Climate-Driven Microbial Communities Regulate Soil Organic Carbon Stocks Along the Elevational Gradient on Alpine Grassland over the Qinghai–Tibet Plateau
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Sampling
2.3. Climate and Soil Factors
2.4. Microbial Community Structure
2.5. Data Analyses
3. Results
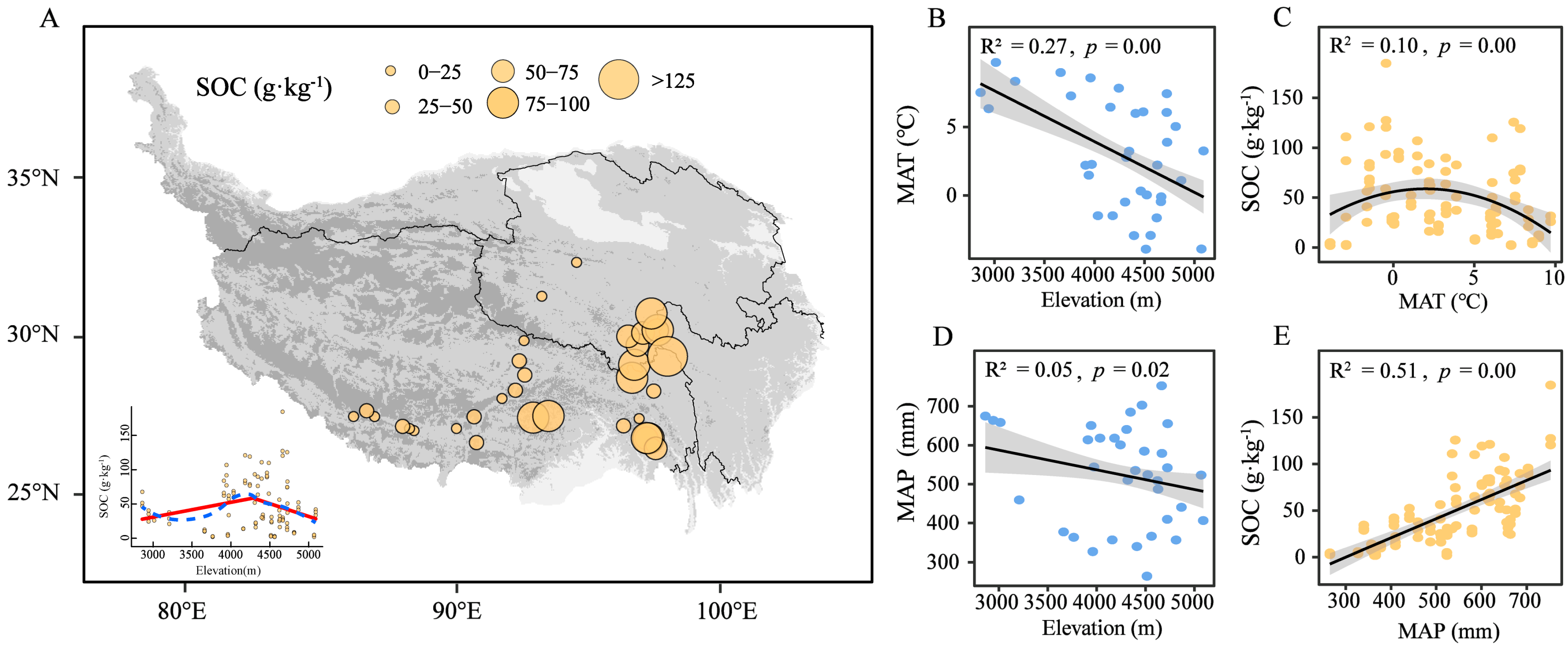
3.1. Relationships Between Climate Factors and Soil Organic Carbon Content
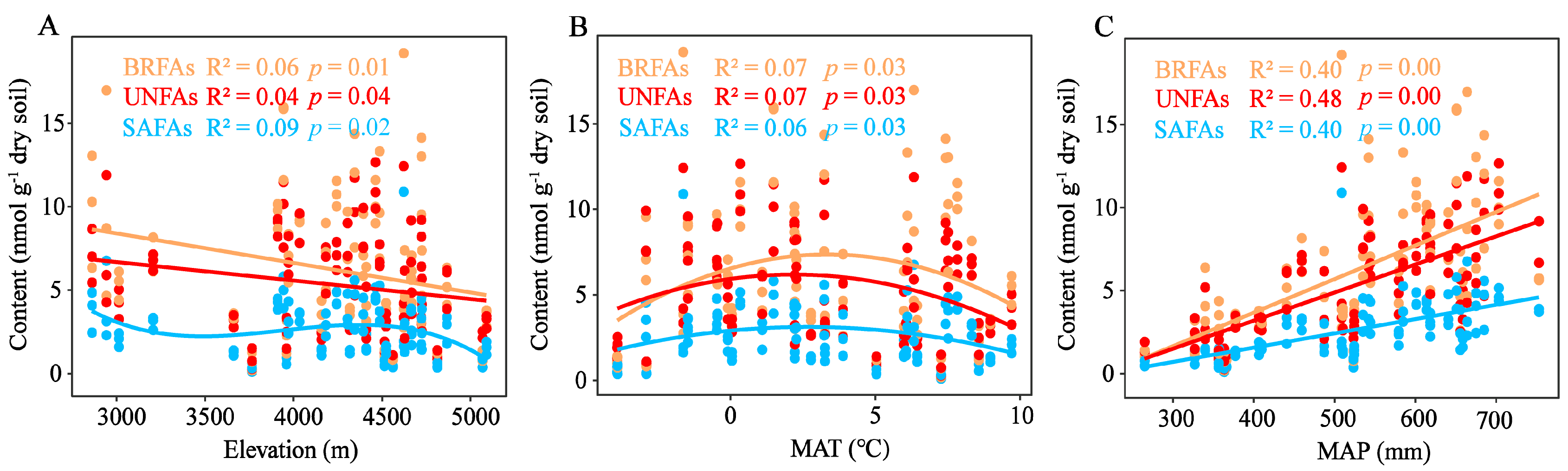
3.2. Relationships Between Environmental Factors and Soil Microbial Biomass
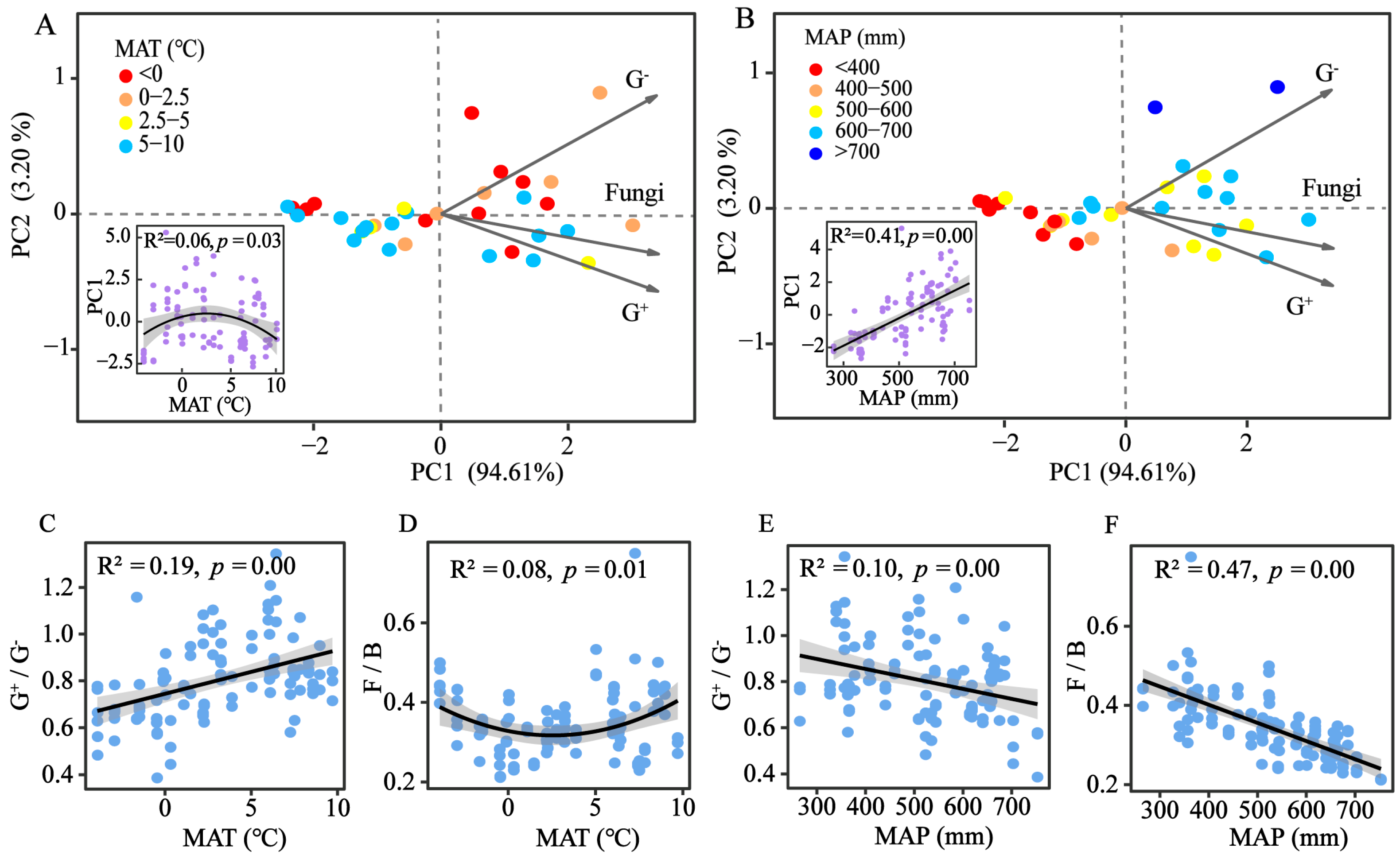
3.3. Soil Microbial Community Composition
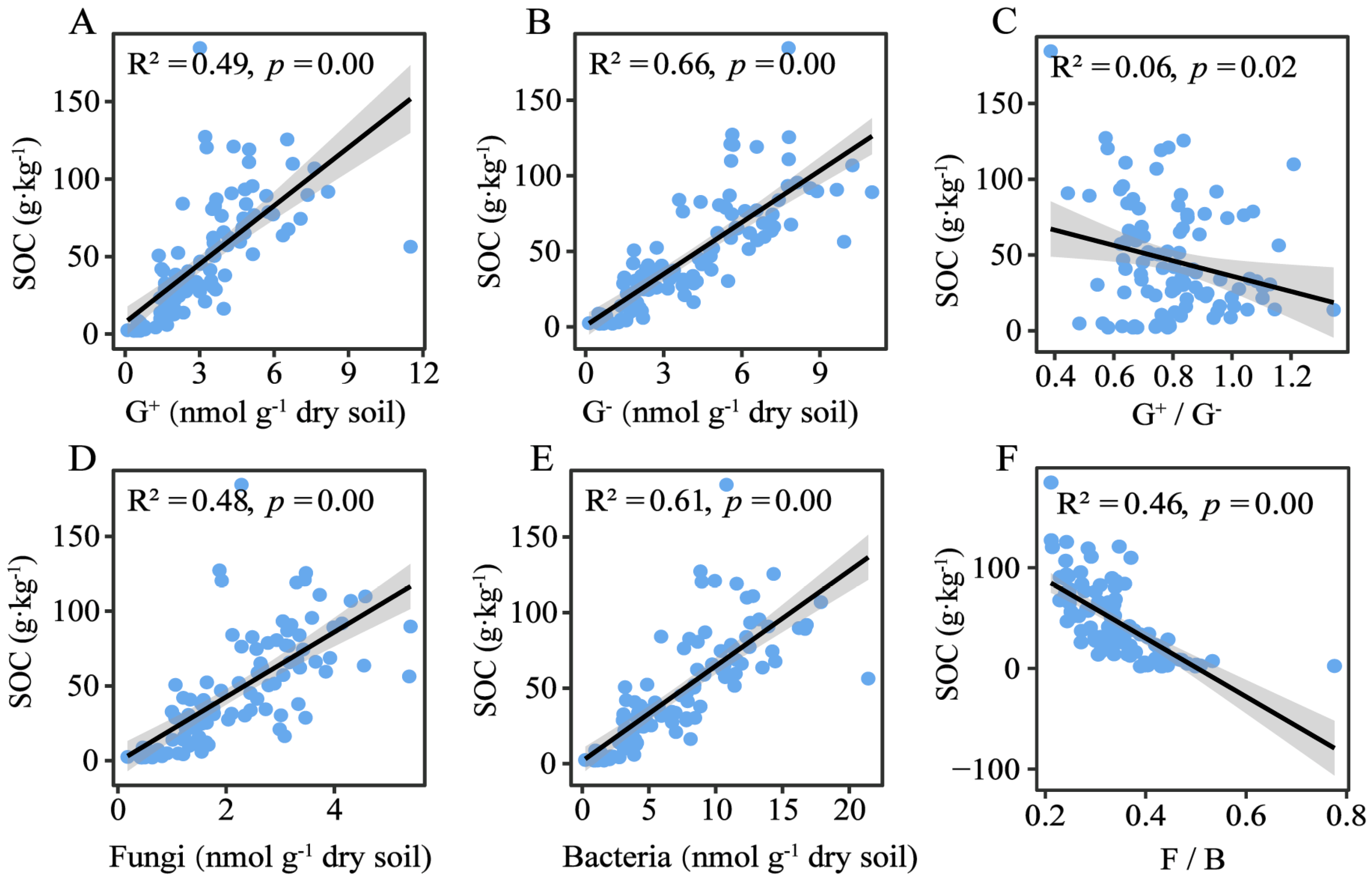
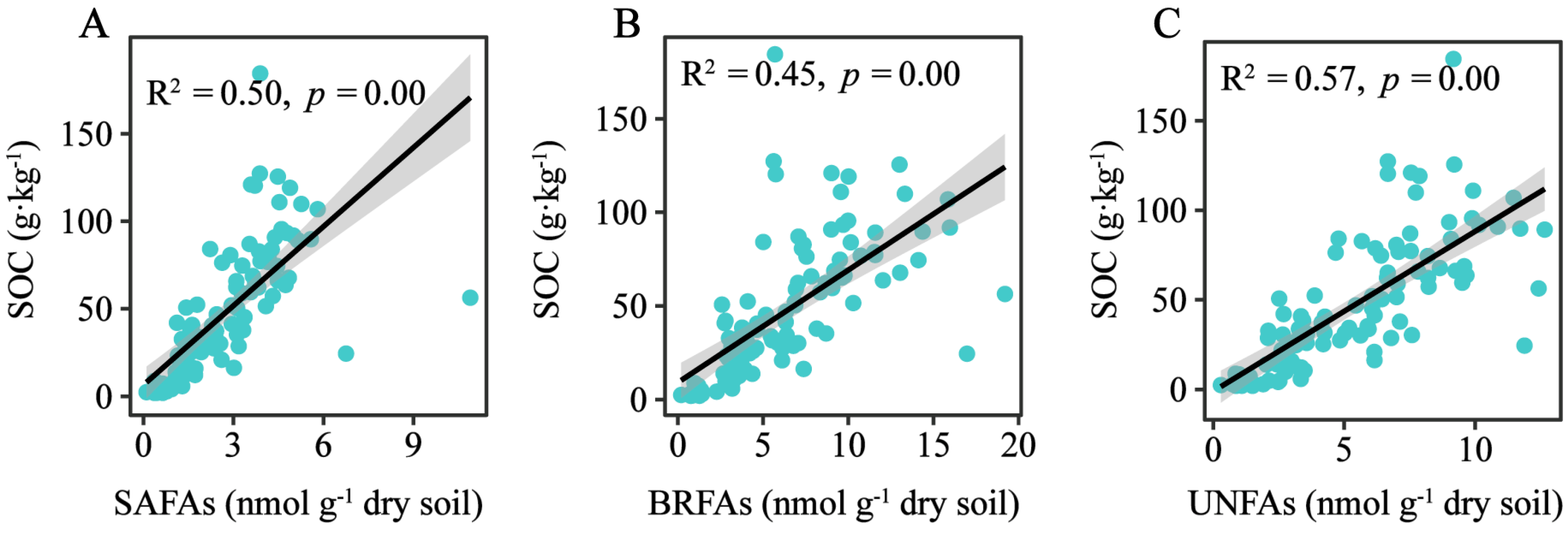
3.4. The Contribution of Microbial Community to Soil Organic Carbon
4. Discussion
4.1. Response of Microbial Biomass to Climatic Gradients
4.2. Response of Soil Organic Carbon to Climatic Gradients
4.3. Shifts in Microbial Community Structure and Implications for Carbon Cycling
4.4. Limitations and Outlook
5. Conclusions
- (1)
- Microbial biomass (indicated by PLFA) increases with mean annual precipitation but shows a unimodal response to mean annual temperature, peaking then declining. Crucially, higher microbial biomass corresponds to increased SOC.
- (2)
- At higher elevations (lower MAP/MAT), shifts in microbial community structure (higher G+/G− ratio and F/B ratio) adapt to harsher conditions. The increased G+/G− and F/B ratios correlate negatively with SOC storage, highlighting their role as key mediators of carbon storage.
- (3)
- Microbial membrane lipid composition also adapts to harsher condition: branched saturated fatty acids (BRFAs) prevail across sites, but the narrowing abundance gap between BRFAs and unsaturated fatty acids (UNFAs) at high elevations reflects costly energy allocation toward maintaining membrane fluidity under cold/dry stress.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Janzen, H.H. Carbon cycling in earth systems—A soil science perspective. Agric. Ecosyst. Environ. 2004, 104, 399–417. [Google Scholar] [CrossRef]
- Lal, R.; Monger, C.; Nave, L.; Smith, P. The role of soil in regulation of climate. Philos. Trans. R. Soc. B 2021, 376, 20210084. [Google Scholar] [CrossRef]
- Gerke, J. The central role of soil organic matter in soil fertility and carbon storage. Soil Syst. 2022, 6, 33. [Google Scholar] [CrossRef]
- Bhattacharya, S.S.; Kim, K.H.; Das, S.; Uchimiya, M.; Jeon, B.H.; Kwon, E.; Szulejko, J.E. A review on the role of organic inputs in maintaining the soil carbon pool of the terrestrial ecosystem. J. Environ. Manag. 2016, 167, 214–227. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Q.; Liu, Y.; Liu, S.; Yu, D.; Shi, X.; Xing, S.; Chen, H.; Fan, X. Combined effects of temperature and precipitation on soil organic carbon changes in the uplands of eastern China. Geoderma 2019, 337, 1105–1115. [Google Scholar] [CrossRef]
- Zhu, X.; Mao, L.; Chen, B. Driving forces linking microbial community structure and functions to enhanced carbon stability in biochar-amended soil. Environ. Int. 2019, 133, 105211. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and Fungal Contributions to Carbon Sequestration in Agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, Y.; Zhou, Z.; Deng, J.; Zhao, F.; Guo, Y.; Han, X.; Yang, G.; Feng, Y.; Ren, G.; et al. Resource limitation and modeled microbial metabolism along an elevation gradient. Catena 2022, 217, 105807. [Google Scholar] [CrossRef]
- Yang, N.; Zhou, C.; Li, Y.; Deng, Y. Microbial specialists in high-altitude forest soils: Environmental sensitivity and ecological significance. Front. Environ. Sci. Eng. 2025, 19, 30. [Google Scholar] [CrossRef]
- Wang, G.X.; Qian, J.; Cheng, G.D.; Lai, Y.M. Soil organic carbon pool of grassland soils on the Qinghai-Tibetan Plateau and its global implication. Sci. Total Environ. 2002, 291, 207–217. [Google Scholar]
- He, M.; Fang, K.; Chen, L.; Feng, X.; Qin, S.; Kou, D.; He, H.; Liang, C.; Yang, Y. Depth-dependent drivers of soil microbial necromass carbon across Tibetan alpine grasslands. Glob. Change Biol. 2022, 28, 936–949. [Google Scholar] [CrossRef]
- Ma, X.; Chen, T.; Zhang, G.; Wang, R. Microbial community structure along an altitude gradient in three different localities. Folia Microbiol. 2004, 49, 105–111. [Google Scholar] [CrossRef]
- Xu, M.; Li, X.; Cai, X.; Gai, J.; Li, X.; Christie, P.; Zhang, J. Soil microbial community structure and activity along a montane elevational gradient on the Tibetan Plateau. Eur. J. Soil Biol. 2014, 64, 6–14. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Niu, B.; Chen, Q.; Hu, Y.; Yang, Y.; Song, L.; Wang, J.; Zhang, G. Temperature and precipitation drive elevational patterns of microbial beta diversity in alpine grasslands. Microb. Ecol. 2021, 82, 1141–1153. [Google Scholar] [CrossRef]
- Donhauser, J.; Niklaus, P.A.; Rousk, J.; Larose, C.; Frey, B. Temperatures beyond the community optimum promote the dominance of heat-adapted, fast growing and stress resistant bacteria in alpine soils. Soil Biol. Biochem. 2020, 148, 107873. [Google Scholar] [CrossRef]
- Roller, B.R.K.; Schmidt, T.M. The physiology and ecological implications of efficient growth. ISME J. 2015, 9, 1481–1492. [Google Scholar] [CrossRef]
- Dhakar, K.; Pandey, A. Microbial ecology from the Himalayan cryosphere perspective. Microorganisms 2020, 8, 257. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Kivlin, S.N.; Rocca, J.D.; Huguet, V.; Thomsen, M.A.; Suttle, K.B. Fungal community responses to precipitation. Glob. Change Biol. 2011, 17, 1637–1645. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Egerton-Warburton, L.M.; Allen, M.F. Topographic position modulates the mycorrhizal response of oak trees to interannual rainfall variability. Ecology 2009, 90, 649–662. [Google Scholar] [CrossRef]
- Fanin, N.; Kardol, P.; Farrell, M.; Nilsson, M.-C.; Gundale, M.J.; Wardle, D.A. The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biol. Biochem. 2019, 128, 111–114. [Google Scholar] [CrossRef]
- He, J.; Tan, X.; Nie, Y.; Ma, L.; Zhou, W.; Shen, W. Enhancement of saturated fatty acid content in soil microbial membranes across natural and experimental warming gradients. Soil Biol. Biochem. 2023, 176, 108866. [Google Scholar] [CrossRef]
- Hall, E.K.; Singer, G.A.; Kainz, M.J.; Lennon, J.T. Evidence for a temperature acclimation mechanism in bacteria: An empirical test of a membrane-mediated trade-off. Funct. Ecol. 2010, 24, 898–908. [Google Scholar] [CrossRef]
- Dong, Z.; Hu, G.; Qian, G.; Lu, J.; Zhang, Z.; Luo, W.; Lyu, P. High-altitude Aeolian research on the Tibetan Plateau. Rev. Geophys. 2017, 55, 864–901. [Google Scholar] [CrossRef]
- Lin, J.; Miao, T.; Zhou, G.; Zhang, Q.; An, J.; Fang, F.; Lv, X.; Dang, H. The Ecological Quality Variation of Vegetation on the Tibetan Plateau from 2001 to 2020 and Its Relationship with Westerly Monsoon Synergy. Agronomy 2025, 15, 1317. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, W.; Xue, K.; Wang, S.; Zhang, L.; Hu, R.; Zeng, H.; Xu, X.; Li, Y.; Jiang, L.; et al. Grassland changes and adaptive management on the Qinghai–Tibetan Plateau. Nat. Rev. Earth Environ. 2022, 3, 668–683. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Li, Y.; Cheng, H. Influences of alpine ecosystem responses to climatic change on soil properties on the Qinghai–Tibet Plateau, China. Catena 2007, 70, 506–514. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- Gehron, M.J.; David, C.W. Sensitive assay of phospholipid glycerol in environmental samples. J. Microbiol. Methods 1983, 1, 23–32. [Google Scholar] [CrossRef]
- Chen, Y.; Du, Z.; Weng, Z.; Sun, K.; Zhang, Y.; Liu, Q.; Yang, Y.; Li, Y.; Wang, Z.; Luo, Y.; et al. Formation of soil organic carbon pool is regulated by the structure of dissolved organic matter and microbial carbon pump efficacy: A decadal study comparing different carbon management strategies. Glob. Change Biol. 2023, 29, 5445–5459. [Google Scholar] [CrossRef]
- Joergensen, R.G. Phospholipid fatty acids in soil—Drawbacks and future prospects. Biol. Fert. Soils 2022, 58, 1–6. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Sun, X.; McLaughlin, N.B.; Jia, S.; Liang, A.; Zhang, S. The driving mechanism of soil organic carbon biodegradability in the black soil region of Northeast China. Sci. Total Environ. 2023, 884, 163835. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Martínez, V.; Mikha, M.M.; Vigil, M.F. Microbial communities and enzyme activities in soils under alternative crop rotations compared to wheat–fallow for the Central Great Plains. Appl. Soil Ecol. 2007, 37, 41–52. [Google Scholar] [CrossRef]
- Swallow, M.; Quideau, S.; MacKenzie, M.; Kishchuk, B. Microbial community structure and function: The effect of silvicultural burning and topographic variability in north ern Alberta. Soil Biol. Biochem. 2009, 41, 770–777. [Google Scholar] [CrossRef]
- Ren, C.; Zhao, F.; Shi, Z.; Chen, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Differential responses of soil microbial biomass and carbon-degrading enzyme activities to altered precipitation. Soil Biol. Biochem. 2017, 115, 1–10. [Google Scholar] [CrossRef]
- Barnard, R.L.; Osborne, C.A.; Firestone, M.K. Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J. 2013, 7, 2229–2241. [Google Scholar] [CrossRef]
- Li, C.; Liu, L.; Zheng, L.; Yu, Y.; Mushinski, R.M.; Zhou, Y.; Xiao, C. Greater soil water and nitrogen availability increase C: N ratios of root exudates in a temperate steppe. Soil Biol. Biochem. 2021, 161, 108384. [Google Scholar] [CrossRef]
- Zogg, G.P.; Zak, D.R.; Ringelberg, D.B.; White, D.C.; MacDonald, N.W.; Pregitzer, K.S. Compositional and functional shifts in microbial communities due to soil warming. Soil Sci. Soc. Am. J. 1997, 61, 475–481. [Google Scholar] [CrossRef]
- Feng, J.; Zeng, X.; Zhang, Q.; Zhou, X.; Liu, Y.; Huang, Q. Soil microbial trait-based strategies drive metabolic efficiency along an altitude gradient. ISME Commun. 2021, 1, 71. [Google Scholar] [CrossRef]
- Slotsbo, S.; Sørensen, J.G.; Holmstrup, M.; Kostal, V.; Kellermann, V.; Overgaard, J. Tropical to subpolar gradient in phospholipid composition suggests adaptive tuning of biological membrane function in drosophilids. Funct. Ecol. 2016, 30, 759–768. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Response of soil microbial communities to altered precipitation: A global synthesis. Glob. Ecol. Biogeogr. 2018, 27, 1121–1136. [Google Scholar] [CrossRef]
- Wilcox, K.R.; Shi, Z.; Gherardi, L.A.; Lemoine, N.P.; Koerner, S.E.; Hoover, D.L.; Bork, E.; Byrne, K.M.; Cahill, J., Jr.; Collins, S.L.; et al. Asymmetric responses of primary productivity to precipitation extremes: A synthesis of grassland precipitation manipulation experiments. Glob. Change Biol. 2017, 23, 4376–4385. [Google Scholar] [CrossRef]
- Wu, Z.; Dijkstra, P.; Koch, G.W.; Penuelas, J.; Hungate, B.A. Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob. Change Biol. 2011, 17, 927–942. [Google Scholar] [CrossRef]
- Wang, C.; Qu, L.; Yang, L.; Liu, D.; Morrissey, E.; Miao, R.; Liu, Z.; Wang, Q.; Fang, Y.; Bai, E. Large-scale importance of microbial carbon use efficiency and necromass to soil organic carbon. Glob. Change Biol. 2021, 27, 2039–2048. [Google Scholar] [CrossRef]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Manzoni, S.; Schaeffer, S.M.; Katul, G.; Porporato, A.; Schimel, J.P. A theoretical analysis of microbial eco-physiological and diffusion limitations to carbon cycling in drying soils. Soil Biol. Biochem. 2014, 73, 69–83. [Google Scholar] [CrossRef]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Responses of soil microbial communities to water stress: Results from a meta-analysis. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Ball, B.A. Impacts of altered precipitation regimes on soil communities and biogeochemistry in arid and semiarid ecosystems. Glob. Change Biol. 2015, 21, 1407–1421. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant-microbial-soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef]
- Zeglin, L.H.; Bottomley, P.J.; Jumpponen, A.; Rice, C.W.; Arango, M.; Lindsley, A.; McGowan, A.; Mfombep, P.; Myrold, D.D. Altered precipitation regime affects the function and composition of soil microbial communities on multiple time scales. Ecology 2013, 94, 2334–2345. [Google Scholar] [CrossRef]
- Federle, T.W.; Dobbins, D.C.; Thortonmanning, J.R.; Jones, D.D. Microbial biomass, activity, and community structure in subsurface soils. Ground Water 1986, 24, 365–374. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Kindler, R.; Miltner, A.; Thullner, M.; Richnow, H.H.; Kästner, M. Fate of bacterial biomass derived fatty acids in soil and their contribution to soil organic matter. Org. Geochem. 2009, 40, 29–37. [Google Scholar] [CrossRef]
- Müller, K.; Marhan, S.; Kandeler, E.; Poll, C. Carbon flow from litter through soil microorganisms: From incorporation rates to mean residence times in bacteria and fungi. Soil Biol. Biochem. 2017, 115, 187–196. [Google Scholar] [CrossRef]
- Kramer, C.; Gleixner, G. Soil organic matter in soil depth profiles: Distinct carbon preferences of microbial groups during carbon transformation. Soil Biol. Biochem. 2008, 40, 425–433. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Firestone, M.K. Response of microbial community composition and function to soil climate change. Microb. Ecol. 2006, 52, 716–724. [Google Scholar] [CrossRef]
- Schimel, J.P.; Balser, T.C.; Wallenstein, M. Microbial stress response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- Sparks, J.P. Ecological ramifications of the direct foliar uptake of nitrogen. Oecologia 2008, 159, 1–13. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, X.; He, J.; Zheng, G.; Tan, X.; Cui, S. Climate-Driven Microbial Communities Regulate Soil Organic Carbon Stocks Along the Elevational Gradient on Alpine Grassland over the Qinghai–Tibet Plateau. Agronomy 2025, 15, 1810. https://doi.org/10.3390/agronomy15081810
Mo X, He J, Zheng G, Tan X, Cui S. Climate-Driven Microbial Communities Regulate Soil Organic Carbon Stocks Along the Elevational Gradient on Alpine Grassland over the Qinghai–Tibet Plateau. Agronomy. 2025; 15(8):1810. https://doi.org/10.3390/agronomy15081810
Chicago/Turabian StyleMo, Xiaomei, Jinhong He, Guo Zheng, Xiangping Tan, and Shuyan Cui. 2025. "Climate-Driven Microbial Communities Regulate Soil Organic Carbon Stocks Along the Elevational Gradient on Alpine Grassland over the Qinghai–Tibet Plateau" Agronomy 15, no. 8: 1810. https://doi.org/10.3390/agronomy15081810
APA StyleMo, X., He, J., Zheng, G., Tan, X., & Cui, S. (2025). Climate-Driven Microbial Communities Regulate Soil Organic Carbon Stocks Along the Elevational Gradient on Alpine Grassland over the Qinghai–Tibet Plateau. Agronomy, 15(8), 1810. https://doi.org/10.3390/agronomy15081810






