Potato Productivity Response to Potassium Fertilizer Source and Rate in Oregon’s Columbia Basin
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Growing Conditions
2.2. Experimental Design
2.3. Statistical Analysis
3. Results
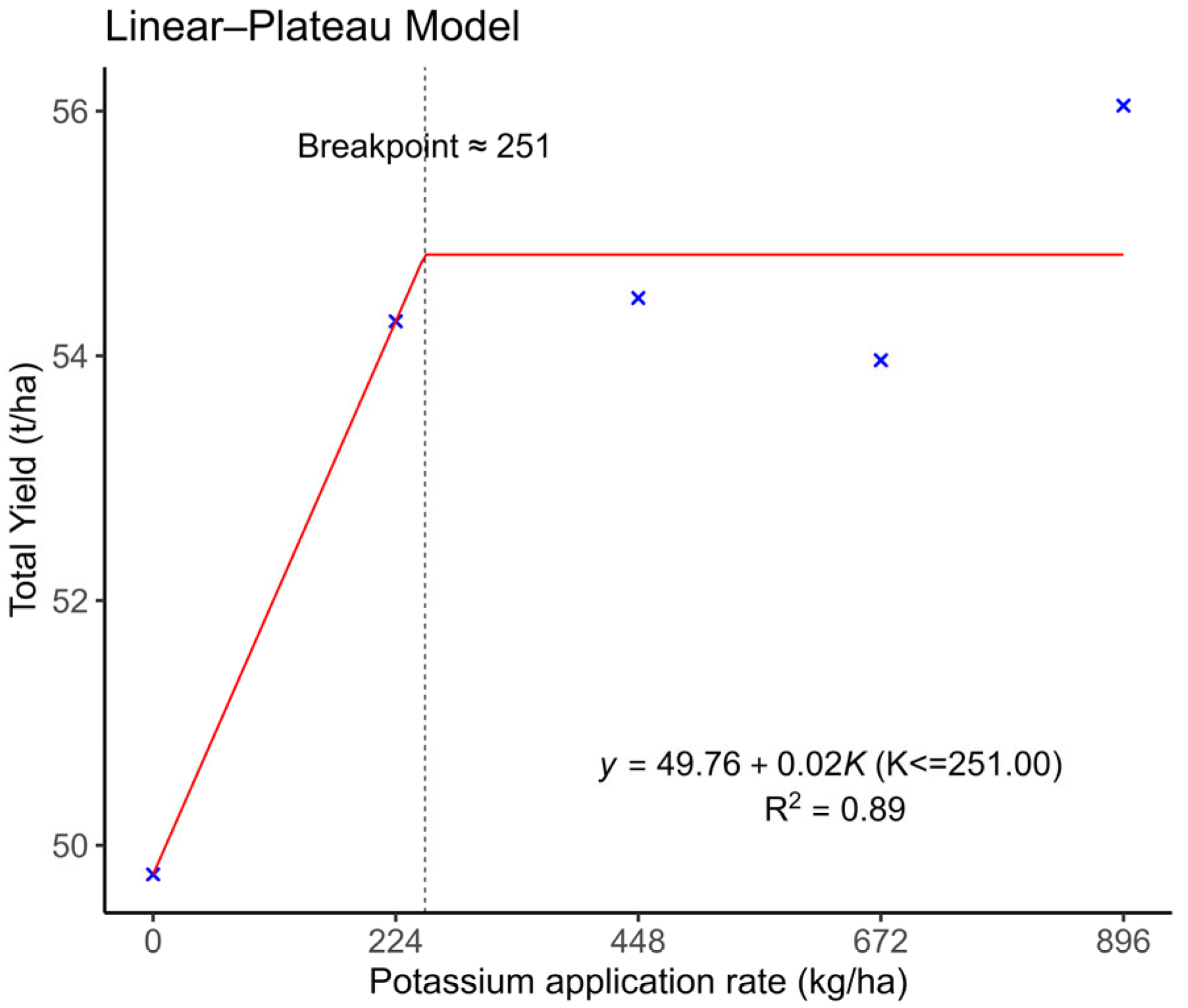
3.1. Total Tuber Yield
3.2. US No. 1 Tubers
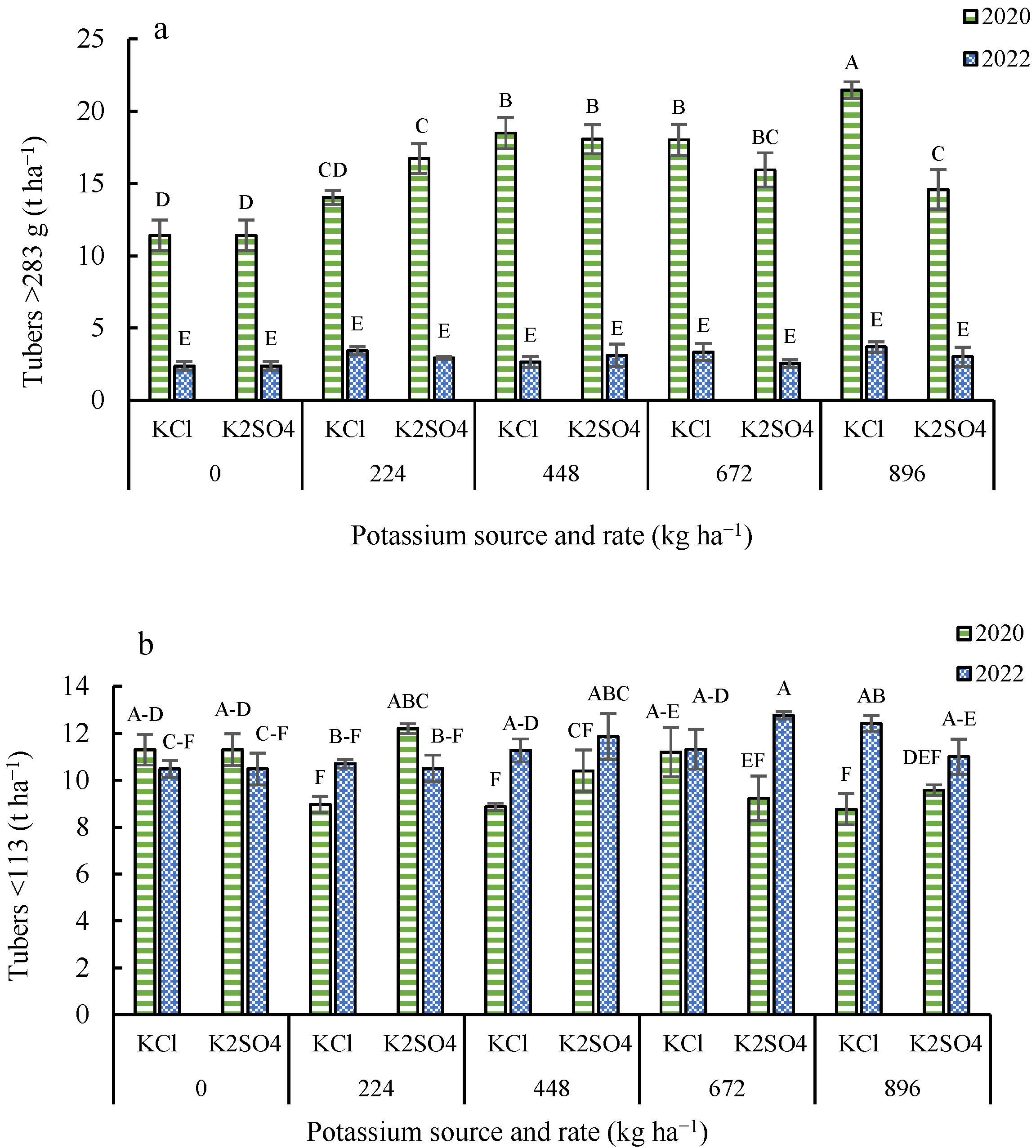
3.3. Tuber Yield by Size Distribution
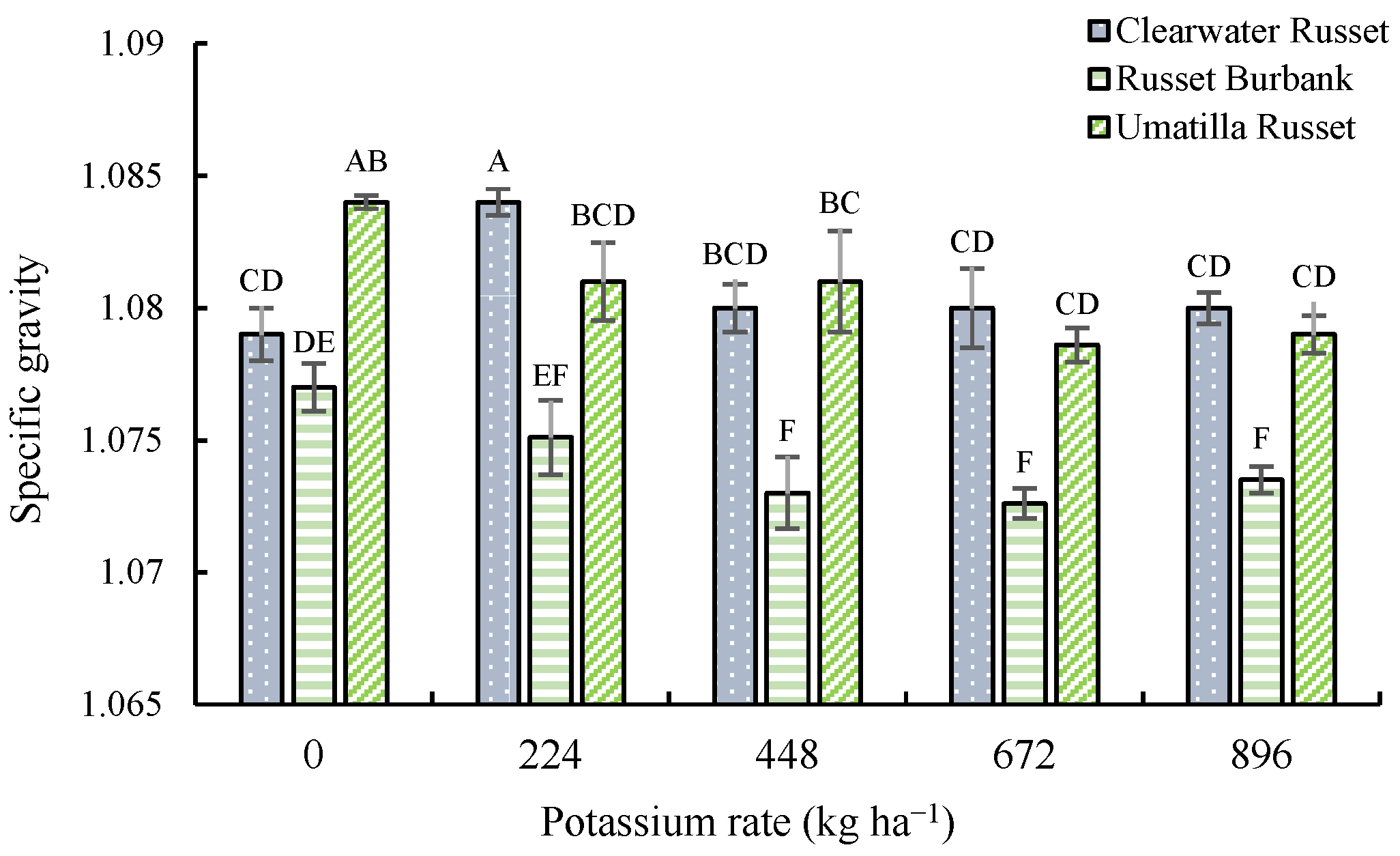
3.4. Specific Gravity and French Fry Color
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| KCl | potassium chloride |
| K2SO4 | potassium sulfate |
| MAP | monoammonium phosphate |
| UAN | urea ammonium nitrate |
References
- Lutaladio, N.; Castaldi, L. Potato: The hidden treasure. J. Food Compos. Anal. 2009, 22, 491–493. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012. [Google Scholar]
- Sardans, J.; Peñuelas, J. Potassium stoichiometry and global change. Glob. Ecol. Biogeogr. 2015, 24, 261–275. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef]
- Gao, Y.; Tang, Z.; Xia, H.; Sheng, M.; Liu, M.; Pan, S.; Li, Z.; Liu, J. Potassium fertilization stimulates sucrose-to-starch conversion and root formation in sweet potato (Ipomoea batatas (L.) Lam.). Int. J. Mol. Sci. 2021, 22, 4826. [Google Scholar] [CrossRef]
- Wang, Y.; Snodgrass, L.B.; Bethke, P.C.; Bussan, A.J.; Holm, D.G.; Novy, R.G.; Pavek, M.J.; Porter, G.A.; Rosen, C.J.; Sathuvalli, V.; et al. Reliability of measurement and genotype × environment interaction for potato specific gravity. Crop Sci. 2017, 57, 1966–1972. [Google Scholar] [CrossRef]
- Kolahchi, Z.; Jalali, M. Effect of water quality on the leaching of potassium from sandy soil. J. Arid Environ. 2007, 68, 624–639. [Google Scholar] [CrossRef]
- Torabian, S.; Farhangi-Abriz, S.; Qin, R.; Noulas, C.; Sathuvalli, V.; Charlton, B.; Loka, D.A. Potassium: A vital macronutrient in potato production—A review. Agronomy 2021, 11, 543. [Google Scholar] [CrossRef]
- Lang, N.S.; Stevens, R.G.; Thornton, R.E.; Victory, S.; Pan, W.L. Potato Nutrient Management for Central Washington; Washington State University Cooperative Extension: Pullman, WA, USA, 1999. [Google Scholar]
- Mikkelsen, R.L.; Roberts, T.L. Inputs: Potassium Sources for Agricultural Systems. In Improving Potassium Recommendations for Agricultural Crops; Springer: Cham, Switzerland, 2021; pp. 47–74. [Google Scholar]
- Prakash, S.; Verma, J.P. Global Perspective of Potash for Fertilizer Production. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: Singapore, 2016; pp. 327–331. [Google Scholar]
- Koch, M.; Naumann, M.; Thiel, H.; Gransee, A.; Pawelzik, E. The importance of nutrient management for potato production. Part II: Plant nutrition and tuber quality. Potato Res. 2020, 63, 121–137. [Google Scholar] [CrossRef]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture—Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Stark, J.C.; Westermann, D.T.; Hopkins, B. Nutrient Management Guidelines for Russet Burbank Potatoes; College of Agricultural and Life Sciences, University of Idaho: Moscow, ID, USA, 2004. [Google Scholar]
- Miller, R.O.; Gavlak, R.; Horneck, D. Soil, Plant and Water Reference Methods for the Western Region, 4th ed.; WREP 125; Western Region Extension Publication; Colorado State University: Fort Collins, CO, USA, 2013. [Google Scholar]
- Chen, C.T.; Setter, T.L. Role of tuber developmental processes in response of potato to high temperature and elevated CO2. Plants 2021, 10, 871. [Google Scholar] [CrossRef]
- Ávila-Valdés, A.; Quinet, M.; Lutts, S.; Martínez, J.P.; Lizana, X.C. Tuber yield and quality responses of potato to moderate temperature increase during tuber bulking under two water availability scenarios. Field Crops Res. 2020, 251, 107786. [Google Scholar] [CrossRef]
- Lal, M.K.; Tiwari, R.K.; Kumar, A.; Dey, A.; Kumar, R.; Kumar, D.; Jaiswal, A.; Changan, S.S.; Raigond, P.; Dutt, S.; et al. Mechanistic concept of physiological, biochemical, and molecular responses of the potato crop to heat and drought stress. Plants 2022, 11, 2857. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.U.; Lee, B.W. Differential mechanisms of potato yield loss induced by high day and night temperatures during tuber initiation and bulking: Photosynthesis and tuber growth. Front. Plant Sci. 2019, 10, 300. [Google Scholar] [CrossRef] [PubMed]
- Davenport, J.R.; Bentley, E.M. Does potassium fertilizer form, source, and time of application influence potato yield and quality in the Columbia Basin? Am. J. Potato Res. 2001, 78, 311–318. [Google Scholar] [CrossRef]
- Mohr, R.M.; Tomasiewicz, D.J. Effect of rate and timing of potassium chloride application on the yield and quality of potato (Solanum tuberosum L. ‘Russet Burbank’). Can. J. Plant Sci. 2012, 92, 783–794. [Google Scholar] [CrossRef]
- Panique, E.; Kelling, K.A.; Schulte, E.E.; Hero, D.E.; Stevenson, W.R.; James, R.V. Potassium rate and source effects on potato yield, quality, and disease interaction. Am. Potato J. 1997, 74, 379–398. [Google Scholar] [CrossRef]
- Wilmer, L.; Pawelzik, E.; Naumann, M. Comparison of the effects of potassium sulphate and potassium chloride fertilisation on quality parameters, including volatile compounds, of potato tubers after harvest and storage. Front. Plant Sci. 2022, 13, 920212. [Google Scholar] [CrossRef]
- Perrenoud, S. Fertilizing for High Yield of Potato. IPI Bulletin 8; International Potash Institute: Berne, Switzerland, 1993. [Google Scholar]
- Koch, M.T.; Pawelzik, E.; Kautz, T. Chloride changes soil–plant water relations in potato (Solanum tuberosum L.). Agronomy 2021, 11, 736. [Google Scholar] [CrossRef]
- Colmenero-Flores, J.M.; Franco-Navarro, J.D.; Cubero-Font, P.; Peinado-Torrubia, P.; Rosales, M.A. Chloride as a beneficial macronutrient in higher plants: New roles and regulation. Int. J. Mol. Sci. 2019, 20, 4686. [Google Scholar] [CrossRef]
- Oliveira, R.C.; Luz, J.M.Q.; Lana, R.M.Q.; Melo, C.M.T.; Campo, D.R.O. Sources of potassic fertilizers in the quality of potato tubers. Rev. Latinoam. Papa 2020, 24, 3–15. [Google Scholar]
- Wang, Y.; Liu, X.; Wang, L.; Li, H.; Zhang, S.; Yang, J.; Liu, N.; Han, X. Effects of long-term application of Cl-containing fertilizers on chloride content and acidification in brown soil. Sustainability 2023, 15, 8801. [Google Scholar] [CrossRef]
- Pereira, D.G.C.; Santana, I.A.; Megda, M.M.; Megda, M.X.V. Potassium chloride: Impacts on soil microbial activity and nitrogen mineralization. Ciênc. Rural 2018. [Google Scholar] [CrossRef]
- Silva, H.R.F.; Fontes, P.C.R. Potassium fertilization and its residual effect on productivity and quality of potato tubers. Pesqui. Agropecu. Bras. 2016, 51, 842–848. [Google Scholar] [CrossRef]
- Chourasia, K.N.; Lal, M.K.; Tiwari, R.K.; Dev, D.; Kardile, H.B.; Patil, V.U.; Kumar, A.; Vanishree, G.; Kumar, D.; Bhardwaj, V.; et al. Salinity stress in potato: Understanding physiological, biochemical and molecular responses. Life 2021, 11, 545. [Google Scholar] [CrossRef]
- Sun, N.; Rosen, C.J.; Thompson, A.L. Nitrogen response of French fry and chip cultivars selected for low tuber reducing sugars. Am. J. Potato Res. 2017, 94, 606–616. [Google Scholar] [CrossRef]
- Blauer, J.M.; Sathuvalli, V.; Charlton, B.A.; Yilma, S.; Shock, C.C.; Baley, N.; Qin, R.; Feibert, E.; Novy, R.G.; Whitworth, J.L.; et al. Rainier Russet: A dual use Russet potato with long tuber dormancy, excellent process quality, and high early harvest packaging efficiency. Am. J. Potato Res. 2024, 101, 17–33. [Google Scholar] [CrossRef]
- Amrein, T.M.; Bachmann, S.; Noti, A.; Biedermann, M.; Barbosa, M.F.; Biedermann-Brem, S.; Grob, K.; Keiser, A.; Realini, P.; Escher, F.; et al. Potential of acrylamide formation, sugars, and free asparagine in potatoes: A comparison of cultivars and farming systems. J. Agric. Food Chem. 2003, 51, 5556–5560. [Google Scholar] [CrossRef]
- De Wilde, T.; De Meulenaer, B.; Mestdagh, F.; Govaert, Y.; Vandeburie, S.; Ooghe, W.; Fraselle, S.; Demeulemeester, K.; Van Peteghem, C.; Calus, A.; et al. Influence of storage practices on acrylamide formation during potato frying. J. Agric. Food Chem. 2005, 53, 6550–6557. [Google Scholar] [CrossRef]
- Thornton, M.; Buhrig, W.; Olsen, N. The relationship between soil temperature and sugar ends in potato. Potato Res. 2010, 53, 289–296. [Google Scholar] [CrossRef]
- Timm, H.; Yamaguchi, M.; Clegg, M.D.; Bishop, J.C. Influence of high-temperature exposure on sugar content and chipping quality of potatoes. Am. Potato J. 1968, 45, 359–365. [Google Scholar] [CrossRef]
- Gause, K. Effect of Nitrogen and Potassium on Potato Yield, Quality and Acrylamide Forming Potential. Master’s Thesis, University of Maine, Orono, ME, USA, 2014. [Google Scholar]
- Gerendás, J.; Heuser, F.; Sattelmacher, B. Influence of nitrogen and potassium supply on contents of acrylamide precursors in potato tubers and on acrylamide accumulation in French fries. J. Plant Nutr. 2007, 30, 1499–1516. [Google Scholar] [CrossRef]


| Organic Matter | NO3-N | NH4-N | P | K | S | pH | |
|---|---|---|---|---|---|---|---|
| (%) | (kg ha−1) | (kg ha−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | ||
| 2020 | 0.85 | 50 | 18 | 65 | 119 | 35 | 6.6 |
| 2022 | 0.79 | 70 | 16 | 22 | 120 | 6.9 | 6.6 |
| 2020 | 2022 | |||||
|---|---|---|---|---|---|---|
| Month | Max. Air Temp. (°C) | Min. Air Temp. (°C) | Rainfall (mm) | Max. Air Temp. (°C) | Min. Air Temp. (°C) | Rainfall (mm) |
| May | 23 | 8.5 | 23.8 | 20 | 7.2 | 27.9 |
| June | 26 | 12 | 11.9 | 26 | 11 | 39.1 |
| July | 32 | 14 | 0 | 34 | 15 | 0 |
| August | 32 | 14 | 0.2 | 35 | 15 | 0 |
| September | 27 | 10 | 2.5 | 28 | 11 | 6.8 |
| N Fertilizers | P Fertilizers | |||
|---|---|---|---|---|
| Fertilizer type | Urea (46-0-0) | Urea Ammonium Nitrate (32-0-0), Ammonium thiosulfate (12-0-0-26) | Monoammonium phosphate (11-52-0) | P Liquid |
| kg N ha−1 | kg P2O5 ha−1 | |||
| 2020 | 125 | 380 | 200 | 75 |
| 2022 | 132 | 275 | 122 | 0 |
| Main Effect | Size Category | US No. 1 | Total Yield | Specific Gravity | Fry Color | ||||
|---|---|---|---|---|---|---|---|---|---|
| <113 g | 113–170 g | 171–283 g | >283 g | Culls | |||||
| Year | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 |
| Cultivar | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 | <0.01 | <0.01 | <0.01 | <0.01 |
| Source | 0.20 | 0.16 | 0.86 | 0.05 | 0.23 | 0.79 | 0.89 | 0.14 | 0.97 |
| Rate | 0.66 | 0.22 | 0.03 | <0.01 | 0.41 | <0.01 | <0.01 | <0.01 | 0.05 |
| year * rate | <0.01 | 0.88 | 0.75 | <0.01 | 0.69 | 0.35 | 0.50 | 0.07 | <0.01 |
| year * cultivar | 0.16 | 0.29 | 0.42 | 0.21 | 0.08 | 0.13 | 0.11 | 0.10 | 0.27 |
| year * source | 0.31 | 0.24 | 0.74 | 0.25 | 0.11 | 0.78 | 0.99 | 0.22 | 0.79 |
| cultivar * rate | 0.57 | 0.96 | 0.72 | 0.40 | 0.43 | 0.93 | 0.88 | 0.03 | 0.10 |
| cultivar * source | 0.07 | 0.81 | 0.37 | 0.90 | 0.22 | 0.37 | 0.14 | 0.17 | 0.58 |
| source * rate | 0.24 | 0.11 | 0.69 | 0.01 | 0.73 | 0.94 | 0.76 | 0.68 | 0.17 |
| year * cultivar * rate | 0.23 | 0.82 | 0.83 | 0.92 | 0.30 | 0.80 | 0.61 | 0.47 | 0.29 |
| year * cultivar * source | 0.51 | 0.43 | 0.12 | 0.87 | 0.65 | 0.41 | 0.23 | 0.53 | 0.55 |
| year * source *rate | 0.01 | 0.36 | 0.45 | 0.03 | 0.12 | 0.09 | 0.02 | 0.66 | 0.34 |
| cultivar * source * rate | 0.54 | 0.67 | 0.91 | 0.67 | 0.94 | 0.95 | 0.96 | 0.67 | 0.66 |
| year * cultivar * source * rate | 0.57 | 0.94 | 0.76 | 0.70 | 0.28 | 0.59 | 0.87 | 0.73 | 0.09 |
| -------------------------- (t ha−1) ----------------------- | - | % | |||||||
| Year | |||||||||
| 2020 | 10.2 B | 14.2 A | 22.3 A | 15.5 A | 0.30 B | 52.1 A | 65.2 A | 1.086 A | 44.3 A |
| 2022 | 11.2 A | 11.6 B | 14.1 B | 2.8 B | 1.30 A | 30.0 B | 42.8 B | 1.070 B | 43.1 B |
| Cultivar | |||||||||
| Clearwater Russet | 8.69 B | 10.8 C | 18.1 A | 14.1 A | 0.60 B | 43.1 A | 53.3 B | 1.080 A | 45.5 A |
| Russet Burbank | 11.8 A | 14.3 B | 16.5 B | 6.4 B | 0.90 AB | 37.3 B | 51.0 C | 1.074 B | 39.4 B |
| Umatilla Russet | 11.7 A | 15.5 A | 19.6 A | 6.6 B | 1.0 A | 41.7 A | 56.5 A | 1.081 A | 46.1 A |
| K Source | |||||||||
| KCl | 10.5 | 13.2 | 18.1 | 9.4 A | 0.80 | 41.0 | 53.7 | 1.078 | 43.7 |
| K2SO4 | 11.0 | 13.9 | 18.0 | 8.5 B | 0.90 | 40.4 | 53.5 | 1.079 | 43.7 |
| K rate (kg ha−1) | |||||||||
| 0 | 10.8 | 13.6 | 16.7 B | 6.8 C | 0.70 | 37.1 C | 49.7 B | 1.080 A | 44.6 A |
| 224 | 10.6 | 14.0 | 18.8 A | 8.8 B | 0.70 | 41.7 AB | 54.2 A | 1.079 AB | 42.8 B |
| 448 | 10.6 | 13.2 | 17.4 AB | 10.4 A | 0.90 | 41.1 AB | 54.4 A | 1.078 BC | 42.8 B |
| 672 | 11.2 | 12.8 | 18.4 AB | 9.2 B | 1.0 | 40.3 B | 53.9 A | 1.076 D | 44.1 AB |
| 896 | 10.6 | 14.2 | 19.2 A | 9.9 AB | 0.80 | 43.4 A | 56.0 A | 1.077 CD | 44.1 AB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torabian, S.; Pieper, J.; Qin, R.; Noulas, C.; Sathuvalli, V.; Charlton, B.; Dara, S.K.; Spear, R. Potato Productivity Response to Potassium Fertilizer Source and Rate in Oregon’s Columbia Basin. Agronomy 2025, 15, 1795. https://doi.org/10.3390/agronomy15081795
Torabian S, Pieper J, Qin R, Noulas C, Sathuvalli V, Charlton B, Dara SK, Spear R. Potato Productivity Response to Potassium Fertilizer Source and Rate in Oregon’s Columbia Basin. Agronomy. 2025; 15(8):1795. https://doi.org/10.3390/agronomy15081795
Chicago/Turabian StyleTorabian, Shahram, Jack Pieper, Ruijun Qin, Christos Noulas, Vidyasagar Sathuvalli, Brian Charlton, Surendra K. Dara, and Rhett Spear. 2025. "Potato Productivity Response to Potassium Fertilizer Source and Rate in Oregon’s Columbia Basin" Agronomy 15, no. 8: 1795. https://doi.org/10.3390/agronomy15081795
APA StyleTorabian, S., Pieper, J., Qin, R., Noulas, C., Sathuvalli, V., Charlton, B., Dara, S. K., & Spear, R. (2025). Potato Productivity Response to Potassium Fertilizer Source and Rate in Oregon’s Columbia Basin. Agronomy, 15(8), 1795. https://doi.org/10.3390/agronomy15081795





_Qin.png)



