Insecticidal Effect of Lemongrass Essential Oil Against Megalurothrips usitatus (Bagnall)
Abstract
1. Introduction
2. Materials and Methods
2.1. Gas Chromatography Mass Spectrometry (GC-MS) Analysis
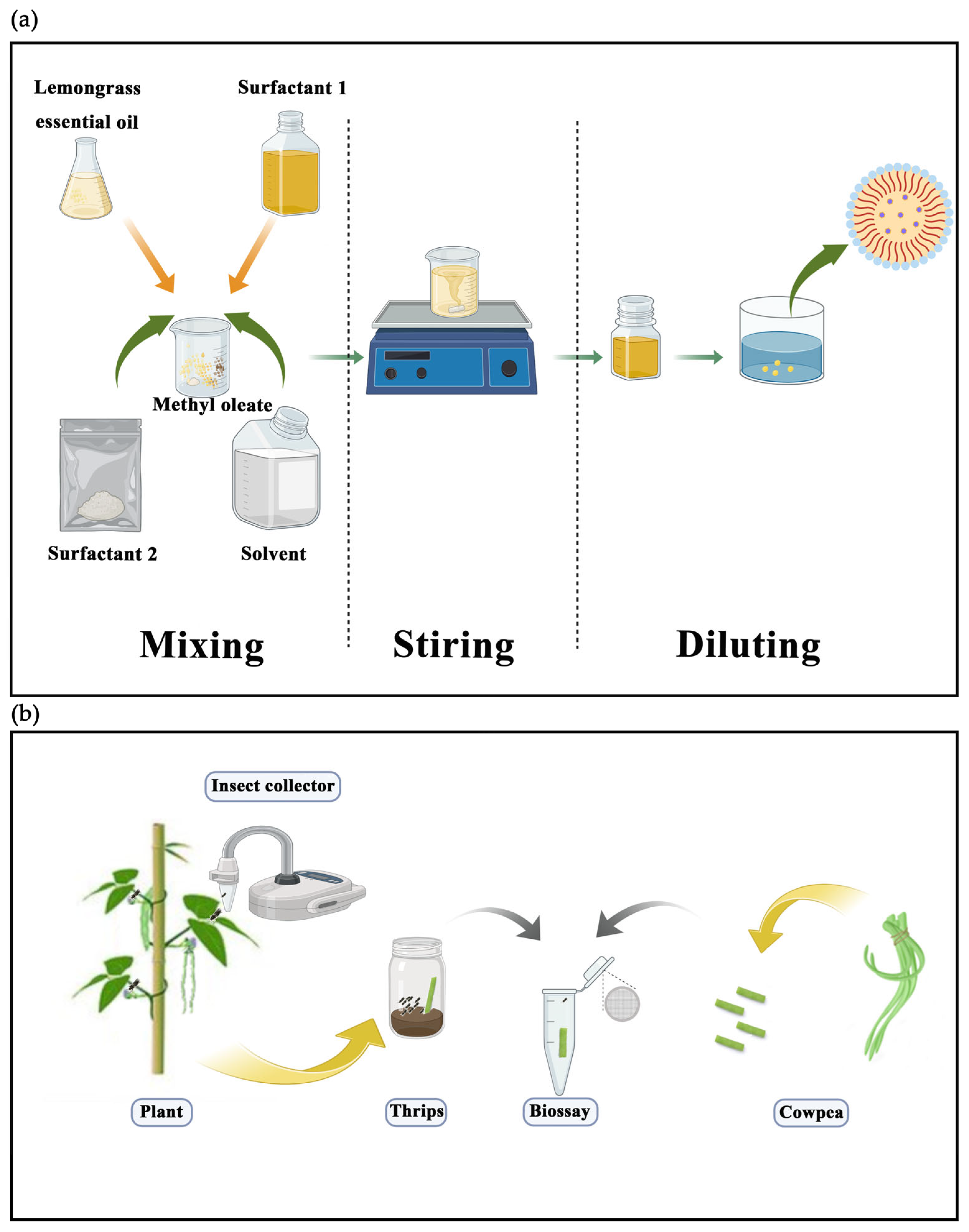
2.2. Preparation of Emulsifiable Concentration (EC)
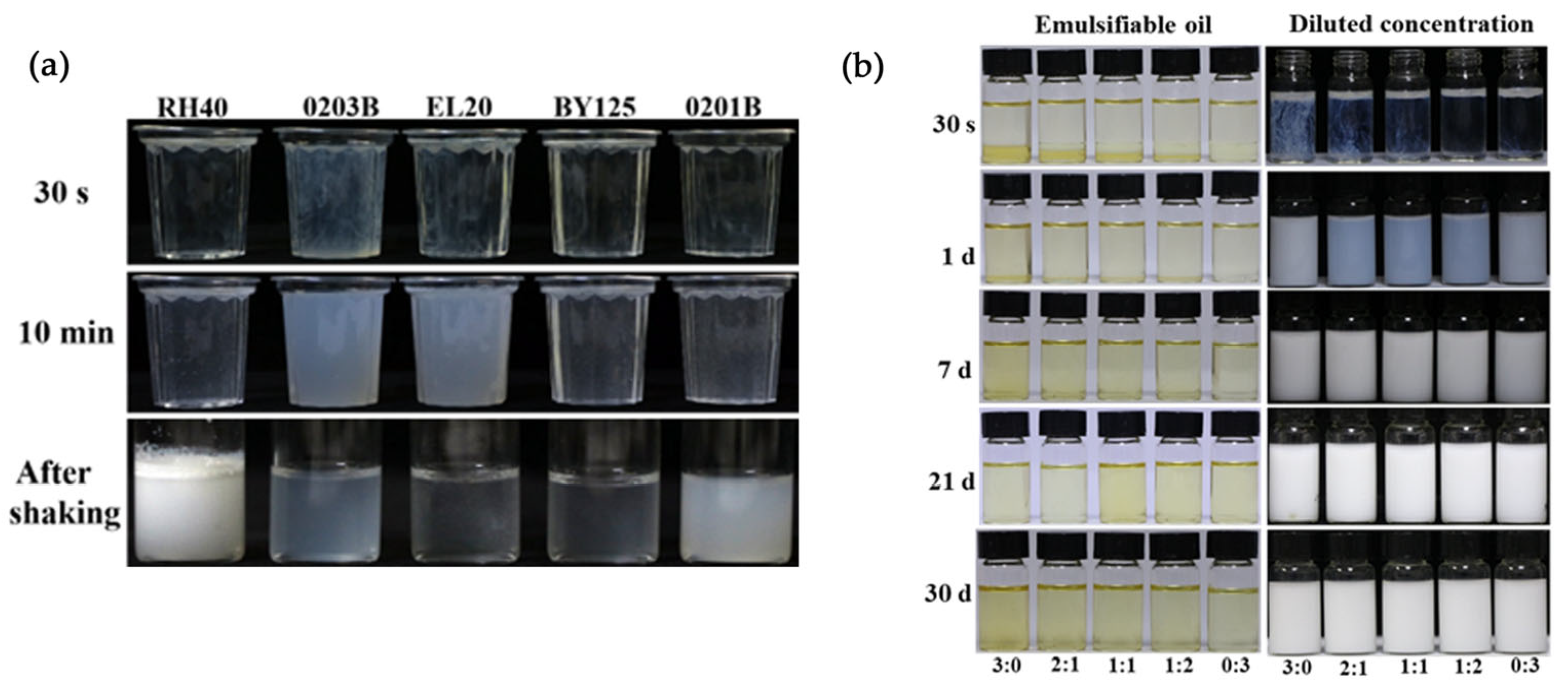
2.3. Characterization of EC
2.4. Thrips Rearing
2.5. Measurement of Insecticidal Activity
2.6. Data Analysis
3. Results
3.1. Chemical Composition of LEO
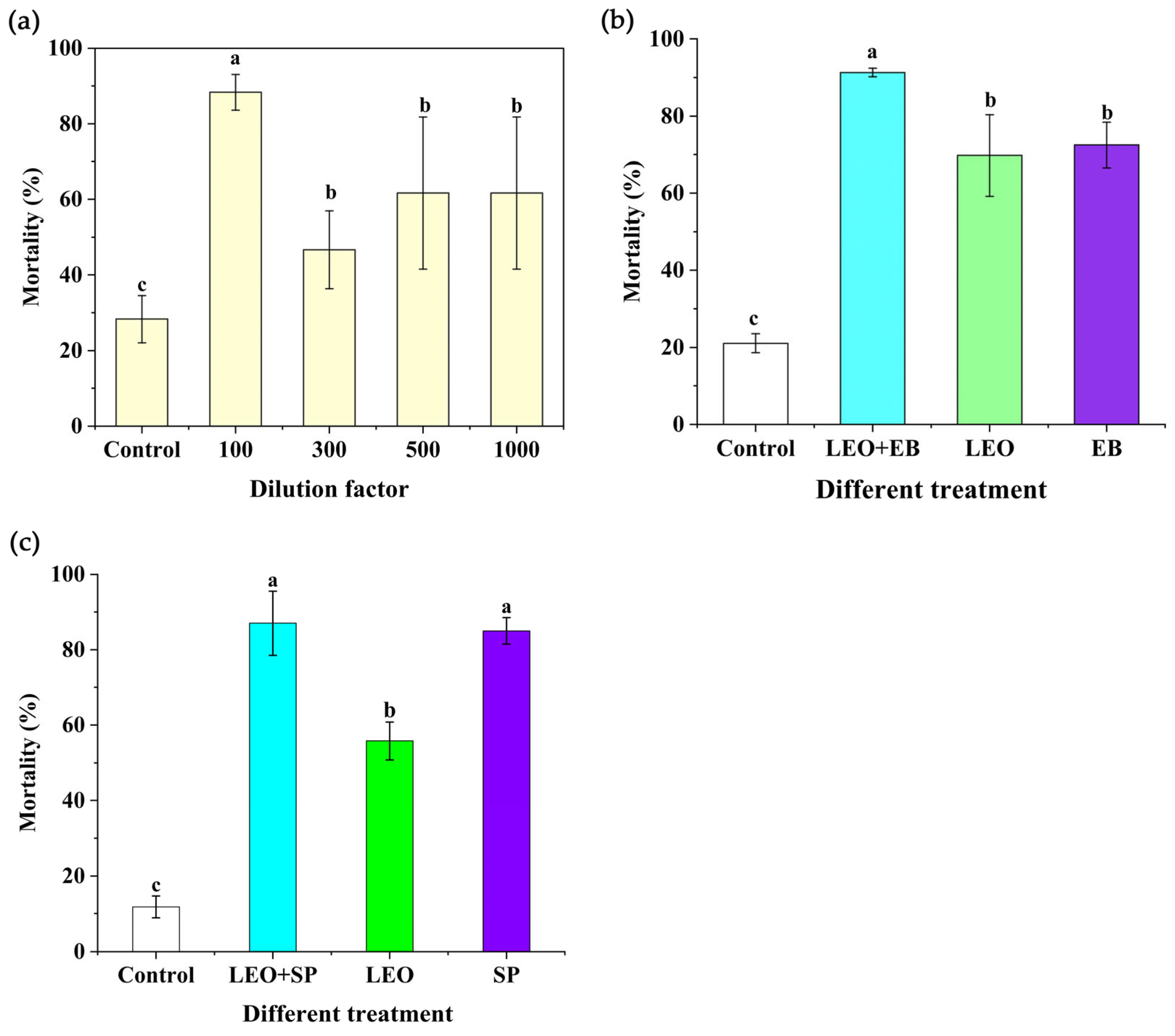
3.2. Insecticidal Activity of LEO and Its Compounds
3.3. Spreadability of EC
3.4. Insecticidal Activity
4. Discussion
4.1. Research on C. citratus
4.2. Analysis of Major Components of LEO
4.3. Insecticidal Activity of LEO ECs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xia, G.; Yang, L.; Li, B.L.; Wang, Q.L.; Huang, L.F.; Tian, X.L.; Zhang, G.H. Genome-wide identification and expression profiling of odorant-binding protein genes in the bean flower thrips Megalurothrips usitatus (Bagnall) (Thysanoptera: Thripidae). Insects 2025, 16, 212. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, S.; Ali, M.; Rahman, M.M.; Akter, M.S.; Latif, M.A. Biological traits of bean flower thrips, Megalurothrips usitatus (Thysanoptera: Thripidae) reared on mung bean. Sci. Herit. J. 2021, 5, 29–33. [Google Scholar] [CrossRef]
- Chi, Y.; Yu, C.; Feng, M.; Zhou, Y.; Li, X.; Zhang, Q. Effects of field releases of Neoseiulus barkeri on Megalurothrips usitatus abundance and arthropod diversity. Sci. Rep. 2024, 14, 14247. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Qin, Z.; Feng, M.; Zhang, L.; Huang, X.; Shi, W. The male-produced aggregation pheromone of the bean flower thrips Megalurothrips usitatus in China: Identification and attraction of conspecifics in the laboratory and field. Pest Manag. Sci. 2020, 76, 2986–2993. [Google Scholar] [CrossRef]
- He, Y.C.; Gao, Y.; Hong, H.N.; Geng, J.M.; Chen, Q.L.; Zhou, Y.; Zhu, Z.R.; Casas, J.L. Megalurothrips usitatus directly causes the black-heads and black-tail symptoms of cowpea along with the production of insect-resistance flavonoids. Plants 2023, 12, 3865. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, L.; Zheng, X.; Gong, R.; Cao, X.; Yang, L. An age-stage, two-sex life table for Megalurothrips usitatus feeding on eight different crop plants. Agronomy 2024, 14, 2283. [Google Scholar] [CrossRef]
- Chen, W.Y.; Li, Z.Y.; Zhou, C.Y.; Ali, A.; Ali, S.; Wu, J.H. RNA interference in cytochrome P450 monooxygenase (CYP) gene results in reduced insecticide resistance in Megalurothrips usitatus Bagnall. Front. Physiol. 2023, 14, 1130389. [Google Scholar] [CrossRef]
- Fu, B.L.; Tao, M.; Xue, H.; Jin, H.F.; Liu, K.; Qiu, H.Y.; Yang, S.Y.; Yang, X.; Gui, L.Y.; Zhang, Y.J.; et al. Spinetoram resistance drives interspecific competition between Megalurothrips usitatus and Frankliniella intonsa. Pest Manag. Sci. 2022, 78, 2129–2140. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.W.; Dong, L.L.; Chen, S.L. Study on botanical pesticides and its application in production of traditional Chinese medicine. China J. Chin. Mater. Med. 2016, 41, 3579–3586. [Google Scholar]
- Hernández-Moreno, D.; Soffers, A.; Wiratno; Falke, H.E.; Rietjens, I.; Murk, A.J. Consumer and farmer safety evaluation of application of botanical pesticides in black pepper crop protection. Food Chem. Toxicol. 2013, 56, 483–490. [Google Scholar] [CrossRef]
- Gostin, I.N.; Popescu, I.E. Evaluation of the essential oils used in the production of biopesticides: Assessing their toxicity toward both arthropod target species and beneficial pollinators. Agriculture 2024, 14, 81. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Kunnath, K.; Alfarhan, A.; Rajagopal, R.; Ramesh, V. Chemical composition of Cinnamomum verum leaf and flower essential oils and analysis of their antibacterial, insecticidal, and larvicidal properties. Molecules 2021, 26, 6303. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Han, H.; Xie, Y.; Li, B.; Zhang, Z.; Zhang, D. Toxicity, behavioral effects, and chitin structural chemistry of Reticulitermes flaviceps exposed to Cymbopogon citratus essential oil and its major constituent citral. Insects 2022, 13, 812. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.Z.; Wu, Z.W.; Chen, Y.Y.; Gong, X.; Yang, S.M.; Zhang, Z.L.; Zhang, D.Y.; Xie, Y.J. Insights into the toxicity, behavioral responses, biochemical activity, and molecular docking of three essential oils and their major constituents. Ind. Crops Prod. 2024, 214, 118563. [Google Scholar] [CrossRef]
- Ngongang, M.D.T.; Eke, P.; Sameza, M.L.; Mback, M.N.L.N.; Lordon, C.D.; Boyom, F.F. Chemical constituents of essential oils from Thymus vulgaris and Cymbopogon citratus and their insecticidal potential against the tomato borer, Tuta absoluta (Lepidoptera: Gelechiidae). Int. J. Trop. Insect Sci. 2022, 42, 31–43. [Google Scholar] [CrossRef]
- Wan, J.; Zhong, S.B.; Schwarz, P.; Chen, B.C.; Rao, J.J. Influence of oil phase composition on the antifungal and mycotoxin inhibitory activity of clove oil nanoemulsions. Food Funct. 2018, 9, 2872–2882. [Google Scholar] [CrossRef]
- Koschier, E.H. Essential Oil Compounds for Thrips Control—A Review. Nat. Prod. Commun. 2008, 3, 1171–1182. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Pei, T.H.; Zhou, X.C.; Xu, M.L.; Gao, H.X.; Wang, L.L.; Gao, Y. An investigation into the biological activities of four Lamiaceae essential oils against Thrips flavus, crops, and weeds. Plants 2025, 14, 448. [Google Scholar] [CrossRef]
- Pei, T.H.; Niu, Y.L.; Liu, B.; Yuan, M.; Zhang, A.G.; Li, X.F.; Wang, L.X.; Xu, M.L.; Gao, Y. Citrus essential oils as potential insecticides against Thrips flavus. Curr. Anal. Chem. 2024, 20, e280224227514. [Google Scholar] [CrossRef]
- Gharbi, K.Z. Fumigant toxicity of essential oils against Frankliniella occidentalis, F. insularis (Thysanoptera: Thripidae), and Solanum lycopersicum (Solanaceae) as affected by polymer release and adjuvants. Insects 2022, 13, 493. [Google Scholar] [CrossRef]
- Riefler, J.; Koschier, E.H. Behaviour-modifying activity of eugenol on Thrips tabaci Lindeman. J. Pest Sci. 2009, 82, 115–121. [Google Scholar] [CrossRef]
- Chahal, K.K.; Singh, B.; Kataria, D.; Dhillon, N.K. Nematotoxicity of lemongrass oil, citral and its derivatives against Meloidogyne incognita. Allelopath. J. 2016, 39, 217–229. [Google Scholar]
- Peach, D.A.H.; Almond, M.; Gries, R.; Gries, G. Lemongrass and cinnamon bark: Plant essential oil blend as a spatial repellent for mosquitoes in a field setting. J. Med. Entomol. 2019, 56, 1346–1352. [Google Scholar] [CrossRef]
- Costa, A.V.; Pinheiro, P.F.; Rondelli, V.M.; de Queiroz, V.T.; Tuler, A.C.; Brito, K.B.; Stinguel, P.; Pratissoli, D. Cymbopogon citratus (Poaceae) essential oil on Frankliniella schultzei (Thysanoptera: Thripidae) and Myzus persicae (Hemiptera: Aphididae). Biosci. J. 2013, 29, 1840–1847. [Google Scholar]
- Moustafa, M.A.M.; Hassan, N.N.; Alfuhaid, N.A.; Amer, A.; Awad, M. Insights into the toxicity, biochemical activity, and molecular docking of Cymbopogon citratus essential oils and citral on Spodoptera littoralis (Lepidoptera: Noctuidae). J. Econ. Entomol. 2023, 116, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.S.; Campos, I.M.; Conde de Brito, D.M.; Cardoso, C.M.; Pontes, E.G.; Alves de Souza, M.A. Efficacy of lemongrass essential oil and citral in controlling Callosobruchus maculatus (Coleoptera: Chrysomelidae), a post-harvest cowpea insect pest. Crop Prot. 2019, 119, 191–196. [Google Scholar] [CrossRef]
- Kim, D.I.; Kim, S.I.; Jung, J.W.; Ilyasov, R.A.; Jang, D.; Lee, S.H.; Kwon, H.W. Spatial releasing properties and mosquito repellency of cellulose-based beads containing essential oils and vanillin. J. Asia-Pac. Entomol. 2019, 22, 409–416. [Google Scholar] [CrossRef]
- Aljedani, D.M. Effects of some insecticides (deltamethrin and malathion) and lemongrass oil on fruit fly (Drosophila melanogaster). Pak. J. Biol. Sci. 2021, 24, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Plata-Rueda, A.; Fiaz, M.; Brugger, B.P.; Canas, V.; Coelho, R.P.; Zanuncio, J.C.; Martinez, L.C.; Serrao, J.E. Lemongrass essential oil and its components cause effects on survival, locomotion, ingestion, and histological changes of the midgut in Anticarsia gemmatalis caterpillars. Toxin Rev. 2022, 41, 208–217. [Google Scholar] [CrossRef]
- Pang, R.; Wang, J.B.; Li, H.L.; Zhong, Z.C.; Li, Z.S.; Qiu, B.; Zhou, C.Y.; Ali, S.; Wu, J.H. Identification of the CYPome associated with acetamiprid resistance based on the chromosome-level genome of Megalurothrips usitatus (Bagnall) (Thysanoptera: Thripidae). Pest Manag. Sci. 2025, 81, 3273–3283. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, S.; Naik, S.N. Biopesticidal value of selected essential oils against pathogenic fungus, termites, and nematodes. Int. Biodeterior. Biodegrad. 2011, 65, 703–707. [Google Scholar] [CrossRef]
- Radunz, A.L.; Radunz, M.; Bizollo, A.R.; Tramontin, M.A.; Radunz, L.L.; Mariot, M.P.; Tempel-Stumpf, E.R.; Calisto, J.F.F.; Zaniol, F.; Albeny-Simoes, D.; et al. Insecticidal and repellent activity of native and exotic lemongrass on Maize weevil. Braz. J. Biol. 2022, 84, e252990. [Google Scholar] [CrossRef] [PubMed]
- Soonwera, M.; Phasomkusolsil, S. Effect of Cymbopogon citratus (lemongrass) and Syzygium aromaticum (clove) oils on the morphology and mortality of Aedes aegypti and Anopheles dirus larvae. Parasitol Res. 2016, 115, 1691–1703. [Google Scholar] [CrossRef]
- Mohsin, A.A.; Al-Salami, W.M.; Abid, A.J. Laboratory Study of the Effect of Lemongrass Leaves (Cymbopogon citratus) on an Onion Thrips Insect (Thrips tabaci Thysanoptera: Thripidae). J. Univ. Babylon Pure Appl. Sci. 2018, 26, 182–192. [Google Scholar] [CrossRef]
- Diabate, S.; Martin, T.; Murungi, L.K.; Fiaboe, K.K.M.; Subramanian, S.; Wesonga, J.; Deletre, E. Repellent activity of Cymbopogon citratus and Tagetes minuta and their specific volatiles against Megalurothrips sjostedti. J. Appl. Entomol. 2019, 143, 855–866. [Google Scholar] [CrossRef]
- Tang, Y.; Li, H.; Song, Q. Lemongrass essential oil and its major component citronellol: Evaluation of larvicidal activity and acetylcholinesterase inhibition against Anopheles sinensis. Parasitol. Res. 2024, 123, 315. [Google Scholar] [CrossRef]
- Tak, J.H.; Jovel, E.; Isman, M.B. Synergistic interactions among the major constituents of lemongrass essential oil against larvae and an ovarian cell line of the cabbage looper, Trichoplusia ni. J. Pest Sci. 2017, 90, 735–744. [Google Scholar] [CrossRef]
- Baranová, B.; Kudláčková, B.; Baral, R.P.; Svojanovská, L.; Javed, Q.; Amato, G.; Caputo, L.; De Martino, L.; Francolino, R.; Chaudhary, S.K.; et al. Potential herbicidal and insecticidal (Beneficial) properties of Nepalese lemongrass essential oil. Chem. Biodivers. 2025, 22, e01095. [Google Scholar] [CrossRef]
- Pinto, Z.T.; Sánchez, F.F.; Santos, A.R.; Amaral, A.C.F.; Ferreira, J.L.P.; Escalona-Arranz, J.C.; Queiroz, M.M.C. Chemical com position and insecticidal activity of Cymbopogon citratus essential oil from Cuba and Brazil against housefly. Rev. Bras. Parasitol. Vet. 2015, 24, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Felipe-Victoriano, M.; Villegas-Luján, R.; Treviño-Cueto, D.; Sánchez-Peña, S.R. Combined activity of natural products and the fungal entomopathogen Cordyceps farinosa against Bagrada hilaris (Hemiptera: Pentatomidae). Fla. Entomol. 2023, 106, 154–160. [Google Scholar] [CrossRef]
- Shaltiel-Harpaz, L.; Kreimer, T.; Dudai, N.; Kaspi, R.; Ben-Yakir, D.; Rytwo, G. Sepiolite-rosemary oil combination as an environmentally oriented insecticide. Appl. Clay Sci. 2023, 234, 106838. [Google Scholar] [CrossRef]
- Abd-Elnabi, A.D.; El-sawy, E.A.F.; Badawy, M.E.I. Plant oil nano-emulsions as a potential solution for pest control in sustainable agriculture. Neotrop. Entomol. 2025, 54, 35. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.; Attard, T.; Calvert, D.; Bullock, J.; Clark, J.H.; Sherwood, J.; McElroy, C.R. Green solvent selection for emulsifiable concentrate agrochemical formulations. Org. Process Res. Dev. 2024, 28, 132–136. [Google Scholar] [CrossRef]
- Wu, J.; Yang, R.; Zheng, Q.; Wei, L.; Wang, B.; Yan, W.; Meng, S.; Cheng, D.; Huang, S.; Zhang, Z.; et al. Effect of Brucea javanica oil on the toxicity of β-cypermethrin emulsifiable concentrate formulation. ACS Appl. Mater. Interfaces 2024, 16, 9713–9724. [Google Scholar] [CrossRef]
- Baldacchino, F.; Tramut, C.; Salem, A.; Lienard, E.; Deletre, E.; Franc, M.; Martin, T.; Duvallet, G.; Jay-Robert, P. The repellency of lemongrass oil against stable flies, tested using video tracking. Parasite 2013, 20, 21. [Google Scholar] [CrossRef]
- Brugger, B.P.; Martinez, L.C.; Plata-Rueda, A.; de Castro e Castro, B.M.; Soares, M.A.; Wilcken, C.F.; Carvalho, A.G.; Serrao, J.E.; Zanuncio, J.C. Bioactivity of the Cymbopogon citratus (Poaceae) essential oil and its terpenoid constituents on the predatory bug, Podisus nigrispinus (Heteroptera: Pentatomidae). Sci. Rep. 2019, 9, 8358. [Google Scholar] [CrossRef]
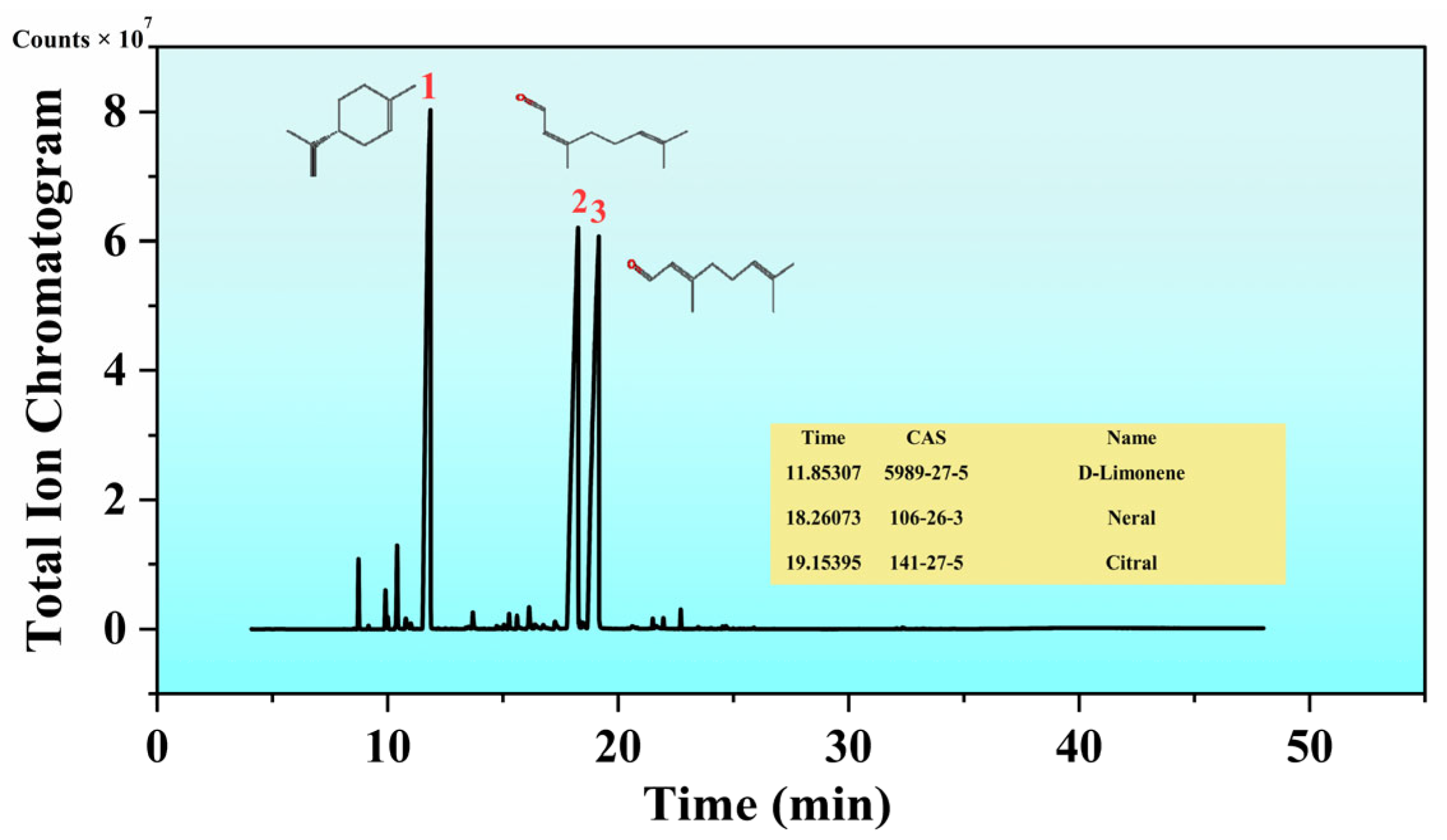


| Retention Time | Compound Name | Matching Factor | Molecular Formula | CAS | Area Ratio (%) |
|---|---|---|---|---|---|
| 19.154 | Citral | 97.66 | C10H16O | 141-27-5 | 31.56 |
| 11.853 | D-Limonene | 98.14 | C10H16 | 5989-27-5 | 31.20 |
| 18.261 | Neral | 99.4 | C10H16O | 106-26-3 | 30.90 |
| 10.409 | β-Myrcene | 97.82 | C10H16 | 123-35-3 | 1.30 |
| 8.735 | α-Pinene | 99.38 | C10H16 | 80-56-8 | 0.85 |
| 9.901 | β-Phellandrene | 98.71 | C10H16 | 555-10-2 | 0.51 |
| 16.132 | 3,6-Octadienal, 3,7-dimethyl- | 98.43 | C10H16O | 55722-59-3 | 0.42 |
| 10.783 | Octanal | 97.41 | C8H16O | 124-13-0 | 0.26 |
| 13.693 | Linalool | 99.38 | C10H18O | 78-70-6 | 0.24 |
| 17.255 | (-)-cis-Isopiperitenol | 98.64 | C10H16O | 96555-02-1 | 0.23 |
| 22.711 | Caryophyllene | 99.42 | C15H24 | 87-44-5 | 0.22 |
| 15.608 | Isoneral | 98.93 | C10H16O | 1000414-18-0 | 0.21 |
| 15.265 | 6-Octenal, 3,7-dimethyl-, (R)- | 99.24 | C10H18O | 2385-77-5 | 0.21 |
| 10.002 | β-Pinene | 99.19 | C10H16 | 127-91-3 | 0.16 |
| 21.957 | 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl- | 86.55 | C15H26O | 4602-84-0 | 0.14 |
| 16.378 | 2,6-Octadienal, 3,7-dimethyl-, (E)- | 87.7 | C10H16O | 141-27-5 | 0.14 |
| 21.497 | 3-Cyclohexene-1-carboxaldehyde, 1,3,4-trimethyl- | 86.3 | C10H16O | 40702-26-9 | 0.12 |
| 18.534 | Geraniol | 94.55 | C10H18O | 106-24-1 | 0.11 |
| 10.997 | 3-Carene | 98.3 | C10H16 | 13466-78-9 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Zhu, M.; Qiu, B.; Ali, S.; Wu, J. Insecticidal Effect of Lemongrass Essential Oil Against Megalurothrips usitatus (Bagnall). Agronomy 2025, 15, 1733. https://doi.org/10.3390/agronomy15071733
Han Y, Zhu M, Qiu B, Ali S, Wu J. Insecticidal Effect of Lemongrass Essential Oil Against Megalurothrips usitatus (Bagnall). Agronomy. 2025; 15(7):1733. https://doi.org/10.3390/agronomy15071733
Chicago/Turabian StyleHan, Yun, Ming Zhu, Bo Qiu, Shaukat Ali, and Jianhui Wu. 2025. "Insecticidal Effect of Lemongrass Essential Oil Against Megalurothrips usitatus (Bagnall)" Agronomy 15, no. 7: 1733. https://doi.org/10.3390/agronomy15071733
APA StyleHan, Y., Zhu, M., Qiu, B., Ali, S., & Wu, J. (2025). Insecticidal Effect of Lemongrass Essential Oil Against Megalurothrips usitatus (Bagnall). Agronomy, 15(7), 1733. https://doi.org/10.3390/agronomy15071733







