Metabolic and Behavioral Impacts of Gustatory Receptor NlGr23 Silencing in the Brown Planthopper
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Preparing and Injecting dsRNA into the BPH
2.3. RNA Extraction and qRT-PCR Analysis
2.4. Measuring Body Weight of and Honeydew Produced by BPHs
2.5. RNA-Seq Analysis
2.6. Protein Extraction and Trypsin Digestion
2.7. Proteomic Analysis
2.8. Triacylglycerol, Glycerol, and Fatty Acid Assays
2.9. Statistical Analysis
3. Results
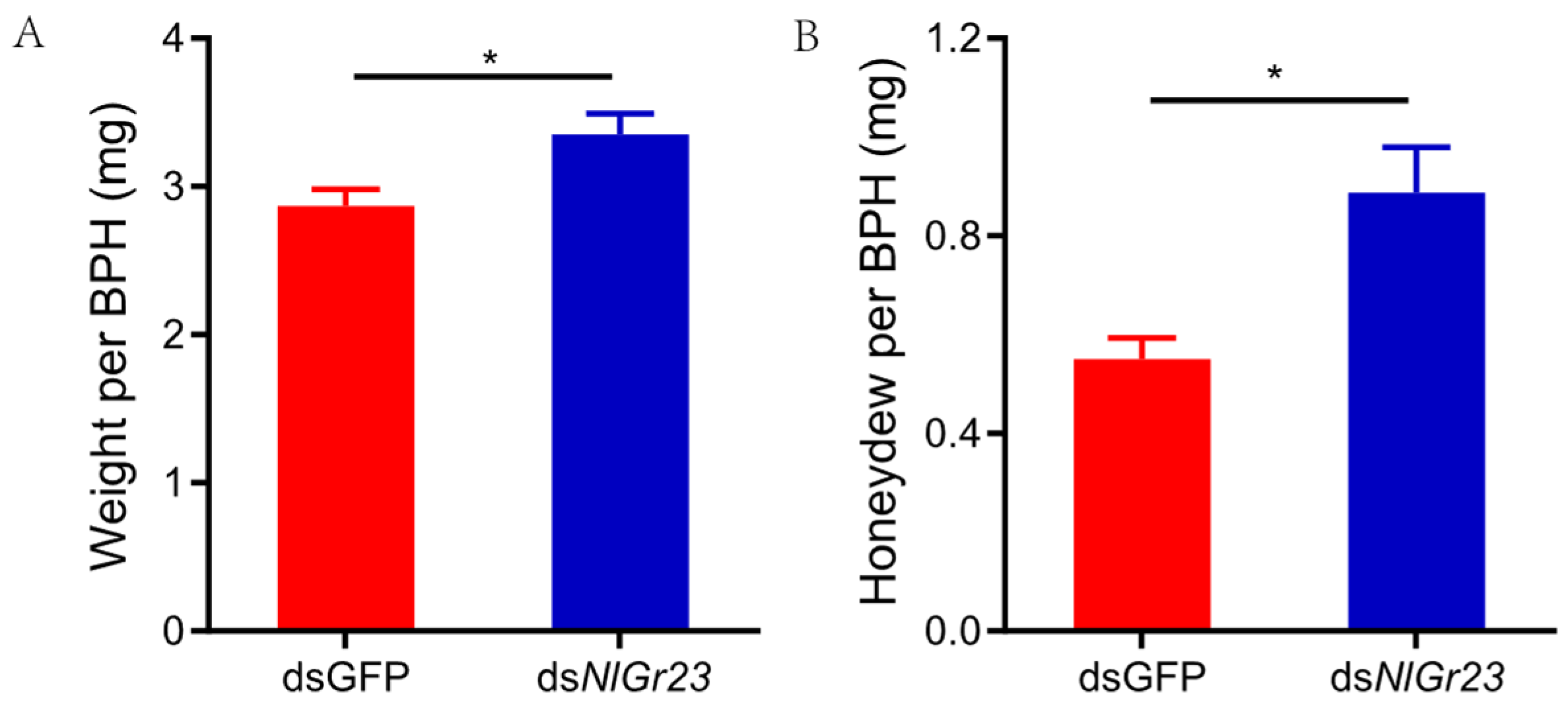
3.1. Effect of NlGr23 Knockdown on BPH Weight and Honeydew Production
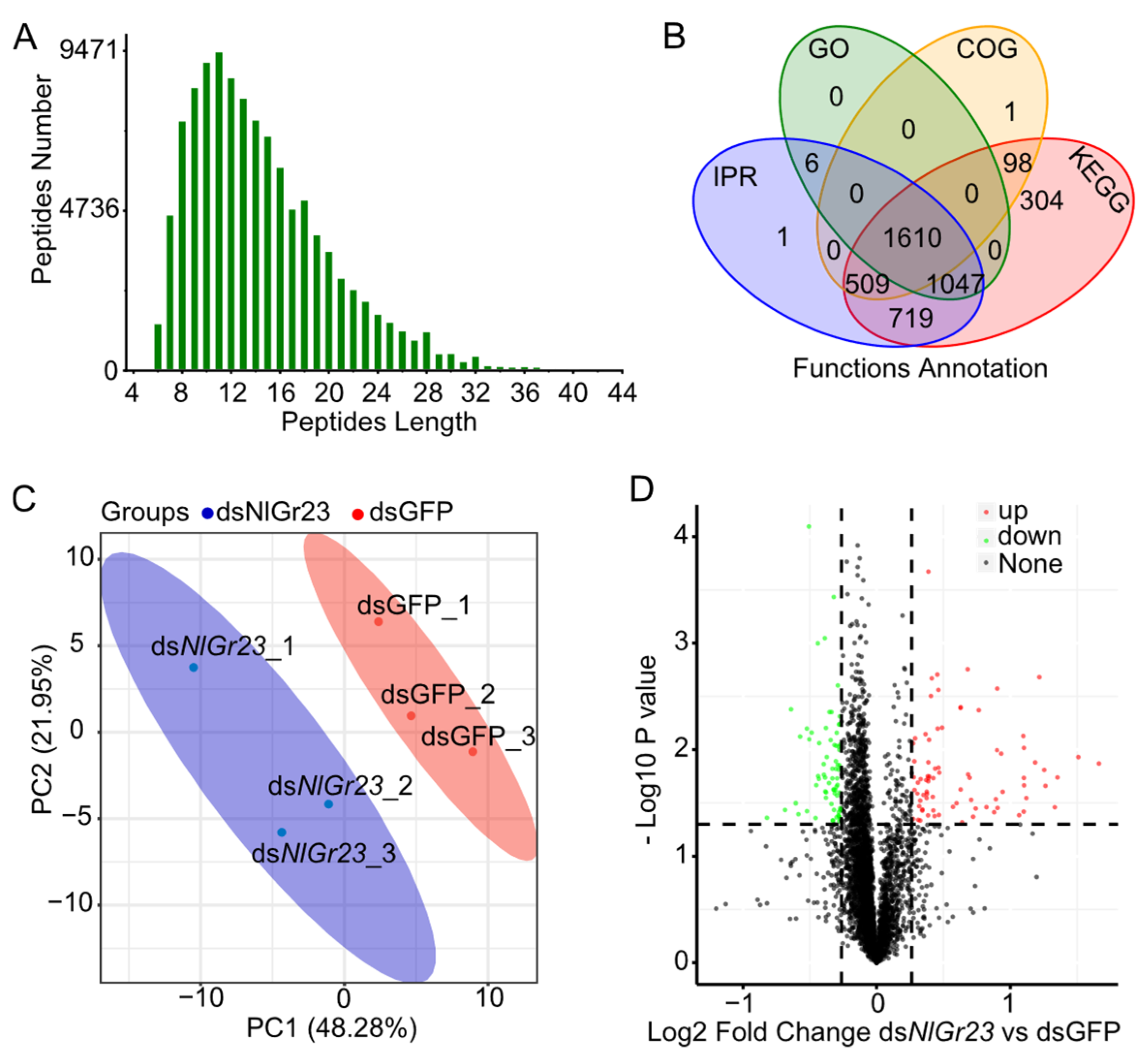
3.2. Transcriptome Analysis of BPHs After Injecting dsNlGr23
3.3. Proteomics Analysis of BPHs After Injecting dsNlGr23
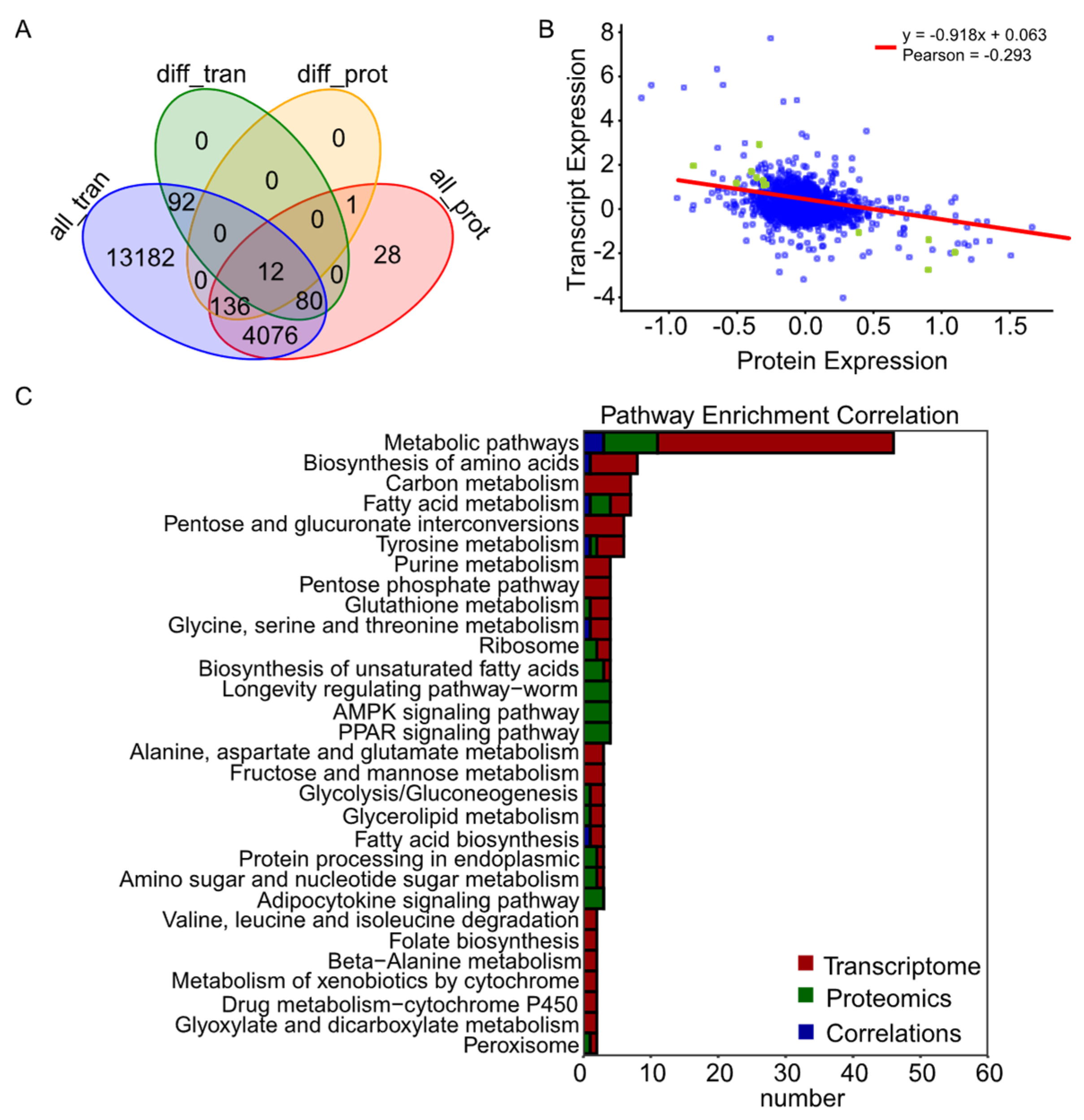
3.4. Proteomics and Transcriptome Coexpression Analyses
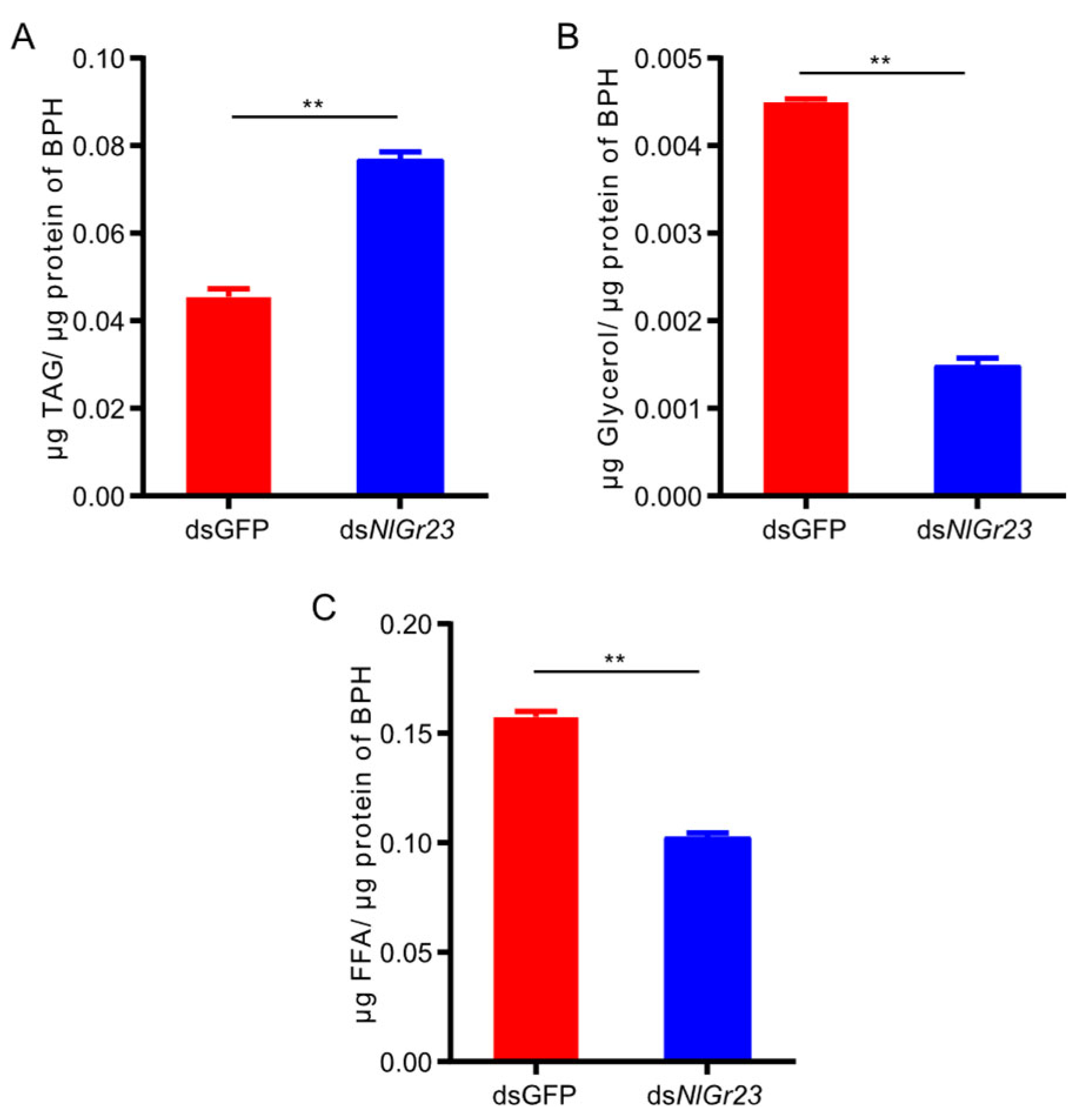
3.5. Effect of NlGr23 Silencing on Lipid Mobilization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPH | Brown planthopper |
| GRs | Gustatory receptors |
| RNAi | RNA interference |
| cDNA | Complementary DNA |
| FDR | False discovery rate |
| DEGS | Differentially expressed genes |
| DEPs | Differentially expressed proteins |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| FC | Fold change |
| qRT-PCR | Quantitative reverse-transcription PCR |
| TAG | Triacylglycerol |
| iRT | Indexed retention time |
| COG | Clusters of Orthologous Groups |
| IPR | Interpro |
References
- Chen, R.; Deng, Y.; Ding, Y.; Guo, J.; Qiu, J.; Wang, B.; Wang, C.; Xie, Y.; Zhang, Z.; Chen, J.; et al. Rice functional genomics: Decades’ efforts and roads ahead. Sci. China Life Sci. 2022, 65, 33–92. [Google Scholar] [CrossRef]
- Ling, Y.; Weilin, Z. Genetic and biochemical mechanisms of rice resistance to planthopper. Plant Cell Rep. 2016, 35, 1559–1572. [Google Scholar] [CrossRef]
- Robertson, H.M.; Warr, C.G.; Carlson, J.R. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2003, 2, 14537–14542. [Google Scholar] [CrossRef]
- Gomes, J.V.; Singh-Bhagania, S.; Cenci, M.; Chacon Cordon, C.; Singh, M.; Butterwick, J.A. The molecular basis of sugar detection by an insect taste receptor. Nature 2024, 629, 228–234. [Google Scholar] [CrossRef]
- Vosshall, L.B.; Stocker, R.F. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 2007, 30, 505–533. [Google Scholar] [CrossRef]
- Ojha, A.; Zhang, W. Characterization of gustatory receptor 7 in the brown planthopper reveals functional versatility. Insect Biochem. Mol. Biol. 2021, 132, 16. [Google Scholar] [CrossRef]
- Clyne, P.J.; Warr, C.G.; Carlson, J.R. Candidate taste receptors in Drosophila. Science 2000, 287, 1830–1834. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.H.; Montell, C. Avoiding DEET through insect gustatory receptors. Neuron 2010, 67, 555–561. [Google Scholar] [CrossRef]
- Rimal, S.; Lee, Y. Molecular sensor of nicotine in taste of Drosophila melanogaster. Insect Biochem. Mol. Biol. 2019, 111, 18. [Google Scholar] [CrossRef]
- Shim, J.; Lee, Y.; Jeong, Y.T.; Kim, Y.; Lee, M.G.; Montell, C.; Moon, S.J. The full repertoire of Drosophila gustatory receptors for detecting an aversive compound. Nat. Commun. 2015, 6, 8867. [Google Scholar] [CrossRef]
- Poudel, S.; Lee, Y. Gustatory Receptors Required for Avoiding the Toxic Compound Coumarin in Drosophila melanogaster. Mol. Cells 2016, 39, 310–315. [Google Scholar] [CrossRef]
- Lee, Y.; Moon, S.J.; Montell, C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc. Natl. Acad. Sci. USA 2009, 106, 4495–4500. [Google Scholar] [CrossRef]
- Kasubuchi, M.; Shii, F.; Tsuneto, K.; Yamagishi, T.; Adegawa, S.; Endo, H.; Sato, R. Insect taste receptors relevant to host identification by recognition of secondary metabolite patterns of non-host plants. Biochem. Biophys. Res. Commun. 2018, 499, 901–906. [Google Scholar] [CrossRef]
- Yang, J.; Guo, H.; Jiang, N.J.; Tang, R.; Li, G.C.; Huang, L.Q.; van Loon, J.J.A.; Wang, C.Z. Identification of a gustatory receptor tuned to sinigrin in the cabbage butterfly Pieris rapae. PLoS Genet. 2021, 17, e1009527. [Google Scholar] [CrossRef]
- Chen, W.W.; Kang, K.; Yang, P.; Zhang, W.Q. Identification of a sugar gustatory receptor and its effect on fecundity of the brown planthopper Nilaparvata lugens. Insect Sci. 2019, 26, 441–452. [Google Scholar] [CrossRef]
- Ojha, A.; Zhang, W. A comparative study of microbial community and dynamics of Asaia in the brown planthopper from susceptible and resistant rice varieties. BMC Microbiol. 2019, 19, 019–1512. [Google Scholar] [CrossRef]
- Yang, K.; Gong, X.L.; Li, G.C.; Huang, L.Q.; Ning, C.; Wang, C.Z. A gustatory receptor tuned to the steroid plant hormone brassinolide in Plutella xylostella (Lepidoptera: Plutellidae). Elife 2020, 11, 64114. [Google Scholar] [CrossRef]
- Ozaki, K.; Ryuda, M.; Yamada, A.; Utoguchi, A.; Ishimoto, H.; Calas, D.; Marion-Poll, F.; Tanimura, T.; Yoshikawa, H. A gustatory receptor involved in host plant recognition for oviposition of a swallowtail butterfly. Nat. Commun. 2011, 2, 542. [Google Scholar] [CrossRef]
- Chen, Y.D.; Dahanukar, A. Recent advances in the genetic basis of taste detection in Drosophila. Cell Mol. Life Sci. 2020, 77, 1087–1101. [Google Scholar] [CrossRef]
- Qiu, J.; He, Y.; Zhang, J.; Kang, K.; Li, T.; Zhang, W. Discovery and functional identification of fecundity-related genes in the brown planthopper by large-scale RNA interference. Insect Mol. Biol. 2016, 25, 724–733. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Zhao, X.T.; Sun, J.T.; Zou, L.F.; Yang, S.X.; Han, X.; Zhu, W.C.; Yin, Q.; Hong, X.Y. Transcriptome and proteome analyses reveal complex mechanisms of reproductive diapause in the two-spotted spider mite, Tetranychus urticae. Insect Mol. Biol. 2017, 26, 215–232. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Li, Y.Y.; An, T.; Huang, F.X.; Wang, M.Q.; Liu, C.X.; Mao, J.J.; Zhang, L.S. Comparative Transcriptome and iTRAQ Proteome Analyses Reveal the Mechanisms of Diapause in Aphidius gifuensis Ashmead (Hymenoptera: Aphidiidae). Front. Physiol. 2018, 9, 1697. [Google Scholar] [CrossRef]
- Liang, Y.; Ma, K.S.; Liang, P.Z.; Yang, L.W.; Zhang, L.; Gao, X.W. Combined Transcriptomic and Proteomic Analysis of Myzus persicae, the Green Peach Aphid, Infected with Cucumber Mosaic Virus. Insects 2021, 12, 372. [Google Scholar] [CrossRef]
- Govaere, L.; Morin, M.D.; Frigault, J.J.; Boquel, S.; Cohen, A.; Lamarre, S.G.; Morin, P.J. Transcriptome and proteome analyses to investigate the molecular underpinnings of cold response in the Colorado potato beetle, Leptinotarsa decemlineata. Cryobiology 2019, 88, 54–63. [Google Scholar] [CrossRef]
- Kang, K.; Zhang, M.; Yue, L.; Chen, W.; Dai, Y.; Lin, K.; Liu, K.; Lv, J.; Guan, Z.; Xiao, S.; et al. Oxalic Acid Inhibits Feeding Behavior of the Brown Planthopper via Binding to Gustatory Receptor Gr23a. Cells 2023, 12, 771. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Choi, M.; Chang, C.Y.; Clough, T.; Broudy, D.; Killeen, T.; MacLean, B.; Vitek, O. MSstats: An R package for statistical analysis of quantitative mass spectrometry-based proteomic experiments. Bioinformatics 2014, 30, 2524–2526. [Google Scholar] [CrossRef]
- Gao, Y.; Su, S.C.; Xing, J.Y.; Liu, Z.Y.; Nässel, D.R.; Bass, C.; Gao, C.F.; Wu, S.F. Pesticide-induced resurgence in brown planthopper is mediated by action on a suite of genes that promote juvenile hormone biosynthesis and female fecundity. ELife 2023, 12, RP91774. [Google Scholar]
- Lee, Y.; Moon, S.J.; Wang, Y.; Montell, C. A Drosophila Gustatory Receptor Required for Strychnine Sensation. Chem. Senses 2015, 40, 525–533. [Google Scholar] [CrossRef]
- Wada-Katsumata, A.; Silverman, J.; Schal, C. Changes in taste neurons support the emergence of an adaptive behavior in cockroaches. Science 2013, 340, 972–975. [Google Scholar] [CrossRef]
- Freeman, E.G.; Dahanukar, A. Molecular neurobiology of Drosophila taste. Curr. Opin. Neurobiol. 2015, 34, 140–148. [Google Scholar] [CrossRef]
- Endo, H.; Tsuneto, K.; Mang, D.; Zhang, W.J.; Yamagishi, T.; Ito, K.; Nagata, S.; Sato, R. Two gustatory receptors involved in host plant recognition of silkworm larvae. J. Insect Physiol. 2024, 154, 104628. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, K.; Zhang, J.; Fang, R.; Lü, J. Metabolic and Behavioral Impacts of Gustatory Receptor NlGr23 Silencing in the Brown Planthopper. Agronomy 2025, 15, 1797. https://doi.org/10.3390/agronomy15081797
Kang K, Zhang J, Fang R, Lü J. Metabolic and Behavioral Impacts of Gustatory Receptor NlGr23 Silencing in the Brown Planthopper. Agronomy. 2025; 15(8):1797. https://doi.org/10.3390/agronomy15081797
Chicago/Turabian StyleKang, Kui, Jie Zhang, Renhan Fang, and Jun Lü. 2025. "Metabolic and Behavioral Impacts of Gustatory Receptor NlGr23 Silencing in the Brown Planthopper" Agronomy 15, no. 8: 1797. https://doi.org/10.3390/agronomy15081797
APA StyleKang, K., Zhang, J., Fang, R., & Lü, J. (2025). Metabolic and Behavioral Impacts of Gustatory Receptor NlGr23 Silencing in the Brown Planthopper. Agronomy, 15(8), 1797. https://doi.org/10.3390/agronomy15081797





