Development and Evaluation of Selenium-Enriched Compound Fertilizers for Remediation of Mercury-Contaminated Agricultural Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.1.1. Soil Sampling, Preparation, and Analysis
2.1.2. Plant Material
2.1.3. Selenium-Enriched Compound Fertilizer Materials
2.2. Development of the Se-Enriched Compound Fertilizer
2.2.1. Granulation Method
2.2.2. Analysis of Particle Properties
2.3. Pot Experiment
2.3.1. Experimental Design
2.3.2. The Hg and Se Analysis
2.3.3. Quality Control
2.4. Data Analysis
3. Results
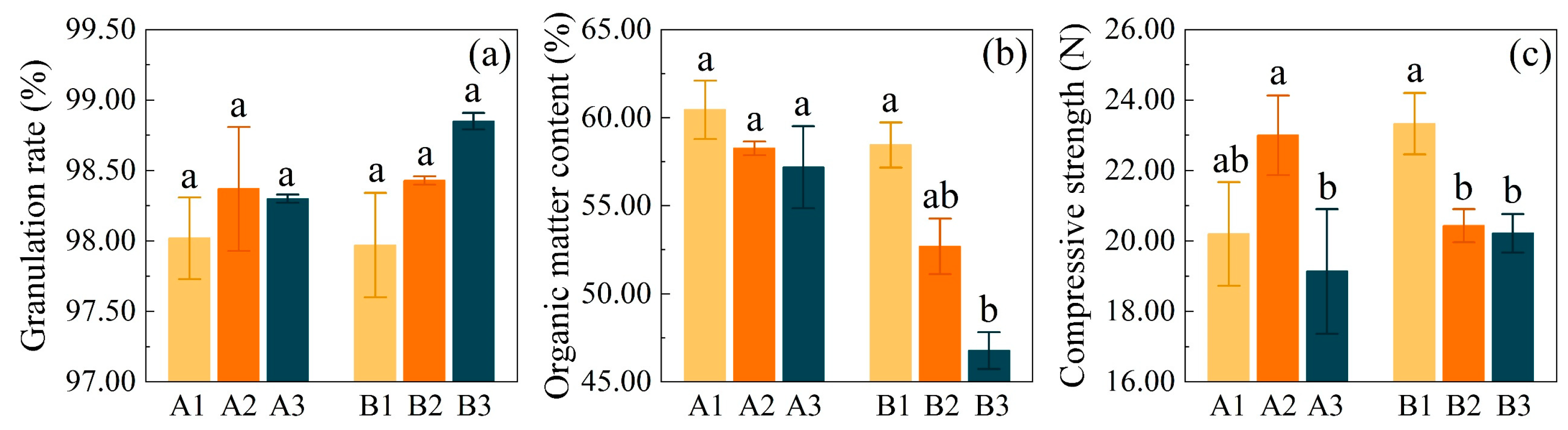
3.1. Binder Dosage Effects on Fertilizer Performance
| Indicator | GB 18877-2002 Standard [40] | Formulation 1 | Formulation 2 | Agronomic Significance |
|---|---|---|---|---|
| N (%) | None | 11.17 ± 0.12 | 12.06 ± 0.06 | Meeting the nutrients requirement of plant |
| P2O5 (%) | None | 4.91 ± 0.08 | 4.67 ± 0.08 | |
| K2O (%) | None | 9.66 ± 0.55 | 9.37 ± 0.21 | |
| Total nutrients (N + P2O5 + K2O) (%) | ≥15.0 | 25.74 ± 0.57 | 26.10 ± 0.23 | |
| Moisture content (%) | ≤10.0 | 2.5 ± 0.03 | 1.7 ± 0.01 | Affecting storage stability |
| Organic matter (%) | ≥20 | 58.26 ± 0.38 | 58.45 ± 1.29 | Improving soil structure |
| Particle size (1.00–4.75 mm) (%) | ≥70 | 93 ± 0.44 | 92 ± 0.37 | Affecting use |
| pH value | 5.5–8.0 | 6.3 ± 0.02 | 6.3 ± 0.02 | Reducing nutrient fixation |
| Chloride ion (%) | ≤3.0 | 0.04 ± 0.00 | 0.11 ± 0.01 | Avoiding salt stress on crops |
| Arsenic (%) | ≤0.0050 | <DL | <DL | Below the ecological risk threshold |
| Cadmium (%) | ≤0.0010 | <DL | <DL | |
| Lead (%) | ≤0.0150 | <DL | <DL | |
| Chromium (%) | ≤0.0500 | 0.0003 ± 0.0000 | 0.0004 ± 0.0000 | |
| Mercury (%) | ≤0.0005 | <DL | <DL | |
| Selenium (%) | None | 0.20 ± 0.01 | 0.20 ± 0.03 | Supplement selenium |
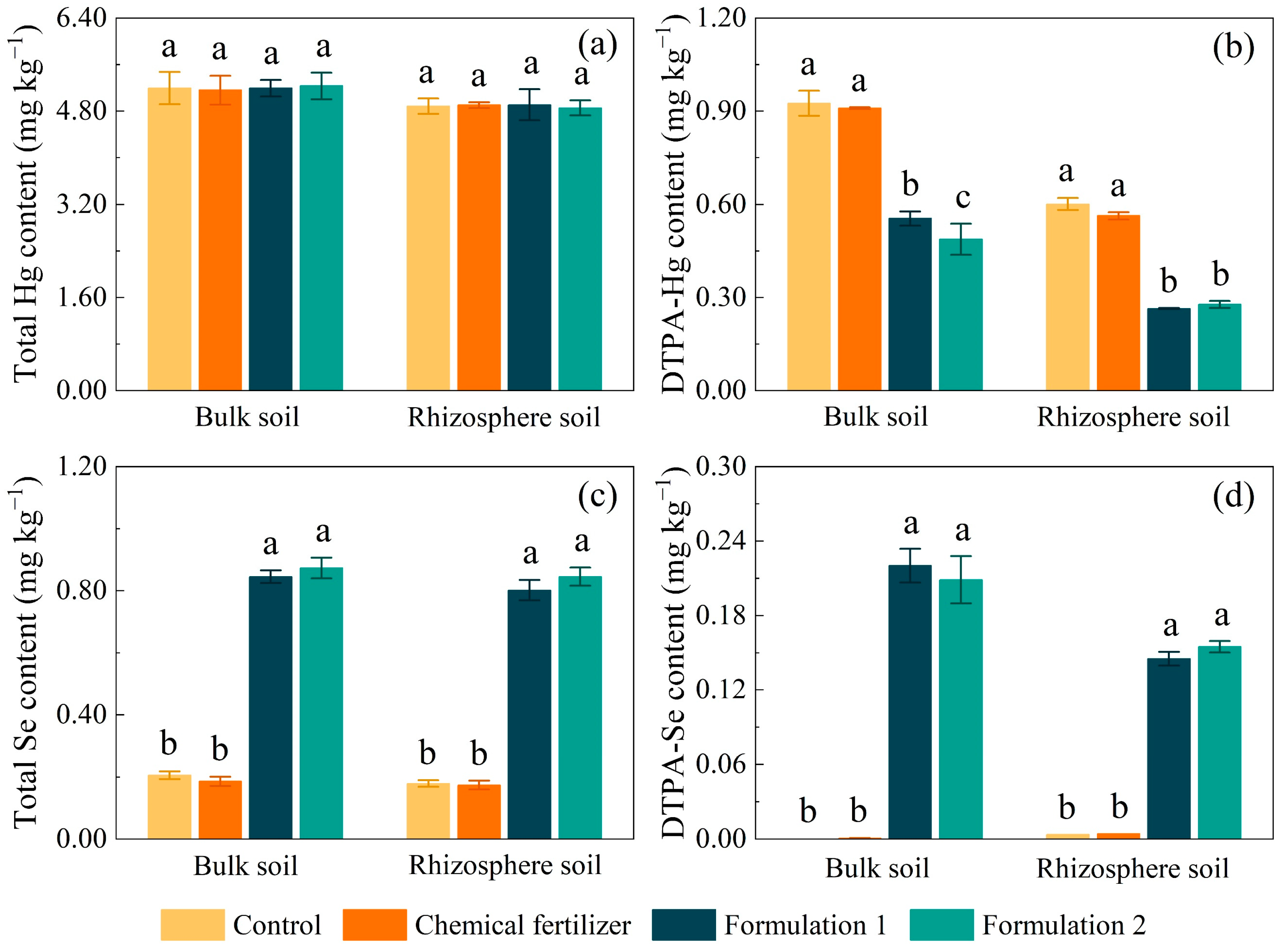
3.2. Soil Available Hg Reduction and Se Increase Caused by Fertilizers
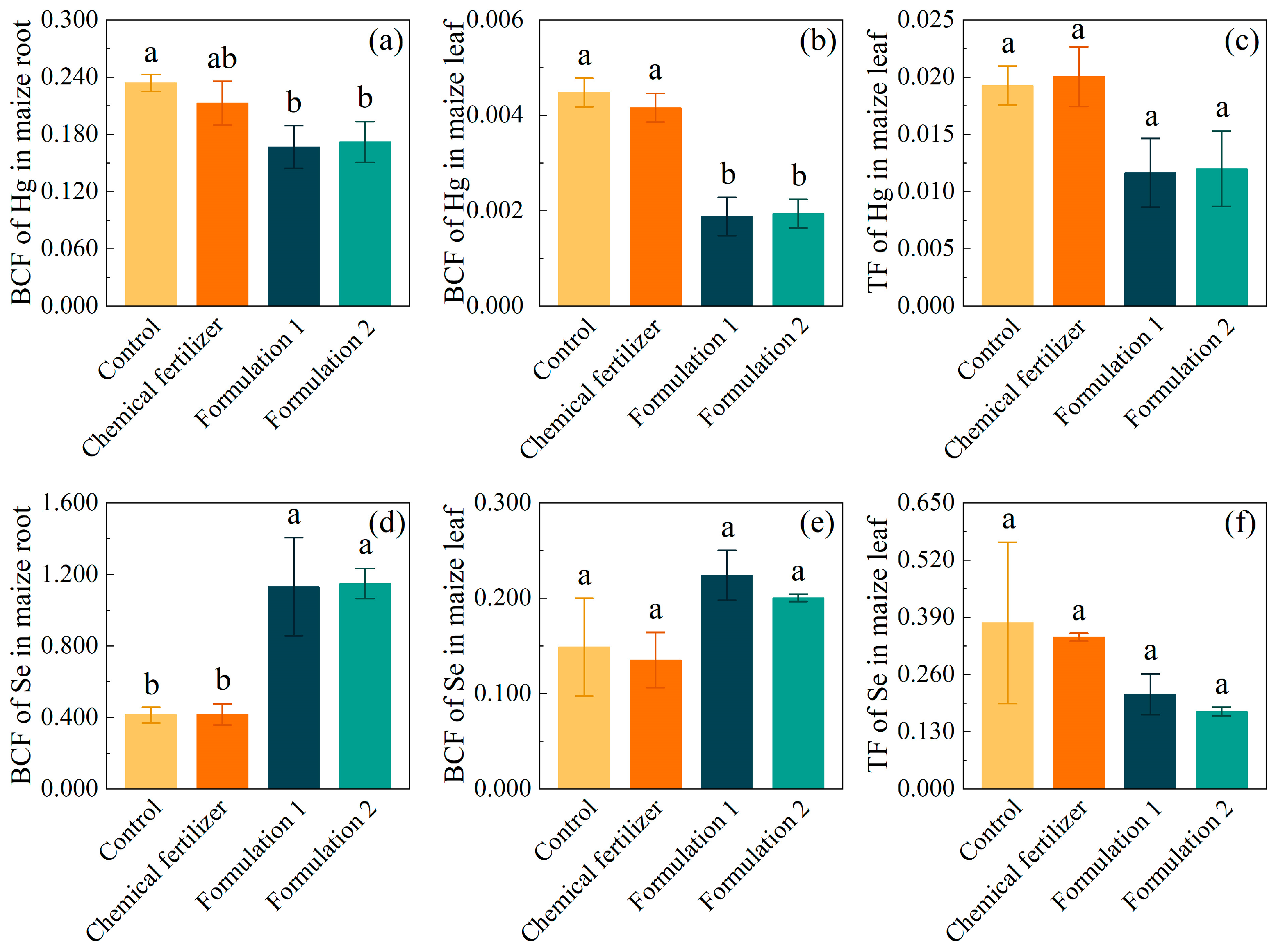
3.3. Maize Hg Uptake Restriction and Se Uptake Enhancement with Fertilizers
4. Discussion
4.1. The Effect of Different Binder Dosages on the Properties of Se-Enriched Compound Fertilizer
4.2. Remediation Effectiveness of Se-Enriched Compound Fertilizer for Hg-Contaminated Soil
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Natasha; Shahid, M.; Khalid, S.; Bibi, I.; Bundschuh, J.; Khan Niazi, N.; Dumat, C. A critical review of mercury speciation, bioavailability, toxicity and detoxification in soil-plant environment: Ecotoxicology and health risk assessment. Sci. Total Environ. 2020, 711, 134749. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.A.T.; Dinh, Q.T.; Zhou, F.; Zhai, H.; Xue, M.Y.; Du, Z.K.; Bañuelos, G.S.; Liang, D.L. Mechanisms underlying mercury detoxification in soil-plant systems after selenium application: A review. Environ. Sci. Pollut. Res. 2021, 28, 46852–46876. [Google Scholar] [CrossRef]
- Liu, Y.R.; Guo, L.; Yang, Z.M.; Xu, Z.; Zhao, J.T.; Wen, S.H.; Delgado-Baquerizo, M.; Chen, L. Multidimensional drivers of mercury distribution in global surface soils: Insights from a global standardized field survey. Environ. Sci. Technol. 2023, 57, 12442–12452. [Google Scholar] [CrossRef]
- Lin, D.J.; Hu, G.Z.; Li, H.B.; Li, L.; Wu, F.; Yang, G.Q.; Zhuang, L.; Gong, Y.Y. Green remediation of mercury-contaminated soil using iron sulfide nanoparticles: Immobilization performance and mechanisms, effects on soil properties, and life cycle assessment. Sci. Total Environ. 2024, 944, 173928. [Google Scholar] [CrossRef]
- Liu, S.J.; Wang, X.D.; Guo, G.L.; Yan, Z.G. Status and environmental management of soil mercury pollution in China: A review. J. Environ. Manag. 2021, 277, 111442. [Google Scholar] [CrossRef]
- Yang, L.X.; Zhang, Y.Y.; Wang, F.F.; Luo, Z.D.; Guo, S.J.; Strähle, U. Toxicity of mercury: Molecular evidence. Chemosphere 2020, 245, 125586. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.C.; Yang, W.C.; Johnson, V.E.; Si, M.Y.; Zhao, F.P.; Liao, Q.; Su, C.Q.; Yang, Z.H. Selenium–sulfur functionalized biochar as amendment for mercury-contaminated soil: High effective immobilization and inhibition of mercury re-activation. Chemosphere 2022, 306, 135552. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Hussain, M.; Ishfaq, M.; Shakoor, N.; Tam, N.F.Y.; Li, X.Y.; Pan, M.; Zhu, Z.J.; Xin, S.; Zhou, H.C. Mercury-induced alterations in soil microbiome: A potential for microbiome stewardship to remediate contaminated soils. J. Clean. Prod. 2025, 512, 145717. [Google Scholar] [CrossRef]
- Teng, D.Y.; Mao, K.; Ali, W.; Xu, G.M.; Huang, G.P.; Niazi, N.K.; Feng, X.B.; Zhang, H. Describing the toxicity and sources and the remediation technologies for mercury-contaminated soil. RSC Adv. 2020, 10, 23221–23232. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; Wang, H.B.; Wang, H.J.; Li, Q.C.; Li, Y. Effect of soil washing on heavy metal removal and soil quality: A two-sided coin. Ecotoxicol. Environ. Saf. 2020, 203, 110981. [Google Scholar] [CrossRef] [PubMed]
- Lwin, C.S.; Seo, B.H.; Kim, H.U.; Owens, G.; Kim, K.R. Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality-a critical review. Soil Sci. Plant Nutr. 2018, 64, 156–167. [Google Scholar] [CrossRef]
- Shi, G.Y.; Hu, J.Y.; Zhang, S.H.; Ni, G.; Shi, W.L.; Hu, C.X.; Zhao, X.H. The application of exogenous Se improved the remediation efficiency of Lolium multiflorum Lam grown in nonylphenol-Cd Co-contaminated soil. J. Environ. Chem. Eng. 2022, 10, 108962. [Google Scholar] [CrossRef]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H.E. Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef]
- Yang, H.; Yang, X.F.; Ning, Z.P.; Kwon, S.Y.; Li, M.L.; Tack, F.M.G.; Kwon, E.E.; Rinklebe, J.; Yin, R.S. The beneficial and hazardous effects of selenium on the health of the soil-plant-human system: An overview. J. Hazard. Mater. 2022, 422, 126876. [Google Scholar] [CrossRef]
- Roman, M. Selenium: Properties and determination. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldr´a, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 734–743. [Google Scholar] [CrossRef]
- Haug, A.; Graham, R.D.; Christophersen, O.A.; Lyons, G.H. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb. Ecol. Health Dis. 2007, 19, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Rong, Q.L.; Chen, J.; Zhang, Y.F.; Tan, Z.B.; Wang, W.J.; Sun, C.X.; Guo, X.; Zhou, C.H.; Cai, H.S.; Zhao, X.M. The interaction between selenium and other elements in soil and rice roots shaped by straw and straw biochar regulated the enrichment of selenium in rice grain. Front. Plant Sci. 2024, 15, 1387460. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Yan, M.; Liang, L.C.; Lu, Q.H.; Han, J.L.; Liu, L.; Feng, X.B.; Guo, J.Y.; Wang, Y.J.; Qiu, G.L. Impacts of selenium supplementation on soil mercury speciation, and inorganic mercury and methylmercury uptake in rice (Oryza sativa L.). Environ. Pollut. 2019, 249, 647–654. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.Y.; Hintelmann, H.; Tang, W.L.; Zhong, H. New insights into Hg-Se antagonism: Minor impact on inorganic Hg mobility while potential impacts on microorganisms. Sci. Total Environ. 2024, 913, 169705. [Google Scholar] [CrossRef]
- Yang, D.Y.; Chen, Y.W.; Gunn, J.M.; Belzile, N. Selenium and mercury in organisms: Interactions and mechanisms. Environ. Rev. 2008, 16, 71–92. [Google Scholar] [CrossRef]
- McNear, D.H.; Afton, S.E.; Caruso, J.A. Exploring the structural basis for selenium/mercury antagonism in Allium fistulosum. Metallomics 2012, 4, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, F.M. Selenium Deficiency and Toxicity in the Environment. In Essentials of Medical Geology; Selinus, O., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 375–416. [Google Scholar] [CrossRef]
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef]
- Li, Z.; Liang, D.L.; Peng, Q.; Cui, Z.W.; Huang, J.; Lin, Z.Q. Interaction between selenium and soil organic matter and its impact on soil selenium bioavailability: A review. Geoderma 2017, 295, 69–79. [Google Scholar] [CrossRef]
- Tran, T.A.T.; Dinh, Q.T.; Cui, Z.W.; Huang, J.; Wang, D.; Wei, T.J.; Liang, D.L.; Sun, X.; Ning, P. Comparing the influence of selenite (Se4+) and selenate (Se6+) on the inhibition of the mercury (Hg) phytotoxicity to pak choi. Ecotoxicol. Environ. Saf. 2018, 147, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Staicu, L.C.; Morin-Crini, N.; Crini, G. Desulfurization: Critical step towards enhanced selenium removal from industrial effluents. Chemosphere 2017, 172, 111–119. [Google Scholar] [CrossRef]
- Niu, Z.R.; Su, Y.Z.; Li, J.; An, F.J.; Liu, T.N. Effect of attapulgite application on aggregate formation and carbon and nitrogen content in sandy Soil. Sustainability 2023, 15, 12511. [Google Scholar] [CrossRef]
- Gu, S.Q.; Kang, X.N.; Wang, L.; Lichtfouse, E.; Wang, C.Y. Clay mineral adsorbents for heavy metal removal from wastewater: A review. Environ. Chem. Lett. 2019, 17, 629–654. [Google Scholar] [CrossRef]
- Rong, Q.; Zhong, K.; Huang, H.; Li, C.Z.; Zhang, C.L.; Nong, X.Y. Humic acid reduces the available cadmium, copper, lead, and zinc in soil and their uptake by tobacco. Appl. Sci. 2020, 10, 1077. [Google Scholar] [CrossRef]
- Li, Y.X.; Pei, G.P.; Zhu, Y.E.; Liu, W.; Li, H. Vinegar residue biochar: A possible conditioner for the safe remediation of alkaline Pb-contaminated soil. Chemosphere 2022, 293, 133555. [Google Scholar] [CrossRef]
- Pei, G.P.; Zhu, Y.E.; Wen, J.G.; Pei, Y.X.; Li, H. Vinegar residue supported nanoscale zero-valent iron: Remediation of hexavalent chromium in soil. Environ. Pollut. 2020, 256, 113407. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environment Quality Risk Control Standard for Soil Contamination of Agriculture Land. China Environment Publishing Group: Beijing, China, 2018.
- NY/T 1121.3-2006; Soil Testing Part 3: Method for Determination of Soil Mechanical Composition. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2006.
- Lu, R.S. Soil Agrochemical Analysis Method; China Agricultural Science and Technology Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- HJ 802-2016; Soil Quality-Determination of Conductivity-Electrode Method. China Environmental Science Press: Beijing, China, 2016.
- Bao, S.D. Soil Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- HJ 889-2017; Soil Quality-Determination of Cation Exchange Capacity (CEC)-Hexamminecobalt Trichloride Solution-Spectrophotometric Method. Ministry of Environmental Protection: Beijing, China, 2017.
- GB/T 22105.1-2008; Soil Quality-Analysis of Total Mercury, Arsenic and Lead Contents Atomic Fluorescence Spectrometry Part 1: Analysis of Total Mercury Contents in Soils. China Standard Publishing House: Beijing, China, 2008.
- NY/T 1104-2006; Determination of Selenium in Soils. China Agricultural Press: Beijing, China, 2006.
- GB 18877-2002; Organic-Inorganic Compound Fertilizers. China Standard Publishing House: Beijing, China, 2002.
- GB/T 17767.1-2008; Determination of Organic-Inorganic Compound Fertilizers Part 1: Total Nitrogen Content. China Standard Publishing House: Beijing, China, 2008.
- GB/T 8573-2017; Determination of Available Phosphorus Content for Compound Fertilizers. China Standard Publishing House: Beijing, China, 2017.
- GB/T 17767.3-2008; Determination of Organic-Inorganic Compound Fertilizers Part 3: Total Potassium Content. China Standard Publishing House: Beijing, China, 2008.
- GB/T 8576-2010; Determination of Free Water for Compound Fertilizers—Vacuum Oven Method. China Standard Publishing House: Beijing, China, 2010.
- GB 15063-2001; Compound Fertilizer (Complex Fertilizer). China Standard Publishing House: Beijing, China, 2001.
- GB/T 23739-2009; Soil Quality-Analysis of Available Lead and Cadmium Contents in Soils-Atomic Absorption Spectrometry. China Standard Publishing House: Beijing, China, 2009.
- GB5009.17-2014; Determination of Total Mercury and Organic Mercury in Food According to the National Food Safety Standard. China Standard Publishing House: Beijing, China, 2014.
- GB 5009.93-2017; National Food Safety Standard Determination of Selenium in Food. China Standard Publishing House: Beijing, China, 2017.
- He, Y.J.; Hou, X.L.; Wu, X.N.; Duan, C.Q.; Liu, C.; Yin, L.Q.; Zhang, M.; Fu, D.G. Fertilization and intercropping reduce Pb accumulation in plants by influencing rhizosphere soil phosphorus forms in soil-plant systems. Ecotox. Environ. Saf. 2025, 293, 118011. [Google Scholar] [CrossRef]
- GBW07403 (GSS-3); Certificate of Certified Reference Material Soil. Institute of Geophysical and Geochemical Exploration: Langfang, China, 2019.
- GBW10012 (GSB-3); Certificate of Certified Reference Material Biological Sample. Institute of Geophysical and Geochemical Exploration: Langfang, China, 2005.
- Siuda, R.; Kwiatek, J.; Szufa, S.; Obraniak, A.; Piersa, P.; Adrian, L.; Modrzewski, R.; Lawinska, K.; Siczek, K.; Olejnik, T.P. Industrial Verification and Research Development of Lime-Gypsum Fertilizer Granulation Method. Minerals 2021, 11, 119. [Google Scholar] [CrossRef]
- Hmeid, H.A.; Akodad, M.; Halim, M.E.; Omdi, F.E.; Baghour, M.; Skalli, A.; Sadik, C.; Gueddari, H.; Chahban, M.; Yousfi, Y.E.; et al. Comparative analysis of Na+ and Ca2+ ion effects on the physical-chemical properties of Bentonite: Implications for industrial applications. Ore Energy Resour. Geol. 2025, 18, 100095. [Google Scholar] [CrossRef]
- Michalek, B.; Ochowiak, M.; Bizon, K.; Wlodarczak, S.; Krupinska, A.; Matuszak, M.; Boro, D.; Gierczyk, B.; Olszewski, R. Effect of Adding Surfactants to a Solution of Fertilizer on the Granulation Process. Energies 2021, 14, 7557. [Google Scholar] [CrossRef]
- Xin, M.J.; Jiang, Z.W.; Song, Y.Q.; Cui, H.G.; Kong, A.J.; Chi, B.W.; Shan, R.B. Compression Strength and Critical Impact Speed of Typical Fertilizer Grains. Agriculture 2023, 13, 2285. [Google Scholar] [CrossRef]
- DZ/T 0295-2016; Specification of Land Quality Geochemical Assessment. China University of Geosciences Press: Beijing, China, 2016.
- Zhang, H.; Feng, X.B.; Zhu, J.M.; Sapkota, A.; Meng, B.; Yao, H.; Qin, H.B.; Larssen, T. Selenium in soil inhibits mercury uptake and translocation in rice (Oryza sativa L.). Environ. Sci. Technol. 2012, 46, 10040–10046. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.X.; Zheng, W.Z.; Lin, J.Y.; Shi, Y.; Xie, W.Z.; Zhou, H.M. Effect of metal ions on the activity of green crab (Scylla serrata) alkaline phosphatase. Int. J. Biochem. Cell Biol. 2000, 32, 879–885. [Google Scholar] [CrossRef]
- Cai, M.M.; Hu, C.X.; Wang, X.; Zhao, Y.Y.; Jia, W.; Sun, X.C.; Elyamine, A.M.; Zhao, X.H. Selenium induces changes of rhizosphere bacterial characteristics and enzyme activities affecting chromium/selenium uptake by pak choi (Brassica campestris L. ssp. Chinensis Makino) in chromium contaminated soil. Environ. Pollut. 2019, 249, 716–727. [Google Scholar] [CrossRef]
- Mroczek-Zdyrska, M.; Wójcik, M. The influence of selenium on root growth and oxidative stress induced by lead in Vicia faba L. minor plants. Biol. Trace Elem. Res. 2012, 147, 320–328. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Hu, C.X.; Wu, Z.C.; Liu, X.W.; Cai, M.M.; Jia, W.; Zhao, X.H. Selenium reduces cadmium accumulation in seed by increasing cadmium retention in root of oilseed rape (Brassica napus L.). Environ. Exp. Bot. 2019, 158, 161–170. [Google Scholar] [CrossRef]
- Li, Y.Y.; Hu, W.J.; Zhao, J.T.; Chen, Q.M.; Wang, W.; Li, B.; Li, Y.F. Selenium decreases methylmercury and increases nutritional elements in rice growing in mercury-contaminated farmland. Ecotoxicol. Environ. Saf. 2019, 182, 109447. [Google Scholar] [CrossRef]
- Pei, G.P.; Li, Y.X.; Li, H. Impacts of Selenium Supplementation on Soil Mercury Speciation, Soil Properties and Mercury-Resistant Microorganisms and Resistant Genes. Agronomy 2024, 14, 1928. [Google Scholar] [CrossRef]
- Shanker, K.; Mishra, S.; Srivastava, S.; Srivastava, R.; Dass, S.; Prakash, S.; Srivastava, M.M. Study of mercury-selenium (Hg-Se) interactions and their impact on Hg uptake by the radish (Raphanus sativus) plant. Food Chem. Toxicol. 1996, 34, 883–886. [Google Scholar] [CrossRef]
- Mounicou, S.; Shah, M.; Meija, J.; Caruso, J.A.; Vonderheide, A.P.; Shann, J. Localization and speciation of selenium and mercury in Brassica juncea—Implications for Se–Hg antagonism. J. Anal. At. Spectrom. 2006, 21, 404–412. [Google Scholar] [CrossRef]
- Li, Y.X.; Pei, G.P.; Qiao, X.L.; Zhu, Y.E.; Li, H. Remediation of cadmium contaminated water and soil using vinegar residue biochar. Environ. Sci. Pollut. Res. 2018, 25, 15754–15764. [Google Scholar] [CrossRef]
- Soares, L.C.; Egreja, F.B.; Linhares, L.A.; Windmoller, C.C.; Yoshida, M.I. Accumulation and oxidation of elemental mercury in tropical soils. Chemosphere 2015, 134, 181–191. [Google Scholar] [CrossRef]
- Qian, C.X.; Chen, Q.W.; Jiang, L.Y.; Yang, X.Y.; Rao, S.; Zhang, W.W.; Xu, F. Physiological Mechanism of Exogenous Selenium in Alleviating Mercury Stress on Pakchoi (Brassica campestris L.). Phyton-Int. J. Exp. Bot. 2024, 93, 951–962. [Google Scholar] [CrossRef]
- Wang, Y.J.; Dang, F.; Evans, R.D.; Zhong, H.; Zhao, J.T.; Zhou, D.M. Mechanistic understanding of MeHg-Se antagonism in soil-rice systems: The key role of antagonism in soil. Sci. Rep. 2016, 6, 19477. [Google Scholar] [CrossRef]
- Leterme, B.; Jacques, D. A reactive transport model for mercury fate in contaminated soil-sensitivity analysis. Environ. Sci. Pollut. Res. 2015, 22, 16830–16842. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, H.E.L.; Nalini, H.A.J.; Leonel, L.V.; Windmoller, C.C.; Santos, R.C.; de Brito, W. Quantification and speciation of mercury in soils from the Tripuí ecological station, Minas Gerais, Brazil. Sci. Total Environ. 2006, 368, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.M.; Zhang, Y.X.; Huang, B.; Zhang, H.D. Source apportionment of selenium and influence factors on its bioavailability in intensively managed greenhouse soil: A case study in the east bank of the Dianchi Lake, China. Ecotoxicol. Environ. Saf. 2019, 170, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Smazíková, P.; Praus, L.; Száková, J.; Tremlová, J.; Hanc, A.; Tlustos, P. Effects of Organic Matter-Rich Amendments on Selenium Mobility in Soils. Pedosphere 2019, 29, 740–751. [Google Scholar] [CrossRef]

| Soil Properties | Value |
|---|---|
| Texture | Loam |
| Clay (%) | 5.14 |
| Silt (%) | 41.78 |
| Sand (%) | 53.08 |
| pH | 7.70 |
| Electrical conductivity (μs cm−1) | 269 |
| Cation exchange capacity (mmol kg−1) | 15.16 |
| Organic matter (g kg−1) | 19.41 |
| Total Hg (mg kg−1) | 5.38 |
| Total Se (mg kg−1) | 0.27 |
| Basic Properties | Vinegar Residue | Biochar | Potassium Humate | Attapulgite | Bentonite (Sodium-Based) |
|---|---|---|---|---|---|
| pH | 4.17 | 9.97 | 9.04 | 5.42 | 10.02 |
| Total Hg (%) | 0 | 0 | 0 | 0 | 0 |
| Total Se (%) | 0 | 0 | 0 | 0 | 0 |
| N (%) | 0.51 | 0.08 | 0.10 | 0.05 | 0.05 |
| P2O5 (%) | 0.12 | 0.03 | 1.42 | 0.01 | 0.79 |
| K2O (%) | - | - | 10.14 | - | - |
| Total nutrients (%) | 0.63 | 0.11 | 1.52 | 0.06 | 0.84 |
| Labels | Urea (%) | KH2PO4 (%) | ZnSO4 (%) | Na2SeO3 (%) | Potassium Humate (%) | Vinegar Residue (%) | Biochar (%) | Attapulgite (%) | Bentonite (%) |
|---|---|---|---|---|---|---|---|---|---|
| A1 | 22.51 | 9.59 | 3.30% | 0.44 | 40.00 | 9.16 | 5.00 | 10.00 | 0.00 |
| A2 | 22.51 | 9.59 | 3.30% | 0.44 | 40.00 | 4.16 | 5.00 | 15.00 | 0.00 |
| A3 | 22.51 | 9.59 | 3.30% | 0.44 | 40.00 | 0.00 | 4.16 | 20.00 | 0.00 |
| B1 | 22.51 | 9.59 | 3.30% | 0.44 | 40.00 | 9.16 | 5.00 | 0.00 | 10.00 |
| B2 | 22.51 | 9.59 | 3.30% | 0.44 | 40.00 | 4.16 | 5.00 | 0.00 | 15.00 |
| B3 | 22.51 | 9.59 | 3.30% | 0.44 | 40.00 | 0.00 | 4.16 | 0.00 | 20.00 |
| Labels | Control | Chemical Fertilizer | Formulation 1 | Formulation 2 |
|---|---|---|---|---|
| Treatment | Received no treatment | Added Chemical fertilizer | Added Formulation 1 of compound fertilizer | Added Formulation 2 of compound fertilizer |
| GBW07403 (GSS-3) | Measured Values | GBW10012 (GSB-3) | Measured Values | |
|---|---|---|---|---|
| Se (mg kg−1) | 0.09 ± 0.02 | 0.08 ± 0.01 | 0.021 ± 0.008 | 0.022 ± 0.004 |
| Hg (mg kg−1) | 0.060 ± 0.004 | 0.062 ± 0.002 | 0.0016 | 0.0017 ± 0.001 |
| Whether it met the standards | Yes | |||
| Labels | A1 | A2 | A3 | B1 | B2 | B3 |
|---|---|---|---|---|---|---|
| pH | 6.31 ± 0.03 b | 6.28 ± 0.02 b | 6.33 ± 0.02 b | 6.32 ± 0.02 b | 6.42 ± 0.01 a | 6.46 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Pei, G.; Zhang, Y.; Guan, S.; Lv, Y.; Li, Z.; Li, H. Development and Evaluation of Selenium-Enriched Compound Fertilizers for Remediation of Mercury-Contaminated Agricultural Soil. Agronomy 2025, 15, 1842. https://doi.org/10.3390/agronomy15081842
Li Y, Pei G, Zhang Y, Guan S, Lv Y, Li Z, Li H. Development and Evaluation of Selenium-Enriched Compound Fertilizers for Remediation of Mercury-Contaminated Agricultural Soil. Agronomy. 2025; 15(8):1842. https://doi.org/10.3390/agronomy15081842
Chicago/Turabian StyleLi, Yuxin, Guangpeng Pei, Yanda Zhang, Shuyun Guan, Yingzhong Lv, Zhuo Li, and Hua Li. 2025. "Development and Evaluation of Selenium-Enriched Compound Fertilizers for Remediation of Mercury-Contaminated Agricultural Soil" Agronomy 15, no. 8: 1842. https://doi.org/10.3390/agronomy15081842
APA StyleLi, Y., Pei, G., Zhang, Y., Guan, S., Lv, Y., Li, Z., & Li, H. (2025). Development and Evaluation of Selenium-Enriched Compound Fertilizers for Remediation of Mercury-Contaminated Agricultural Soil. Agronomy, 15(8), 1842. https://doi.org/10.3390/agronomy15081842







