Comparison of Nutritional and Medicinal Ingredients Between Ganoderma leucocontextum and G. lucidum
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Sample
2.3. Determination of Protein Content
2.4. Determination of Amino Acids Composition
2.5. Determination of Triterpenoid Content
2.6. Determination of Phenolic Content
2.7. Analysis of Polysaccharide
2.7.1. Extraction of Crude Polysaccharide
2.7.2. Purification of Polysaccharide
2.7.3. Characterization of Polysaccharide
2.8. Antioxidant Capacity In Vitro
2.9. Statistical Analysis
3. Results
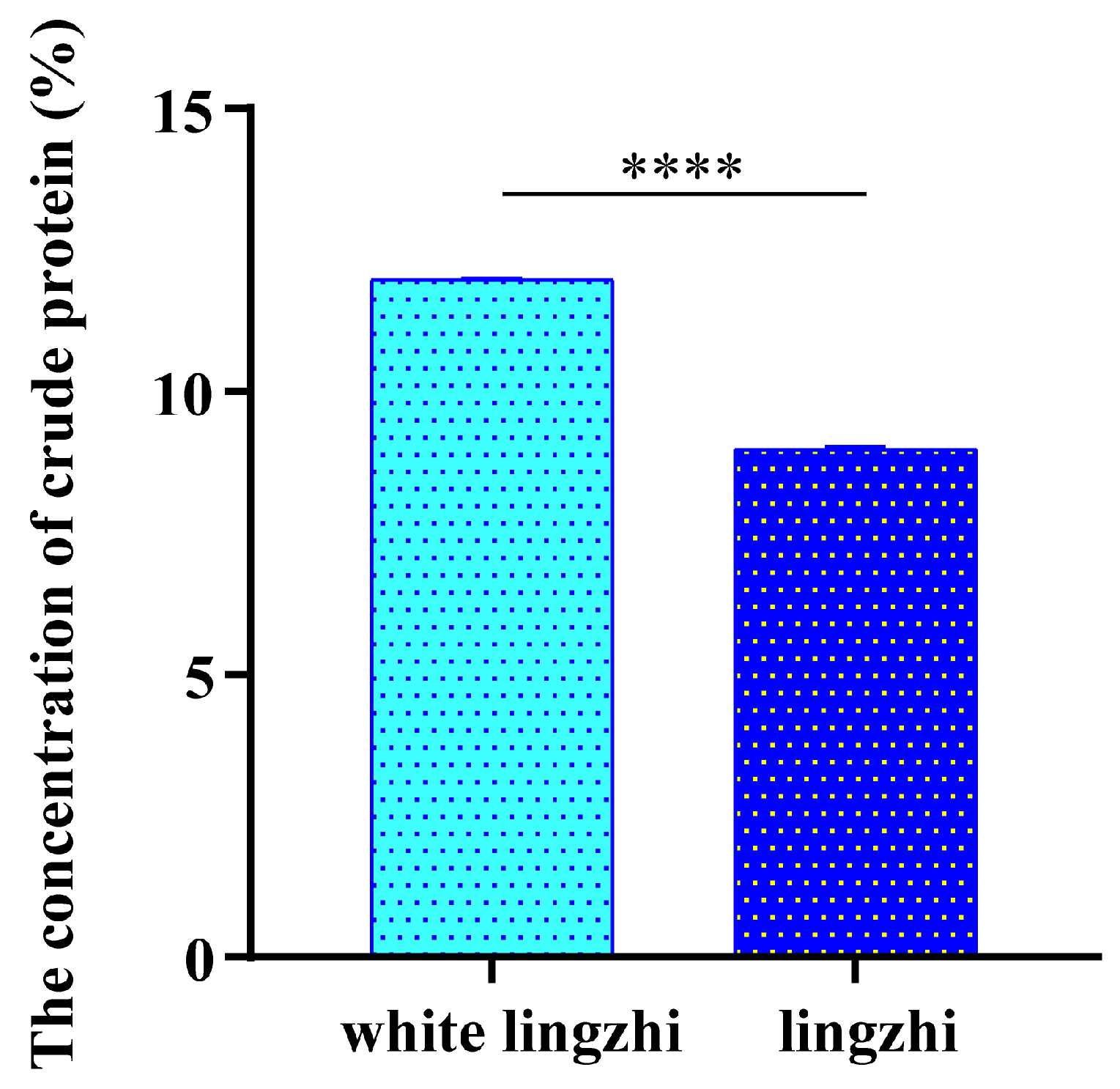
3.1. Difference in Crude Protein Concentration
3.2. Differences in Amino Acid Composition
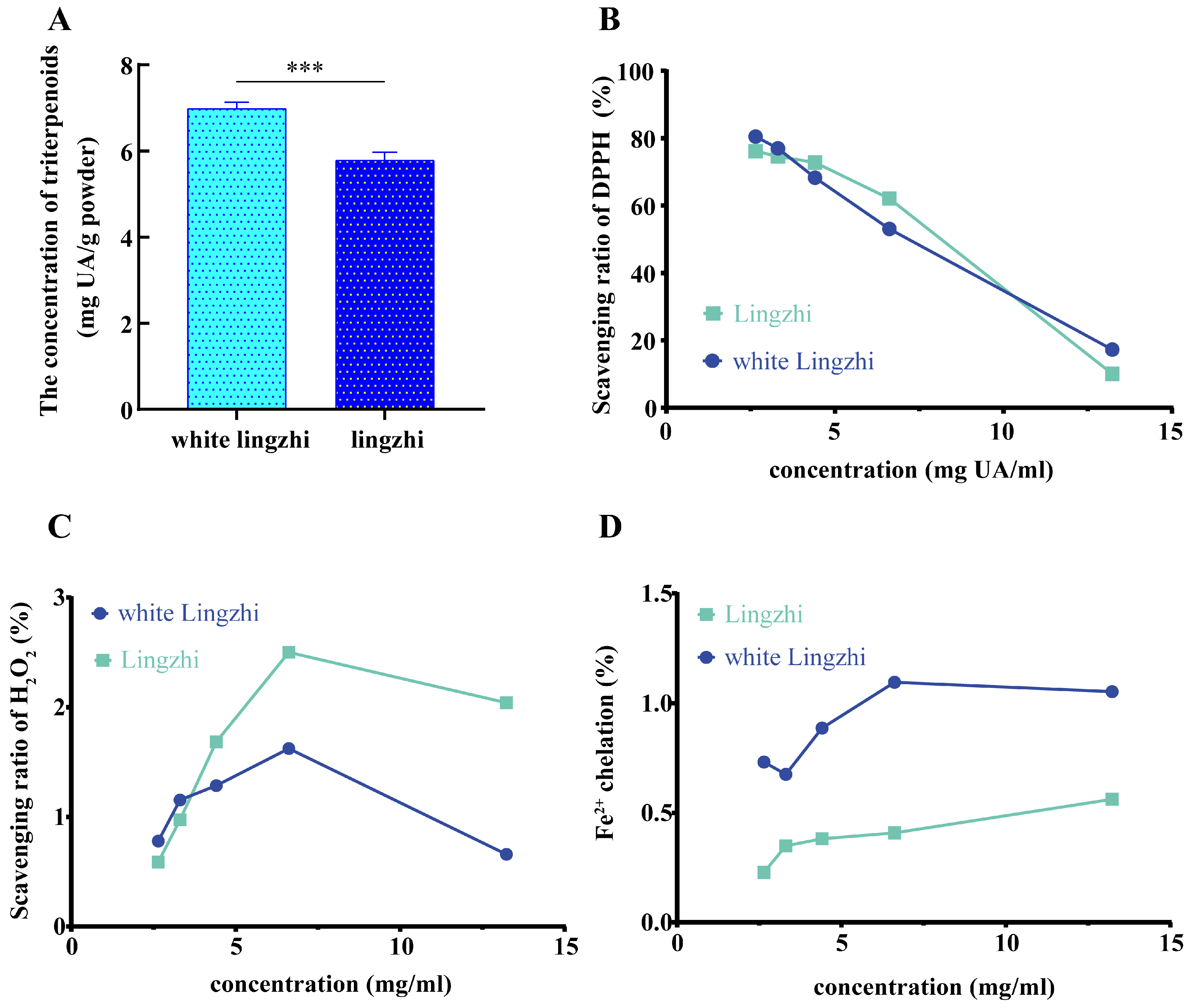
3.3. Differences in Concentrations and Antioxidant Activities of Triterpenoids
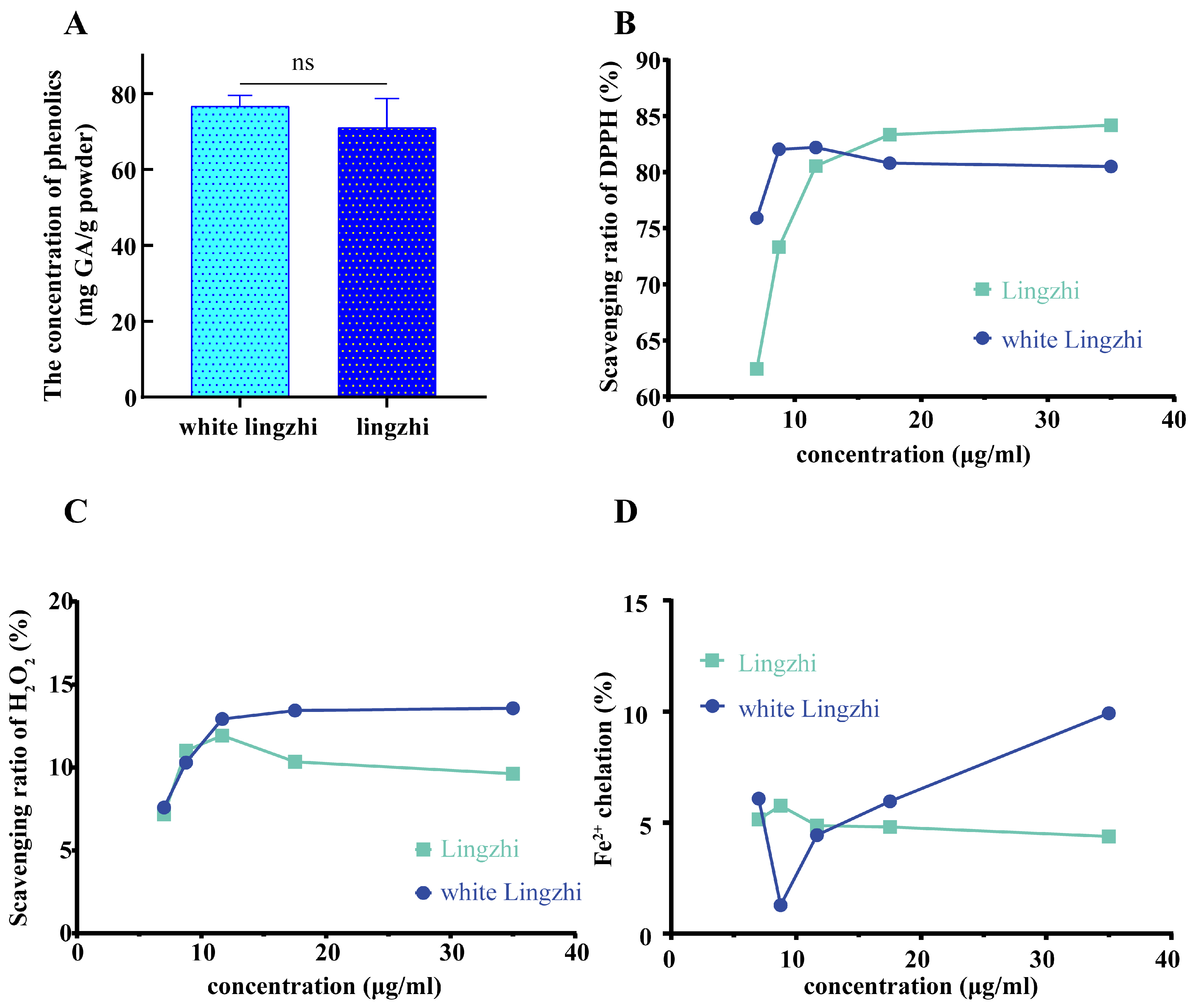
3.4. Differences in Concentrations and Antioxidant Activities of Total Phenolics
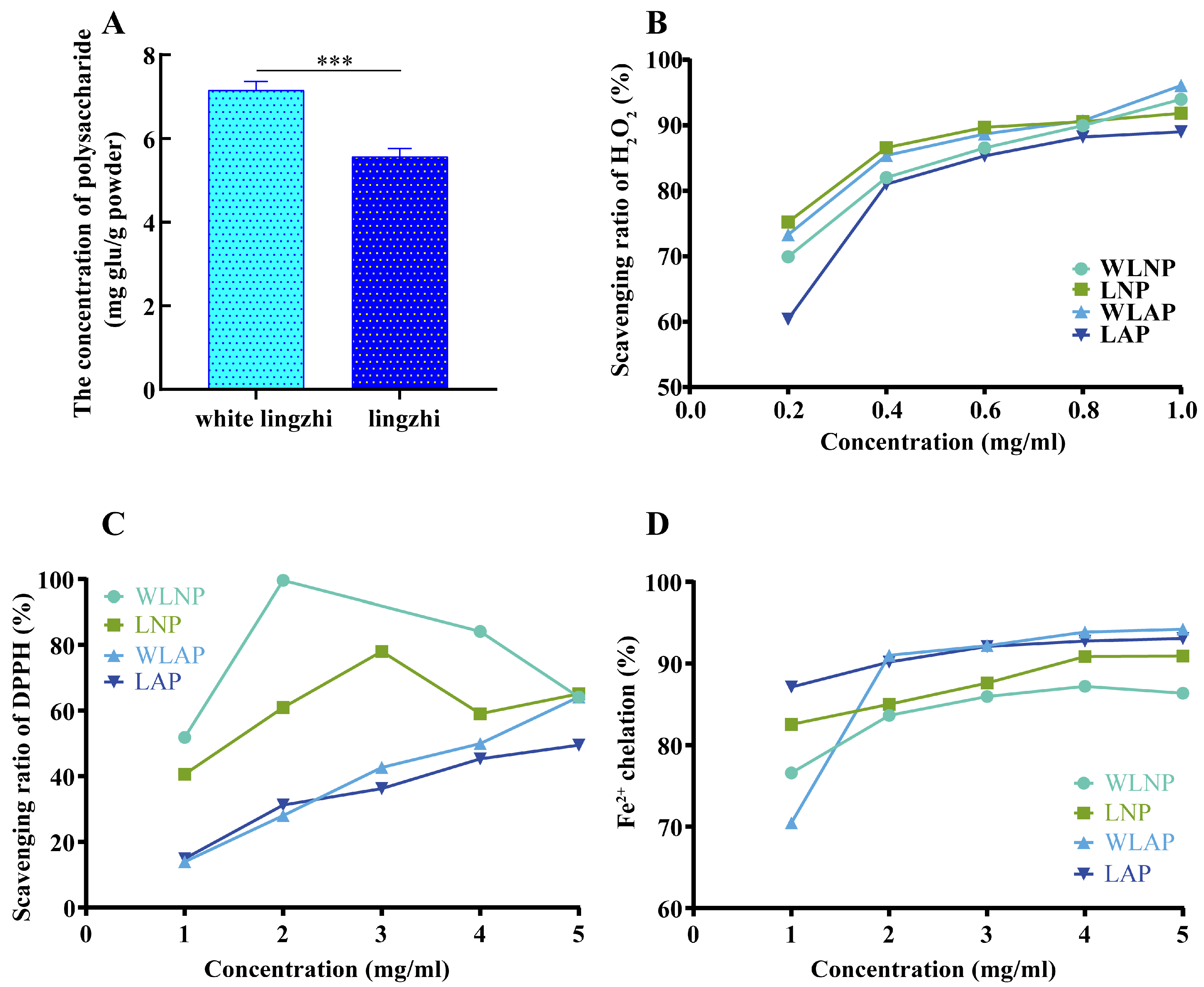
3.5. Differences in Characters and Antioxidant Activities of Polysaccharides
3.5.1. The Molecular Weights of Polysaccharides
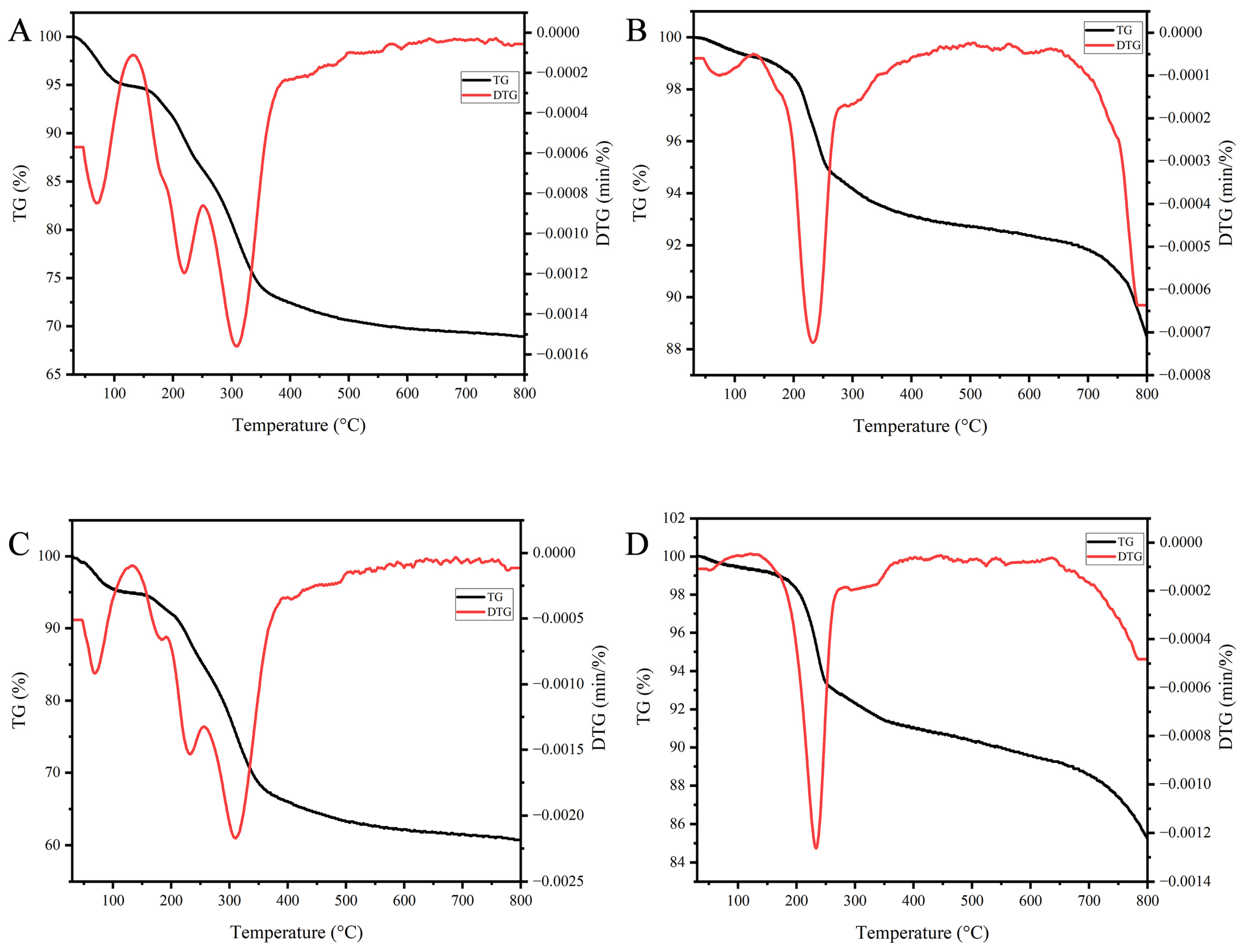
3.5.2. Thermogravimetric Analysis
3.5.3. Monosaccharide Components of Polysaccharides
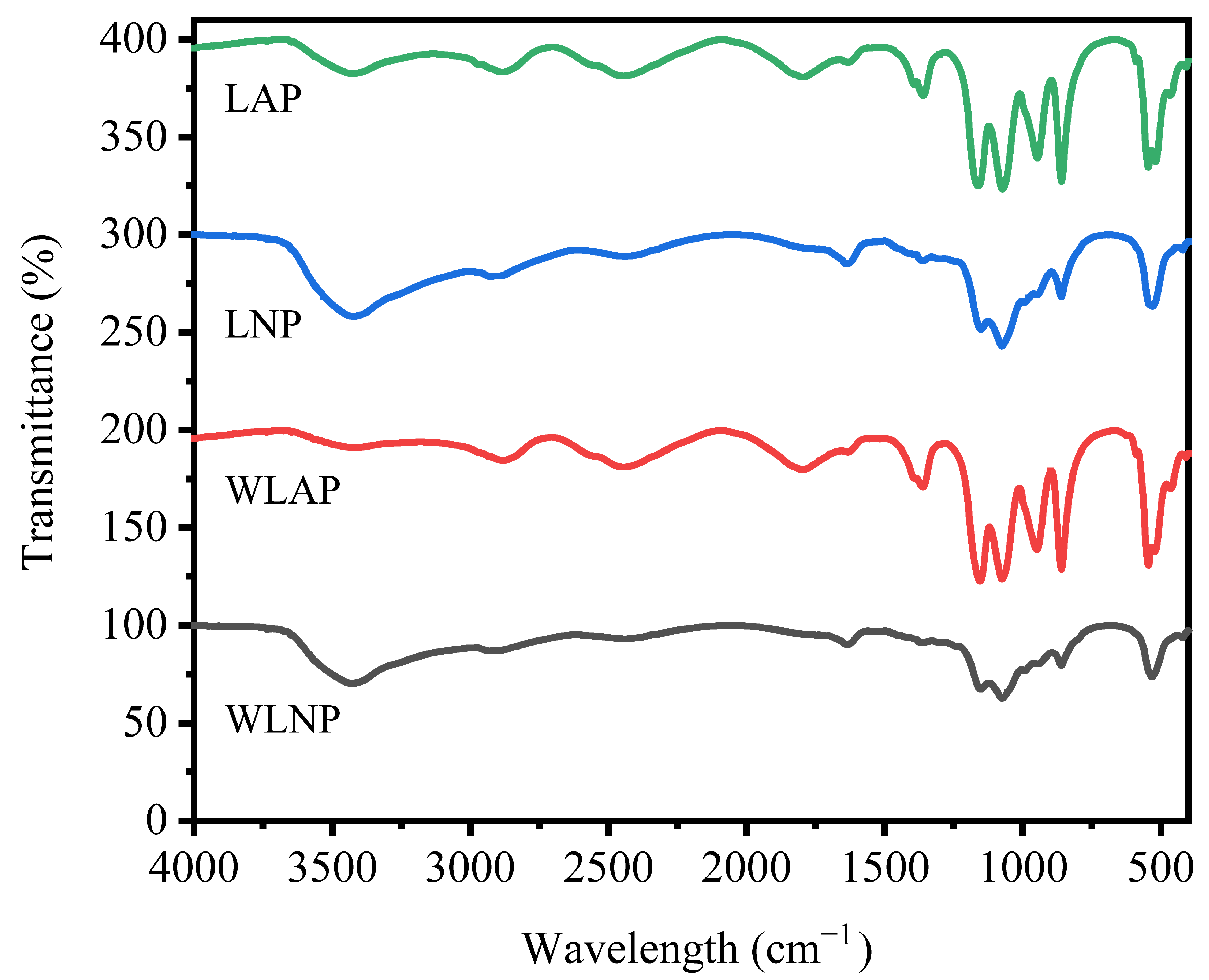
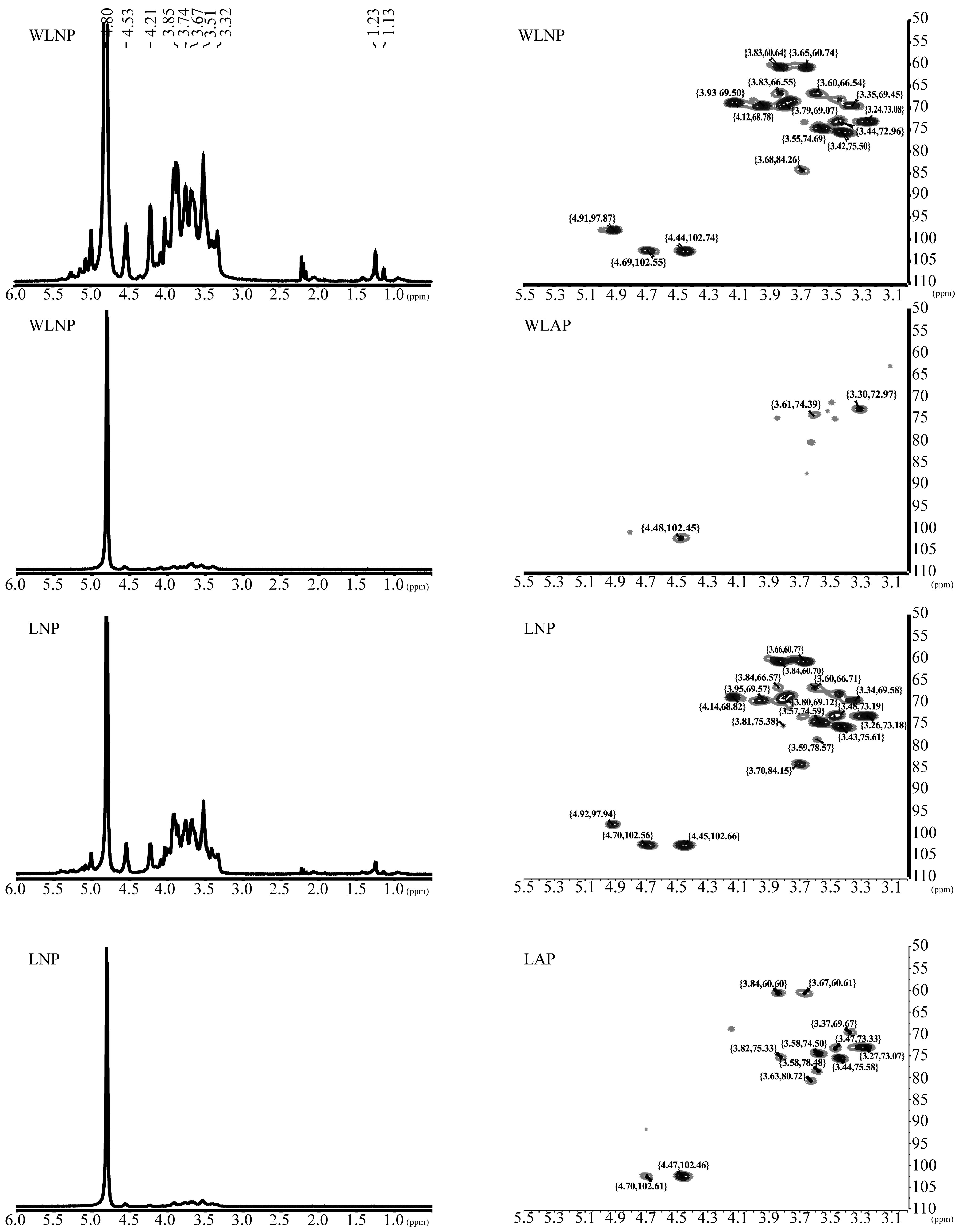
3.5.4. Polysaccharide Structural Analysis
3.5.5. Antioxidant Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jin, X.; Ruiz Beguerie, J.; Sze, D.M.; Chan, G.C. Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst. Rev. 2016, 4, Cd007731. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Y.; Li, Y.; Pan, Y.; Sun, Z.; Zhao, S.; Hou, Y. Ganoderma lucidum fruiting body extracts inhibit colorectal cancer by inducing apoptosis, autophagy, and G0/G1 phase cell cycle arrest in vitro and in vivo. Am. J. Transl. Res. 2020, 12, 2675–2684. [Google Scholar] [PubMed]
- Lin, Z.B. Cellular and molecular mechanisms of immuno-modulation by Ganoderma lucidum. J. Pharmacol. Sci. 2005, 99, 144–153. [Google Scholar] [CrossRef]
- Wu, S.J.; Zhang, S.Y.; Peng, B.; Tan, D.C.; Wu, M.Y.; Wei, J.C.; Wang, Y.T.; Luo, H. Ganoderma lucidum: A comprehensive review of phytochemistry, efficacy, safety and clinical study. Food Sci. Hum. Wellness 2024, 13, 568–596. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Zeyaullah, M.; Alsayegh, A.A.; Mahmood, S.E.; AlShahrani, A.M.; Khan, M.S.; Shama, E.; Hamouda, A.; Elbendary, E.Y.; et al. Ganoderma lucidum: Novel insight into hepatoprotective potential with mechanisms of action. Nutrients 2023, 15, 1874. [Google Scholar] [CrossRef]
- Wang, J.; Cao, B.; Zhao, H.; Feng, J. Emerging roles of Ganoderma lucidum in anti-aging. Aging Dis. 2017, 8, 691–707. [Google Scholar] [CrossRef]
- Ma, H.T.; Hsieh, J.F.; Chen, S.T. Anti-diabetic effects of Ganoderma lucidum. Phytochemistry 2015, 114, 109–113. [Google Scholar] [CrossRef]
- Li, T.H.; Hu, H.P.; Deng, W.Q.; Wu, S.H.; Wang, D.M.; Tsering, T. Ganoderma leucocontextum, a new member of the G. lucidum complex from southwestern China. Mycoscience 2015, 56, 81–85. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Ren, J.; Wang, W.; Xiong, W.; Zhang, Y.; Bao, L.; Liu, H. Triterpenes and meroterpenes with neuroprotective effects from Ganoderma leucocontextum. Chem. Biodivers. 2018, 15, e1700567. [Google Scholar] [CrossRef]
- Gao, X.; Qi, J.; Ho, C.T.; Li, B.; Mu, J.; Zhang, Y.; Hu, H.; Mo, W.; Chen, Z.; Xie, Y. Structural characterization and immunomodulatory activity of a water-soluble polysaccharide from Ganoderma leucocontextum fruiting bodies. Carbohydr. Polym. 2020, 249, 116874. [Google Scholar] [CrossRef]
- Batra, P.; Sharma, A.K.; Khajuria, R. Probing Lingzhi or Reishi medicinal mushroom Ganoderma lucidum (higher Basidiomycetes): A bitter mushroom with amazing health benefits. Int. J. Med. Mushrooms 2013, 15, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Klupp, N.L.; Kiat, H.; Bensoussan, A.; Steiner, G.Z.; Chang, D.H. A double-blind, randomised, placebo-controlled trial of Ganoderma lucidum for the treatment of cardiovascular risk factors of metabolic syndrome. Sci. Rep. 2016, 6, 29540. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Z.P.; Yang, X.X.; Huang, M.; Gao, Y.; Tang, W.; Chan, S.Y.; Dai, X.; Ye, J.; Ho, P.C.; et al. Monitoring of immune responses to a herbal immuno-modulator in patients with advanced colorectal cancer. Int. Immunopharmacol. 2006, 6, 499–508. [Google Scholar] [CrossRef]
- Chu, T.T.; Benzie, I.F.; Lam, C.W.; Fok, B.S.; Lee, K.K.; Tomlinson, B. Study of potential cardioprotective effects of Ganoderma lucidum (Lingzhi): Results of a controlled human intervention trial. Br. J. Nutr. 2012, 107, 1017–1027. [Google Scholar] [CrossRef]
- Nascimento, F.d.S.; Santos, C.A.F.; Figueiredo Neto, A.; Bezerra, L.G.V.; da Silva, S.A.M.F. Crude proteion content in pigeon pea grain lines. Rev. Contemp. 2024, 4, e3615. [Google Scholar] [CrossRef]
- Fraile-Fabero, R.; Ozcariz-Fermoselle, M.V.; Oria-de-Rueda-Salgueiro, J.A.; Garcia-Recio, V.; Cordoba-Diaz, D.; Del P Jiménez-López, M.; Girbés-Juan, T. Differences in antioxidants, polyphenols, protein digestibility and nutritional profile between Ganoderma lingzhi from industrial crops in asia and Ganoderma lucidum from cultivation and Iberian origin. Foods 2021, 10, 1750. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, M.; Lahm, H.W. Hydrolysis and amino acid composition of proteins. J. Chromatogr. A 1998, 826, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.Z.; Fang, H. Spectrophotometric determination of L-tryptophan by condensation with p-dimethylaminobenzaldehyde. J. Cap. Norm. Univ. 1999, 1999, 48–52. [Google Scholar]
- Oludemi, T.; Barros, L.; Prieto, M.A.; Heleno, S.A.; Barreiro, M.F.; Ferreira, I. Extraction of triterpenoids and phenolic compounds from Ganoderma lucidum: Optimization study using the response surface methodology. Food Funct. 2018, 9, 209–226. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Martins, A.; Queiroz, M.; Santos-Buelga, C.; Ferreira, I. Fruiting body, spores and in vitro produced mycelium of Ganoderma lucidum from Northeast Portugal: A comparative study of the antioxidant potential of phenolic and polysaccharidic extracts. Food Res. Int. 2012, 46, 135–140. [Google Scholar] [CrossRef]
- Ding, J.; Du, H.; Tan, H.; Li, J.; Wang, L.; Li, L.; Zhang, Y.; Liu, Y. Optimization of protein removal process of Lonicera japonica polysaccharide and its immunomodulatory mechanism in cyclophosphamide-induced mice by metabolomics and network pharmacology. Food Sci. Nutr. 2022, 11, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Cong, G.; Zhang, J. Effects of exogenous salicylic acid on polysaccharides production of Dendrobium officinale. S. Afr. J. Bot. 2014, 95, 78–84. [Google Scholar] [CrossRef]
- Feng, X.; Wang, P.; Lu, Y.; Zhang, Z.; Yao, C.; Tian, G.; Liu, Q. A novel polysaccharide from Heimioporus retisporus displays hypoglycemic activity in a diabetic mouse model. Front. Nutr. 2022, 9, 964948. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, Y. Purification, chemical characterization and antioxidant activities of a novel polysaccharide from Auricularia polytricha. Int. J. Biol. Macromol. 2018, 120, 1087–1092. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, L.; Xu, Y.; Wang, L.; Teng, X.; Li, X.; Dai, J. Characterization and biological activities of a novel polysaccharide isolated from raspberry (Rubus idaeus L.) fruits. Carbohydr. Polym. 2015, 132, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Feng, X.; Lv, Z.; Liu, J.; Teng, Q.; Chen, T.; Liu, Q. Temporal dynamics of lignin degradation in Quercus acutissima sawdust during Ganoderma lucidum cultivation. Int. J. Biol. Macromol. 2024, 268, 131686. [Google Scholar] [CrossRef] [PubMed]
- Abeygunawardana, C.; Williams, T.C.; Sumner, J.S.; Hennessey, J.P., Jr. Development and validation of an NMR-based identity assay for bacterial polysaccharides. Anal. Biochem. 2000, 279, 226–240. [Google Scholar] [CrossRef]
- Ganie, S.A.; Haq, E.; Masood, A.; Hamid, A.; Zargar, M.A. Antioxidant and protective effect of ethyl acetate extract of podophyllum hexandrum rhizome on carbon tetrachloride induced rat liver injury. Evid.-Based Complement. Altern. Med. 2011, 2011, 238020. [Google Scholar] [CrossRef]
- Li, C.; Huang, Q.; Fu, X.; Yue, X.J.; Liu, R.H.; You, L.J. Characterization, antioxidant and immunomodulatory activities of polysaccharides from Prunella vulgaris Linn. Int. J. Biol. Macromol. 2015, 75, 298–305. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Stojković, D.S.; Barros, L.; Calhelha, R.C.; Glamočlija, J.; Ćirić, A.; van Griensven, L.J.; Soković, M.; Ferreira, I.C. A detailed comparative study between chemical and bioactive properties of Ganoderma lucidum from different origins. Int. J. Food Sci. Nutr. 2014, 65, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Hu, Q.; Wang, X.; Li, L.; Dong, Y.; Sun, C.; Hu, C.; Gu, X. Thermal kinetics of a lignin-based flame retardant. Polymers 2020, 12, 2123. [Google Scholar] [CrossRef]
- Rozi, P.; Abuduwaili, A.; Mutailifu, P.; Gao, Y.; Rakhmanberdieva, R.; Aisa, H.A.; Yili, A. Sequential extraction, characterization and antioxidant activity of polysaccharides from Fritillaria pallidiflora Schrenk. Int. J. Med. Mushrooms 2019, 131, 97–106. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.; Lai, Q.; Yang, Q.; Dong, Y.; Liu, X.; Wang, W.; Zhang, J.; Jia, L. Antioxidant and hepatoprotective activities of modified polysaccharides from Coprinus comatus in mice with alcohol-induced liver injury. Int. J. Biol. Macromol. 2019, 127, 476–485. [Google Scholar] [CrossRef]
- Liu, D.; Sun, Q.; Xu, J.; Li, N.; Lin, J.; Chen, S.; Li, F. Purification, characterization, and bioactivities of a polysaccharide from mycelial fermentation of Bjerkandera fumosa. Carbohydr. Polym. 2017, 167, 115–122. [Google Scholar] [CrossRef]
- Seedevi, P.; Moovendhan, M.; Viramani, S.; Shanmugam, A. Bioactive potential and structural chracterization of sulfated polysaccharide from seaweed (Gracilaria corticata). Carbohydr. Polym. 2017, 155, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, T.; Du, Y.; Yang, H.; Wei, L.; Bi, H.; Ni, W. Anticancer and immunostimulating activities of a novel homogalacturonan from Hippophae rhamnoides L. berry. Carbohydr. Polym. 2015, 131, 288–296. [Google Scholar] [CrossRef]
- Bociek, S.M.; Welti, D. The quantitative analysis of uronic acid polymers by infrared spectroscopy. Carbohydr. Res. 1975, 42, 217–226. [Google Scholar] [CrossRef]
- Yang, J.P.; Hsu, T.; Lin, F.; Hsu, W.; Chen, Y. Potential antidiabetic activity of extracellular polysaccharides in submerged fermentation culture of Coriolus versicolor LH1. Carbohydr. Polym. 2012, 90, 174–180. [Google Scholar] [CrossRef]
- Jiang, J.; Kong, F.; Li, N.; Zhang, D.; Yan, C.; Lv, H. Purification, structural characterization and in vitro antioxidant activity of a novel polysaccharide from Boshuzhi. Carbohydr. Polym. 2016, 147, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Ru, Y.; Chen, X.; Wang, J.; Guo, L.; Lin, Z.; Peng, X.; Qiu, B.; Wong, W.-L. Structural characterization, hypoglycemic effects and mechanism of a novel polysaccharide from Tetrastigma hemsleyanum Diels et Gilg. Int. J. Med. Mushrooms 2019, 123, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.F.; Stevenson, A.; Reuter, D.C.; Sirota, J.M. Absolute band intensities in the ν19/ν23 (530 cm−1) and ν7 (777 cm−1) bands of acetone ((CH3)2CO) from 232 to 295 K. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 1111–1116. [Google Scholar] [CrossRef]
- Li, M.F.; Fan, Y.M.; Xu, F.; Sun, R.C. Structure and thermal stability of polysaccharide fractions extracted from the ultrasonic irradiated and cold alkali pretreated bamboo. J. Appl. Polym. Sci. 2011, 121, 176–185. [Google Scholar] [CrossRef]
- Choi, J.W.; Synytsya, A.; Capek, P.; Bleha, R.; Pohl, R.; Park, Y.I. Structural analysis and anti-obesity effect of a pectic polysaccharide isolated from Korean mulberry fruit Oddi (Morus alba L.). Carbohydr. Polym. 2016, 146, 187–196. [Google Scholar] [CrossRef]
- Kono, H.; Kondo, N.; Hirabayashi, K.; Ogata, M.; Totani, K.; Ikematsu, S.; Osada, M. NMR spectroscopic structural characterization of a water-soluble β-(1→3, 1→6)-glucan from Aureobasidium pullulans. Carbohydr. Polym. 2017, 174, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, D.; Chen, Y.; Zhong, R.; Gao, L.; Yang, C.; Ai, C.; El-Seedi, H.R.; Zhao, C. Physicochemical characterization of a polysaccharide from Agrocybe aegirita and its anti-ageing activity. Carbohydr. Polym. 2020, 236, 116056. [Google Scholar] [CrossRef]
- Ooi, L.S.M.; Ooi, V.E.C.; Fung, M.C. Induction of gene expression of immunomodulatory cytokines in the mouse by a polysaccharide from Ganoderma lucidum (Curt.: Fr.) P. Karst. (Aphyllophoromycetideae). Int. J. Med. Mushrooms 2002, 4, 27–35. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Yan, X.H.; Zhang, J.L.; Wang, L.Y.; Xue, H.; Jiang, G.C.; Ma, X.T.; Liu, X.J. Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2019, 135, 706–716. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, Z.; Li, L.; Vidyarthi, S.K.; Zheng, Z.; Zhang, R. Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from Ziziphus jujuba cv. Hamidazao: A comparison. Carbohydr. Polym. 2021, 261, 117879. [Google Scholar] [CrossRef]
- Kang, Q.; Chen, S.; Li, S.; Wang, B.; Liu, X.; Hao, L.; Lu, J. Comparison on characterization and antioxidant activity of polysaccharides from Ganoderma lucidum by ultrasound and conventional extraction. Int. J. Biol. Macromol. 2019, 124, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yang, C.; Mao, D. Fraction and chemical analysis of antioxidant active polysaccharide isolated from flue-cured tobacco leaves. Pharmacogn. Mag. 2014, 10, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.S.; Liu, H.; Han, C.-R.; Zeng, S.J.; Xu, X.J.; Lu, D.J.; He, H.J. Extraction, characterization and antioxidant activity of polysaccharide from Pouteria campechiana seed. Carbohydr. Polym. 2020, 229, 115409. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.L.; Cheng, H.R.; Xu, Z.; Feng, S.C.; Yuan, M.; Huang, Y.; Liao, J.Q.; Ding, C.B. Antioxidant and anti-aging activities and structural elucidation of polysaccharides from Panax notoginseng root. Process Biochem. 2019, 78, 189–199. [Google Scholar] [CrossRef]
- Li, S.; Liu, H.; Wang, W.; Wang, X.; Zhang, C.; Zhang, J.; Jing, H.; Ren, Z.; Gao, Z.; Song, X.; et al. Antioxidant and anti-aging effects of acidic-extractable polysaccharides by Agaricus bisporus. Int. J. Biol. Macromol. 2018, 106, 1297–1306. [Google Scholar] [CrossRef]
- Ma, C.; Feng, M.; Zhai, X.; Hu, M.; You, L.; Luo, W.; Zhao, M. Optimization for the extraction of polysaccharides from Ganoderma lucidum and their antioxidant and antiproliferative activities. J. Taiwan Inst. Chem. Eng. 2013, 44, 886–894. [Google Scholar] [CrossRef]
- Ruthes, A.C.; Smiderle, F.R.; Iacomini, M. Mushroom heteropolysaccharides: A review on their sources, structure and biological effects. Carbohydr. Polym. 2016, 136, 358–375. [Google Scholar] [CrossRef]
- Zhu, X.-l.; Lin, Z.-b. Effects of Ganoderma lucidum polysaccharides on proliferation and cytotoxicity of cytokine-induced killer cells. Acta Pharmacol. Sin. 2005, 26, 1130–1137. [Google Scholar] [CrossRef]
- Liang, X.; Liu, M.; Wei, Y.; Tong, L.; Guo, S.; Kang, H.; Zhang, W.; Yu, Z.; Zhang, F.; Duan, J.-a. Structural characteristics and structure-activity relationship of four polysaccharides from Lycii fructus. Int. J. Biol. Macromol. 2023, 253, 127256. [Google Scholar] [CrossRef]
- Hennicke, F.; Cheikh-Ali, Z.; Liebisch, T.; Maciá-Vicente, J.G.; Bode, H.B.; Piepenbring, M. Distinguishing commercially grown Ganoderma lucidum from Ganoderma lingzhi from Europe and East Asia on the basis of morphology, molecular phylogeny, and triterpenic acid profiles. Phytochemistry 2016, 127, 29–37. [Google Scholar] [CrossRef]
- Bohin, M.C.; Roland, W.S.; Gruppen, H.; Gouka, R.J.; van der Hijden, H.T.; Dekker, P.; Smit, G.; Vincken, J.P. Evaluation of the bitter-masking potential of food proteins for EGCG by a cell-based human bitter taste receptor assay and binding studies. J. Agric. Food Chem. 2013, 61, 10010–10017. [Google Scholar] [CrossRef] [PubMed]
| Amino Acid | Amino Acid Concentration (mg g−1) | ||
|---|---|---|---|
| White Lingzhi | Lingzhi | ||
| MSG-like | Aspartic acid | 0.96 ± 0.02 | 0.77 ± 0.02 |
| Glutamic acid | 1.44 ± 0.02 | 0.80 ± 0.02 | |
| Total/percentage | 2.40/25.2% | 1.57/22.6% | |
| Sweet | Alanine | 0.64 ± 0.00 | 0.50 ± 0.01 |
| Glycine | 0.50 ± 0.01 | 0.40 ± 0.02 | |
| Serine | 0.47 ± 0.01 | 0.40 ± 0.02 | |
| Threonine | 0.51 ± 0.01 | 0.47 ± 0.02 | |
| Total/percentage | 2.12/22.3% | 1.77/25.4% | |
| Bitter | Arginine | 0.65 ± 0.02 | 0.32 ± 0.01 |
| Histidine | 0.17 ± 0.00 | 0.13 ± 0.01 | |
| Leucine | 0.88 ± 0.01 | 0.69 ± 0.02 | |
| Phenylalanine | 0.45 ± 0.00 | 0.34 ± 0.01 | |
| Methionine | 0.15 ± 0.01 | 0.10 ± 0.00 | |
| Cysteine | 0.15 ± 0.00 | 0.18 ± 0.00 | |
| Valine | 0.60 ± 0.01 | 0.48 ± 0.01 | |
| Total/percentage | 3.05/32.0% | 2.24/32.2% | |
| Tasteless | Isoleucine | 0.44 ± 0.01 | 0.35 ± 0.01 |
| Lysine | 0.55 ± 0.01 | 0.36 ± 0.01 | |
| Proline | 0.47 ± 0.01 | 0.35 ± 0.01 | |
| Tryptophan | 0.15 ± 0.00l | 0.09 ± 0.00 | |
| Tyrosine | 0.34 ± 0.00 | 0.23 ± 0.01 | |
| Total/percentage | 1.95/20.5% | 1.38/19.8% | |
| Total amino acids | 9.52 | 6.96 | |
| Polysaccharide | Mw | Mn | PD |
|---|---|---|---|
| WLNP | 131,127 | 76,372 | 1.72 |
| WLAP | 123,869 | 74,105 | 1.67 |
| LNP | 139,141 | 88,465 | 1.57 |
| LAP | 104,006 | 61,417 | 1.69 |
| Sample | Fucose (Fuc) | Galactose (Gal) | Glucose (Glc) | Xylose (Xyl) | Glucuronic Acid (GlcA) |
|---|---|---|---|---|---|
| LAP | 0.00 | 0.00 | 78.07% | 0.00 | 21.93% |
| WLAP | 0.00 | 0.00 | 66.91% | 0.00 | 33.09% |
| LNP | 3.65% | 12.79% | 70.84% | 7.51% | 5.21% |
| WLNP | 5.05% | 19.21% | 66.32% | 6.00% | 3.42% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Fang, F.; Yao, C.; Teng, Q.; Tian, G.; Hong, L.; Bin, Y.; Liu, Q. Comparison of Nutritional and Medicinal Ingredients Between Ganoderma leucocontextum and G. lucidum. Agronomy 2024, 14, 2523. https://doi.org/10.3390/agronomy14112523
Wang P, Fang F, Yao C, Teng Q, Tian G, Hong L, Bin Y, Liu Q. Comparison of Nutritional and Medicinal Ingredients Between Ganoderma leucocontextum and G. lucidum. Agronomy. 2024; 14(11):2523. https://doi.org/10.3390/agronomy14112523
Chicago/Turabian StyleWang, Peng, Fei Fang, Chunxin Yao, Qian Teng, Guoting Tian, Linhai Hong, Yalan Bin, and Qinghong Liu. 2024. "Comparison of Nutritional and Medicinal Ingredients Between Ganoderma leucocontextum and G. lucidum" Agronomy 14, no. 11: 2523. https://doi.org/10.3390/agronomy14112523
APA StyleWang, P., Fang, F., Yao, C., Teng, Q., Tian, G., Hong, L., Bin, Y., & Liu, Q. (2024). Comparison of Nutritional and Medicinal Ingredients Between Ganoderma leucocontextum and G. lucidum. Agronomy, 14(11), 2523. https://doi.org/10.3390/agronomy14112523






