Transcriptome and Metabolome Analyses Reveal the Physiological Variations of a Gradient-Pale-Green Leaf Mutant in Sorghum
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Measurement of Agronomic Traits and Chlorophyll Content
2.3. Measurement of Photosynthesis-Related Parameters
2.4. Total Nitrogen, Phosphorus, Potassium, and Mineral Element Content Measurement
2.5. RNA-Seq Analysis
2.6. Widely Targeted Metabolome Analysis
2.7. Data Statistics
3. Results
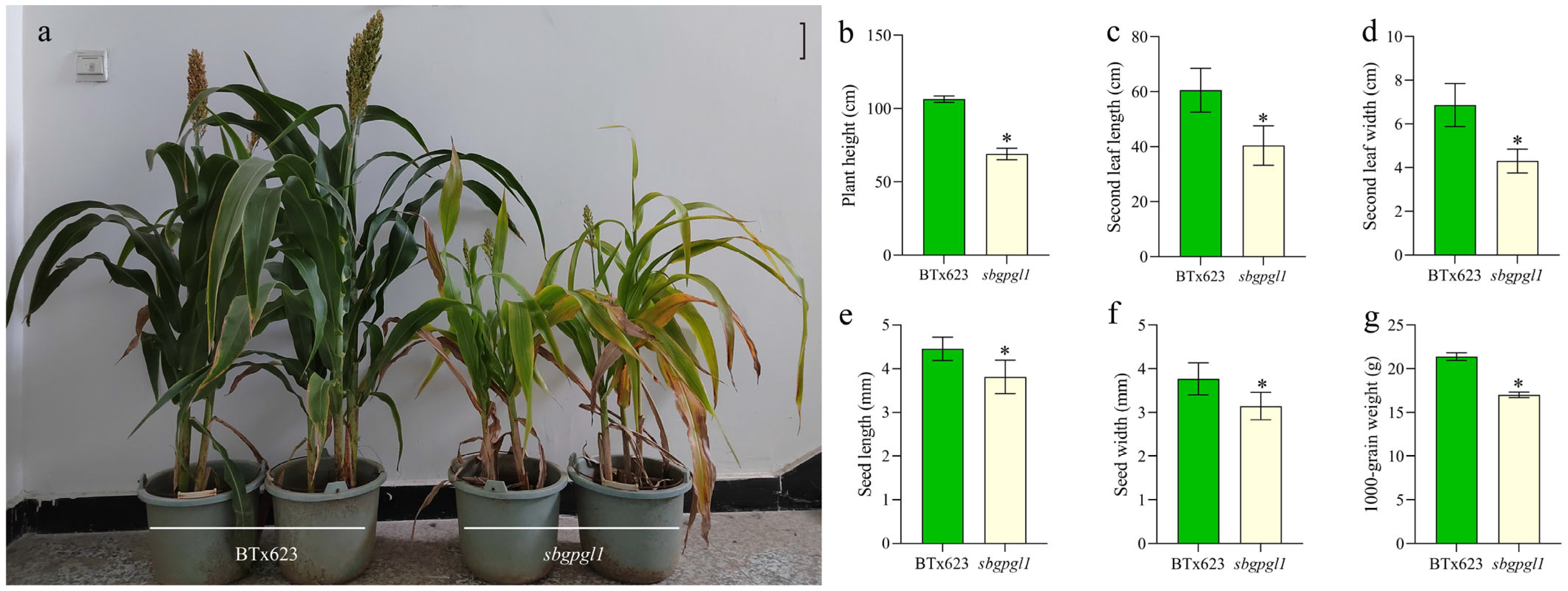
3.1. Phenotypic Characterization of sbgpgl1 Mutant
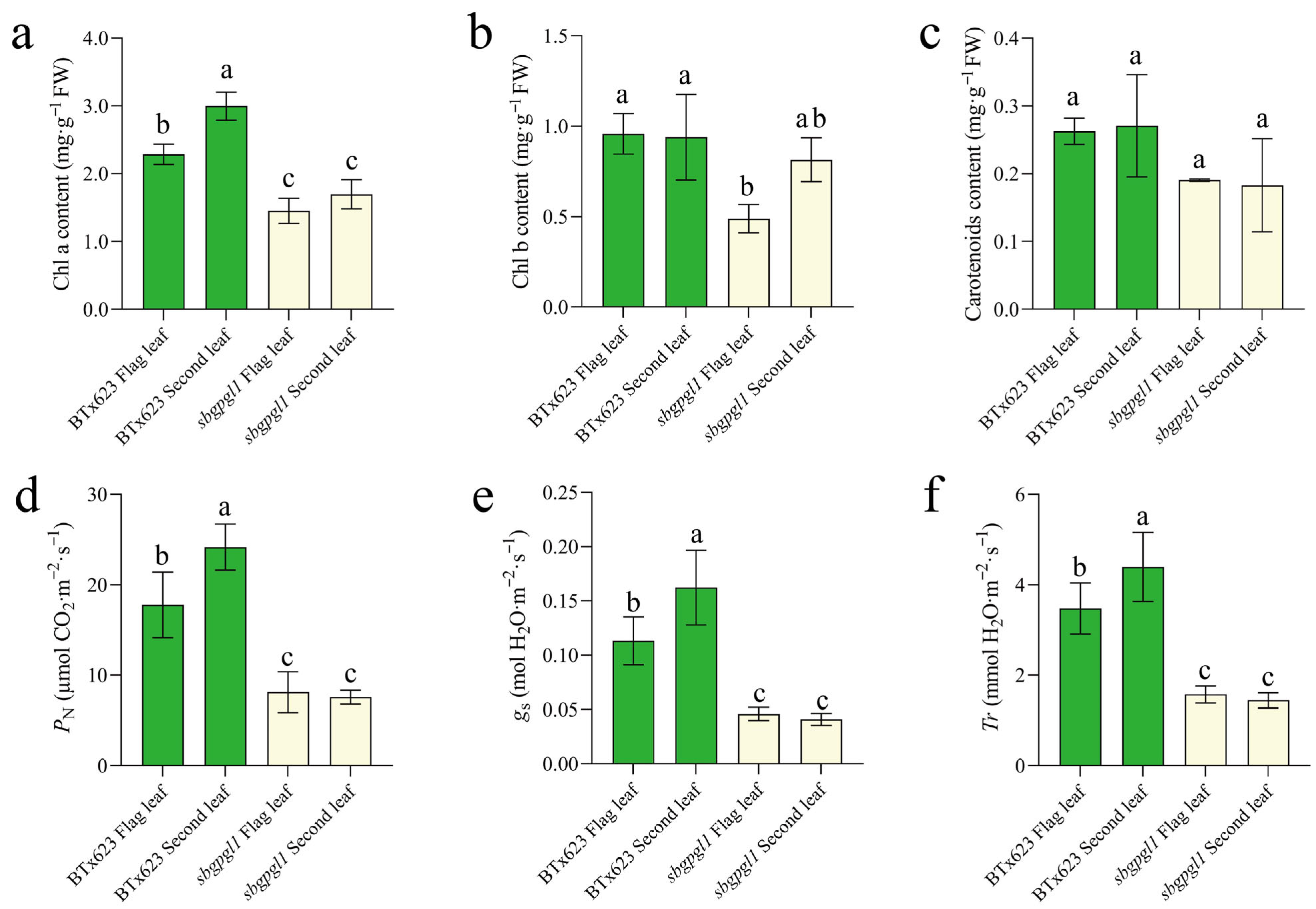
3.2. Chlorophyll Content and Photosynthesis Reduced in sbgpgl1 Mutant
3.3. Mineral Element Content Analysis of sbgpgl1 Leaves
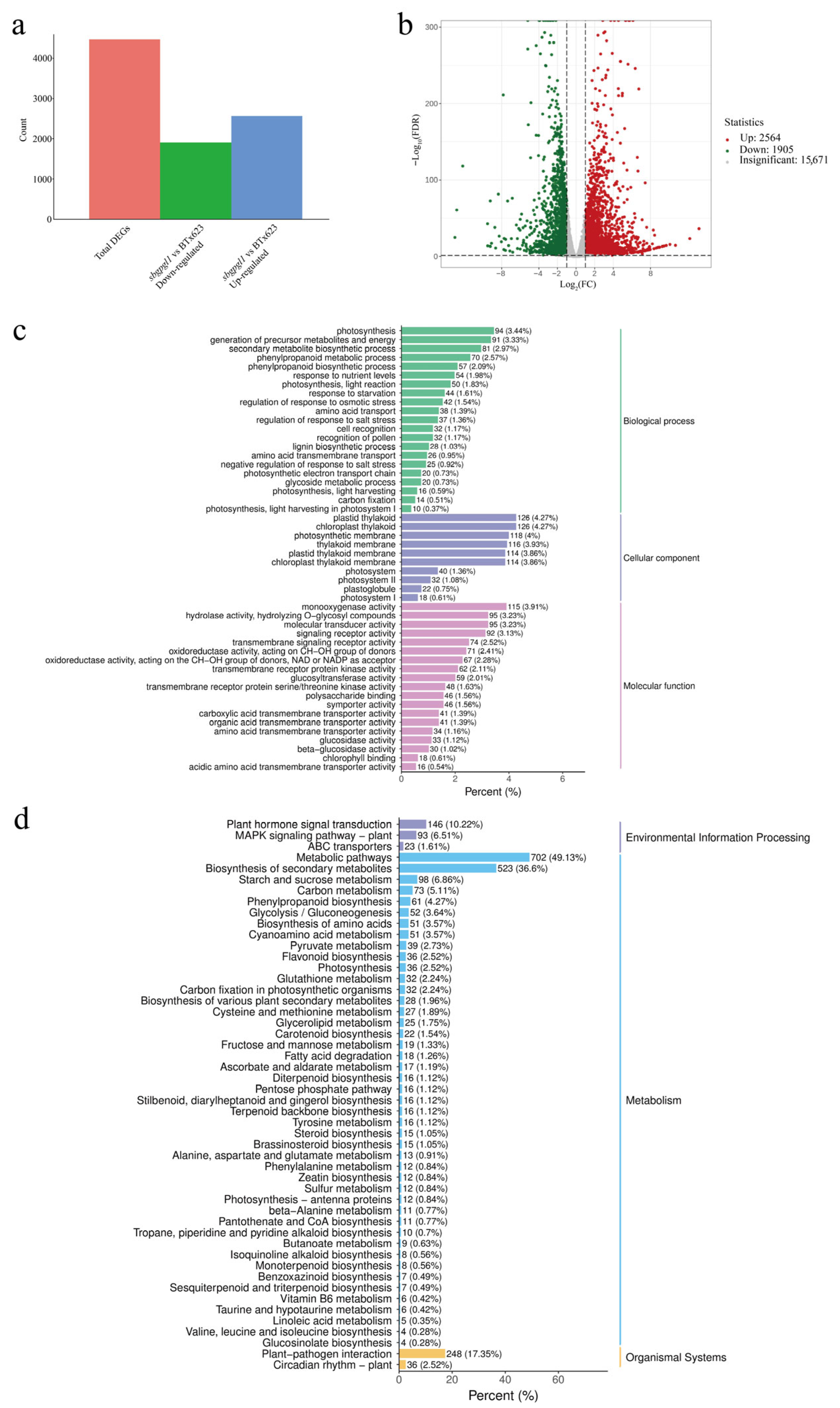
3.4. Transcriptome Analysis of sbgpgl1 Compared with BTx623
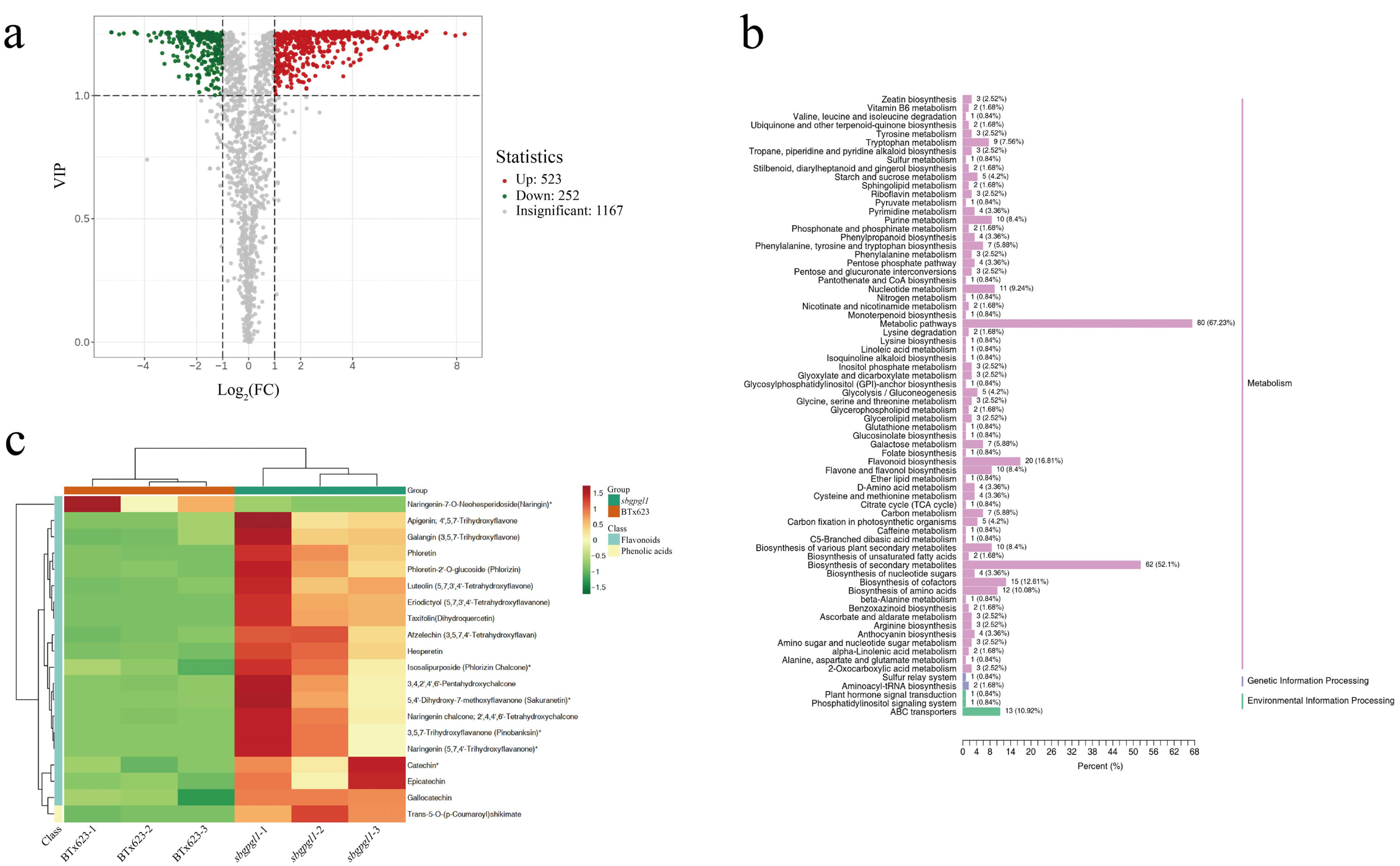
3.5. Metabolome Analysis of sbgpgl1 Compared with BTx623
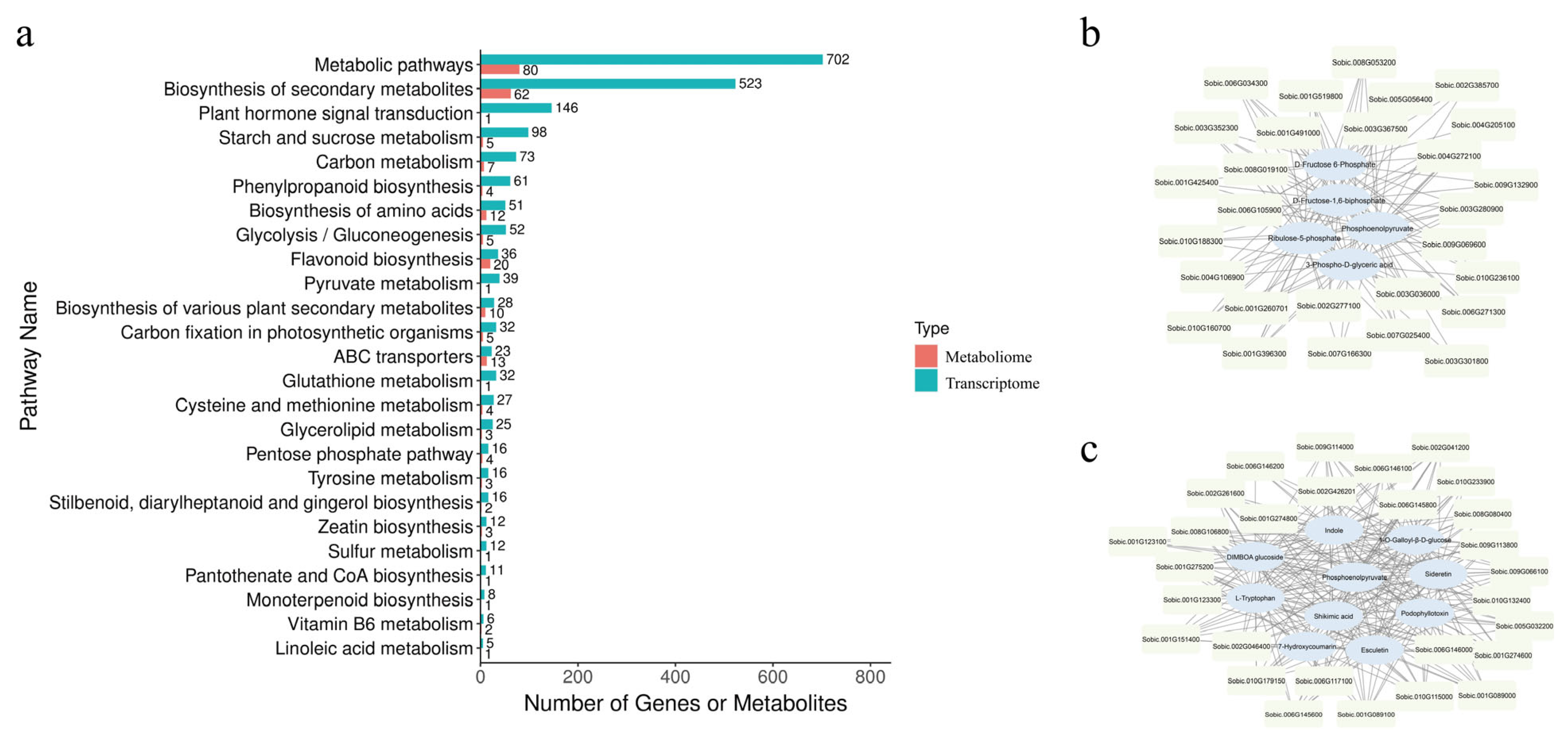
3.6. Integrated Transcriptome and Metabolome Analyses of sbgpgl1 Mutant
4. Discussion
4.1. sbgpgl1 Showed Reduced Chlorophyll Pigment Content and Agronomic Trait Parameters
4.2. sbgpgl1 Mutant Presented Reduced Photosynthesis and Mineral Element Contents
4.3. DEGs and DAMs Showed Potential Mechanisms of Pale-Green Leaf in sbgpgl1
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dahlberg, J. The Role of Sorghum in Renewables and Biofuels. Methods Mol. Biol. 2019, 1931, 269–277. [Google Scholar]
- Wang, C.; Zhou, L.; Zhang, G.; Xu, Y.; Zhang, L.; Gao, X.; Gao, J.; Jiang, N.; Shao, M. Optimal fertilization for high yield and good quality of waxy sorghum (Sorghum bicolor L. Moench). Field Crops Res. 2017, 203, 1–7. [Google Scholar] [CrossRef]
- Lopes, L.D.; Chai, Y.N.; Marsh, E.L.; Rajewski, J.F.; Dweikat, I.; Schachtman, D.P. Sweet Sorghum Genotypes Tolerant and Sensitive to Nitrogen Stress Select Distinct Root Endosphere and Rhizosphere Bacterial Communities. Microorganisms 2021, 9, 1329. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.V.; Ottosen, C.O.; Rosenqvist, E.; Medanha, T.; Abdelhakim, L.; Machado, E.C.; Struik, P.C. Elevated CO2 concentration increases photosynthetic sensitivity to nitrogen supply of sorghum in a genotype-dependent manner. Plant Physiol. Biochem. 2021, 168, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Maw, M.J.W.; Houx, J.H.; Fritschi, F.B. Nitrogen fertilization of high biomass sorghum affects macro- and micronutrient accumulation and tissue concentrations. Ind. Crops Prod. 2020, 156, 112819. [Google Scholar] [CrossRef]
- Makino, Y.; Ueno, O. Structural and physiological responses of the C4 grass Sorghum bicolor to nitrogen limitation. Plant Prod. Sci. 2018, 21, 39–50. [Google Scholar] [CrossRef]
- Yong, S.; Chen, Q.; Xu, F.; Fu, H.; Liang, G.; Guo, Q. Exploring the interplay between angiosperm chlorophyll metabolism and environmental factors. Planta 2024, 260, 25. [Google Scholar] [CrossRef]
- Hu, X.; Gu, T.; Khan, I.; Zada, A.; Jia, T. Research Progress in the Interconversion, Turnover and Degradation of Chlorophyll. Cells 2021, 10, 3134. [Google Scholar] [CrossRef]
- Takabayashi, A.; Kurihara, K.; Kuwano, M.; Kasahara, Y.; Tanaka, R.; Tanaka, A. The oligomeric states of the photosystems and the light-harvesting complexes in the Chl b-less mutant. Plant Cell Physiol. 2011, 52, 2103–2114. [Google Scholar] [CrossRef]
- Bryant, D.A.; Hunter, C.N.; Warren, M.J. Biosynthesis of the modified tetrapyrroles-the pigments of life. J. Biol. Chem. 2020, 295, 6888–6925. [Google Scholar] [CrossRef]
- Tamiaki, H.; Kichishima, S. Chlorophyll Pigments and Their Synthetic Analogs. Plant Cell Physiol. 2025, 66, 153–167. [Google Scholar] [CrossRef]
- Sun, M.; Shen, Y. Integrating the multiple functions of CHLH into chloroplast-derived signaling fundamental to plant development and adaptation as well as fruit ripening. Plant Sci. 2024, 338, 111892. [Google Scholar] [CrossRef]
- Ibata, H.; Nagatani, A.; Mochizuki, N. CHLH/GUN5 Function in Tetrapyrrole Metabolism Is Correlated with Plastid Signaling but not ABA Responses in Guard Cells. Front. Plant Sci. 2016, 7, 1650. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lin, L.; Liu, X.; Wangzha, M.; Pang, X.; Feng, L.; Wan, B.; Wu, G.Z.; Yu, J.; Rochaix, J.D.; et al. Characterization of a tomato chlh mis-sense mutant reveals a new function of ChlH in fruit ripening. Plant Biotechnol. J. 2025, 23, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, L.; Ying, Y.; Wang, J.; Shao, J.F.; Yamaji, N.; Whelan, J.; Ma, J.F.; Shou, H. A transcription factor OsbHLH156 regulates Strategy II iron acquisition through localising IRO2 to the nucleus in rice. New Phytol. 2020, 225, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Xia, H.; Li, Q.; Li, Z.; Zhai, C.; Weng, L.; Mi, H.; Yan, S.; Datla, R.; Wang, H.; et al. PALE-GREEN LEAF 1, a rice cpSRP54 protein, is essential for the assembly of the PSI-LHCI supercomplex. Plant Direct 2022, 6, e436. [Google Scholar] [CrossRef]
- Frick, G.; Su, Q.; Apel, K.; Armstrong, G.A. An Arabidopsis porB porC double mutant lacking light-dependent NADPH: Protochlorophyllide oxidoreductases B and C is highly chlorophyll-deficient and developmentally arrested. Plant J. 2003, 35, 141–153. [Google Scholar] [CrossRef]
- Kumar, A.M.; SoÈll, D. Antisense HEMA1 RNA expression inhibits heme and chlorophyll biosynthesis in arabidopsis. Plant Physiol. 2000, 122, 49–55. [Google Scholar] [CrossRef]
- Luo, W.G.; Liang, Q.W.; Su, Y.; Huang, C.; Mo, B.X.; Yu, Y.; Xiao, L.T. Auxin inhibits chlorophyll accumulation through ARF7-IAA14-mediated repression of chlorophyll biosynthesis genes in Arabidopsis. Front. Plant Sci. 2023, 14, 1172059. [Google Scholar] [CrossRef]
- Talla, S.K.; Panigrahy, M.; Kappara, S.; Nirosha, P.; Neelamraju, S.; Ramanan, R. Cytokinin delays dark-induced senescence in rice by maintaining the chlorophyll cycle and photosynthetic complexes. J. Exp. Bot. 2016, 67, 1839–1851. [Google Scholar] [CrossRef]
- Nehnevajova, E.; Ramireddy, E.; Stolz, A.; Gerdemann-Knorck, M.; Novak, O.; Strnad, M.; Schmulling, T. Root enhancement in cytokinin-deficient oilseed rape causes leaf mineral enrichment, increases the chlorophyll concentration under nutrient limitation and enhances the phytoremediation capacity. BMC Plant Biol. 2019, 19, 83. [Google Scholar] [CrossRef]
- Keawmanee, N.; Ma, G.; Zhang, L.; Yahata, M.; Murakami, K.; Yamamoto, M.; Kojima, N.; Kato, M. Exogenous gibberellin induced regreening through the regulation of chlorophyll and carotenoid metabolism in Valencia oranges. Plant Physiol. Biochem. 2022, 173, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Gao, G.; Yu, C.; Zhu, A.; Chen, J.; Chen, K.; Wang, X.; Abubakar, A.S.; Chen, P. Transcriptome and metabolome analysis reveals anthocyanin biosynthesis pathway associated with ramie (Boehmeria nivea (L.) Gaud.) leaf color formation. BMC Genom. 2021, 22, 684. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chang, C.; Li, H.; Huang, L.; Zhou, Z.; Zhu, J.; Liu, D. Metabolome analysis reveals flavonoid changes during the leaf color transition in Populus x euramericana ‘Zhonghuahongye’. Front. Plant Sci. 2023, 14, 1162893. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Chen, J.; Zhu, M.; Liu, Y.; Wu, X.; Xiao, X.; Yuyama, N.; Liu, F.; Jing, H.; Cai, H. Wall-associated kinase-like gene RL1 contributes to red leaves in sorghum. Plant J. 2022, 112, 135–150. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Z.; Qu, K.; Li, J.; Ma, D.; Lu, X. A coiled-coil domain mutation in the NLR receptor SbYR1 coordinates plant growth and stress tolerance in sorghum. Plant Sci. 2025, 350, 112246. [Google Scholar] [CrossRef]
- Li, W.; Yang, S.; Lu, Z.; He, Z.; Ye, Y.; Zhao, B.; Wang, L.; Jin, B. Cytological, physiological, and transcriptomic analyses of golden leaf coloration in Ginkgo biloba L. Hortic. Res. 2018, 5, 12. [Google Scholar]
- Zhu, Z.; Qu, K.; Li, D.; Zhang, L.; Wang, C.; Cong, L.; Bai, C.; Lu, X. SbPHO2, a conserved Pi starvation signalling gene, is involved in the regulation of the uptake of multiple nutrients in sorghum. Plant Sci. 2023, 327, 111556. [Google Scholar] [CrossRef]
- Zheng, H.; Dang, Y.; Sui, N. Sorghum: A Multipurpose Crop. J. Agric. Food Chem. 2023, 71, 17570–17583. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, J.; Huang, L.; Leng, Y.; Dai, L.; Rao, Y.; Chen, L.; Wang, Y.; Tu, Z.; Hu, J.; et al. PGL, encoding chlorophyllide a oxygenase 1, impacts leaf senescence and indirectly affects grain yield and quality in rice. J. Exp. Bot. 2016, 67, 1297–1310. [Google Scholar] [CrossRef]
- Han, H.; Zhou, Y.; Liu, H.; Chen, X.; Wang, Q.; Zhuang, H.; Sun, X.; Ling, Q.; Zhang, H.; Wang, B.; et al. Transcriptomics and Metabolomics Analysis Provides Insight into Leaf Color and Photosynthesis Variation of the Yellow-Green Leaf Mutant of Hami Melon (Cucumis melo L.). Plants 2023, 12, 1623. [Google Scholar] [CrossRef] [PubMed]
- Sakowska, K.; Alberti, G.; Genesio, L.; Peressotti, A.; Delle Vedove, G.; Gianelle, D.; Colombo, R.; Rodeghiero, M.; Panigada, C.; Juszczak, R.; et al. Leaf and canopy photosynthesis of a chlorophyll deficient soybean mutant. Plant Cell Environ. 2018, 41, 1427–1437. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Mao, Y.; Yin, Y.; Shen, X. Physiological analysis and transcriptome sequencing of a delayed-green leaf mutant ‘Duojiao’ of ornamental crabapple (Malus sp.). Physiol. Mol. Biol. Plants 2022, 28, 1833–1848. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, H.; Wei, L.; Guo, R.; Liu, X.; Zhang, M.; Fan, J.; Liu, S.; Liao, J.; Huang, Y.; et al. Yellow-Green Leaf 19 Encoding a Specific and Conservative Protein for Photosynthetic Organisms Affects Tetrapyrrole Biosynthesis, Photosynthesis, and Reactive Oxygen Species Metabolism in Rice. Int. J. Mol. Sci. 2023, 24, 16762. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Zhang, H.; Leng, B.; Cao, B.; Shan, J.; Yan, Z.; Guan, H.; Cheng, W.; Liu, X.; Mu, C. A large deletion conferring pale green leaves of maize. BMC Plant Biol. 2023, 23, 360. [Google Scholar] [CrossRef]
- Gu, J.; Zhou, Z.; Li, Z.; Chen, Y.; Wang, Z.; Zhang, H.; Yang, J. Photosynthetic Properties and Potentials for Improvement of Photosynthesis in Pale Green Leaf Rice Under High Light Conditions. Front. Plant Sci. 2017, 8, 1082. [Google Scholar] [CrossRef]
- Qu, K.Z.; Lu, P.; Li, D.; Cong, L.; Lu, X.C.; Zhu, Z.X. Phenotypic Identification of Pale-green Leaf Mutant sbpgl1 During Seedling Stage. Liaoning Agric. Sci. 2024, 3, 66–69. [Google Scholar]
- Zhao, Y.; Zhu, M.; Gao, H.; Zhou, Y.; Yao, W.; Zhao, Y.; Zhang, W.; Feng, C.; Li, Y.; Jin, Y.; et al. Photosynthetic characteristics and genetic mapping of a yellow-green leaf mutant jym165 in soybean. BMC Plant Biol. 2024, 24, 1009. [Google Scholar] [CrossRef]
- Liang, G. Iron uptake, signaling, and sensing in plants. Plant Commun. 2022, 3, 100349. [Google Scholar] [CrossRef]
- Gao, F.; Dubos, C. Transcriptional integration of plant responses to iron availability. J. Exp. Bot. 2021, 72, 2056–2070. [Google Scholar] [CrossRef]
- Dong, W.; Liu, L.; Sun, Y.; Xu, X.; Guo, G.; Heng, W.; Jiao, H.; Wei, S.; Jia, B. PbbHLH155 enhances iron deficiency tolerance in pear by directly activating PbFRO2 and PbbHLH38. Plant Physiol. Biochem. 2024, 213, 108786. [Google Scholar] [CrossRef]
- Yang, X.; Cai, J.; Xue, J.; Luo, X.; Zhu, W.; Xiao, X.; Xue, M.; An, F.; Li, K.; Chen, S. Magnesium chelatase subunit D is not only required for chlorophyll biosynthesis and photosynthesis, but also affecting starch accumulation in Manihot esculenta Crantz. BMC Plant Biol. 2023, 23, 258. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhang, X.; Tian, G.; Liu, C.; Xing, Y.; Feng, Z.; Lyu, M.; Liu, J.; Xu, X.; Zhu, Z.; et al. Magnesium alleviates growth inhibition under low potassium by enhancing photosynthesis and carbon-nitrogen metabolism in apple plants. Plant Physiol. Biochem. 2024, 214, 108875. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.X.; Zhang, M.Y.; Han, Y.Y.; Hao, J.H.; Liu, C.J.; Fan, S.X. Beneficial Phytochemicals with Anti-Tumor Potential Revealed through Metabolic Profiling of New Red Pigmented Lettuces (Lactuca sativa L.). Int. J. Mol. Sci. 2018, 19, 1165. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Y.; Chen, H.; Chen, C.; Liu, Z.; Han, C.; Wu, Q.; Yu, F. Transcriptomic Analyses Reveal Key Genes Involved in Pigment Biosynthesis Related to Leaf Color Change of Liquidambar formosana Hance. Molecules 2022, 27, 5433. [Google Scholar] [CrossRef]
- Li, G.; Liang, H.; Ren, X.; Ma, W.; Lu, Y.; Zhang, Z.; Wang, Z.; Zhao, T.; Zhao, J. Transcriptome Analysis Reveals Association of Photosynthesis and Phytohormone Pathways with Leaf Color in Chinese Cabbage. Agronomy 2023, 13, 2273. [Google Scholar] [CrossRef]
- Dumas, L.; Chazaux, M.; Peltier, G.; Johnson, X.; Alric, J. Cytochrome b6f function and localization, phosphorylation state of thylakoid membrane proteins and consequences on cyclic electron flow. Photosynth. Res. 2016, 129, 307–320. [Google Scholar] [CrossRef]
- Liu, C.J. Cytochrome b5: A versatile electron carrier and regulator for plant metabolism. Front. Plant Sci. 2022, 13, 984174. [Google Scholar] [CrossRef]
- Gou, M.; Yang, X.; Zhao, Y.; Ran, X.; Song, Y.; Liu, C.J. Cytochrome b5 Is an Obligate Electron Shuttle Protein for Syringyl Lignin Biosynthesis in Arabidopsis. Plant Cell 2019, 31, 1344–1366. [Google Scholar] [CrossRef]
- Schiphorst, C.; Achterberg, L.; Gomez, R.; Koehorst, R.; Bassi, R.; van Amerongen, H.; Dall’Osto, L.; Wientjes, E. The role of light-harvesting complex I in excitation energy transfer from LHCII to photosystem I in Arabidopsis. Plant Physiol. 2022, 188, 2241–2252. [Google Scholar] [CrossRef]
- Ye, J.J.; Lin, X.Y.; Yang, Z.X.; Wang, Y.Q.; Liang, Y.R.; Wang, K.R.; Lu, J.L.; Lu, P.; Zheng, X.Q. The light-harvesting chlorophyll a/b-binding proteins of photosystem II family members are responsible for temperature sensitivity and leaf color phenotype in albino tea plant. J. Adv. Res. 2024, 66, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Du, Y.; Zhang, J.; Feng, H.; Liu, J.; Yang, G.; Zhu, Y. Unraveling the Mechanism of Purple Leaf Formation in Brassica napus by Integrated Metabolome and Transcriptome Analyses. Front. Plant Sci. 2022, 13, 945553. [Google Scholar] [CrossRef]
- Jiang, L.; Lyu, S.; Yu, H.; Zhang, J.; Sun, B.; Liu, Q.; Mao, X.; Chen, P.; Pan, D.; Chen, W.; et al. Transcription factor encoding gene OsC1 regulates leaf sheath color through anthocyanidin metabolism in Oryza rufipogon and Oryza sativa. BMC Plant Biol. 2024, 24, 147. [Google Scholar] [CrossRef]
- Feder, A.; Burger, J.; Gao, S.; Lewinsohn, E.; Katzir, N.; Schaffer, A.A.; Meir, A.; Davidovich-Rikanati, R.; Portnoy, V.; Gal-On, A.; et al. A Kelch Domain-Containing F-Box Coding Gene Negatively Regulates Flavonoid Accumulation in Muskmelon. Plant Physiol. 2015, 169, 1714–1726. [Google Scholar] [PubMed]
- Li, D.; Qu, K.Z.; Ma, D.R.; Zhu, Z.X.; Lu, X.C. Transcriptome and Metabolome Analysis of the Leaf Colour Mutation Mechanism in a Light-Green Leaf Mutant of Maize. Agronomy 2025, 15, 1364. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, K.; Li, D.; Zhu, Z.; Lu, X. Transcriptome and Metabolome Analyses Reveal the Physiological Variations of a Gradient-Pale-Green Leaf Mutant in Sorghum. Agronomy 2025, 15, 1841. https://doi.org/10.3390/agronomy15081841
Qu K, Li D, Zhu Z, Lu X. Transcriptome and Metabolome Analyses Reveal the Physiological Variations of a Gradient-Pale-Green Leaf Mutant in Sorghum. Agronomy. 2025; 15(8):1841. https://doi.org/10.3390/agronomy15081841
Chicago/Turabian StyleQu, Kuangzheng, Dan Li, Zhenxing Zhu, and Xiaochun Lu. 2025. "Transcriptome and Metabolome Analyses Reveal the Physiological Variations of a Gradient-Pale-Green Leaf Mutant in Sorghum" Agronomy 15, no. 8: 1841. https://doi.org/10.3390/agronomy15081841
APA StyleQu, K., Li, D., Zhu, Z., & Lu, X. (2025). Transcriptome and Metabolome Analyses Reveal the Physiological Variations of a Gradient-Pale-Green Leaf Mutant in Sorghum. Agronomy, 15(8), 1841. https://doi.org/10.3390/agronomy15081841





