Transcriptomic and Metabolomic Profiling of Pleurotus eryngii Cultivated on Olive Mill Solid Waste-Enriched Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Pleurotus eryngii Cultivation
2.2. RNA Extraction and Sequencing
2.3. Metabolite Profiling
2.4. Real-Time qPCR Analysis of Gene Expression
2.5. Nitrogen Analysis
2.6. Protein Quantification
2.7. α- and β-Glucan Determination
2.8. Statistical Analysis
3. Results
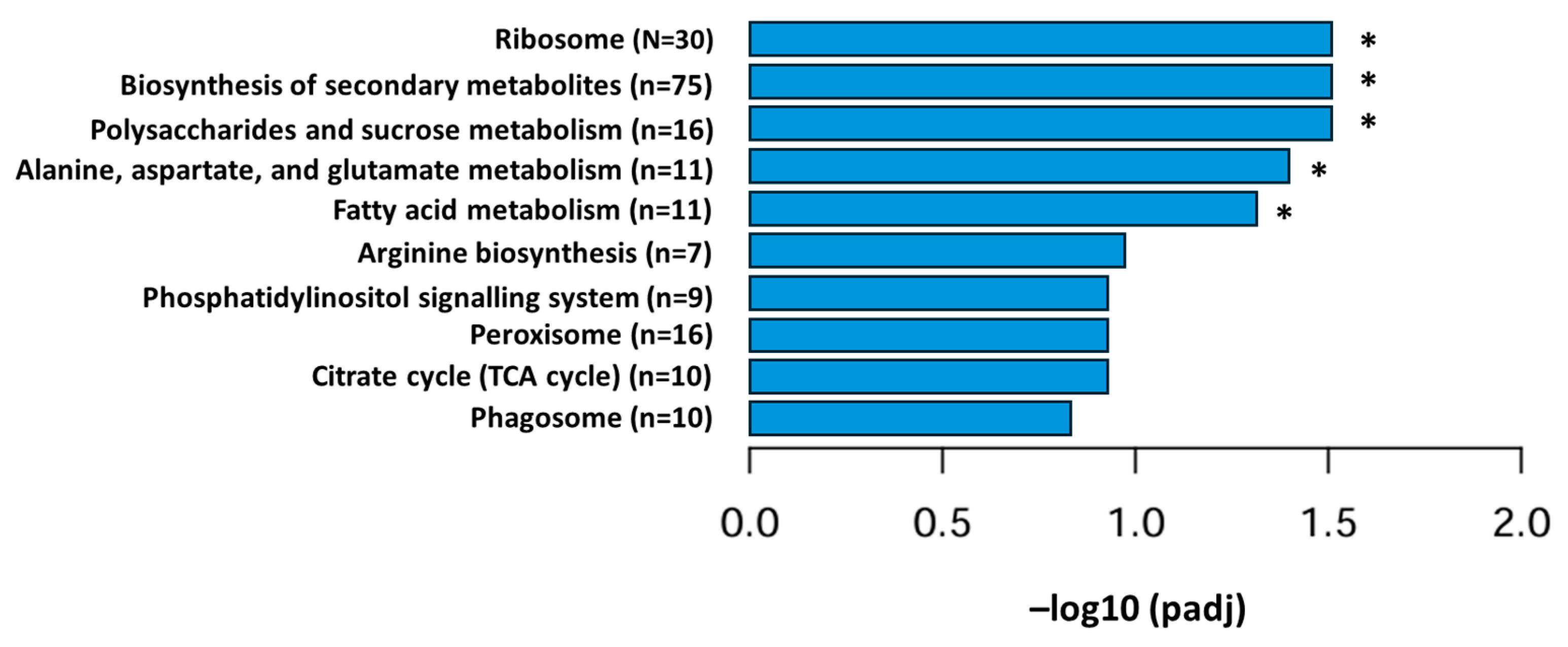
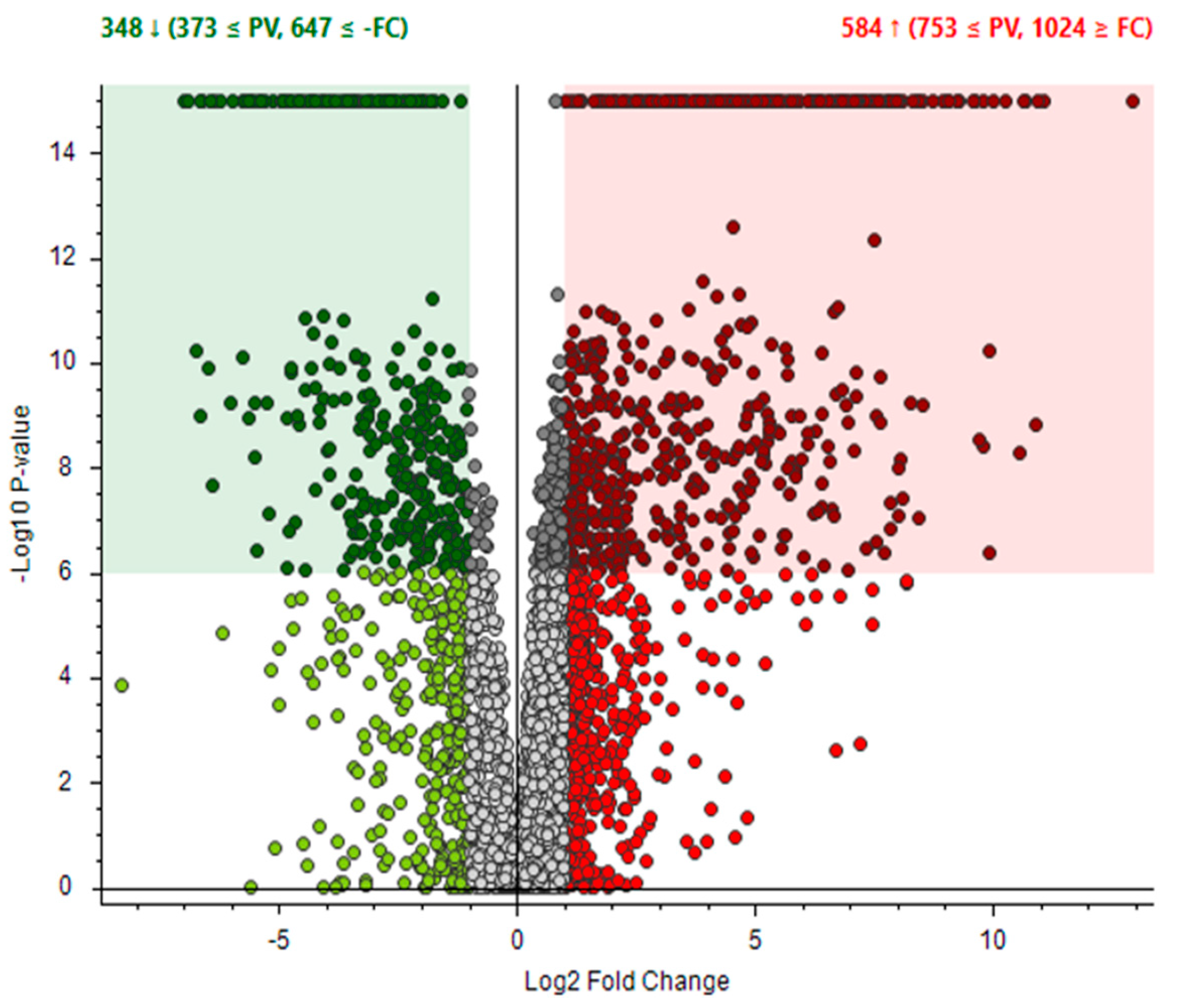
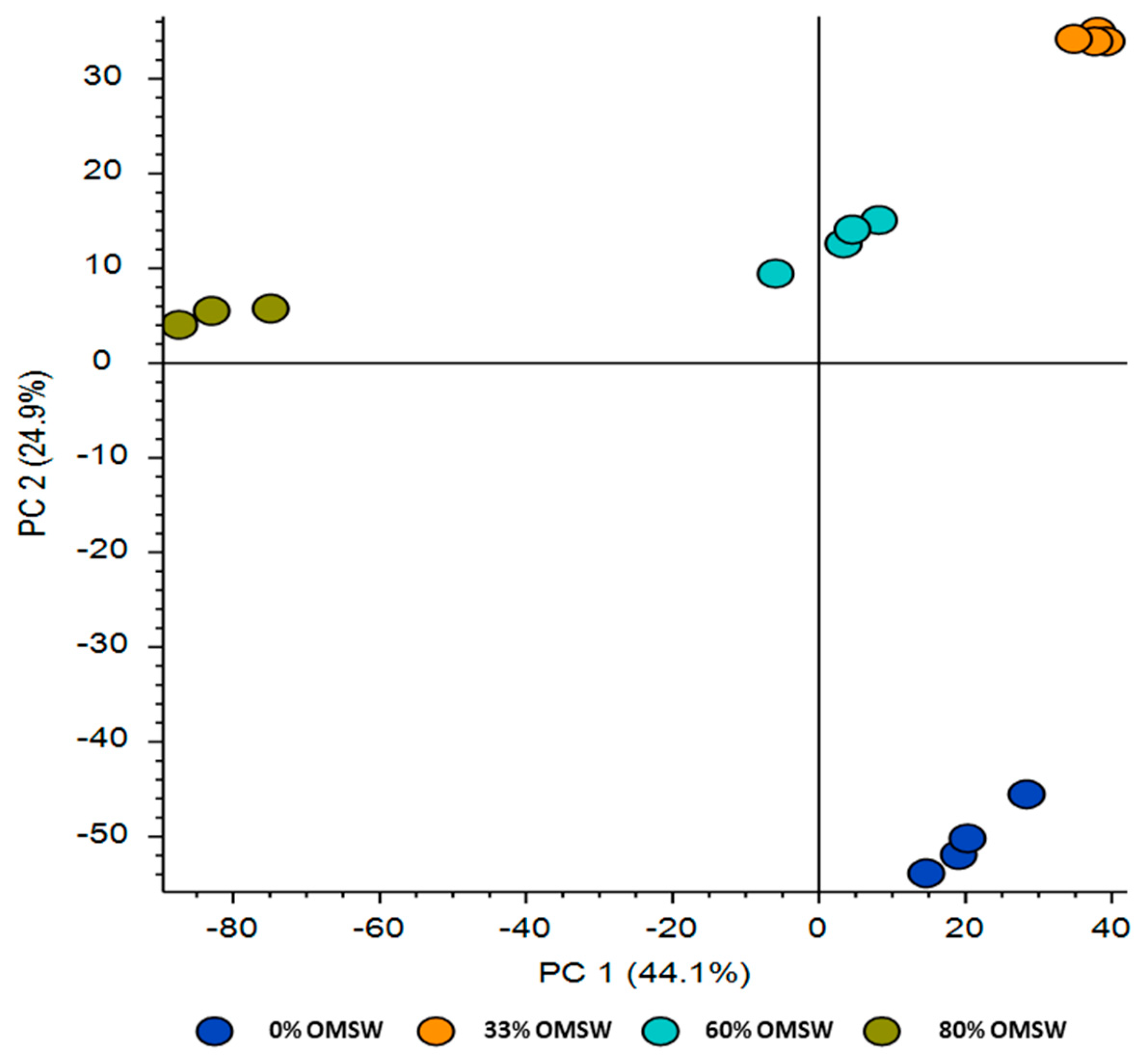
3.1. Transcriptional Profiling of P. eryngii Grown on OMSW-Enriched Substrates
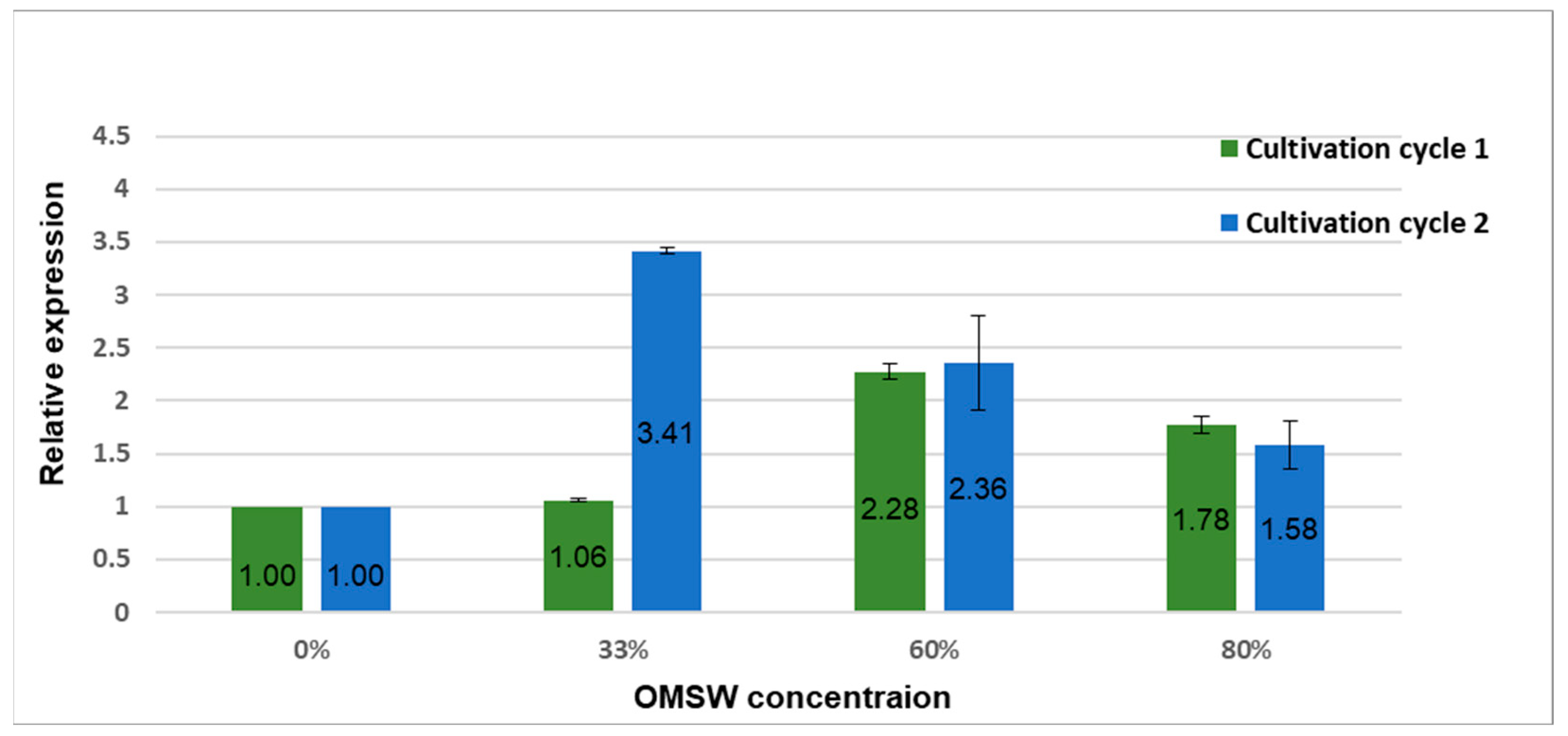
3.2. Expression of β-glucan Synthase Is Elevated in High-OMSW Substrates
3.3. Metabolomic Profiling of Fruiting Bodies Cultivated on High-OMSW Substrates
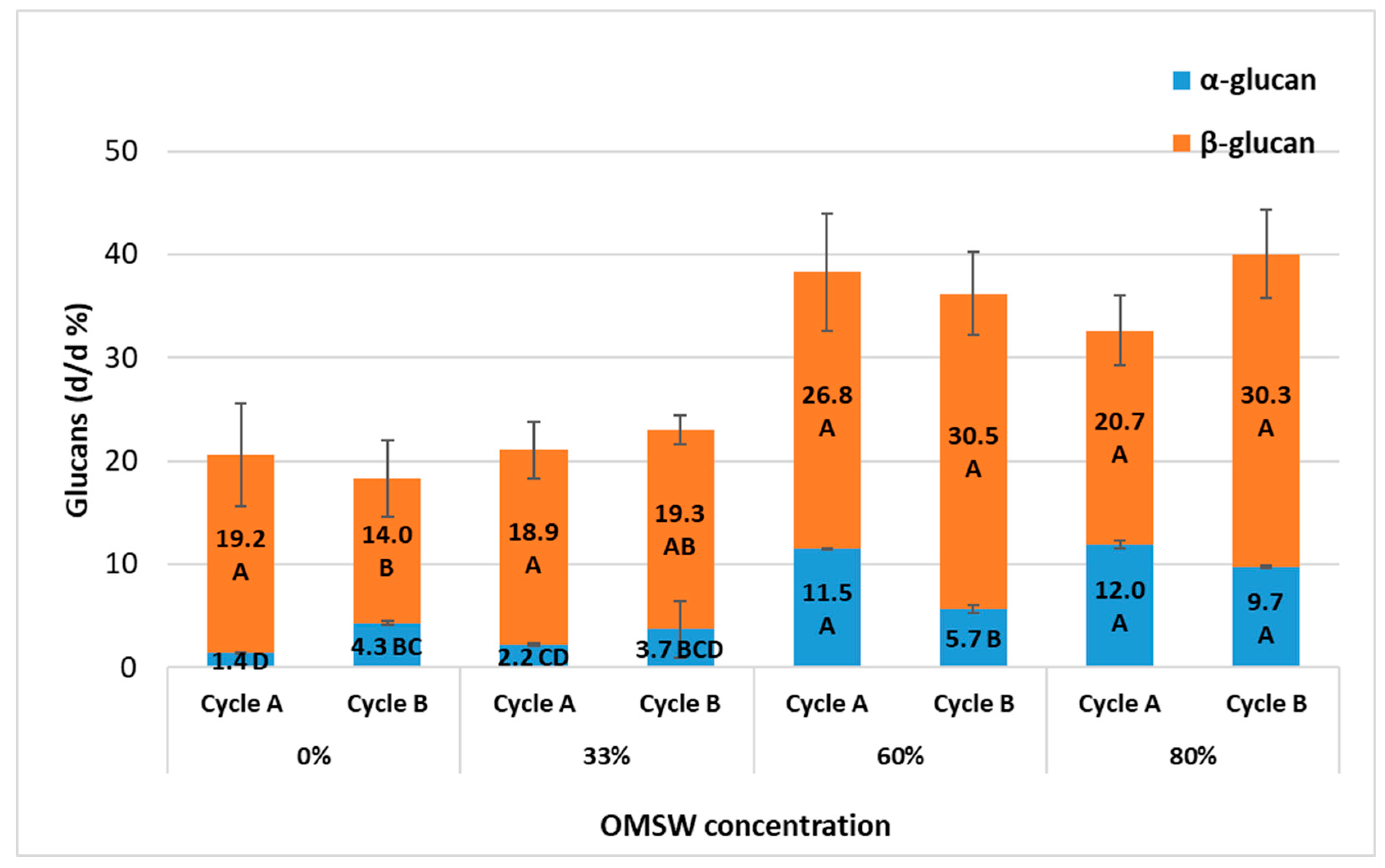
3.4. Glucan Content Increases with Higher OMSW Substrate Concentrations
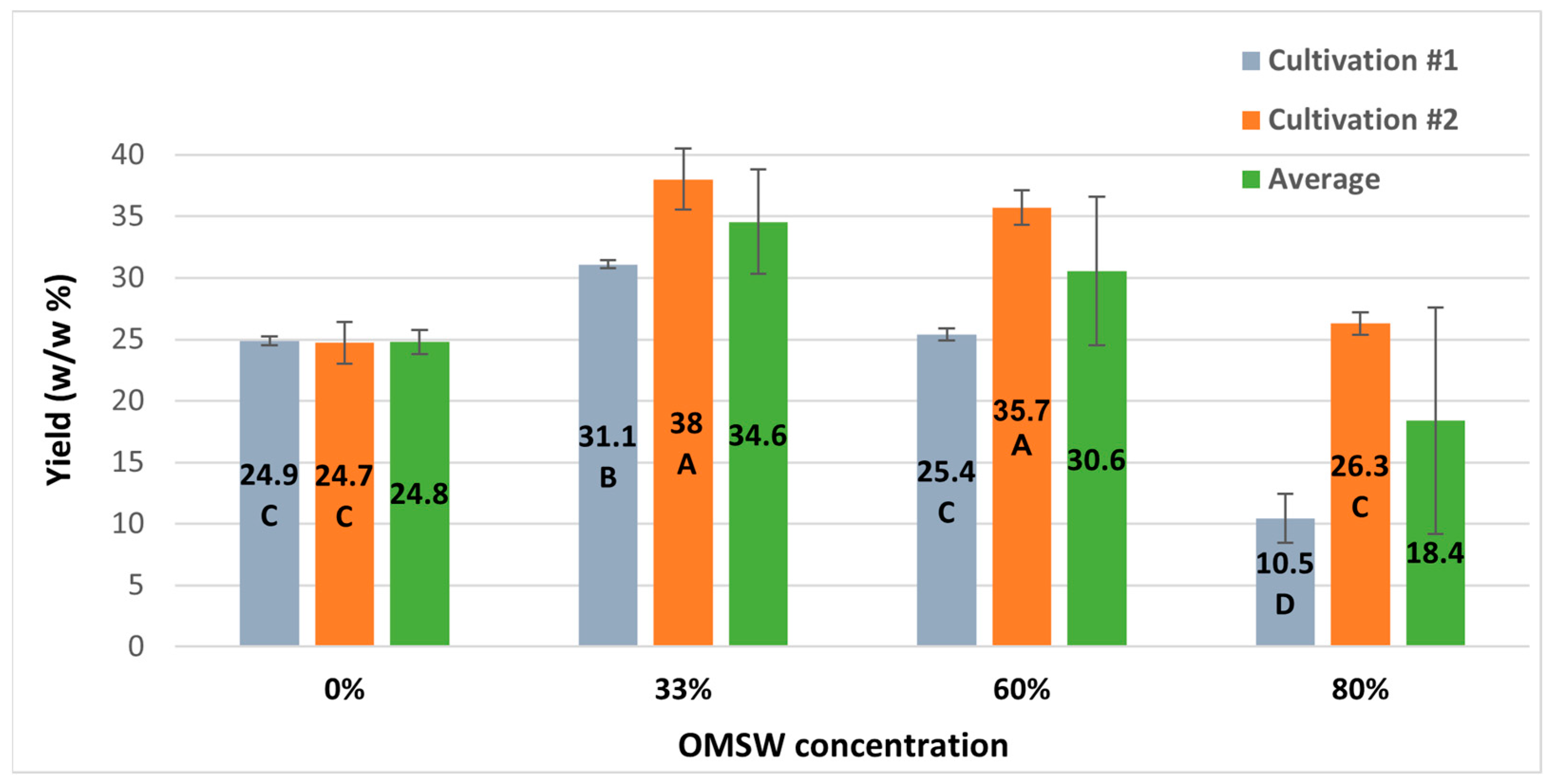
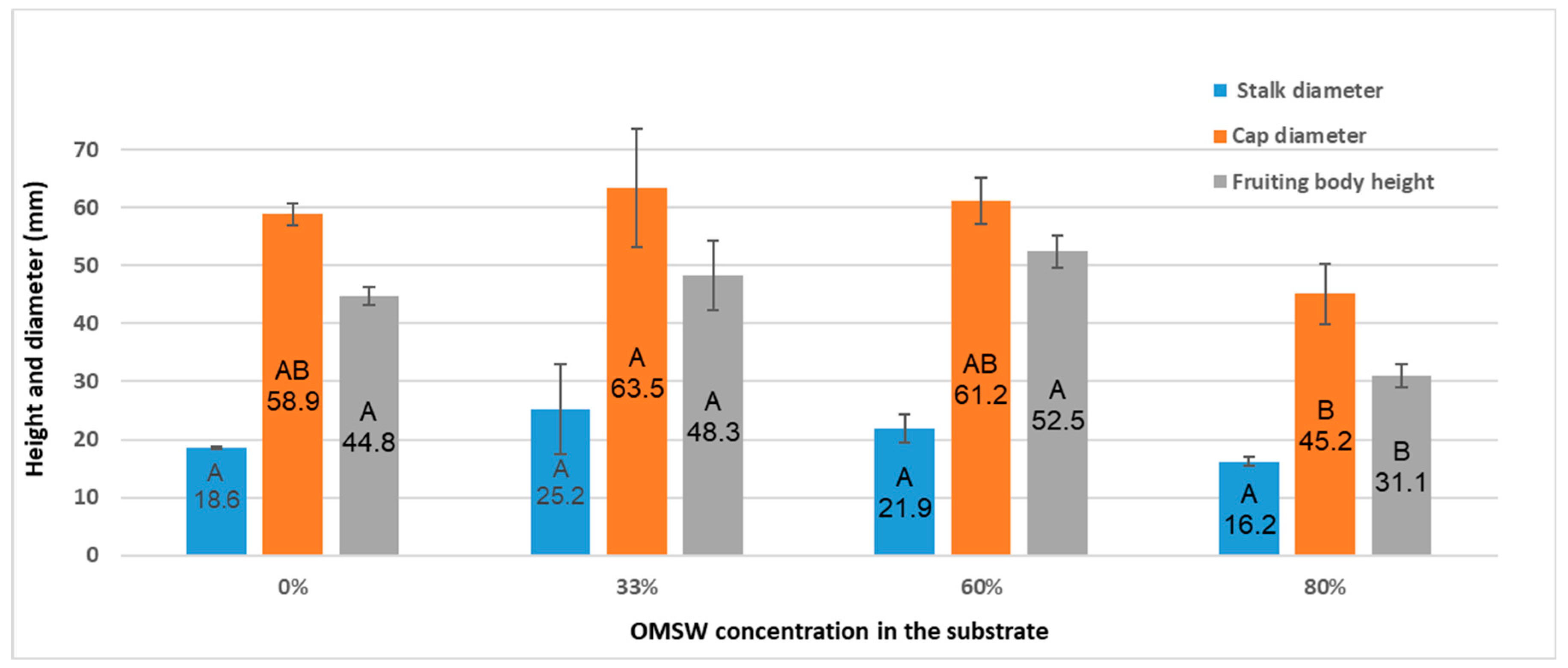
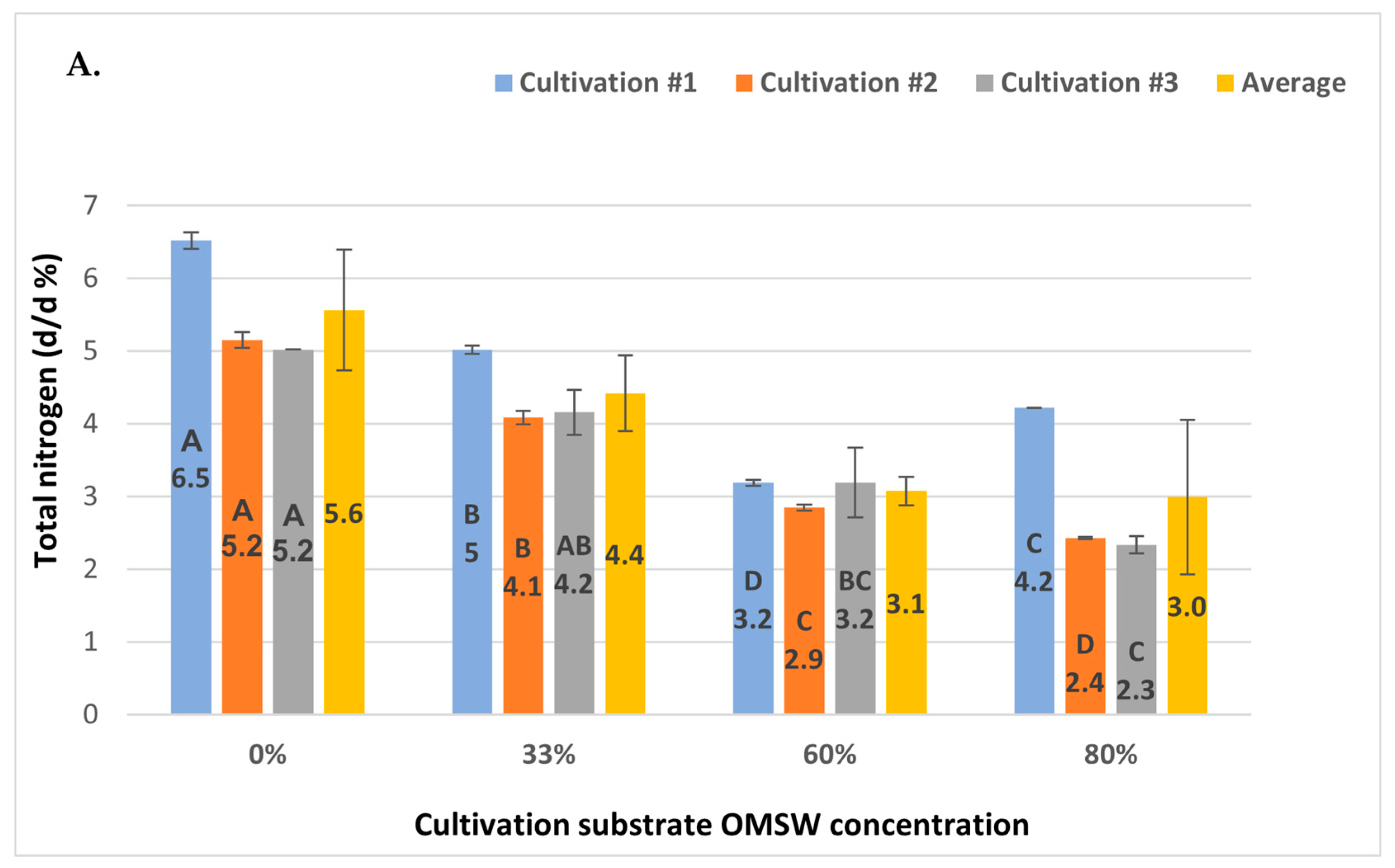
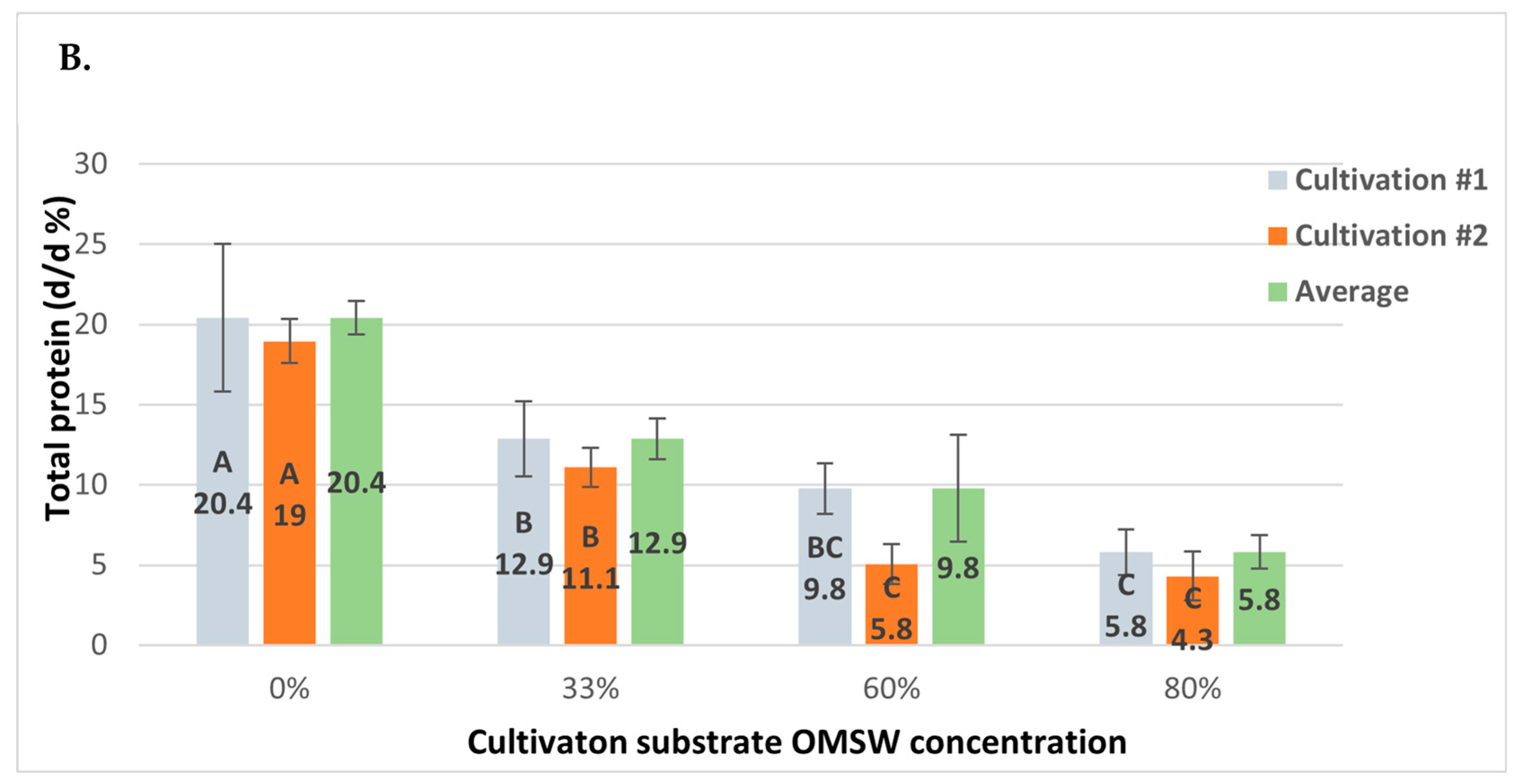
3.5. Protein and Nitrogen Content Decline with Increasing OMSW Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maamir, W.; Ouahabi, Y.; Poncin, S.; Li, H.Z.; Bensadok, K. Effect of Fenton Pretreatment on Anaerobic Digestion of Olive Mill Wastewater and Olive Mill Solid Waste in Mesophilic Conditions. Int. J. Green Energy 2017, 14, 555–560. [Google Scholar] [CrossRef]
- Ouazzane, H.; Laajine, F.; El Yamani, M.; El Hilaly, J.; Rharrabti, Y.; Amarouch, M.Y.; Mazouzi, D. Olive Mill Solid Waste Characterization and Recycling Opportunities: A Review. J. Mater. Environ. Sci. 2017, 8, 2632–2650. [Google Scholar]
- Aviani, I.; Laor, Y.; Medina, S.; Krassnovsky, A.; Raviv, M. Co-Composting of Solid and Liquid Olive Mill Wastes: Management Aspects and the Horticultural Value of the Resulting Composts. Bioresour. Technol. 2010, 101, 6699–6706. [Google Scholar] [CrossRef]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An Overview on Olive Mill Wastes and Their Valorisation Methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef]
- Wzorek, M.; Junga, R.; Yilmaz, E.; Bozhenko, B. Thermal Decomposition of Olive-Mill Byproducts: A TG-FTIR Approach. Energies 2021, 14, 4123. [Google Scholar] [CrossRef]
- Giardina, P.; Autore, F.; Faraco, V.; Festa, G.; Palmieri, G.; Piscitelli, A.; Sannia, G. Structural Characterization of Heterodimeric Laccases from Pleurotus ostreatus. Appl. Microbiol. Biotechnol. 2007, 75, 1293–1300. [Google Scholar] [CrossRef]
- Salame, T.M.; Knop, D.; Levinson, D.; Yarden, O.; Hadar, Y. Redundancy among Manganese Peroxidases in Pleurotus ostreatus. Appl. Environ. Microbiol. 2013, 79, 2405–2415. [Google Scholar] [CrossRef]
- da Luz, J.M.R.; Nunes, M.D.; Paes, S.A.; Torres, D.P.; da Silva, M.D.C.S.; Kasuya, M.C.M. Lignocellulolytic Enzyme Production of Pleurotus ostreatus Growth in Agroindustrial Wastes. Braz. J. Microbiol. 2012, 43, 1508–1515. [Google Scholar] [CrossRef]
- Mane, V.P.; Patil, S.S.; Syed, A.A.; Baig, M.M.V. Bioconversion of Low Quality Lignocellulosic Agricultural Waste into Edible Protein by Pleurotus Sajor-Caju (Fr.) Singer. J. Zhejiang Univ. Sci. B 2007, 8, 745–751. [Google Scholar] [CrossRef]
- Chanakya, H.N.; Malayil, S.; Vijayalakshmi, C. Cultivation of Pleurotus Spp. on a Combination of Anaerobically Digested Plant Material and Various Agro-Residues. Energy Sustain. Dev. 2015, 27, 84–92. [Google Scholar] [CrossRef]
- Stajić, M.; Vukojević, J.; Duletić-Lauević, S. Biology of Pleurotus eryngii and Role in Biotechnological Processes: A Review. Crit. Rev. Biotechnol. 2009, 29, 55–66. [Google Scholar] [CrossRef]
- Chai, R.; Qiu, C.; Liu, D.; Qi, Y.; Gao, Y.; Shen, J.; Qiu, L. β-Glucan Synthase Gene Overexpression and β-Glucans Overproduction in Pleurotus ostreatus Using Promoter Swapping. PLoS ONE 2013, 8, e61693. [Google Scholar] [CrossRef]
- Vetvicka, V.; Gover, O.; Karpovsky, M.; Hayby, H.; Danay, O.; Ezov, N.; Hadar, Y.; Schwartz, B. Immune-Modulating Activities of Glucans Extracted from Pleurotus ostreatus and Pleurotus eryngii. J. Funct. Foods 2019, 54, 81–91. [Google Scholar] [CrossRef]
- Reverberi, M.; Di Mario, F.; Tomati, U. β-Glucan Synthase Induction in Mushrooms Grown on Olive Mill Wastewaters. Appl. Microbiol. Biotechnol. 2004, 66, 217–225. [Google Scholar] [CrossRef]
- Vetvicka, V.; Gover, O.; Hayby, H.; Danay, O.; Ezov, N.; Hadar, Y.; Schwartz, B. Spatial Distribution of Glucan Type and Content between Caps and Stalks in Pleurotus eryngii: Impact on the Anti-Inflammatory Functionality. Int. J. Mol. Sci. 2018, 19, 3371. [Google Scholar] [CrossRef]
- Avni, S.; Ezove, N.; Hanani, H.; Yadid, I.; Karpovsky, M.; Hayby, H.; Gover, O.; Hadar, Y.; Schwartz, B.; Danay, O. Olive Mill Waste Enhances α-Glucan Content in the Edible Mushroom Pleurotus eryngii. Int. J. Mol. Sci. 2017, 18, 1564. [Google Scholar] [CrossRef]
- Zerva, A.; Papaspyridi, L.M.; Christakopoulos, P.; Topakas, E. Valorization of Olive Mill Wastewater for the Production of β-Glucans from Selected Basidiomycetes. Waste Biomass Valorization 2017, 8, 1721–1731. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Patsou, M.; Mitsou, E.K.; Bekiaris, G.; Kotsou, M.; Tarantilis, P.A.; Pletsa, V.; Kyriacou, A.; Zervakis, G.I. Valorization of Olive By-Products as Substrates for the Cultivation of Ganoderma Lucidum and Pleurotus ostreatus Mushrooms with Enhanced Functional and Prebiotic Properties. Catalysts 2019, 9, 537. [Google Scholar] [CrossRef]
- Hoa, H.T.; Wang, C.L.; Wang, C.H. The Effects of Different Substrates on the Growth, Yield, and Nutritional Composition of Two Oyster Mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef]
- Chang, S.T.; Wasser, S.P. The Role of Culinary-Medicinal Mushrooms on Human Welfare with a Pyramid Model for Human Health. Int. J. Med. Mushrooms 2012, 14, 95–134. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhang, J.; Wu, L.H.; Zhao, Y.L.; Li, T.; Li, J.Q.; Wang, Y.Z.; Liu, H.G. A Mini-Review of Chemical Composition and Nutritional Value of Edible Wild-Grown Mushroom from China. Food Chem. 2014, 151, 279–285. [Google Scholar] [CrossRef]
- Zervakis, G.I.; Koutrotsios, G.; Katsaris, P. Composted versus Raw Olive Mill Waste as Substrates for the Production of Medicinal Mushrooms: An Assessment of Selected Cultivation and Quality Parameters. BioMed Res. Int. 2013, 2013, 546830. [Google Scholar] [CrossRef]
- Yu, L.; Ahmad, N.; Meng, W.; Zhao, S.; Chang, Y.; Wang, N.; Zhang, M.; Yao, N.; Liu, X.; Zhang, J. Integrated Metabolomics and Transcriptomics Provide Key Molecular Insights into Floral Stage-Driven Flavonoid Pathway in Safflower. Int. J. Mol. Sci. 2024, 25, 11903. [Google Scholar] [CrossRef]
- Shankar, A.; Sharma, K.K. Fungal Secondary Metabolites in Food and Pharmaceuticals in the Era of Multi-Omics. Appl. Microbiol. Biotechnol. 2022, 106, 3465–3488. [Google Scholar] [CrossRef]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors Affecting Mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef]
- Liu, J.; Peng, C.; Han, Q.; Wang, M.; Zhou, G.; Ye, B.; Xiao, Y.; Fang, Z.; Kües, U. Coprinopsis Cinerea Uses Laccase Lcc9 as a Defense Strategy to Eliminate Oxidative Stress during Fungal-Fungal Interactions. Appl. Environ. Microbiol. 2022, 88, e0176021. [Google Scholar] [CrossRef]
- de Jesus, L.I.; Smiderle, F.R.; Cordeiro, L.M.C.; de Freitas, R.A.; Van Griensven, L.J.L.D.; Iacomini, M. Simple and Effective Purification Approach to Dissociate Mixed Water-Insoluble α- and β-D-Glucans and Its Application on the Medicinal Mushroom Fomitopsis betulina. Carbohydr. Polym. 2018, 200, 353–360. [Google Scholar] [CrossRef]
- Akramiene, D.; Kondrotas, A.; Didziapetriene, J.; Kevelaitis, E. Effects of Beta-Glucans on the Immune System. Medicina 2007, 43, 597. [Google Scholar] [CrossRef]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary Modulation of Immune Function by β-Glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef]
- Khatib, S.; Pereman, I.; Kostanda, E.; Zdouc, M.M.; Ezov, N.; Schweitzer, R.; Van Der Hooft, J.J.J. Olive Mill Solid Waste Induces Beneficial Mushroom-Specialized Metabolite Diversity: A Computational Metabolomics Study. BioRxiv 2024. [Google Scholar] [CrossRef]
| Treatment | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| OMSW (%) | 0 | 33 | 60 | 80 |
| Beer brewery grains (%) | 50 | 33 | 20 | 10 |
| Eucalyptus shavings (%) | 50 | 33 | 20 | 10 |
| Water content (%) | 60 | 58 | 54 | 52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezov, N.; Amiram, A.; Khatib, S.; Danay, O.; Levanon, D.; Pereman, I. Transcriptomic and Metabolomic Profiling of Pleurotus eryngii Cultivated on Olive Mill Solid Waste-Enriched Substrates. Agronomy 2025, 15, 1811. https://doi.org/10.3390/agronomy15081811
Ezov N, Amiram A, Khatib S, Danay O, Levanon D, Pereman I. Transcriptomic and Metabolomic Profiling of Pleurotus eryngii Cultivated on Olive Mill Solid Waste-Enriched Substrates. Agronomy. 2025; 15(8):1811. https://doi.org/10.3390/agronomy15081811
Chicago/Turabian StyleEzov, Nirit, Adir Amiram, Soliman Khatib, Ofer Danay, Dan Levanon, and Idan Pereman. 2025. "Transcriptomic and Metabolomic Profiling of Pleurotus eryngii Cultivated on Olive Mill Solid Waste-Enriched Substrates" Agronomy 15, no. 8: 1811. https://doi.org/10.3390/agronomy15081811
APA StyleEzov, N., Amiram, A., Khatib, S., Danay, O., Levanon, D., & Pereman, I. (2025). Transcriptomic and Metabolomic Profiling of Pleurotus eryngii Cultivated on Olive Mill Solid Waste-Enriched Substrates. Agronomy, 15(8), 1811. https://doi.org/10.3390/agronomy15081811







