Root-Emitted Volatile Organic Compounds from Daucus carota Modulate Chemotaxis in Phasmarhabditis and Oscheius Nematodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection, Isolation, and Storage Preparation of Nematodes
2.2. Tested Volatile Compounds
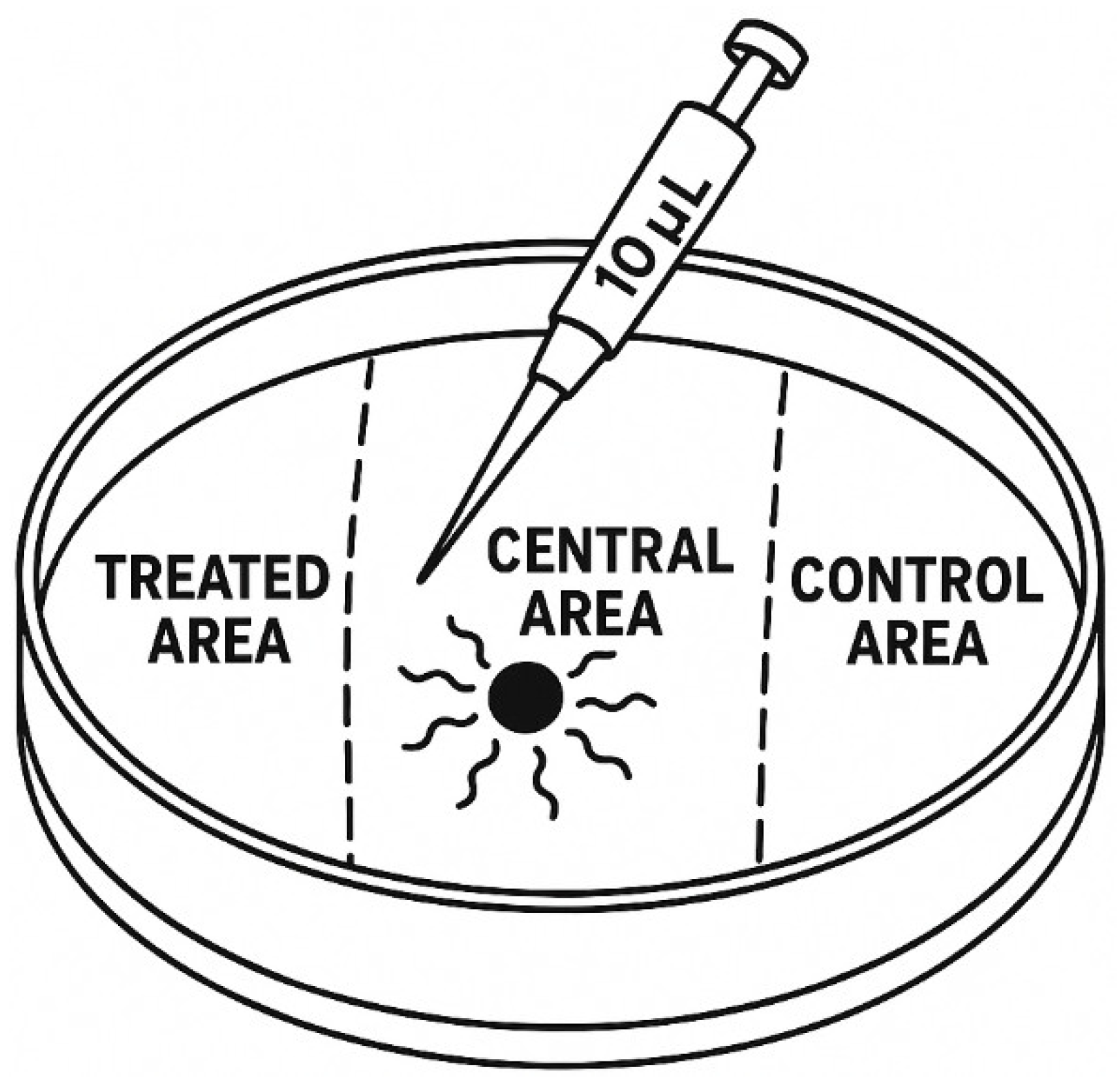
2.3. Chemotaxis Assay
2.4. Statistical Analysis
3. Results
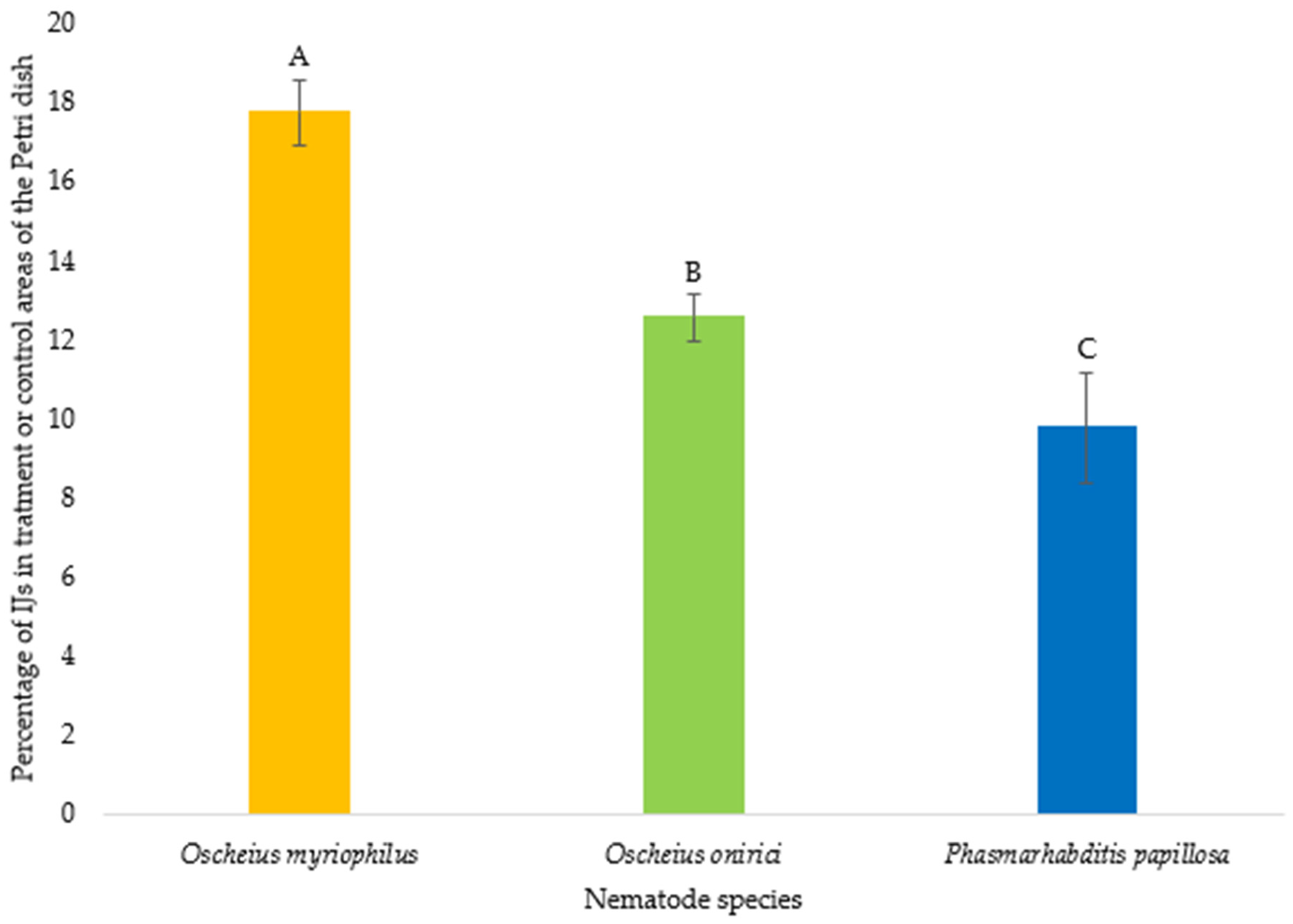
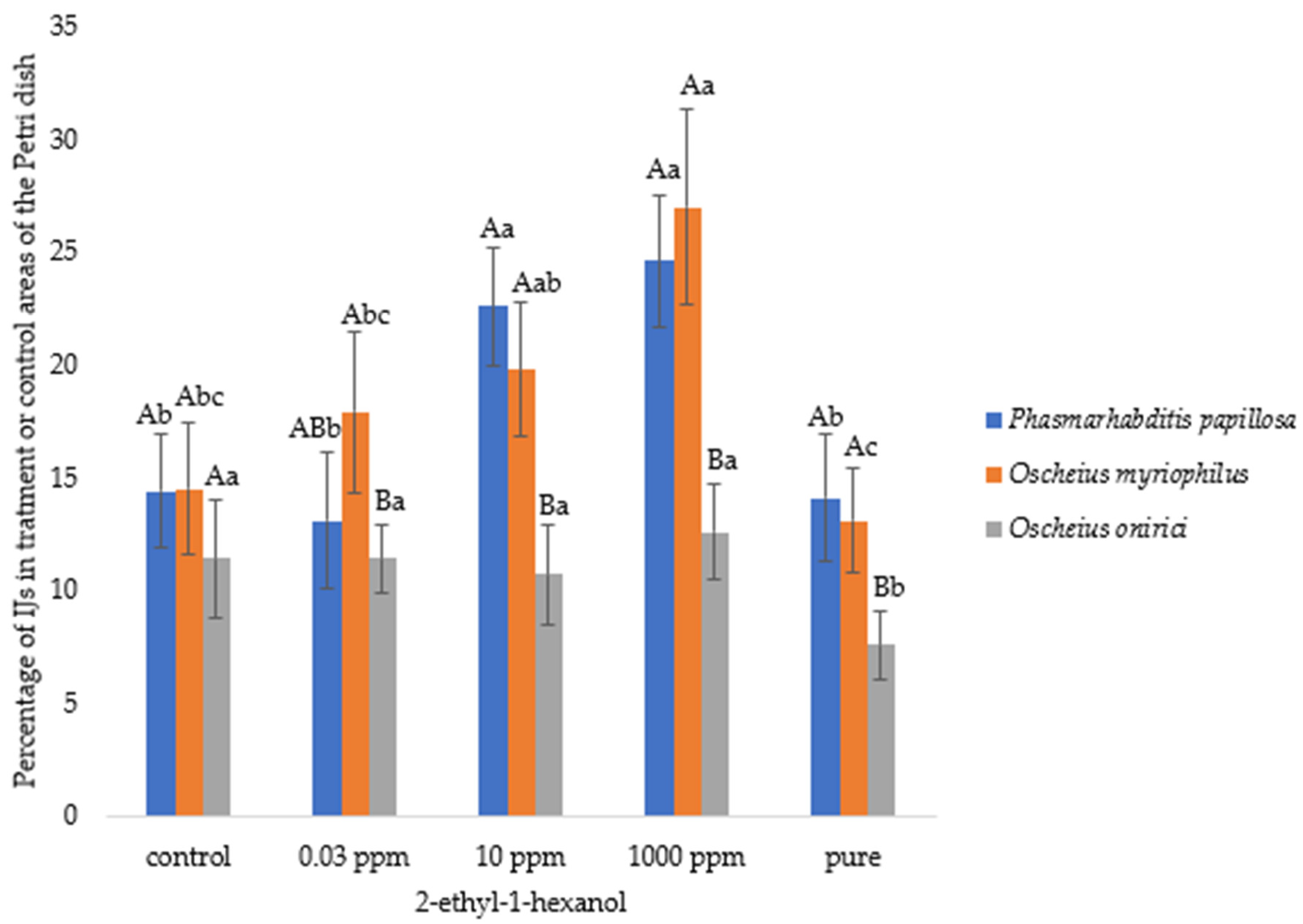
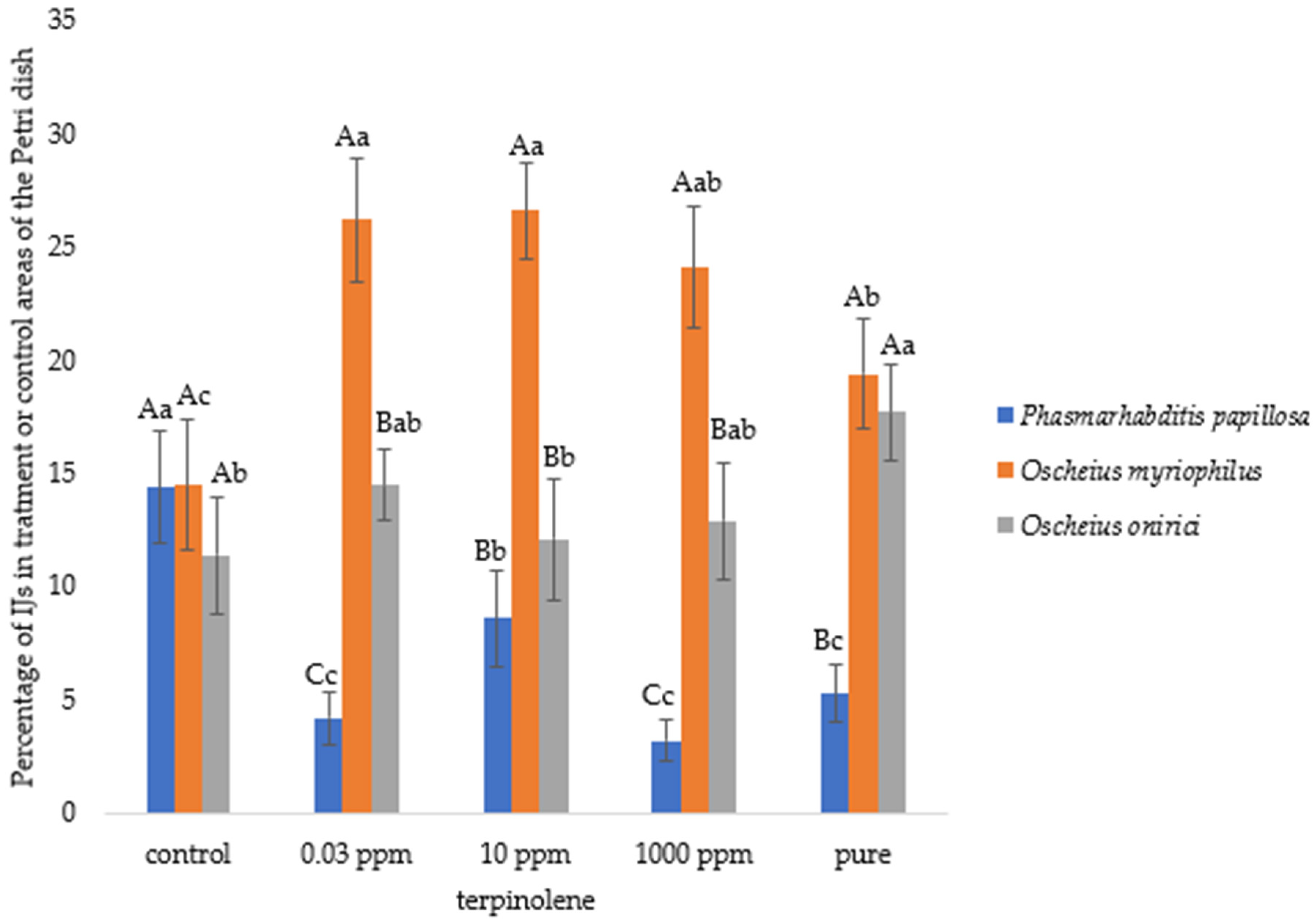
3.1. Nematode Motility
3.2. Chemotaxis Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, P.P.G.; Bhattacharyya, B.; Bhagawnti, S.; Dev, E.B.; Manpoong, N.S.; Bhairavi, K.S. Slug: An emerging menace in agriculture: A review. J. Entomol. Zool. Stud. 2020, 8, 1–6. [Google Scholar]
- Kozłowski, J.; Kozłowski, R.J. Expansion of the invasive slug species Arion lusitanicus Mabille, 1868 and dangers to garden crops—A literature review with some new data. Folia Malacol. 2011, 19, 249–258. [Google Scholar] [CrossRef]
- Dörler, D.; Scheucher, A.; Zaller, J.G. Efficacy of chemical and biological slug control measures in response to watering and earthworms. Sci. Rep. 2019, 9, 2954. [Google Scholar] [CrossRef]
- Askary, T.H.; Khan, A.A.; Waliullah, M.I.S.; Banday, S.A.; Iqbal, U.; Mir, M.M. Slug Pest Management Through Nematodes in Agricultural and Horticultural Crops. In Nematodes: Morphology, Functions and Management Strategies; Boeri, F., Chung, J.A., Eds.; Nova Publishers: New York, NY, USA, 2012; pp. 197–211. [Google Scholar]
- Wenke, K.; Kai, M.; Piechulla, B. Belowground volatiles facilitate interactions between plant roots and soil organisms. Planta 2010, 231, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Delory, B.M.; Delaplace, P.; Fauconnier, M.L.; Du Jardin, P. Root-emitted volatile organic compounds: Can they mediate belowground plant-plant interactions? Plant Soil. 2016, 402, 1–26. [Google Scholar] [CrossRef]
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Laznik, Ž.; Tóth, T.; Ádám, S.; Trdan, S.; Majić, I.; Lakatos, T. Responses of parasitic nematodes to volatile organic compounds emitted by Brassica nigra roots. Agronomy 2025, 15, 664. [Google Scholar] [CrossRef]
- Sheehy, L.; Cutler, J.; Weedall, G.D.; Rae, R. Microbiome analysis of malacopathogenic nematodes suggests no evidence of a single bacterial symbiont responsible for gastropod mortality. Front. Immunol. 2022, 13, 878783. [Google Scholar] [CrossRef] [PubMed]
- Torrini, G.; Mazza, G.; Carletti, B.; Benvenuti, C.; Roversi, P.F.; Fanelli, E.; De Luca, F.; Troccoli, A.; Tarasco, E. Oscheius onirici sp. n. (Nematoda: Rhabditidae): A new entomopathogenic nematode from an Italian cave. Zootaxa 2015, 3, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Laznik, Ž.; Trdan, S.; Tóth, T.; Ádám, S.; Lakatos, T.; Majić, I. Discovery of Oscheius myriophilus (Nematoda: Rhabditidae) in Gastropods and Its Similar Virulence to Phasmarhabditis papillosa against Arion vulgaris, Deroceras reticulatum, and Cernuella virgata. Agronomy 2023, 13, 1386. [Google Scholar] [CrossRef]
- Laznik, Ž.; Trdan, S.; Yonesi, M. Chemotactic responses of slug-parasitic nematodes to potato-tuber-emitted volatile organic compounds. Agronomy 2025, 15, 951. [Google Scholar] [CrossRef]
- Ghavamabad, R.G.; Talebi, A.A.; Mehrabadi, M.; Farashiani, M.E.; Pedram, M. First record of Oscheius myriophilus (Poinar, 1986) (Rhabditida: Rhabditidae) from Iran; and its efficacy against two economic forest tree pests in laboratory condition. J. Nematol. 2021, 53, e2021–e2035. [Google Scholar] [CrossRef] [PubMed]
- Laznik, Ž.; Trdan, S.; Šavli, K. Chemotactic responses of Oscheius myriophilus to mollusk mucus. Agronomy 2024, 14, 3049. [Google Scholar] [CrossRef]
- Ye, W.; Foye, S.; MacGuidwin, A.E.; Steffan, S. Incidence of Oscheius onirici (Nematoda: Rhabditidae), a potentially entomopathogenic nematode from the marshlands of Wisconsin, USA. J. Nematol. 2018, 50, 9. [Google Scholar] [CrossRef] [PubMed]
- Barua, A.; Williams, C.D.; Ross, J.L. A literature review of biological and bio-rational control strategies for slugs: Current research and future prospects. Insects 2021, 12, 541. [Google Scholar] [CrossRef] [PubMed]
- Rae, R.; Sheehy, L.; McDonald-Howard, K. Thirty years of slug control using the parasitic nematode Phasmarhabditis hermaphrodita and beyond. Pest. Manag. Sci. 2023, 79, 3408–3424. [Google Scholar] [CrossRef] [PubMed]
- Schurkman, J.; De Ley, I.T.; Dillman, A.R. Dose dependence of Phasmarhabditis isolates on the mortality of adult invasive white garden snails (Theba pisana). PLoS ONE 2022, 17, e0270185. [Google Scholar] [CrossRef] [PubMed]
- Cutler, J.; Rae, R. Natural variation in host-finding behaviour of gastropod parasitic nematodes (Phasmarhabditis spp.) exposed to host-associated cues. J. Helminthol. 2021, 95, e10. [Google Scholar] [CrossRef] [PubMed]
- Keilwagen, J.; Lehnert, H.; Berner, T.; Budahn, H.; Nothnagel, T.; Ulrich, D.; Dunemann, F. The terpene synthase gene family of carrot (Daucus carota L.): Identification of QTLs and candidate genes associated with terpenoid volatile compounds. Front. Plant Sci. 2017, 8, 1930. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, S.; Budahn, H.; Lamprecht, D.; Riewe, D.; Ulrich, D.; Dunemann, F.; Kopertekh, L. The carrot monoterpene synthase gene cluster on chromosome 4 harbours genes encoding flavour-associated sabinene synthases. Hortic. Res. 2020, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Muchlinski, A.; Ibdah, M.; Ellison, S.; Yahyaa, M.; Nawade, B.; Laliberte, S.; Senalik, D.; Simon, P.; Whitehead, S.R.; Tholl, D. Diversity and function of terpene synthases in the production of carrot aroma and flavor compounds. Sci. Rep. 2020, 10, 9989. [Google Scholar] [CrossRef] [PubMed]
- van Roon, A.; Parsons, J.R.; te Kloeze, A.M.; Govers, H.A.J. Fate and transport of monoterpenes through soils. Part I. Prediction of temperature dependent soil fate model input-parameters. Chemosphere 2005, 61, 599–609. [Google Scholar] [CrossRef] [PubMed]
- van Roon, A.; Krap, J.R.P.L.; Govers, H.A.J. Fate and transport of monoterpenes through soils. Chemosphere 2005, 61, 159. [Google Scholar] [PubMed]
- Kleinheinz, G.T.; Bagley, S.T.; St John, W.P.; Rughani, J.R.; McGinnis, G.D. Characterization of alpha-pinene-degrading microorganisms and application to a bench-scale biofiltration system for VOC degradation. Arch. Environ. Contam. Toxicol. 1999, 37, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Laznik, Ž.; Trdan, S. Attraction behaviors of entomopathogenic nematodes to synthetic volatiles emitted by insect-damaged carrot roots. J. Pest. Sci. 2016, 89, 597–606. [Google Scholar] [CrossRef]
- Weissteiner, S. The Effect of Root Volatiles on the Orientation Behavior of Cockchafer Larvae in the Soil. Ph.D. Thesis, Georg-August-University Göttingen, Göttingen, Germany, 2010; p. 182. [Google Scholar]
- O’Halloran, D.M.; Burnell, A.M. An Investigation of Chemotaxis in the Insect Parasitic Nematode Heterorhabditis bacteriophora. Parasitology 2003, 127, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Bargmann, C.I.; Horvitz, H.R. Chemosensory Neurons with Overlapping Functions Direct Chemotaxis to Multiple Chemicals in C. elegans. Neuron 1991, 7, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, X.; Pei, Z.; Sun, J.; Xi, J.; Li, X.; Shapiro-Ilan, D.; Ruan, W. Volatile organic compounds released from entomopathogenic nematode-infected insect cadavers for the biocontrol of Meloidogyne incognita. Pest. Manag. Sci. 2024, 80, 5400–5411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, L.; Kesner, L.; Robert, C.A.M. Chemical host-seeking cues of entomopathogenic nematodes. Curr. Opin. Insect Sci. 2021, 44, 72–81. [Google Scholar] [CrossRef] [PubMed]
- De Ley, I.T.; Holovachov, O.; McDonnell, R.J.; Bert, W.; Paine, T.D.; De Ley, P. Description of Phasmarhabditis californica n. sp. and first report of P. papillosa (Nematoda: Rhabditidae) from invasive slugs in the USA. Nematology 2016, 18, 175–193. [Google Scholar] [CrossRef]
- Pieterse, A.; Tiedt, L.R.; Malan, A.P.; Ross, J.L. First record of Phasmarhabditis papillosa (Nematoda: Rhabditidae) in South Africa, and its virulence against the invasive slug, Deroceras panormitanum. Nematology 2017, 19, 1035–1050. [Google Scholar] [CrossRef]
- Köllner, T.G.; Held, M.; Lenk, C.; Hiltpold, I.; Turlings, T.C.J.; Gershenzon, J.; Degenhardt, J. A maize (E)-β-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell 2008, 20, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, J.; Gershenzon, J.; Baldwin, I.T.; Kessler, A. Attracting friends to feast on foes: Engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr. Opin. Biotechnol. 2009, 20, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Hiltpold, I.; Turlings, T.C.J. Belowground chemical signaling in maize: When simplicity rhymes with efficiency. J. Chem. Ecol. 2008, 34, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Hallem, E.A.; Dillman, A.R.; Hong, A.V.; Zhang, Y.; Yano, J.M.; DeMarco, S.F.; Sternberg, P.W. A sensory code for host seeking in parasitic nematodes. Curr. Biol. 2011, 21, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.G.; Alborn, H.T.; Stelinski, L.L. Subterranean herbivore-induced volatiles released by citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes. J. Chem. Ecol. 2010, 36, 361–368. [Google Scholar] [CrossRef] [PubMed]
- van Dam, N.M.; Bouwmeester, H.J. Metabolomics in the rhizosphere: Tapping into belowground chemical communication. Trends Plant Sci. 2016, 21, 256–265. [Google Scholar] [CrossRef] [PubMed]
- El-Borai, F.E.; Campos-Herrera, R.; Stuart, R.J.; Duncan, L.W. Substrate modulation, group effects and the behavioral responses of entomopathogenic nematodes to nematophagous fungi. J. Invertebr. Pathol. 2011, 106, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Sanchez-Moreiras, A.M.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193, 997–1008. [Google Scholar] [CrossRef] [PubMed]


| Factor | Sum of Squares | Df | F | p |
|---|---|---|---|---|
| Nematode species (S) | 6553.79 | 2 | 16.50 | <0.0001 |
| VOCs (V) | 5187.43 | 4 | 6.53 | <0.0001 |
| VOCs concentration (Vc) | 3193.48 | 4 | 4.02 | 0.0032 |
| Temporal replication | 2949.21 | 9 | 1.65 | 0.0987 |
| Spatial replication | 421.03 | 2 | 1.06 | 0.3455 |
| S × V | 13,600.11 | 8 | 8.56 | <0.0001 |
| S × Vc | 3320.59 | 8 | 2.09 | 0.0350 |
| V × Vc | 6672.95 | 16 | 2.10 | 0.0156 |
| S × V × Vc | 11,820.66 | 32 | 1.86 | 0.0081 |
| Residual | 104,860.66 | 528 | ||
| Total (corrected) | 150,484.60 | 599 |
| Factor | Sum of Squares | Df | F | p |
|---|---|---|---|---|
| Nematode species (S) | 0.44 | 2 | 26.24 | <0.0001 |
| VOCs (V) | 0.08 | 4 | 2.51 | 0.0410 |
| VOCs concentration (Vc) | 0.16 | 4 | 4.72 | 0.0010 |
| Temporal replication | 0.10 | 9 | 1.37 | 0.1970 |
| Spatial replication | 0.01 | 2 | 0.65 | 0.5211 |
| S × V | 0.12 | 8 | 1.74 | 0.0315 |
| S × Vc | 0.16 | 8 | 2.42 | 0.0140 |
| V × Vc | 0.51 | 16 | 3.82 | <0.0001 |
| S × V × Vc | 1.35 | 32 | 5.09 | <0.0001 |
| Residual | 4.39 | 528 | ||
| Total (corrected) | 7.32 | 599 |
| 2-ethyl-1-hexanol | |||||
| Pure | 1000 ppm | 10 ppm | 0.03 ppm | Control | |
| PP | −0.10 ± 0.02 Aab | −0.11 ± 0.01 Aa | −0.05 ± 0.02 Ac | −0.07 ± 0.02 Abc | 0.02 ± 0.01 Ad |
| OM | −0.01 ± 0.02 Bbc | −0.08 ± 0.04 Aa | −0.03 ± 0.01 Aab | −0.06 ± 0.02 Aa | 0.01 ± 0.01 Ac |
| OO | 0.04 ± 0.01 Cb | 0.00 ± 0.02 Ba | 0.02 ± 0.02 Bab | 0.01 ± 0.01 Ba | 0.00 ± 0.01 Aa |
| α-pinene | |||||
| Pure | 1000 ppm | 10 ppm | 0.03 ppm | Control | |
| PP | 0.02 ± 0.01 Bb | −0.01 ± 0.01 Ba | −0.01 ± 0.01 Aa | 0.00 ± 0.00 Aa | 0.02 ± 0.01 Ab |
| OM | −0.05 ± 0.02 Ab | −0.20 ± 0.04 Aa | −0.03 ± 0.01 Ab | 0.03 ± 0.01 Bc | 0.01 ± 0.01 Ac |
| OO | −0.03 ± 0.01 Aa | −0.01 ± 0.01 Bab | 0.04 ± 0.01 Bc | −0.02 ± 0.02 Aab | 0.00 ± 0.01 Ab |
| bornyl acetate | |||||
| Pure | 1000 ppm | 10 ppm | 0.03 ppm | Control | |
| PP | −0.02 ± 0.01 Ba | −0.02 ± 0.01 Ba | −0.01 ± 0.01 Ba | −0.01 ± 0.02 Bab | 0.02 ± 0.01 Ab |
| OM | −0.15 ± 0.03 Aab | −0.20 ± 0.03 Aa | −0.13 ± 0.02 Ab | −0.19 ± 0.03 Aa | 0.01 ± 0.01 Ac |
| OO | 0.04 ± 0.02 Cb | 0.01 ± 0.03 Bab | 0.05 ± 0.02 Cb | 0.02 ± 0.02 Bab | 0.00 ± 0.01 Aa |
| Terpinolene | |||||
| Pure | 1000 ppm | 10 ppm | 0.03 ppm | Control | |
| PP | 0.00 ± 0.00 Bb | 0.00 ± 0.00 Bb | −0.05 ± 0.02 Aa | 0.00 ± 0.00 Bb | 0.02 ± 0.01 Ac |
| OM | −0.03 ± 0.02 Ac | −0.10 ± 0.04 Aab | −0.08 ± 0.03 Abc | −0.14 ± 0.02 Aa | 0.01 ± 0.01 Ad |
| OO | 0.05 ± 0.03 Cbc | 0.03 ± 0.02 Cb | 0.05 ± 0.03 Bbc | 0.09 ± 0.02 Cc | 0.00 ± 0.01 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sen, E.; Lakatos, T.; Tóth, T.; Trdan, S.; Laznik, Ž. Root-Emitted Volatile Organic Compounds from Daucus carota Modulate Chemotaxis in Phasmarhabditis and Oscheius Nematodes. Agronomy 2025, 15, 1793. https://doi.org/10.3390/agronomy15081793
Sen E, Lakatos T, Tóth T, Trdan S, Laznik Ž. Root-Emitted Volatile Organic Compounds from Daucus carota Modulate Chemotaxis in Phasmarhabditis and Oscheius Nematodes. Agronomy. 2025; 15(8):1793. https://doi.org/10.3390/agronomy15081793
Chicago/Turabian StyleSen, Emre, Tamás Lakatos, Tímea Tóth, Stanislav Trdan, and Žiga Laznik. 2025. "Root-Emitted Volatile Organic Compounds from Daucus carota Modulate Chemotaxis in Phasmarhabditis and Oscheius Nematodes" Agronomy 15, no. 8: 1793. https://doi.org/10.3390/agronomy15081793
APA StyleSen, E., Lakatos, T., Tóth, T., Trdan, S., & Laznik, Ž. (2025). Root-Emitted Volatile Organic Compounds from Daucus carota Modulate Chemotaxis in Phasmarhabditis and Oscheius Nematodes. Agronomy, 15(8), 1793. https://doi.org/10.3390/agronomy15081793








