Phacelia tanacetifolia Benth. as a Multifunctional Plant: Support for Pollinators and Sustainable Agricultural Practices
Abstract
1. Introduction
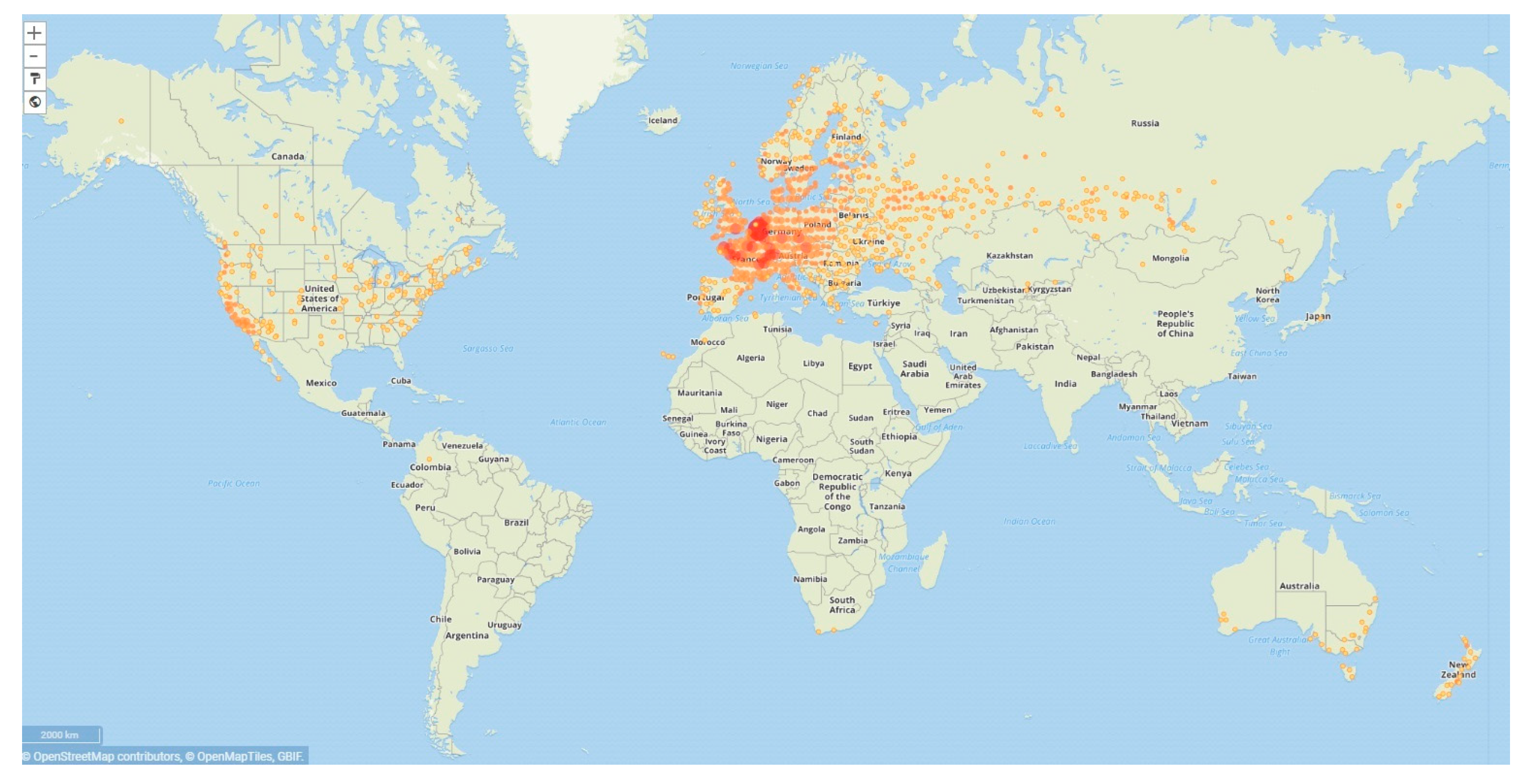
2. Materials and Methods
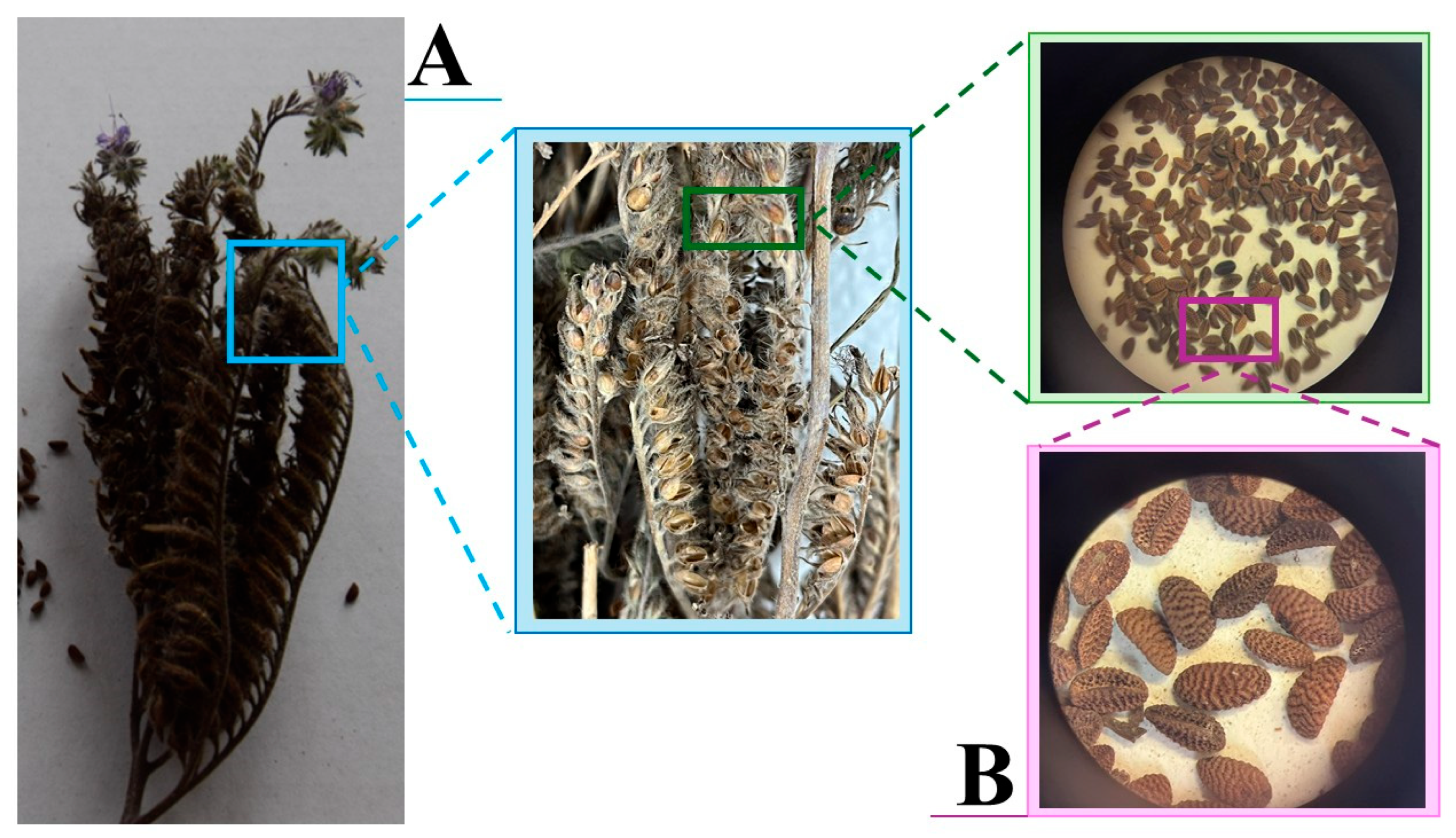
3. Morphology
4. Soil Requirements and Impact on Soil Properties
5. Agronomic Management—Cultivation Recommendations
6. The Role of Phacelia in the Aspect of Beekeeping
7. Suitability for Livestock
8. The Healing Role of Phacelia
9. Reclamation of Degraded Areas and Ecosystem Services
10. Energy Generation
11. Directions for Further Research on Phacelia tanacetifolia Benth.
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AES | Agri-Environmental Schemes |
| FM | Fresh Matter |
| DM | Dry Matter |
References
- FAO. How to Feed the World in 2050. Available online: https://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 30 April 2025).
- Yang, T.; Siddique, K.H.M.; Liu, K. Cropping systems in agriculture and their impact on soil health—A review. Glob. Ecol. Conserv. 2020, 23, e01118. [Google Scholar] [CrossRef]
- Gawdiya, S.; Sharma, R.K.; Singh, H.; Kumar, D. Crop diversification as a cornerstone for sustainable agroecosystems: Tackling biodiversity loss and global food system challenges. Discov. Appl. Sci. 2025, 7, 373. [Google Scholar] [CrossRef]
- Nikolova, T.; Petrova, V. The climate factors impact on the nectariferous qualities of Phacelia tanacetifolia Benth. Sci. Papers Ser. D Anim. Sci. 2019, 62, 267–271. [Google Scholar]
- Cîrlig, N.; Ţîţei, V.; Iurcu-Străistaru, E. Morphobiological features and the significance of the species Phacelia tanacetifolia Benth. as honey plant. Lucr. Ştiinţifice Ser. Agron. 2023, 6, 55–60. Available online: https://repository.iuls.ro/xmlui/handle/20.500.12811/4028 (accessed on 30 April 2025).
- Kavassilas, Z.; Mittmannsgruber, M.; Gruber, E.; Zaller, J.G. Artificial light at night reduces the surface activity of earthworms, increases the growth of a cover crop and reduces water leaching. Land 2024, 13, 1698. [Google Scholar] [CrossRef]
- Pruszyński, G.; Mrówczyński, M. Metodyka Integrowanej Ochrony Facelii Błękitnej dla Producentów, 1st ed.; Instytut Ochrony Roślin—Państwowy Instytut Badawczy: Poznań, Poland, 2015; pp. 1–25. Available online: https://www.ior.poznan.pl/plik,2303,metodyka-integrowanej-ochrony-facelii-blekitnej-dla-producentow-pdf.pdf (accessed on 30 April 2025).
- Pinke, G.; Giczi, Z.; Vona, V.; Dunai, É.; Vámos, O.; Kulmány, I.; Koltai, G.; Varga, Z.; Kalocsai, R.; Botta-Dukát, Z.; et al. Weed composition in Hungarian Phacelia (Phacelia tanacetifolia Benth.) seed production: Could tine harrow take over chemical management? Agronomy 2022, 12, 891. [Google Scholar] [CrossRef]
- Smit, B.; Janssens, B.; Haagsma, W.; Hennen, W.; Adrados, J.; Kathage, J. Adoption of Cover Crops for Climate Change Mitigation in the EU; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar] [CrossRef]
- Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The problem of weed infestation of agricultural plantations vs. the assumptions of the European biodiversity strategy. Agronomy 2022, 12, 1808. [Google Scholar] [CrossRef]
- Kosolapov, V.M.; Cherniavskih, V.I.; Dumacheva, E.V.; Konoplev, V.V.; Tseiko, V.I.; Markova, E.I. The search for source material of Phacelia tanacetifolia Benth for breeding for fodder productivity. IOP Conf. Ser. Earth Environ. Sci. 2021, 901, 012006. [Google Scholar] [CrossRef]
- Żarczyński, P.J.; Krzebietke, S.J.; Sienkiewicz, S.; Wierzbowska, J. The Role of Fallows in Sustainable Development. Agriculture 2023, 13, 2174. [Google Scholar] [CrossRef]
- Sienkiewicz, S.; Żarczyński, P.J.; Wierzbowska, J.; Krzebietke, S.J. The impact of long-term fallowing on the yield and quality of winter rape and winter and spring wheat. Agriculture 2024, 14, 567. [Google Scholar] [CrossRef]
- Mackiewicz-Walec, E.; Żarczyński, P.J.; Krzebietke, S.J.; Żarczyńska, K. Smooth Brome (Bromus inermis L.)—A Versatile Grass: A Review. Agriculture 2024, 14, 854. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development. 2015. A/RES/70/1. Available online: https://sdgs.un.org/2030agenda (accessed on 30 April 2025).
- GBIF—The Global Biodiversity Information Facility. Available online: https://doi.org/10.48580/dgrrq (accessed on 22 April 2024).
- Garen, H.; Avcioglu, R.; Kaymakkavak, D. Effects of different row spacings on the seed yield and some other characteristics of phacelia (Phacelia tanacetifolia Bentham.) varieties. J. Food Agric. Environ. 2009, 7, 383–386. [Google Scholar]
- Jasińska, Z.; Koteckiego, A. Szczegółowa Uprawa Roślin, 2nd ed.; Uniwersytet Przyrodniczy we Wrocławiu: Poland, 1999; Volume 2, pp. 305–306. [Google Scholar]
- Petanidou, T. Introducing plants for bee-keeping at any cost?—Assessment of Phacelia tanacetifolia as nectar source plant under xeric Mediterranean conditions. Plant Syst. Evol. 2003, 238, 155–168. [Google Scholar] [CrossRef]
- Kirk, W.D.J. Phacelia. Bee World 2005, 86, 14–16. [Google Scholar] [CrossRef]
- Kliszcz, A.; Puła, J.; Możdżeń, K.; Tatoj, A.; Zandi, P.; Stachurska-Swakoń, A.; Barabasz-Krasny, B. Wider use of honey plants in farming: Allelopathic potential of Phacelia tanacetifolia Benth. Sustainability 2023, 15, 3061. [Google Scholar] [CrossRef]
- Giovanetti, M.; Malabusini, S.; Zugno, M.; Lupi, D. Influence of flowering characteristics, local environment, and daily temperature on the visits paid by Apis mellifera to the exotic crop Phacelia tanacetifolia. Sustainability 2022, 14, 10186. [Google Scholar] [CrossRef]
- Walden, G.K.; Patterson, R.; Garrison, L.M.; Hansen, D.R. Phacelia tanacetifolia. In Jepson Flora Project; Jepson eFlora, 2016; Revision 1; Available online: http://ucjeps.berkeley.edu/cgi-bin/get_IJM.pl?tid=37579 (accessed on 30 April 2025).
- Cǐrlig, N. Some aspects of the study on the species Phacelia tanacetifolia Benth. A honey plant in the Republik of Moldova. J. Bot. 2020, 2, 167–168. [Google Scholar] [CrossRef]
- Kowalska, J.; Antkowiak, M.; Tymoszuk, A. Effect of plant seed mixture on overwintering and floristic attractiveness of the flower strip in Western Poland. Agriculture 2023, 13, 467. [Google Scholar] [CrossRef]
- Lipiński, M. Pożytki Pszczele. Zapylanie i Miododajność, 4th ed.; Kotłowski, Z., Ed.; Powszechne Wydawnictwo Rolnicze i Leśne: Warsaw, Poland, 2010; pp. 24–29. [Google Scholar]
- Walden, G.K.; Patterson, R.W.; Garrison, L.M.; Hansen, D.R. Phacelia tanacetifolia. In Jepson Flora Project; Jepson eFlora, 2023; Revision 12; Available online: https://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=37579 (accessed on 1 November 2024).
- Tiryaki, I.; Keles, H. Reversal of the inhibitory effect of light and high temperature ongermination of Phacelia tanacetifolia seeds by melatonin. J. Pineal Res. 2012, 52, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Tworkowski, J.; Szczukowski, S.; Kwiatkowski, J. Plon i wartość siewna nasion facelii błękitnej (Phacelia tanacaetifolia Benth.) w zależności od wybranych czynników agrotechnicznych. Zesz. Probl. Post. Nauk. Roln. 1999, 468, 241–247. [Google Scholar]
- Gentsch, N.; Riechers, F.L.; Boy, J.; Schweneker, D.; Feuerstein, U.; Heuermann, D.; Guggenberger, G. Cover crops improve soil structure and change organic carbon distribution in macroaggregate fractions. Soil 2024, 10, 139–150. [Google Scholar] [CrossRef]
- Bacq-Labreuil, A.; Crawford, J.; Mooney, S.J.; Neal, A.L.; Ritz, K. Phacelia (Phacelia tanacetifolia Benth.) affects soil structure differently depending on soil texture. Plant Soil 2019, 441, 543–554. [Google Scholar] [CrossRef]
- Lermi, A.G.; Palta, Ş. The effects of different sowing dates of fiddleneck (Phacelia tanacetifolia) during the autumn and spring sowing periods. J. Bartin Fac. For. 2014, 16, 11–18. [Google Scholar]
- Hajzler, M.; Klimesova, J.; Streda, T.; Vejrazka, K.; Marecek, V.; Cholastova, T. Root system production and aboveground biomass production of chosen cover crops. WASET 2012, 6, 651–656. [Google Scholar]
- Bełkot, M.; Pięta, D. Effect of organic matter of tansy phacelia, winter wheat, white mustard, rye, turnip and soybean on the healthiness and yielding of soybean [Glycine max [L.] Merrill]. Ann. Univ. Mariae Curie-Skłodowska. Sect. EEE: Hortic. 2005, 15, 91–104. [Google Scholar]
- Kubíková, Z.; Smejkalová, H.; Hutyrová, H.; Kintl, A.; Elbl, J. Effect of sowing date on the development of Lacy Phacelia (Phacelia tanacetifolia Benth.). Plants 2022, 11, 3177. [Google Scholar] [CrossRef] [PubMed]
- Lalewicz, P.; Domagała-Świątkiewicz, I.; Siwek, P. Phacelia and Buckwheat cover crops’ effects on soil quality in organic vegetable production in a high tunnel system. Agronomy 2024, 14, 1614. [Google Scholar] [CrossRef]
- Lami, F.; Vuerich, M.; Fabro, M.; Zandigiacomo, P.; Braidot, E.; Petrussa, E.; Barbieri, S.; Volpe, V.; Sigura, M.; Vedove, D.G.; et al. Sustaining multiple ecosystem functions in agricultural landscapes: Effect of summer cover crops on weed control, soil quality and support to pollinators. Crop Prot. 2024, 184, 106832. [Google Scholar] [CrossRef]
- Harasim, E.; Antonkiewicz, J.; Kwiatkowski, C.A. The effects of catch crops and tillage systems on selected physical properties and enzymatic activity of loess soil in a spring wheat monoculture. Agronomy 2020, 10, 334. [Google Scholar] [CrossRef]
- Rosa, R.; Zaniewicz-Bajkowska, A.; Kosterna, E.; Franczuk, J. Phacelia and Amaranth catach crops in Sweet corn cultivation. Part I. Corn yields. Acta Sci. Pol. Hortorum Cultus. 2012, 11, 145–159. Available online: https://czasopisma.up.lublin.pl/asphc/article/view/3013/2081 (accessed on 30 April 2025).
- Willmott, R.; Valdez-Herrera, J.; Mitchell, J.P.; Shrestha, A. Potential of cover crop use and termination with a roller-crimper in a strip-till silage maize (Zea mays L.) production system in the Central Calley of California. Agronomy 2025, 15, 132. [Google Scholar] [CrossRef]
- Jacobs, A.A.; Stout Evans, R.; Allison, J.K.; Kingery, W.L.; McCulley, R.L.; Brye, K.R. Tillage and cover crop systems alter soil particle size distribution in raised-bed-and-furrow row-crop agroecosystems. Soil Syst. 2024, 8, 6. [Google Scholar] [CrossRef]
- Rossi, G.; Beni, C.; Neri, U. Organic mulching: A sustainable technique to improve soil quality. Sustainability 2024, 16, 10261. [Google Scholar] [CrossRef]
- Svensson, S.E.; Johansson, E.; Kreuger, E.; Prade, T. Evaluating intermediate crops for biogas production—Effects of nitrogen fertilization and harvest timing on biomass yield, methane output and economic viability. Biomass Bioenergy 2024, 192, 107497. [Google Scholar] [CrossRef]
- Wilczewski, E.; Skinder, Z. Content and accumulation of macroelements in the biomass of non papilionaceous plants grown in stubble intercrop. Acta Sci. Pol. 2005, 4, 163–173. [Google Scholar]
- Butcaru, A.C.; Stanica, F.; Matei, G.M.; Matei, S. Influences of soil ameliorative plant species on organic edible rose culture. Acta Hortic. 2019, 1242, 107–114. [Google Scholar] [CrossRef]
- De Baets, S.; Poesen, J.; Meersmans, J.; Serlet, L. Cover crops and their erosion-reducing effects during concentrated flow erosion. Catena 2011, 85, 237–244. [Google Scholar] [CrossRef]
- Żuk-Gołaszewska, K.; Wanic, M.; Orzech, K. The role of catch crops in the field plant production—A review. J. Elem. 2019, 24, 575–587. [Google Scholar] [CrossRef]
- Haberle, J.; Chuchma, F.; Raimanova, I.; Wollnerova, J. Agroclimatic zoning of temperature limitations for growth of stubble cover crops. Climate 2025, 13, 15. [Google Scholar] [CrossRef]
- Mitropolova, L.; Korotkikh, E.; Pavlova, O.; Ivleva, O. Study of Phacelia tanacetifolia Benth as a Green Manure Crop in the Conditions of Primorsky Krai. In XV International Scientific Conference “INTERAGROMASH 2022”; Lecture Notes in Networks and Systems; Beskopylny, A., Shamtsyan, M., Artiukh, V., Eds.; Springer: Cham, Switzerland, 2023; Volume 574. [Google Scholar] [CrossRef]
- Titov, V.N.; Mamonov, A.N. Role of sweetclover and phacelia in agriculture ecology improvement for droughty left-bank areas of Saratov region. Russ. Agric. Sci. 2013, 39, 338–341. [Google Scholar] [CrossRef]
- Yakimovich, A.; Kwiatkowski, C.A.; Haliniarz, M. Effectiveness of the application of metamitron in lacy phacelia (Phacelia tanacetifolia Benth.) crops. Ann. UMCS Sect. E Agric. 2017, 72, 125–135. [Google Scholar] [CrossRef]
- Olofsson, F.; Ernfors, M. Frost killed cover crops induced high emissions of nitrous oxide. Sci. Total Environ. 2022, 837, 155634. [Google Scholar] [CrossRef] [PubMed]
- Błażewicz-Woźniak, M.; Konopiński, M. Influence of ridge cultivation and phacelia intercrop on weed infestation of root vegetables of the Asteraceae family. Folia Hort. 2012, 24, 21–32. Available online: https://intapi.sciendo.com/pdf/10.2478/v10245-012-0003-3 (accessed on 30 April 2025). [CrossRef]
- Schappert, A.; Schumacher, M.; Gerhards, R. Weed control ability of single sown cover crops compared to species mixtures. Agronomy 2019, 9, 294. [Google Scholar] [CrossRef]
- Büchi, L.; Wendling, M.; Amossé, C.; Jeangros, B.; Charles, R. Cover crops to secure weed control strategies in a maize crop with reduced tillage. Field Crops Res. 2020, 247, 107583. [Google Scholar] [CrossRef]
- Tursun, N.; Işık, D.; Demir, Z.; Jabran, K. Use of living, mowed, and soil-incorporated cover crops for weed control in apricot orchards. Agronomy 2018, 8, 150. [Google Scholar] [CrossRef]
- Dhima, K.; Vasilakoglou, I.; Garane, V.; Ritzoulis, C.; Lianopoulou, V.; Panou-Philotheou, E. Competitiveness and essential oil phytotoxicity of seven annual aromatic plants. Weed Sci. 2010, 58, 457–465. Available online: http://www.jstor.org/stable/40891262 (accessed on 30 April 2025). [CrossRef]
- Wooliver, R.; Jagadamma, S. Response of soil organic carbon fractions to cover cropping: A meta-analysis of agroecosystems. Agric. Ecosyst. Environ. 2023, 351, 108497. [Google Scholar] [CrossRef]
- Launay, C.; Houot, S.; Frédéric, S.; Girault, R.; Levavasseur, F.; Marsac, S.; Constantin, J. Incorporating energy cover crops for biogas production into agricultural systems: Benefits and environmental impacts. A review. Agron. Sustain. Dev. 2022, 42, 57. [Google Scholar] [CrossRef]
- Kosewska, A.; Kordan, B. The cultivation of phacelia (Phacelia tanacetifolia benth.) as a habitat for the occurrence of beneficial ground beetles (coleoptera, carabidae). Zeszyty Naukowe OTN 2016, XXX, 243–251. [Google Scholar]
- Szymczak-Nowak, J.; Nowakowski, M. Antinematode effect and yielding of white mustard, tansy phacelia and oil radish cultivated as a main crop. Rośliny Oleiste 2000, 21, 285–291. [Google Scholar]
- Didyk, N.; Ivanytska, B. Potential of some medicinal and fodder crops to alleviate soil sickness in the old Prunus persica var. persica (L.) Batsch and Malus domestica Borkh. Tree Monocultures. Agrobiodivers Improv. Nutr. Health Life Qua. 2020, 4, 1–12. Available online: https://agrobiodiversity.uniag.sk/scientificpapers/article/view/309 (accessed on 30 April 2025).
- Kliszcz, A.; Danel, A.; Puła, J.; Barabasz-Krasny, B.; Możdżeń, K. Fleeting Beauty—The World of Plant Fragrances and Their Application. Molecules 2021, 26, 2473. [Google Scholar] [CrossRef]
- Stępniewska-Jarosz, S.; Kierzek, R.; Wojczyńska, J.; Sadowska, K.; Tyrakowska, M.; Łukaszewska-Skrzypniak, N.; Rataj-Guranows, M. Fungi colonizing tansy phacelia plants (Phacelia tanacetifolia Benth.) after fungicides treatments. Prog. Plant Prot. 2017, 57, 239–244. [Google Scholar] [CrossRef]
- Zaniewicz-Bajkowska, A.; Rosa, R.; Kosterna, E.; Franczuk, J. Catch crops for green manure: Biomass yield and macroelement content depending on the sowing date. Acta Sci. Pol. Agric. 2013, 12, 65–79. [Google Scholar]
- Siwek, P.; Bucki, P.; Domagała-Świątkiewicz, I.; Lalewicz, P. Effect of cover crops integration in crop rotation on the yield and chemical composition of edible parts of vegetables grown in an organic system in high tunnel. Sci. Hortic. 2024, 332, 113191. [Google Scholar] [CrossRef]
- Kęsik, T.; Mirosław Konopiński, M.; Marzena Błażewicz-Woźniak, M. Response of onion and carrot on soil mulching and no-tillage cultivation system. Acta Agroph. 2001, 45, 95–104. [Google Scholar]
- Kubíková, Z.; Hutyrová, H.; Smejkalová, H.; Kintl, A.; Elbl, J. Application of extended BBCH scale for studying the development of Phacelia tanacetifolia Benth. Ann. Appl. Biol. 2022, 181, 332–346. [Google Scholar] [CrossRef]
- Cîrlig, N.; Iurcu-Străistaru, E.; Ţîţei, V. The impact of the entomofauna on the plants of Phacelia tanacetifolia Benth in the collection of the ‘’al. ciubotaru’’ National Botanical Garden (Institute). Sci. Pap-Ser. A-Agron. 2021, 64, 185–191. Available online: https://agronomyjournal.usamv.ro/pdf/2021/issue_2/Art26.pdf (accessed on 30 April 2025).
- Owayss, A.A.; Shebl, M.A.; Iqbal, J.; Awad, A.M.; Raweh, H.S.; Alqarni, A.S. Phacelia tanacetifolia can enhance conservation of honey bees and wild bees in the drastic hot-arid subtropical Central Arabia. J. Apic. Res. 2020, 59(4), 569–582. [Google Scholar] [CrossRef]
- Popović, V.; Vučković, S.; Dolijanović, Ž.; Mihailović, V.; Ignjatov, M.; Ljubičić, N.; Aćimović, M. Phacelia honey productivity in relation to locality of cultivation. In Proceedings of the GEA (Geo Eco-Eco Agro) International Conference, Podgorica, Montenegro, 28–29 May 2020; pp. 79–95. Available online: https://www.researchgate.net/publication/343376128_Phacelia_Honey_Productivity_in_Relation_to_Locality_of_Cultivation (accessed on 30 April 2025).
- Bryś, M.S.; Strachecka, A. The key role of amino acids in pollen quality and honey bee physiology—A review. Molecules 2024, 29, 2605. [Google Scholar] [CrossRef]
- Bryś, M.S.; Staniec, B.; Strachecka, A. The effect of pollen monodiets on fat body morphology parameters and energy substrate levels in the fat body and hemolymph of Apis mellifera L. workers. Sci. Rep. 2024, 14, 15177. [Google Scholar] [CrossRef] [PubMed]
- Vergun, O.; Grygorieva, O.; Lidiková, J.; Hauptvogel, P.; Brindza, J. Nutritional Composition of Phacelia tanacetifolia Benth. Bee Pollen and Inflorescences. Agrobiodivers. Improv. Nutr. Health Life Qua. 2023, 7, 95–104. Available online: https://agrobiodiversity.uniag.sk/scientificpapers/article/view/485 (accessed on 30 April 2025). [CrossRef]
- Liolios, V.; Tananaki, C.; Papaioannou, A.; Kanelis, D.; Rodopoulou, M.A.; Argena, A. Mineral content in monofloral bee pollen: Investigation of the effect of the botanical and geographical origin. J. Food Meas. Charact. 2019, 13, 1674–1682. [Google Scholar] [CrossRef]
- Bryś, M.S.; Olszewski, K.; Bartoń, M.; Strachecka, A. Changes in the activities of antioxidant enzymes in the fat body and hemolymph of Apis mellifera L. due to pollen monodiets. Antioxidants 2025, 14, 69. [Google Scholar] [CrossRef]
- Sprague, R.; Boyer, S.; Stevenson, G.M.; Wratten, S.D. Assessing pollinators’ use of floral resource subsidies in agri-environment schemes: An illustration using Phacelia tanacetifolia and honeybees. PeerJ 2016, 4e, 2677. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, J.; Antkowiak, M.; Sienkiewicz, P. Flower Strips and Their Ecological Multifunctionality in Agricultural Fields. Agriculture 2022, 12, 1470. [Google Scholar] [CrossRef]
- Totland, Ø.; Nielsen, A.; Bjerknes, A.L.; Ohlson, M. Effects of an exotic plant and habitat disturbance on pollinator visitation and reproduction in a boreal forest herb. Am. J. Bot. 2006, 93, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Barry, C.; Hodge, S. You reap what you sow: A botanical and economic assessment of wildflower seed mixes available in Ireland. Conservation 2023, 3, 73–86. [Google Scholar] [CrossRef]
- Dumanoğlu, Z. General characteristics and importance of phacelia (Phacelia tanacetifolia Bentham.) and some studies in Turkey. TURSTEP 2019, 7, 365–369. [Google Scholar] [CrossRef]
- Ates, E.; Coskuntuna, L.; Tekeli, A.S. Plant growth stage effects on the yield, feeding value and some morphological characters of the fiddleneck (Phacelia tanacetifolia Benth.). Cuban J. Agric. Sci. 2010, 44, 425–428. Available online: https://cjascience.com/index.php/CJAS/article/view/210 (accessed on 30 April 2025).
- Cerempei, V.; Țiței, V.; Vlăduț, V.; Moiceanu, G.A. Comparative study on the characteristics of seeds and phytomass of new high-potential fodder and energy crops. Agriculture 2023, 13, 1112. [Google Scholar] [CrossRef]
- Omokanye, A.; Hernandez, G.; Lardner, H.A.; Al-Maqtari, B.; Gill, K.S.; Lee, A. Alternative forage feeds for beef cattle in Northwestern Alberta, Canada: Forage yield and nutritive value of forage brassicas and forbs. J. Appl. Anim. Res. 2021, 49, 203–210. [Google Scholar] [CrossRef]
- Muneer, F.; Hovmalm, H.P.; Svensson, S.E.; Newson, W.R.; Johansson, E.; Prade, T. Economic viability of protein concentrate production from green biomass of intermediate crops: A pre-feasibility study. J. Clean. Prod. 2021, 294, 126304. [Google Scholar] [CrossRef]
- Grecka, K.; Kuś, P.M.; Worobo, R.W.; Szweda, P. Study of the anti-staphylococcal potential of honeys produced in northern Poland. Molecules 2018, 23, 260. [Google Scholar] [CrossRef]
- Kuś, P.M.; Igor Jerković, I.; Aladić, K.; Jokić, S. Supercritical CO2 and ultrasound extraction and characterization of lipids and polyphenols from Phacelia tanacetifolia Benth. pollen. Ind. Crops Prod. 2023, 206, 117646. [Google Scholar] [CrossRef]
- Sheykhmagomedova, P.A.; Sergeeva, E.O.; Abisalova, I.L.; Sajaya, L.A.; Popova, O.I.; Popov, I.V. Hypolipidemic activity of the phytocomplex of the herb Phacelia tanacetifolia Benth. Probl. Biol. Med. Pharm. Chem. 2024, 27, 69–74. [Google Scholar] [CrossRef]
- Jaśkiewicz, K.; Szczęsna, T.; Jachuła, J. How phenolic compounds profile and antioxidant activity depend on botanical origin of honey—A case of Polish varietal honeys. Molecules 2025, 30, 360. [Google Scholar] [CrossRef] [PubMed]
- Aylanc, V.; Tomás, A.; Russo-Almeida, P.; Falcão, S.I.; Vilas-Boas, M. Assessment of Bioactive Compounds under Simulated Gastrointestinal Digestion of Bee Pollen and Bee Bread: Bioaccessibility and Antioxidant Activity. Antioxidants 2021, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Benavides-Guevara, R.M.; Quicazan, M.C.; Ramírez-Toro, C. Digestibility and availability of nutrients in beepollen applying different pretreatments. Ing. compet. 2017, 19, 119–128. [Google Scholar]
- Popović, V.; Mihailović, V.; Vasiljević, S.; Kiprovski, B.; Rajičić, V.; Tabaković, M.; Šašić Zorić, L.; Tatić, M. Seed quality of the facelia-variety Ns priora grown in Serbia. In Proceedings of the VIII International Scientific Agricultural Symposium “Agrosym 2017”, Jahorina, Bosnia and Herzegovina, 5–8 October 2017; pp. 974–981. Available online: https://fiver.ifvcns.rs/handle/123456789/2345 (accessed on 30 April 2025).
- Pătruică, S.; Alexa, E.; Obiștioiu, D.; Cocan, I.; Radulov, I.; Berbecea, A.; Lazăr, R.N.; Simiz, E.; Vicar, N.M.; Hulea, A.; et al. Chemical composition, antioxidant and antimicrobial activity of some types of honey from Banat Region, Romania. Molecules 2022, 27, 4179. [Google Scholar] [CrossRef]
- Sakač, M.; Jovanov, P.; Marić, A.; Četojević-Simin, D.; Novaković, A.; Plavšić, D.; Škrobot, D.; Kovač, R. Antioxidative, antibacterial and antiproliferative properties of honey types from the Western Balkans. Antioxidants 2022, 11, 1120. [Google Scholar] [CrossRef]
- Kunat-Budzyńska, M.; Rysiak, A.; Wiater, A.; Grąz, M.; Andrejko, M.; Budzyński, M.; Bryś, M.S.; Sudziński, M.; Tomczyk, M.; Gancarz, M.; et al. Chemical composition and antimicrobial activity of new honey varietals. Int. J. Environ. Res. Public Health 2023, 20, 2458. [Google Scholar] [CrossRef] [PubMed]
- Brant, V.; Pivec, J.; Fuksa, P.; Neckář, K.; Kocourková, D.; Venclová, V. Biomass and energy production of catch crops in areas with deficiency of precipitation during summer period in central Bohemia. Biomass Bioenergy 2011, 35, 1286–1294. [Google Scholar] [CrossRef]
- Żarczyński, P.J.; Sienkiewicz, S.; Wierzbowska, J.; Mackiewicz-Walec, E.; Jankowski, K.J.; Krzebietke, S.J. Effect of land protection on the content of mineral nitrogen in soil. Fresen Environ. Bull. 2019, 28, 4506–4513. [Google Scholar]
- Wilczewski, E. Utilization of nitrogen and other microelements by non-papilionaeceous plants cultivated in stubble intercop. Ecol. Chem. Eng. A 2010, 17, 689–698. [Google Scholar]
- Cherniavskih, V.I.; Dumacheva, E.V.; Gorbacheva, A.A.; Vorobyova, O.V.; Ermakova, L.R. The use of morphobiological characteristics in the selection of Phacelia tanacetifolia Benth. Int. J. Green Pharm. 2018, 11, 433–436. [Google Scholar] [CrossRef]
- Kumar, U.; Thomsen, I.K.; Eriksen, J.; Vogeler, I.; Mäenpää, M.; Hansen, E.M. Delaying sowing of cover crops decreases the ability to reduce nitrate leaching. Agric. Ecosyst. Environ. 2023, 355, 108598. [Google Scholar] [CrossRef]
- Fernando, M.; Scott, N.; Shrestha, A.; Gao, S.; Hale, L. A native plant species cover crop positively impacted vineyard water dynamics, soil health, and vine vigor. Agric. Ecosyst. Environ. 2024, 367, 108972. [Google Scholar] [CrossRef]
- Perlein, A.; Bert, V.; de Souza, M.F.; Papin, A.; Meers, E. Field evaluation of industrial non-food crops for phytomanaging a metal-contaminated dredged sediment. Environ. Sci. Pollut. Res. 2023, 30, 44963–44984. [Google Scholar] [CrossRef]
- Klimont, K.; Bulińska-Radomska, Z. Evaluating the usability of selected honey plant species and various willow (Salix spp.) forms for land reclamation savaged by sulphur industry. Zesz. Probl. Post. Nauk Rol. 2010, 555, 517–528. Available online: http://agro.icm.edu.pl/agro/element/bwmeta1.element.dl-catalog-467facc5-1005-45a2-b5ac-84632c0d2a8e (accessed on 30 April 2025).
- Klimont, K.; Bulińska-Radomska, Z.; Górka, J. Utilization possibility of selected honeybee plants for land reclamation of sulfur post-exploitation area. Pol. J. Agron. 2013, 12, 17–25. [Google Scholar]
- Predikaka, T.C.; Kralj, T.; Jerman, M.S.; Mastnak, T. A full-scale bioremediation study of diesel fuel-contaminated soil: The effect of plant species and soil amendments. Int. J. Environ. Sci. Technol. 2024, 21, 4319–4330. [Google Scholar] [CrossRef]
- Słomka, A.; Pawłowska, M. Catch and cover crops’ use in the energy sector via conversion into biogas—Potential benefits and disadvantages. Energies 2024, 17, 600. [Google Scholar] [CrossRef]
- Herrmann, C.; Idler, C.; Heiermann, M. Biogas crops grown in energy crop rotations: Linking chemical composition and methane production characteristics. Bioresour. Technol. 2016, 206, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Bohra, A.; Kilian, B.; Sivasankar, S.; Caccamo, M.; Mba, C.; McCouch, S.R.; Varshney, R.K. Reap the crop wild relatives for breeding future crops. Trends Biotechnol. 2022, 40, 412–431. [Google Scholar] [CrossRef] [PubMed]





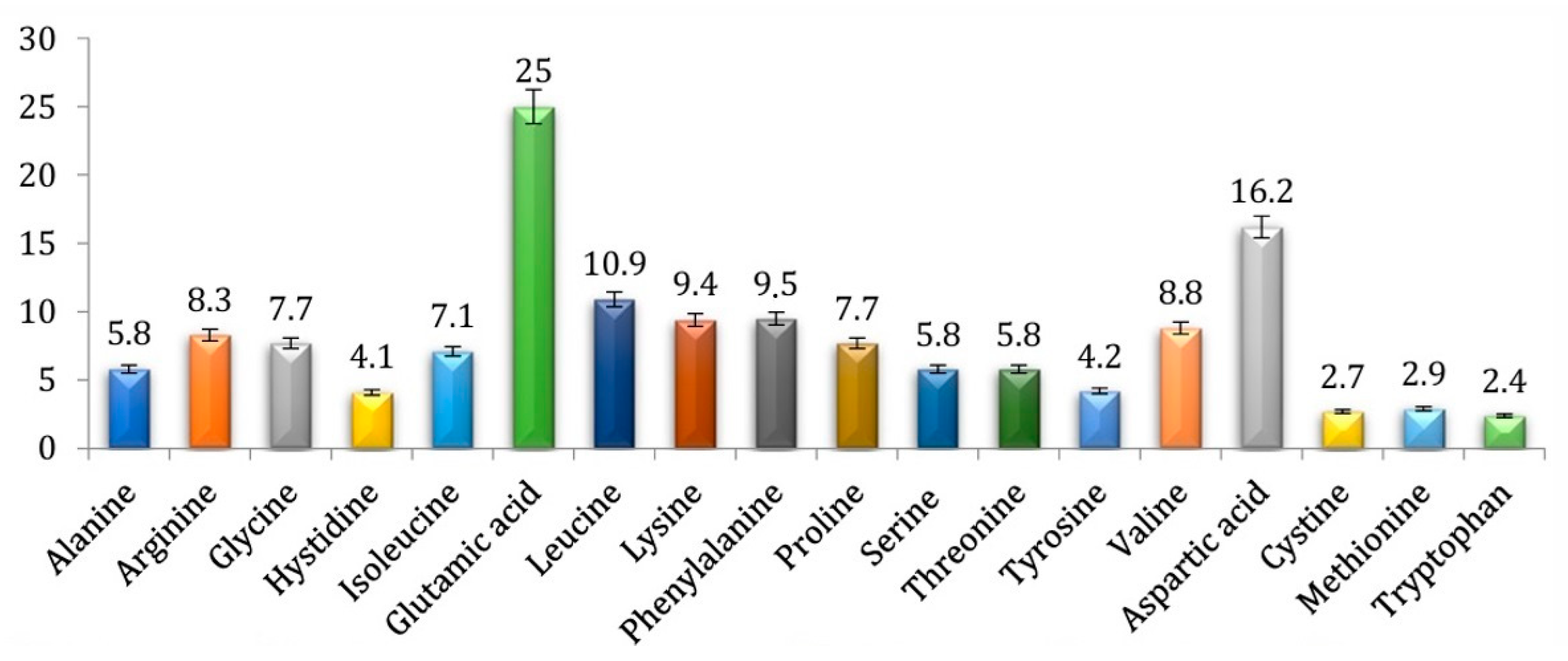
| Ingredient | Unit | Content | Source |
|---|---|---|---|
| Dry matter (DM) | % | 11.8 | [36] |
| Acid detergent fiber (ADF) | g/kg | 346.3–388.0 | [32,82,84] |
| Neutral detergent fiber (NDF) | 425.1–553.0 | ||
| Total digestible nutrients (TDN) | 588.0–601.2 | ||
| Acid detergent lignin (ADL) | 224.3–237.0 | ||
| Crude protein (CP) | g/kg | 132.2–170.0 | [82,83,84] |
| Crude fiber (CF) | 324.0 | [83] | |
| Celulose | 303.0 | ||
| Crude ash | 93.8–104.7 | [32,83] | |
| Carbon (C) | g/kg | 499.0 | [83] |
| Nitrogen (N) | 23.7–27.9 | [44,83,84] | |
| Phosphorus (P) | 4.9–5.5 | [44,83,84] | |
| Potassium (K) | 38.8–39.6 | [44,83,84] | |
| Calcium (Ca) | 16.3–29.0 | [44,83,84] | |
| Magnesium (Mg) | 2.0–3.9 | [82,84] | |
| Sodium (Na) | 2.0–3.1 | [36,84] | |
| Sulfur (S) | 2.3 | [36] | |
| Boron (B) | mg/kg | 26.8 | [36] |
| Copper (Cu) | 7.57 | ||
| Iron (Fe) | 158.0 | ||
| Zink (Zn) | 24.1 | ||
| Manganese (Mn) | 27.9 | ||
| Titanium (Ti) | 3.44 | ||
| Biomethane potential | l/kg | 324.0 | [83] |
| Digestible energy | MJ/kg | 12.05 | [83] |
| Metabolizable energy | 9.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żarczyński, P.J.; Mackiewicz-Walec, E.; Krzebietke, S.J.; Sienkiewicz, S.; Żarczyńska, K. Phacelia tanacetifolia Benth. as a Multifunctional Plant: Support for Pollinators and Sustainable Agricultural Practices. Agronomy 2025, 15, 1843. https://doi.org/10.3390/agronomy15081843
Żarczyński PJ, Mackiewicz-Walec E, Krzebietke SJ, Sienkiewicz S, Żarczyńska K. Phacelia tanacetifolia Benth. as a Multifunctional Plant: Support for Pollinators and Sustainable Agricultural Practices. Agronomy. 2025; 15(8):1843. https://doi.org/10.3390/agronomy15081843
Chicago/Turabian StyleŻarczyński, Piotr Jarosław, Ewa Mackiewicz-Walec, Sławomir Józef Krzebietke, Stanisław Sienkiewicz, and Katarzyna Żarczyńska. 2025. "Phacelia tanacetifolia Benth. as a Multifunctional Plant: Support for Pollinators and Sustainable Agricultural Practices" Agronomy 15, no. 8: 1843. https://doi.org/10.3390/agronomy15081843
APA StyleŻarczyński, P. J., Mackiewicz-Walec, E., Krzebietke, S. J., Sienkiewicz, S., & Żarczyńska, K. (2025). Phacelia tanacetifolia Benth. as a Multifunctional Plant: Support for Pollinators and Sustainable Agricultural Practices. Agronomy, 15(8), 1843. https://doi.org/10.3390/agronomy15081843







