 25, 29366–29378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, M.C.; Zhang, H.Y.; Tan, W.M.; Li, Z.H.; Duan, L.S. Effects of chlormequat-uniconazole 30% micro-emulsion on stem physical and chemical characteristics of rice. Acta Agron. Sin. 2013, 39, 1089–1095. [Google Scholar] [CrossRef]
- Hou, J.F.; Xu, Y.; Wang, Z.X.; Chen, F.; Yuan, L.Y.; Zhu, S.D.; Shan, G.L.; Wang, C.G. Exogenous paclobutrazol can relieve the low irradiance stress in Capsicum annuum seedlings. Biol. Plant. 2021, 65, 297–306. [Google Scholar] [CrossRef]
- Liao, P.; Bell, S.M.; Chen, L.; Huang, S.; Wang, H.Y.; Miao, J.H.; Qi, Y.M.; Sun, Y.N.; Liao, B.; Zeng, Y.J.; et al. Improving rice grain yield and reducing lodging risk simultaneously: A meta-analysis. Eur. J. Agron. 2023, 143, 126709. [Google Scholar] [CrossRef]
- Wu, L.M. Effects of Shading and Exogenous Regulators on Lodging Resistance of Japonica Rice Stalks and their Physiological and Molecular Mechanisms; Nanjing Agricultural University: Nanjing, China, 2017; pp. 66–69. [Google Scholar]
- Leroux, O. Collenchyma: A versatile mechanical tissue with dynamic cell walls. Ann. Bot. 2012, 110, 1083–1098. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, J.; Lin, Q.; Li, X.; Teng, N.; Li, Z.; Li, B.; Zhang, A.; Lin, J. Effects of stem structure and cell wall components on bending strength in wheat. Chin. Sci. Bull. 2006, 51, 815–823. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, S.; Hou, Q.; Gao, J.; Wang, H.; Chen, X.; Liao, X.; Zhang, F.; Zhao, C.; Qin, Z. Transcriptomic and metabolomic analysis provides insights into lignin biosynthesis and accumulation and differences in lodging resistance in hybrid wheat. J. Integr. Agr. 2024, 23, 1105–1117. [Google Scholar] [CrossRef]
- Begovic, L.; Abicic, I.; Lalic, A.; Lepedus, H.; Cesar, V.; Leljak-Levanic, D. Lignin synthesis and accumulation in barley cultivars differing in their resistance to lodging. Plant Physiol. Biochem. 2018, 133, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, L.; Ding, Y.; Weng, F.; Wu, X.; Li, G.; Liu, Z.; Tang, S.; Ding, C.; Wang, S. Top-dressing nitrogen fertilizer rate contributes to decrease culm physical strength by reducing structural carbohydrate content in japonica rice. J. Integr. Agric. 2016, 15, 992–1004. [Google Scholar] [CrossRef]
- Cai, T.; Peng, D.; Wang, R.; Jia, X.; Qiao, D.; Liu, T.; Jia, Z.; Wang, Z.; Ren, X. Can intercropping or mixed cropping of two genotypes enhance wheat lodging resistance? Field Crops Res. J. 2019, 239, 10–18. [Google Scholar] [CrossRef]
- Koyama, K.; Ikeda, H.; Poudel, P.R.; Goto-Yamamoto, N. Light quality affects flavonoid biosynthesis in young berries of Cabernet Sauvignon grape. Phytochemistry 2012, 78, 54–64. [Google Scholar] [CrossRef]
- Li, C.H.; Li, W.Q.; Luo, Y.L.; Jin, M.; Chang, Y.L.; Cui, H.X.; Sun, S.F.; Li, Y.; Wang, Z.L. Mixed cropping increases grain yield and lodging resistance by improving the canopy light environment of wheat populations. Eur. J. Agron. 2023, 147, 126849. [Google Scholar] [CrossRef]
- Luo, Y.L.; Ni, J.; Pang, D.W.; Jin, M.; Chen, J.; Kong, X.; Li, W.; Chang, Y.L.; Wang, Z. Regulation of lignin composition by nitrogen rate and density and its relationship with stem mechanical strength of wheat. Field Crops Res. 2019, 241, 107572. [Google Scholar] [CrossRef]
- Zhang, M.C.; Duan, L.S.; Tian, X.L.; He, Z.P.; Li, J.M.; Wang, B.M.; Li, Z.H. Uniconazole-induced tolerance of soybean to water deficit stress in relation to changes in photosynthesis, hormones and antioxidant system. J. Plant Physiol. 2007, 164, 709–717. [Google Scholar] [CrossRef]
- Weng, J.; Chapple, C. The origin and evolution of lignin biosynthesis. New Phytol. 2010, 187, 273–285. [Google Scholar] [CrossRef]
- Halpin, C.; Knight, M.E.; Foxon, G.A.; Campbell, M.M.; Boudet, A.M.; Boon, J.J.; Schuch, W. Manipulation of lignin quality by downregulation of cinnamyl alcohol dehydrogenase. Plant J. 1994, 6, 339–350. [Google Scholar] [CrossRef]
- Cheng, B.; Raza, A.; Wang, L.; Xu, M.; Lu, J.; Gao, Y.; Qin, S.; Zhang, Y.; Ahmad, I.; Zhou, T.; et al. Effects of multiple planting densities on lignin metabolism and lodging resistance of the strip intercropped soybean stem. Agronomy 2020, 10, 1177. [Google Scholar] [CrossRef]
- Kuai, J.; Li, X.; Ji, J.; Li, Z.; Xie, Y.; Wang, B.; Zhou, G. The physiological and proteomic characteristics of oilseed rape stem affect seed yield and lodging resistance under different planting densities and row spacing. J. Agron. Crop Sci. 2021, 207, 840–856. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Xie, Y.; Wang, B.; Kuai, J.; Zhou, G. An improvement in oilseed rape (Brassica napus L.) productivity through optimization of rice-straw quantity and plant density. Field Crops Res. 2021, 273, 108290. [Google Scholar] [CrossRef]
- Yu, N.N.; Ren, B.Z.; Zhao, B.; Liu, P.; Zhang, J.W. Optimized agronomic management practices narrow the yield gap of summer maize through regulating canopy light interception and nitrogen distribution. Eur. J. Agron. 2022, 137, 126520. [Google Scholar] [CrossRef]
- Wen, B.; Zhang, Y.; Hussain, S.; Wang, S.; Zhang, X.; Yang, J.; Xu, M.; Qin, S.; Yang, W.; Liu, W. Slight shading stress at seedling stage does not reduce lignin biosynthesis or affect lodging resistance of soybean stems. Agronomy 2020, 10, 544. [Google Scholar] [CrossRef]


| Trait/ Source of Variation | SNP | GNS | TGW | GY(+net) | GY(−net) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2020–2021 | 2021–2022 | 2020–2021 | 2021–2022 | 2020–2021 | 2021–2022 | 2020–2021 | 2021–2022 | 2020–2021 | 2021–2022 | |
| Cultivar (C) | *** | *** | *** | ** | *** | *** | *** | *** | *** | *** |
| Shading (S) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Paclobutrazol (P) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| C × S | ns | ns | *** | *** | ns | ns | * | ** | *** | *** |
| C × P | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| S × P | ns | ns | ns | * | ns | ns | ns | ns | ns | ns |
| C × S × P | ns | ns | ns | ns | ns | ns | ns | ns | * | ns |
| Cultivar | Treatments | 2020–2021 | 2021–2022 | ||||
|---|---|---|---|---|---|---|---|
| Time of Lodging | Lodging Angle (°) | Lodging Rate (%) | Time of Lodging | Lodging Angle (°) | Lodging Rate (%) | ||
| YZM | NL | EA (GS60) | 55.64b | 62.96c | AS(GS65) | 49.73c | 71.11c |
| NP | ED (GS82) | 32.27c | 34.81d | ED (GS82) | 35.50d | 35.92d | |
| SH | JS (GS39) | 74.00a | 90.74a | JS (GS39) | 82.21a | 98.55a | |
| SP | LH (GS55) | 63.12b | 78.89b | EA (GS60) | 64.53b | 83.33b | |
| S28 | NL | -- | 0c | 0c | -- | 0c | 0c |
| NP | -- | 0c | 0c | -- | 0c | 0c | |
| SH | AS (GS65) | 30.39a | 35.55a | AS (GS65) | 29.65a | 45.18a | |
| SP | DS (GS85) | 17.73b | 21.85b | DS (GS85) | 10.33b | 26.66b | |
| Analysis of variance | |||||||
| Cultivar (C) | -- | *** | *** | -- | *** | *** | |
| Shading (S) | -- | *** | *** | -- | *** | *** | |
| Paclobutrazol (P) | -- | *** | *** | -- | *** | *** | |
| C × S | -- | ns | ns | -- | ** | ns | |
| C × P | -- | *** | ** | -- | ns | ** | |
| S × P | -- | ns | ns | -- | ** | ns | |
| C × S × P | -- | ** | ** | -- | * | * | |
| Trait/ Source of Variation | PH | GCH | BILP | LC | ||||
|---|---|---|---|---|---|---|---|---|
| 2020–2021 | 2021–2022 | 2020–2021 | 2021–2022 | 2020–2021 | 2021–2022 | 2020–2021 | 2021–2022 | |
| Cultivar (C) | *** | *** | *** | *** | *** | *** | *** | *** |
| Shading (S) | *** | *** | *** | *** | *** | *** | *** | *** |
| Paclobutrazol (P) | *** | *** | *** | *** | *** | *** | *** | *** |
| C × S | *** | *** | ns | ** | ns | ns | *** | ns |
| C × P | ** | *** | ns | ns | ns | ns | ns | ns |
| S × P | ** | * | ns | ns | ns | ns | ** | ns |
| C × S × P | * | ns | * | ns | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, D.; Xu, H.; Guo, Z.; Cao, W.; Zhang, J.; Liu, M.; Wang, X.; Tang, Y.; Cai, T. Characteristics of Lodging Resistance of Wheat Cultivars from Different Breeding Decades as Affected by the Application of Paclobutrazol Under Shading Stress. Agronomy 2025, 15, 1848. https://doi.org/10.3390/agronomy15081848
Peng D, Xu H, Guo Z, Cao W, Zhang J, Liu M, Wang X, Tang Y, Cai T. Characteristics of Lodging Resistance of Wheat Cultivars from Different Breeding Decades as Affected by the Application of Paclobutrazol Under Shading Stress. Agronomy. 2025; 15(8):1848. https://doi.org/10.3390/agronomy15081848
Chicago/Turabian StylePeng, Dianliang, Haicheng Xu, Zhen Guo, Wenchao Cao, Jingmin Zhang, Mei Liu, Xingcui Wang, Yuhai Tang, and Tie Cai. 2025. "Characteristics of Lodging Resistance of Wheat Cultivars from Different Breeding Decades as Affected by the Application of Paclobutrazol Under Shading Stress" Agronomy 15, no. 8: 1848. https://doi.org/10.3390/agronomy15081848
APA StylePeng, D., Xu, H., Guo, Z., Cao, W., Zhang, J., Liu, M., Wang, X., Tang, Y., & Cai, T. (2025). Characteristics of Lodging Resistance of Wheat Cultivars from Different Breeding Decades as Affected by the Application of Paclobutrazol Under Shading Stress. Agronomy, 15(8), 1848. https://doi.org/10.3390/agronomy15081848






