Common Ragweed—Ambrosia artemisiifolia L.: A Review with Special Regards to the Latest Results in Protection Methods, Herbicide Resistance, New Tools and Methods
Abstract
1. Introduction
2. Protection Methods Against Ragweed Under Cultivated Areas
2.1. Agrotechnical Protection
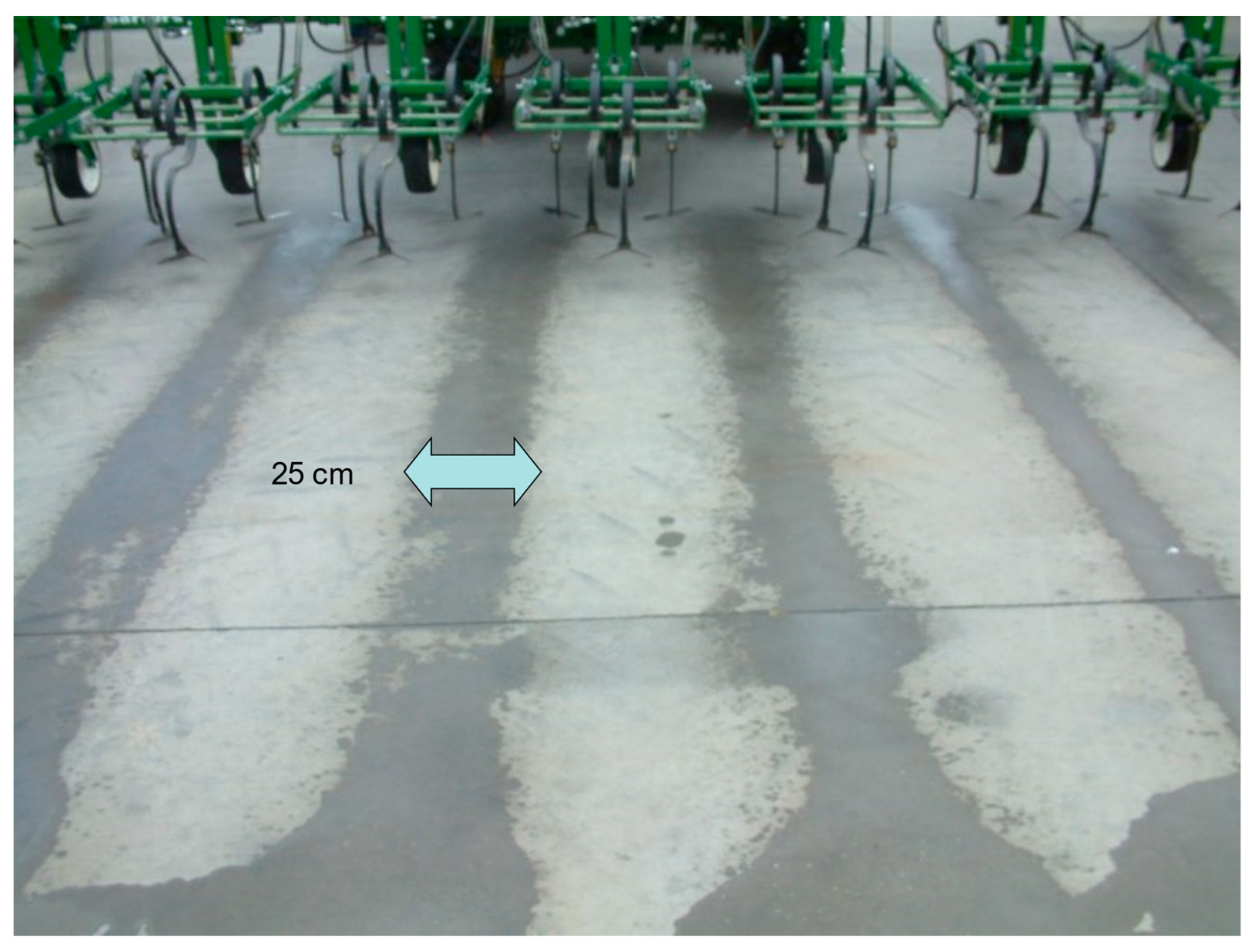
2.2. Mechanical Weed Management
2.3. Thermal Weed Control
2.4. Biological Weed Control
2.4.1. Viruses
2.4.2. Fungi
2.4.3. Insects

2.5. Chemical Weed Control
2.5.1. Stubble Treatment
2.5.2. Herbicide Resistance
| Site of Action | Active Ingredient |
|---|---|
| Auxin mimics (HRAC Group 4) [110] | clopyralid |
| ALS inhibitors (HRAC Group 2) [104,111] | amidosulfuron |
| chlorimuron-ethyl | |
| cloransulam-methyl | |
| diclosulam | |
| flazasulfuron | |
| flumetsulam | |
| foramsulfuron | |
| halosulfuron-methyl | |
| imazamethabenz-methyl | |
| imazamox | |
| imazaquin imazapyr imazethapyr iodosulfuron-methyl-Na metsufuron-methyl nicosulfuron | |
| primisulfuron-methyl prosulfuron pyriothiobac-sodium thiencarbazone-methyl tribenuron-methyl trifloxysulfuron-Na | |
| PPO inhibitors (HRAC Group 14) [104] | acifluorfen carfentrazone-ethyl flumiclorac-pentyl flumioxan fomesafen lactofen oxyfluorfen pyraflufen-ethyl sulfentrazone |
| PSII inhibitors (HRAC Group 5) [104] | atrazine cyanazine linuron metribuzin simazine |
| EPSPS inhibitor (HRAC Group 9) [104] | glyphosate |
3. Ragweed Control on Non-Cultivated Areas
3.1. Ruderals
3.2. Industrial Parks, Construction Sites
3.3. Populated Areas
3.4. Along Linear Constructions
3.5. Natural and Semi-Natural Habitats
4. New Tools and Methods of Weed Survey and Control
5. Official Measures and Pollen Monitoring in HU
6. Discussion
6.1. Comparative Effectiveness of Control Methods
6.1.1. Chemical Methods
6.1.2. Biological Control
6.1.3. Mechanical and Thermal Methods
6.1.4. Precision Technologies
6.2. Trade-Offs and Limitations of Different Weed Control Technologies
6.3. Future Research
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knolmajer, B.; Jócsák, I.; Taller, J.; Keszthelyi, S.; Kazinczi, G. Common Ragweed—Ambrosia artemisiifolia L.: A Review with Special Regards to the Latest Results in Biology and Ecology. Agronomy 2024, 14, 497. [Google Scholar] [CrossRef]
- Kolejanisz, T.; Pinke, G. Az ürömlevelű parlagfű (Ambrosia artemisiifolia L.) térfoglalását befolyásoló ökológiai és agrotechnikai tényezők. Magy. Gyomkutatás és Technológia 2018, 19, 3–20. [Google Scholar]
- Farkas, A. A parlagfű (Ambrosia artemisiifolia L.) jelenléte és borítási százalékának változása különböző művelési eljárások hatására. Növényvédelem 2003, 39, 111–121. [Google Scholar]
- Kazinczi, G.; Novák, R. (Eds.) Integrated Methods for Suppression of Common Ragweed; National Food Chain Safety Office, Directorata of Plant Protection Soil Conservation and Agri-Environment: Budapest, Hungary, 2014. [Google Scholar]
- Pinke, G. Abiotikus és Gazdálkodási Tényezők Hatása Magyarország Szántóföldi Gyomnövényzetének Fajösszetételére. Ph.D. Thesis, Hungarian Academy of Sciences, Mosonmagyaróvár, Hungary, 2017. [Google Scholar]
- Benécsné Bárdi, G. Integrált védelem a parlagfű ellen. Nem vegyszeres védekezési módszerek. Növényvédelem 2009, 45, 459–464. [Google Scholar]
- Saulic, M.; Oveisi, M.; Djalovic, I.; Bozic, D.; Pishyar, A.; Savić, A.; Vrbničanin, S. How do long term crop rotations influence weed populations: Exploring the impacts of more than 50 Years of crop management in Serbia. Agronomy 2022, 12, 1772. [Google Scholar] [CrossRef]
- Rueda-Ayala, V.; Rasmussen, J.; Gerhards, R. Mechanical Weed Control. In Precision Crop Protection—The Challenge and Use of Heterogeneity; Oerke, E.-C., Gerhards, R., Menz, G., Sikora, R.A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 279–294. [Google Scholar]
- Kismányoky, A. Agrotechnikai Tényezők Hatása a Kultúrnövényekre és a Gyomosodásra. PhD Thesis, Pannon University, Keszthely, Hungary, 2010. [Google Scholar]
- Van Der Weide, R.Y.; Bleeker, P.O.; Achten, V.T.J.M.; Lotz, A.T.; Fogelberg, F.; Melander, B. Innovation in mechanical weed control in crop rows. Weed Res. 2008, 48, 215–224. [Google Scholar] [CrossRef]
- Reisinger, P.; Borsiczky, I. A parlagfű (Ambrosia artemisiifolia) elleni védekezés precíziós gyomszabályozási módszerekkel. Növényvédelem 2009, 45, 445–448. [Google Scholar]
- Melander, B.; McCollough, M.R. Advances in Mechanical Weed Control; Burleigh Dodds Science Publishing: Sawston, UK, 2021; pp. 1–29. [Google Scholar]
- Kazinczi, G.; Béres, I.; Novák, R.; Karmán, J. Újra fókuszban az ürömlevelű parlagfű. Növényvédelem 2009, 45, 389–403. [Google Scholar]
- Reznik, S.Y.; Spasskaya, I.A.; Dolgovskaya, M.Y.; Volkovitsh, M.G.; Zaitzev, V.F. The ragweed leaf beetle Zygogramma suturalis F. (Coleoptera: Chrysomelidae) in Russia: Current distribution, abundance and implication for biological control of common ragweed, Ambrosia artemisiifolia L. In Proceedings of the 7th International Symposium on Biological Control of Weeds, La Grande Motte, France, 22–27 April 2007; Julien, M.H., Sforza, R., Bon, M.C., Evans, H.C., Hatcher, P.E., Hinz, H.E., Rector, B.G., Eds.; CAB International: Wallingford, UK, 2007; pp. 614–619. [Google Scholar]
- Béres, I.; Hunyadi, K. A parlagfű (Ambrosia elatior L.) biológiája. Növényvédelem 1980, 16, 109–116. [Google Scholar]
- Basky, Z. Effect of native aphid species on the development of invasive ragweed Ambrosia artemisiifolia (L.) in Hungary. Redia 2009, 92, 211–213. [Google Scholar]
- Hódi, L. Integrált védelem a parlagfű ellen. Növényvédelem 2009, 45, 485–489, (In Hungarian with an English summary). [Google Scholar]
- Kőmíves, T.; Béres, I.; Reisinger, P.; Lehoczky, É.; Berke, J.; Tamás, J.; Páldy, A.; Csornai, G.; Nádor, G.; Kardeván, P.; et al. A parlagfű elleni integrált védekezés új stratégiai programja. Magy. Gyomkutatás és Technológia 2006, 7, 5–49. [Google Scholar]
- Takács, A.; Jenser, G.; Kazinczi, G.; Horváth, J. Natural weed hosts of tomato spotted wilt virus (TSWV) in Hungary. In Proceedings of the 5th Alps-Adria Scientific Workshop, Opatija, Croatia, 6–10 March 2006. [Google Scholar]
- Didovich, S.V.; Berestetskiy, A.O.; Gasich, E.L.; Pas, A.N.; Alekseenko, O.P. Biologically active microorganisms for inhibition of Ambrosia artemisiifolia L. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 022086. [Google Scholar]
- Farr, D.F.; Bills, G.F.; Chamuris, G.P.; Rossman, A.Y. Fungi on Plants and Plant Products in the United States; APS Press: St. Paul, MN, USA, 1989. [Google Scholar]
- Runion, G.B.; Prior, S.A.; Price, A.J.; McElroy, J.S.; Torbert, H.A. Effects of elevated CO2 on biomass and fungi associated with two ecotypes of ragweed (Ambrosia artemisiifolia L.). Front. Plant Sci. 2014, 5, 500. [Google Scholar] [CrossRef] [PubMed]
- Bohár, G. Biocontrol opportunities against common ragweed (Ambrosia artemisiifolia var. elatior (L.) Descourt.) using plant pathogenic fungi. Növényvédelem 1996, 32, 489–492. [Google Scholar]
- Kiss, L.; Bohár, G. Felhasználható-e a Puccinia xanthii rozsdagomba az ürömlevelű parlagfű (Ambrosia artemisiifolia) elleni védekezés egyik elemeként Európában? Növényvédelem 2009, 45, 419–425. [Google Scholar]
- Kiss, L.; Vajna, L.; Bohár, G. Biological control of common ragweed (Ambrosia artemisiifolia L.). Növényvédelem 2003, 39, 319–331. [Google Scholar]
- Bohár, G. Két polifág kórokozó bioherbicidként történő kísérleti alkalmazása a parlagfű ellen. Növényvédelem 2009, 45, 409–419. [Google Scholar]
- Kiss, L.; Kovács, G.M.; Bóka, K.; Bohár, G.; Bohárné, K.V.; Németh, M.Z.; Takamatsu, S.; Shin, H.-D.; Hayova, V.; Nischwitz, C.; et al. Deciphering the biology of Cryptophyllachora eurasiatica gen. et sp. nov., an often cryptic pathogen of an allergenic weed, Ambrosia artemisiifolia. Sci. Rep. 2018, 8, 10806. [Google Scholar] [CrossRef] [PubMed]
- Kazinczi, G.; Novák, R. (Eds.) A Parlagfű Visszaszorításának Integrált Módszerei; Vidékfejlesztési Minisztérium Élelmiszerlánc-felügyeleti Főosztály, Növény-és Talajvédelmi Osztály: Budapest, Hungary, 2012. [Google Scholar]
- Dorner, Z.; Osman, M.G.A.; Kukorellyné Szénási, Á.; Zalai, M. Assessment of Common Ragweed (Ambrosia artemisiifolia L.) Seed Predation in Crop Fields and Their Adjacent Semi-Natural Habitats in Hungary. Diversity 2024, 16, 609. [Google Scholar] [CrossRef]
- Szigetvári, C.; Benkő, Z.R. Common ragweed (Ambrosia elatior L.). In The Most Important Invasive Plants in Hungary; Botta-Dukát, Z., Balogh, L., Eds.; Institute of Ecology and Botany, Hungarian Academy of Sciences: Vácrátot, Hungary, 2008; pp. 183–203. [Google Scholar]
- Pipper, G.L. The biology and immature stages of Zygogramma suturalis (Fabricius) (Coleoptera: Chrysomelidae). Ohio J. Sci. 1974, 75, 19–22. [Google Scholar]
- Wan, F.; Wang, R.; Ding, J. Biological control of Ambrosia artemisiifolia with introduced insect agents, Zygogramma suturalis and Epiblema strenuana, in China. In Proceedings of the Eighth International Symposium on Biological Control of Weeds, Cordoba, Spain, 3–6 April 1995; Delfosse, E.S., Scott, R.R., Eds.; DSIR/CSIRO: Melbourne, Australia, 1995; pp. 193–200. [Google Scholar]
- Zhou, Z.S.; Chen, H.S.; Zheng, X.W.; Guo, J.Y.; Guo, W.; Li, M.; Luo, M.; Wan, F.H. Control of the invasive weed Ambrosia artemisiifolia with Ophraella communa and Epiblema strenuana. Biocontrol Sci. Technol. 2014, 24, 950–964. [Google Scholar] [CrossRef]
- Iqbal, M.F.; Feng, W.W.; Guan, M.; Xiang, L.Z.; Feng, Y.L. Biological control of natural herbivores on Ambrosia species at Liaoning Province in Northeast China. Appl. Ecol. Environ. Res. 2020, 18, 1419–1436. [Google Scholar] [CrossRef]
- Litto, M.; Bouchemousse, S.; Schaffner, U.; Müller-Schärer, H. Population differentiation in response to temperature in Ophraella communa: Implication for the biological control of Ambrosia artemisiifolia. Biol. Control 2021, 164, 104777. [Google Scholar] [CrossRef]
- Müller-Schärer, H.; Lommen, S.T.E.; Rossinelli, S.; Bonini, M.; Boriani, M.; Bosio, G.; Schaffner, U. Ophraella communa, the ragweed leaf beetle, has successfully landed in Europe: Fortunate coincidence or threat? Weed Res. 2014, 54, 109–119. [Google Scholar] [CrossRef]
- Nduwayo, P.; Sundusin, A.I.B.; Toepfer, S.; Tarigan, S.I.; Doan, N.P.Y.; Dorner, Z.; Iványi, D.; Kiss, J.; Schaffner, U. Host specificity assessments of Ophraella communa for the biocontrol of Ambrosia artemisiifolia in the Pannonian Basin. In Proceedings of the 66th Plant Protection Scientific Days, Budapest, Hungary, 18 February 2025. [Google Scholar]
- Augustinus, B.A.; Guarino, M.F.; Colombo, F.; Citterio, S.; Schaffner, U.; Müller-Schärer, H.; Gentili, R. Spread of Ambrosia artemisiifolia L. and Ophraella communa LeSage in Valtellina (Central Alps, Lombardia). Nat. Brescia. 2015, 39, 45–48. [Google Scholar]
- Mouttet, R.; Augustinus, B.; Bonini, M.; Chauvel, B.; Desneux, N.; Gachet, E.; Le Bourgeois, T.; Müller-Schärer, H.; Thibaudon, M.; Schaffner, U. Estimating economic benefits of biological control of Ambrosia artemisiifolia by Ophraella communa in southeastern France. Basic Appl. Ecol. 2018, 33, 14–24. [Google Scholar] [CrossRef]
- Zadravec, M.; Horvatić, B.; Prpić, P. The Balkans invaded—First record of Ophraella communa LeSage, 1986 (Coleoptera: Chrysomelidae) in Croatia. BioInvasions Rec. 2019, 8, 521–529. [Google Scholar] [CrossRef]
- Zandigiacomo, P.; Boscutti, F.; Buian, F.M.; Villani, A.; Wiedemeier, P.; Cargnus, E. Occurrence of the non-native species Ophraella communa on Ambrosia artemisiifolia in north-eastern Italy, with records from Slovenia and Croatia. Bull. Insectol. 2020, 73, 87–94. [Google Scholar]
- Karrer, G.; Zadravec, M.; Augustinus, B.A.; Müller-Schärer, H.; Sun, Y.; Horvatić, B.; Prpić, P.; Kulijer, D. Eastward spread of the ragweed leaf beetle Ophraella communa towards the Pannonian Plain and the Balkans. Res. Prepr. 2020.
- Petrović-Obradović, O.; Smiljanić, D.; Čkrkić Matijević, M. Ophraella communa (Coleoptera: Chrysomelidae) has arrived in Serbia. Acta Entomol. Serb. 2020, 25, 101–104. [Google Scholar]
- Horváth, D.; Lukátsi, M. First record of Ophraella communa in Hungary (Coleoptera: Chrysomelidae). Folia Entomol. Hung. 2020, 81, 73–79. [Google Scholar] [CrossRef]
- Bosio, G.; Massobrio, V.; Chersi, C.; Scavarda, G.; Clark, S. Spread of the ragweed leaf beetle, Ophraella communa LeSage, 1986 (Coleoptera: Chrysomelidae), in Piedmont Region (northwestern Italy). Boll. Soc. Entomol. Ital. 2014, 146, 17–30. [Google Scholar] [CrossRef]
- Keszthelyi, S.; Kazinczi, G.; Somfalvi-Tóth, K. Geographical dispersion of ragweed leaf beetle (Ophraella communa) based on climatic and biological characters in the Palearctic habitats. Agric. For. Entomol. 2022, 24, 165–185. [Google Scholar] [CrossRef]
- Mattia, I.; De Simone, W.; D’Alessandro, P.; Console, G.; Biondi, M. Investigating the current and future co-occurrence of Ambrosia artemisiifolia and Ophraella communa in Europe through ecological modelling and remote sensing data analysis. Int. J. Environ. Res. Public Health 2019, 16, 3416. [Google Scholar]
- Müller-Schärer, H.; Bouchemousse, S.; Litto, M.; McEvoy, P.B.; Roderick, G.K.; Sun, Y. How to better predict long-term benefits and risks in weed biocontrol: An evolutionary perspective. Curr. Opin. Insect Sci. 2020, 38, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, U.; Steinbach, S.; Sun, Y.; Skjøth, C.A.; de Weger, L.A.; Lommen, S.T.; Augustinus, B.A.; Bonini, M.; Karrer, G.; Šikoparija, B.; et al. Biological weed control to relieve millions from Ambrosia allergies in Europe. Nat. Commun. 2020, 11, 1745. [Google Scholar] [CrossRef] [PubMed]
- Hinz, H.L.; Winston, R.L.; Schwarzländer, M. A global review of target impact and direct nontarget effects of classical weed biological control. Curr. Opin. Insect Sci. 2020, 38, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Paynter, Q.; Paterson, I.D.; Kwong, R.M. Predicting non-target impacts. Curr. Opin. Insect Sci. 2020, 38, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.S.; Weng, X.W.; Will, G.; Wan, F.H. Relationship between host searching and wind direction in Ophraella communa (Coleoptera: Chrysomelidae). Fla. Entomol. 2018, 101, 536–539. [Google Scholar] [CrossRef]
- Dernovici, S.A.; Teshler, M.P.; Watson, A.K. Is sunflower (Helianthus annuus) at risk to damage from Ophraella communa, a natural enemy of common ragweed (Ambrosia artemisiifolia)? Biocontrol Sci. Technol. 2006, 16, 669–686. [Google Scholar] [CrossRef]
- Kontschán, J.; Kerezsi, V.; Bozsik, G.; Kiss, B. New occurrences of the ragweed leaf beetle (Ophraella communa LeSage, 1986) (Coleoptera: Chrysomelidae) in Hungary. Acta Phytopathol. Entomol. Hung. 2021, 56, 181–185. [Google Scholar]
- Iványi, D.; Magyar, B.I.; Dorner, Z.; Schaffner, U.; Zalai, M.; Kiss, J.; Kontschán, J.; Modic, Š.; Nekrep, I.; Razinger, J.; et al. Population dynamics of the rageweed leaf beetle (Ophraella communa) in different climatic regions of central Europe. In Proceedings of the 66th Plant Protection Scientific Days, Budapest, Hungary, 20–21 February 2024. [Google Scholar]
- Wang, C.L.; Chiang, M.Y. New record of a fastidious chrysomelid, Ophraella communa LeSage (Coleoptera: Chrysomelidae), in Taiwan. Plant Prot. Bull. 1998, 40, 185–188. [Google Scholar]
- Takizawa, H.; Saito, A.; Sato, K.; Hirano, Y.; Ohno, M. Invading insect, Ophraella communa LeSage, 1986. Range expansion and life history in Kanto District, Japan. Gekkanlushi 1999, 338, 26–30. [Google Scholar]
- Sohn, J.C.; An, S.L.; Lee, J.E.; Park, K.T. Notes on exotic species, Ophraella communa LeSage (Coleoptera: Chrysomelidae) in Korea. Korean J. Appl. Entomol. 2002, 41, 145–150. [Google Scholar]
- Zhang, L.; Yang, X.; Li, W.; Cui, J. A new record of Ophraella communa of mainland China. Chin. Bull. Entomol. 2005, 42, 227–228. [Google Scholar]
- Tian, T.; Chen, G.; Zhang, Y.; Ma, C.; Tian, Z.; Gao, X.; Chen, H.; Guo, J.; Zhou, Z. Rapid evolution of Ophraella communa cold tolerance in new low-temperature environments. J. Pest Sci. 2022, 95, 1233–1244. [Google Scholar] [CrossRef]
- Horváth, D.; Kazinczi, G.; Keszthelyi, S. A karcsú répabarkó (Coniocleonus nigrosuturatus, Goeze, 1777) A parlagfû természetes ellensége. Növényvédelem 2014, 50, 371–374. [Google Scholar]
- Kiss, L. Hazai parlagfűfogyasztó rovarok. Növényvédelem 2009, 45, 419–425. [Google Scholar]
- Basky, Z. A Magyarországon őshonos levéltetvek hatása a parlagfű (Ambrosia artemisiifolia L.) fejlődésére. Magy. Gyomkut. Technol. 2007, 8, 21–40. [Google Scholar]
- Magyar, D.; Basky, Z. Levéltetűfajok táplálkozásának hatása a parlagfű (Ambrosia artemisiifolia) fejlődésére és pollen kibocsátására üvegházi és szabadföldi kísérletekben. Egészségtudomány 2008, 52, 26–36. [Google Scholar]
- Bálint, J.; Balog, A.; Nyárádi, I. Amit a Növényvédőszer Hatóanyagokról Tudni Kell; Editura University Press-Târgu-Mureş: Târgu-Mureș, Romania, 2012. [Google Scholar]
- Csóka, G.; Szalczer, B.; Hirkó, A. A gyapottok-bagolylepke (Helicoverpa armigera Hbn.), mint a parlagfű (Ambrosia artemisiifolia L.) fogyasztója. Növényvédelem 2009, 45, 433–434. [Google Scholar]
- Jhala, A.J.; Sandell, L.D.; Kruger, G.R. Control of glyphosate-resistant giant ragweed (Ambrosia trifida L.) with 2,4-D followed by pre-emergence or post-emergence herbicides in glyphosate-resistant soybean (Glycine max L.). Am. J. Plant Sci. 2014, 5, 2289–2297. [Google Scholar] [CrossRef]
- Hunyadi, K.; Béres, I.; Kazinczi, G. (Eds.) Gyomnövények, Gyombiológia, Gyomirtás; Mezőgazda Kiadó: Budapest, Hungary, 2011. [Google Scholar]
- Ganie, Z.A.; Jugulam, M.; Jhala, A.J. Temperature influences efficacy, absorption, and translocation of 2,4-D or glyphosate in glyphosate-resistant and glyphosate-susceptible common ragweed (Ambrosia artemisiifolia) and giant ragweed (Ambrosia trifida). Weed Sci. 2017, 65, 588–602. [Google Scholar] [CrossRef]
- Chandi, A.; Jordan, D.L.; York, A.C.; Lassiter, B.R. Confirmation and management of common ragweed (Ambrosia artemisiifolia) resistant to diclosulam. Weed Technol. 2012, 26, 29–36. [Google Scholar] [CrossRef]
- Mueller, T.C.; Main, C.L.; Thompson, M.A.; Steckel, L.E. Comparison of glyphosate-resistant and -susceptible giant ragweed (Ambrosia trifida) populations and response to glyphosate. Weed Sci. 2005, 53, 826–833. [Google Scholar]
- Barnes, E.R.; Knezevic, S.Z.; Sikkema, P.H.; Lindquist, J.L.; Jhala, A.J. Control of glyphosate-resistant common ragweed (Ambrosia artemisiifolia L.) in glufosinate-resistant soybean [Glycine max (L.) Merr.]. Front. Plant Sci. 2017, 8, 1445. [Google Scholar] [CrossRef] [PubMed]
- Nedelcu, C.A.; Lauer, K.F.; Ştef, R. Chemical control with herbicides at species Ambrosia artemisiifolia in Timișoara. Res. J. Agric. Sci. 2010, 42, 122–128. [Google Scholar]
- Ştef, R. Chemical control of the invasive species Ambrosia artemisiifolia L. in sunflower agroecosystem. In Proceedings of the 17th International Multidisciplinary Scientific GeoConference SGEM, Vienna, Austria, 27–29 November 2017. [Google Scholar]
- Týr, S.; Vereš, T.; Lacko-Bartošová, R. Efficacy of herbicides control of common ragweed (Ambrosia artemisiifolia L.). Res. J. Agric. Sci. 2009, 41, 337–340. [Google Scholar]
- Pinke, G.; Karácsony, P.; Botta-Dukát, Z.; Czúcz, B. Relating Ambrosia artemisiifolia and other weeds to the management of Hungarian sunflower crops. J. Pest Sci. 2013, 86, 621–631. [Google Scholar] [CrossRef]
- Kádár, A. Vegyszeres Gyomirtás és Termésszabályozás, 6th ed.; Kádár Aurél: Budapest, Hungary, 2019. [Google Scholar]
- Pinke, G.; Karácsony, P. Napraforgóvetéseink gyomnövényzetének vizsgálata. Növényvédelem 2010, 46, 425–429. [Google Scholar]
- Kolejanisz, T.; Nagy, K.; Bede-Fazekas, Á.; Vér, A.; Pinke, G. Nyárutói gyomnövényzet összetétele az osztrák–magyar határ térségének szántóföldjein. Magy. Gyomkut. Technol. 2020, 21, 3–17. [Google Scholar]
- Kukorelli, G. Weed Management of Herbicide Tolerant Crops, and Their Position in the Hungarian crop Production System. Ph.D. Thesis, University of West-Hungary Faculty of Agricultural- and Food Sciences, Mosonmagyaróvár, Hungary, 2012. [Google Scholar]
- Kömives, T.; Reisinger, P.; Bittsánszky, A. Control of common ragweed in ALS herbicide-resistant sunflower hybrids (Helianthus annuus). Jul.-Kühn-Arch. 2016, 455, 161–165. [Google Scholar]
- Kömives, T.; Simončič, A. Complex Research on Methods to Halt the Ambrosia Invasion in Europe. HALT Ambrosia Deliv. 2012. Available online: https://circabc.europa.eu/sd/a/b2c00515-33e7-41ec-8ab2-442e2a75bfba/F%20Fighting%20Ambrosia%20in%20different%20scenarios.pdf (accessed on 12 July 2025).
- Dávid, I.; Kovács, E. Gyomirtó szerek összehasonlítása napraforgóban kétszikű gyomnövények ellen. J. Agric. Sci. 2012, 50, 137–142. [Google Scholar]
- Kádár, A. (Ed.) Vegyszeres Gyomirtás és Termésszabályozás; Kádár Aurél: Budapest, Hungary, 2024. [Google Scholar]
- Novák, R.; Magyar, M.; Simon, G.; Kadaravek, B.; Kadaravekné Guttyán, A.; Blazsek, K.; Erdélyi, K.; Farkas, G.; Gyulai, B.; Hornyák, A.; et al. A Hatodik Országos Szántóföldi Gyomfelvételezés előzetes eredményei. In Proceedings of the 66th Plant Protection Scientific Days, Budapest, Hungary, 18–19 February 2020. (In Hungarian). [Google Scholar]
- Bonny, S. Genetically modified herbicide-tolerant crops, weeds, and herbicides: Overview and impact. Environ. Dev. Sustain. 2016, 18, 981–1010. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, K.; VanGessel, M. Control of Large Palmer Amaranth and Common Ragweed in Soybean or Corn. Available online: https://extension.umd.edu/resource/control-large-palmer-amaranth-and-common-ragweed-soybean-or-corn-fs-1192 (accessed on 3 April 2025).
- Vivian, R.; Reis, A.; Kálnay, P.A.; Vargas, L.; Ferreira, A.C.C.; Mariani, F. Weed management in soybean—Issues and practices. In Soybean—Pest Resistance; El-Shemy, H.A., Ed.; InTech: Rijeka, Croatia, 2013; pp. 47–84. [Google Scholar]
- Lehoczky, É.; Kerekes, B.; Szabó, R.; Busznyák, J.; Gólya, G. Study on the biomass and seed production of ragweed (Ambrosia artemisiifolia L.) on winter wheat stubble. Növénytermelés 2011, 60, 57–60. [Google Scholar]
- Knolmajer, B. Szőlőültetvények gyomvegetációja és gyomszabályozásának sajátosságai. Magy. Gyomkutatás Technológia 2023, 24, 45–65. [Google Scholar]
- Doma, C. A szőlő növényvédelme II.: Gyomirtás. Növényvédelem 2023, 84, 207–211. [Google Scholar]
- Palma-Bautista, C.; Tahmasebi, B.K.; Fernández-Moreno, P.T.; Rojano-Delgado, A.M.; de la Cruz, R.A.; De Prado, R. First Case of Conyza canadensis from Hungary with Multiple Resistance to Glyphosate and Flazasulfuron. Agronomy 2018, 8, 157. [Google Scholar] [CrossRef]
- Kazinczi, G. A parlagfű biológiai sajátosságaira épülő integrált védekezési eljárások. Magy. Gyomkut. Technol. 2019, 20, 80–81. [Google Scholar]
- Peterson, M.A.; Collavo, A.; Ovejero, R.; Shivrain, V.; Walsh, M.J. The challenge of herbicide resistance around the world: A current summary. Pest Manag. Sci. 2018, 74, 2246–2259. [Google Scholar] [CrossRef] [PubMed]
- Ofosu, R.; Agyemang, E.D.; Márton, A.; Pásztor, G.; Taller, J.; Kazinczi, G. Herbicide resistance: Managing weeds in a changing world. Agronomy 2023, 13, 1595. [Google Scholar] [CrossRef]
- Stephenson, G.R.; Dykstra, M.D.; McLaren, R.D.; Hamill, A.S. Agronomic practices influencing triazine-resistant weed distribution in Ontario. Weed Technol. 1990, 4, 199–207. [Google Scholar] [CrossRef]
- Rios, R.D.; Saione, H.; Robredo, C.; Acevedo, A.; Colombo, N.; Prina, A.R. Isolation and molecular characterization of atrazine tolerant barley mutants. Theor. Appl. Genet. 2003, 106, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Cseh, A.; Taller, J. Genetic diversity of ragweed (Ambrosia artemisiifolia L.): A comparison of the maternally inherited cpDNA and mtDNA. J. Plant Dis. Prot. 2008, 389–394. [Google Scholar]
- Cseh, A.; Cernák, I.; Taller, J. Molecular characterization of atrazine resistance in common ragweed (Ambrosia artemisiifolia L.). J. Appl. Genet. 2009, 50, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Mátyás, K.; Taller, J.; Cseh, A.; Poczai, P.; Cernák, I. Development of a simple PCR-based assay for the identification of triazine resistance in the noxious plant common ragweed (Ambrosia artemisiifolia) and its applicability in higher plants. Biotechnol. Lett. 2011, 33, 2509–2515. [Google Scholar] [CrossRef] [PubMed]
- Saint-Louis, S.; DiTommaso, A.; Watson, A.K. A common ragweed (Ambrosia artemisiifolia) biotype in southwestern Québec resistant to linuron. Weed Technol. 2005, 19, 737–743. [Google Scholar] [CrossRef]
- Solymosi, P. Weeds with sub-specific herbicide resistance. (Szubspecifikus herbicidrezisztenciájú gyomfajok). Növényvédelem 2003, 39, 617–625. [Google Scholar]
- Kutasy, B.; Farkas, Z.; Kolics, B.; Decsi, K.; Hegedűs, G.; Kovács, J.; Taller, J.; Tóth, Z.; Kálmán, N.; Kazinczi, G.; et al. Detection of target-site herbicide resistance in the common ragweed: Nucleotide polymorphism genotyping by targeted amplicon sequencing. Diversity 2021, 13, 118. [Google Scholar] [CrossRef]
- Rousonelos, S.L.; Lee, R.M.; Moreira, M.S.; VanGessel, M.J.; Tranel, P.J. Characterization of a Common Ragweed (Ambrosia artemisiifolia) Population Resistant to ALS- and PPO-Inhibiting Herbicides. Weed Sci. 2012, 60, 335–344. [Google Scholar] [CrossRef]
- Loubet, I.; Caddoux, L.; Fontaine, S.; Michel, S.; Pernin, F.; Barrès, B.; Le Corre, V.; Délye, C. A high diversity of mechanisms endows ALS-inhibiting herbicide resistance in the invasive common ragweed (Ambrosia artemisiifolia L.). Sci. Rep. 2021, 11, 19904. [Google Scholar] [CrossRef] [PubMed]
- Nandula, V.K.; Tehranchian, P.; Bond, J.A.; Norsworthy, J.K.; Eubank, T.W. Glyphosate resistance in common ragweed (Ambrosia artemisiifolia L.) from Mississippi, USA. Weed Biol. Manag. 2017, 17, 45–53. [Google Scholar] [CrossRef]
- Byker, H.P.; Soltani, N.; Nissen, S.J. Mechanisms of glyphosate resistance in common ragweed (Ambrosia artemisiifolia): Patterns of absorption, translocation, and metabolism. Weed Sci. 2022, 70, 151–159. [Google Scholar] [CrossRef]
- Délye, C.; Michel, S.; Pernin, F.; Gautier, V.; Gislard, M.; Poncet, C.; Le Corre, V. Harnessing the power of next-generation sequencing technologies to the purpose of high-throughput pesticide resistance diagnosis. Pest. Manag. Sci. 2020, 76, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Loubet, I.; Meyer, L.; Michel, S.; Pernin, F.; Carrère, S.; Barrès, B.; Le Corre, V.; Délye, C. A high diversity of non-target site resistance mechanisms to acetolactate-synthase (ALS) inhibiting herbicides has evolved within and among field populations of common ragweed (Ambrosia artemisiifolia L.). BMC Plant Biol. 2023, 23, 510. [Google Scholar] [CrossRef] [PubMed]
- Gallina, G.; Cregg, B.; Patterson, E.; Hill, E.; Saha, D. Alternative Integrated Weed Management Options for Clopyralid-Resistant Common Ragweed. Horticulturae 2023, 9, 985. [Google Scholar] [CrossRef]
- Taylor, J.B.; Loux, M.M.; Harrison, S.K.; Regnier, E. Response of ALS-Resistant Common Ragweed (Ambrosia artemisiifolia) and Giant Ragweed (Ambrosia trifida) to ALS-Inhibiting and Alternative Herbicides. Weed Technol. 2002, 16, 815–825. [Google Scholar] [CrossRef]
- Doan Nhu, P.Y.; Toepfer, S.; Dorner, Z.; Tarigan, S.I.; Mmaka, P.; Sundusin, A.I.B.; Kiss, J.; Schaffner, U. Preliminary assessments of integrated weed management options against the invasive ragweed, Ambrosia artemisiifolia, including biological control. Population dynamics of the ragweed leaf beetle (Ophraella communa) in different climatic regions of Central Europe. In Proceedings of the 66th Plant Protection Scientific Days, Budapest, Hungary, 18 February 2025. [Google Scholar]
- Mohácsi, E.; Tóth, Á. Az allergiát kiváltó növényi pollenszennyezés elleni védekezés mezőgazdasági és egészségügyi szempontjai, lehetőségei. In Háttértanulmány; A TEP Egészség és Élettudományok, Valamint Mezőgazdaság, Élelmiszeripar Munkacsoportok Közös Megbízásából: Budapest, Hungary, 1998. [Google Scholar]
- Mahlein, A.K. Plant disease detection by imaging sensors—Parallels and specific demands for precision agriculture and plant phenotyping. Plant Dis. 2016, 100, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.C.; Oliveira Neto, A.M.; Bottega, E.L.; Guerra, N.; Rocha, R.P.; Vilar, C.C. Weed mapping using techniques of precision agriculture. Planta Daninha 2015, 33, 157–164. [Google Scholar] [CrossRef]
- Esposito, M.; Crimaldi, M.; Cirillo, V.; Sarghini, F.; Maggio, A. Drone and sensor technology for sustainable weed management: A review. Chem. Biol. Technol. Agric. 2021, 8, 18. [Google Scholar] [CrossRef]
- Raj, R.; Kar, S.; Nandan, R.; Jagarlapudi, A. Precision agriculture and unmanned aerial vehicles (UAVs). In Unmanned Aerial Vehicle: Applications in Agriculture and Environment; Avtar, R., Watanabe, T., Eds.; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Hunter, J.E.; Gannon, T.W.; Richardson, R.J.; Yelverton, F.H.; Leon, R.G. Integration of remote-weed mapping and an autonomous spraying unmanned aerial vehicle for site-specific weed management. Pest Manag. Sci. 2020, 76, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, P. A precíziós növénytermesztés hazai helyzete, eddig elért fejlesztési eredmények és perspektívák. Magy. Gyomkut. Technol. 2012, 13, 3–19. [Google Scholar]
- Bai, A.; Kovách, I.; Czibere, I.; Megyesi, B.; Balogh, P. Examining the adoption of drones and categorisation of precision elements among Hungarian precision farmers using a trans-theoretical model. Drones 2022, 6, 200. [Google Scholar] [CrossRef]
- Balogh, P.; Bai, A.; Czibere, I.; Kovách, I.; Fodor, L.; Bujdos, Á.; Sulyok, D.; Gabnai, Z.; Birkner, Z. Economic and social barriers of precision farming in Hungary. Agronomy 2021, 11, 1112. [Google Scholar] [CrossRef]
- Reisinger, P.; Borsiczky, I. An overview of the precision plant protection in Hungary, theory and practice. Part II. Növényvédelem 2018, 54, 431–440. [Google Scholar]
- Lottes, P.; Khanna, R.; Pfeifer, J.; Siegwart, R.; Stachniss, C. UAV-based crop and weed classification for smart farming. In Proceedings of the IEEE International Conference on Robotics and Automation (ICRA), Singapore, 29 May–3 June 2017; IEEE: Piscataway, NJ, USA, 2017. [Google Scholar]
- Westwood, J.H.; Charudattan, R.; Duke, S.O.; Fennimore, S.A.; Marrone, P.; Slaughter, D.C.; Swanton, C.; Zollinger, R. Weed management in 2050: Perspectives on the future of weed science. Weed Sci. 2018, 66, 275–285. [Google Scholar] [CrossRef]
- Lanz, B.; Dietz, S.; Swanson, T. The expansion of modern agriculture and global biodiversity decline: An integrated assessment. Ecol. Econ. 2018, 144, 260–277. [Google Scholar] [CrossRef]
- McAllister, W.; Osipychev, D.; Davis, A.; Chowdhary, G. AgBots: Weeding a field with a team of autonomous robots. Comput. Electron. Agric. 2019, 163, 104842. [Google Scholar] [CrossRef]
- Anderson, K.; Gaston, K.J. Lightweight unmanned aerial vehicles will revolutionize spatial ecology. Front. Ecol. Environ. 2013, 11, 138–146. [Google Scholar] [CrossRef]
- Erisman, J.W.; van Eekeren, N.; de Wit, J.; Koopmans, C.; Cuijpers, W.; Oerlemans, N. Agriculture and biodiversity: A better balance benefits both. AIMS Agric. Food 2016, 1, 157–174. [Google Scholar] [CrossRef]
- Ngom, R.; Gosselin, P. Development of a remote sensing-based method to map likelihood of common ragweed (Ambrosia artemisiifolia) presence in urban areas. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 126–140. [Google Scholar] [CrossRef]
- Adhinata, F.D.; Wahyono; Sumiharto, R.S. A comprehensive survey on weed and crop classification using machine learning and deep learning. Artif. Intell. Agric. 2024, 13, 45–63. [Google Scholar] [CrossRef]
- Plaščak, I.; Jurišić, M.; Šiljeg, A.; Jeftić, L.; Zimmer, D.; Barač, Ž. Remote detection of ragweed (Ambrosia artemisiifolia L.). Tech. J. 2018, 12, 226–230. [Google Scholar] [CrossRef]
- Farkas, I.; Erdei, E.; Magyar, D.; Fehér, Z. Anti-ragweed campaign in Hungary in the frame of the National Health Programme. In Ragweed in Europe; Spieksma, M., Ed.; Alk-Abelló A/S: Hørsholm, Denmark, 1998; p. 46. [Google Scholar]
- Thibaudon, M.; Šikoparija, B.; Oliver, G.; Smith, M.; Skjøth, C.A. Ragweed pollen source inventory for France: The second largest centre of Ambrosia in Europe. Atmos. Environ. 2014, 83, 62–71. [Google Scholar] [CrossRef]
- Udvardy, O.; Kajtor-Apatini, D.; Magyar, D.; Szigeti, T. A Magyarországi Aerobiológiai Hálózat Tájékoztatója 2019; Nemzeti Népegészségügyi Központ: Budapest, Hungary, 2020. [Google Scholar]
- Páldy, A.; Bobvos, J.; Apatini, D.; Józsa, E.; Magyar, D.; Mányoki, G.; Novák, E. A klímaváltozás várható hatásának becslése a parlagfű pollenszezon, valamint a kapcsolódó allergiás betegségek jellemzőinek változására 2021–2050 és 2071–2100 között. Assessment of the predicted impact of climate change on the ragweed pollen season and the changes of characteristics of allergic diseases for the periods of 2021–2050 and 2071–2100. Egészségtudomány 2012, 74–97. [Google Scholar]
- Csépe, Z.; Magyar, D.; Mányoki, G.; Bobvos, J.; Elekes, P.; Páldy, A. A polleninformációs szolgáltatás fejlődése Magyarországon. Egészségtudomány 2013, 57, 24–34. [Google Scholar]
- Bodon, D.; Reisinger, P.; Borsiczky, I. A parlagfű (Ambrosia artemisiifolia L.) többszöri kaszálásának és glyphosate-tal történő vegyszeres gyomirtásának hatásvizsgálata. Növényvédelem 2009, 45, 440. [Google Scholar]
- Patkó, Z.; Bozsik, N.; Koncz, G.; Láposi, R. A parlagfű elleni hatósági védekezés vizsgálata Komárom-Esztergom megyében. Acta Carolus Robertus 2018, 8, 193–207. [Google Scholar]
- D’Amico, F.; Besanҫon, T.; Koehler, A.; Shergill, L.; Ziegler, M.; VanGessel, M. Common ragweed (Ambrosia artemisiifolia L.) accessions in the Mid-Atlantic region resistant to ALS-, PPO-, and EPSPS-inhibiting herbicides. Weed Technol. 2024, 38, 30. [Google Scholar] [CrossRef]
- Jin, J.; Zhao, M.; Zhou, Z.; Wang, R.; Guo, J.; Wan, F. Host-Plant Selection Behavior of Ophraella communa, a Biocontrol Agent of the Invasive Common Ragweed Ambrosia artemisiifolia. Insects 2023, 14, 334. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.; Santos, S. Sustainable Approach to Weed Management: The Role of Precision Weed Management. Agronomy 2022, 12, 118. [Google Scholar] [CrossRef]








| Active Ingredient | Sensitivity of Ragweed 1 | Application Time |
|---|---|---|
| fluorochloridone | 8 | PPI, Pre |
| flumioxazin | 7 | Pre, post |
| metobromuron | 6 | Pre |
| haluxifen-methyl (not in just HT hybrids) | 9 | Post |
| imazamox (IMI sunflower) | 8 | Post |
| tribenuron-methyl (SU sunflower) | 8 | Post |
| tribenuron-methyl + thiensulfuron-methyl (SU sunflower) | 8 | Post |
| glyphosate | 9 | Desiccation |
| Active Ingredient | Sensitivity of Ragweed 1 | Application Time |
|---|---|---|
| isoxaflutole + cyprosulfamide | 9 | PP, Pre, Post |
| florasulam + mesotrion | 8 | Pre, Post |
| isoxaflutole + thiencarbazone-methyl + cyprosulfamide | 9 | Pre, Post |
| mesotrion | 7 | Pre |
| sulcotrion + terbuthylazine | 8 | Post |
| sulcotrion | 8 | Post |
| mesotrion + piridate | 7 | Post |
| flufenacet + terbuthylazine | 6 | Post |
| florasulam + fluroxipir | 7 | Post |
| 2,4-D | 7 | Post |
| 2,4-D + dicamba | 8 | Post |
| bentazon + dicamba | 9 | Post |
| dicamba | 9 | Post |
| dicamba+nicosulfuron + prosulfuron | 8 | Post |
| florasulam + 2,4-D | 8 | Post |
| floramsulfurom + izoxadifeni-ethyl | 9 | Post |
| floramsulfurom + izoxadifen-ethyl+iodoszulfuron-methyl-Na | 9 | Post |
| foramsulfuron + thiencarbazone-methyl + iodoszulfuron+cyprosulfamide | 9 | Post |
| clopyralid | 8 | Post |
| foramsulfuron-Na + thiencarbazone-methyl + cyprosulfamide | 9 | Post |
| clopyralid + picrolam | 9 | Post |
| mesotrion + dicambaa | 9 | Post |
| mesotrion + dicamba + nicosulfuron | 8 | Post |
| mesotrion + nicosulfuron | 6 | Post |
| mesotrion+nicosulfuron + prosulfuron | 8 | Post |
| mesotrion + nicosulfuron + rimsulfuron | 6 | Post |
| mesotrion + terbuthylazine | 8 | Post |
| thifensulfuron | 7 | Post |
| nicosulfuron + dicamba | 9 | Post |
| nicosulfuron + rimulfuron + dicamba | 8 | Post |
| prorsulfuron | 9 | Post |
| pyridatee | 6 | Post |
| prosulfuron + dicamba | 9 | Post |
| rimsulfuron + dicamba | 8 | Post |
| tembotrione + izoxadifen-ethyl | 9 | Post |
| tembotrione + thiencarbazone-methyl + izoxadifen-ethyl | 9 | Post |
| thifensulfuron-methyl | 7 | Post |
| tritosulfuron + dicamba | 8 | Post |
| glyphosate | 9 | Desiccation |
| Active Ingredient | Sensitivity of Ragweed 1 | Application Time |
|---|---|---|
| dimethenamid-P + pendimethalin | 6 | Pre |
| flumioxazin | 6 | Pre |
| clomazone | 6 | Pre |
| metribuzin | 6 | Pre |
| metobromuron | 6 | Pre |
| Bentazon + imazamox | 8 | Post |
| imazamox | 8 | Post |
| thifensulfuron-methyl | 7 | Post |
| Active Ingredient | Sensitivity of Ragweed 1 | Application Time |
|---|---|---|
| metsulfuron-methyl + tifensulfuron-methyl | 8 | autumn post |
| flufenacet + metribuzin | 6 | autumn post |
| flumioxazin | 6 | autumn post |
| diflufenican + metsulfuron | 7 | autumn post |
| 2,4-D | 8 | spring post |
| 2,4-D + dicamba | 9 | spring post |
| MCPA | 8 | spring post |
| florasulam + 2,4-D | 8 | spring post |
| florasulam + 2,4-D + aminopyralid | 9 | spring post |
| amidosulfuron + iodosulfuron + mefenipir-diethyl | 9 | spring post |
| aminopyralid + florasulam | 8 | spring post |
| aminopyralid + florasulam + cloquintocet-mexil + pyroxsulam | 6 | spring post |
| bifenox + mecoprop-P | 9 | spring post |
| diflufenican + florasulam | 6 | spring post |
| dicamba | 9 | spring post |
| dicamba + 2,4-D | 9 | spring post |
| dicamba + tritosulfuron | 9 | spring post |
| dichlorprop-P | 8 | spring post |
| dichlorprop-P + MCPA + mecoprop-P | 9 | spring post |
| florasulam | 7 | spring post |
| florasulam + fluroxypyr | 6 | spring post |
| florasulam + haluxifen-methyl | 9 | spring post |
| florasulam + haluxifen-methyl + piroxsulam | 9 | spring post |
| florasulam + cloquintocet-mexil + pinoxaden | 6 | spring post |
| florasulam + metsulfuron-metil + tribenuron | 9 | spring post |
| florasulam + tribenuron-methyl | 8 | spring post |
| florasulam + tritosulfuron | 7 | spring post |
| fluroxipir + clopyralid + MCPA | 9 | spring post |
| fluroxipir + metsulfuron-metil + tribenuron-methyl | 9 | spring post |
| fluroxipir + metsulfuron | 7 | spring post |
| fluroxipir + metsulfuron + thifensulfuron-metil | 8 | spring post |
| mecoprop-P | 9 | spring post |
| iodosulfuron + mefenpir-diethyl + thiencarbazon-methyl | 6 | spring post |
| clopyralid | 8 | spring post |
| cloquintocet-mexil + pinoxaden + piroxsulam | spring post | |
| metsulfuron-methyl | 7 | spring post |
| pyraflufen-ethyl | 6 | spring post |
| metsulfuron-methyl, tribenuron-methyl | 9 | spring post |
| thifensulfuron-methyl + tribenuron-methyl | 7 | spring post |
| tribenuron-methyl | 7 | spring post |
| tritosulfuron | 7 | spring post |
| glyphosate | 9 | PP, stubble treatment |
| Active Ingredient | Sensitivity of Ragweed 1 | Application Time |
|---|---|---|
| fluorochloridone | 8 | Pre |
| flumioxazin | 7 | Pre |
| metobromuron | 6 | Pre |
| prosulfocarb | 6 | Pre |
| clomazone + metribuzin | 7 | Pre |
| glyphosate | 9 | Pre/post |
| metribuzin | 6 | Pre, Post |
| prosulfocarb + metribuzin | 7 | Pre, Post |
| pyraflufen-ethyl | 8 | Desiccant |
| Active Ingredient | Sensitivity of Ragweed 1 | Application Time |
|---|---|---|
| flumioxazin | 7 | Pre (just fruit), Post |
| glyphosate | 9 | Post |
| glyphosate + flazasulfuron | 9 | Post |
| MCPA | 7 | Post |
| flazasulfuron | 8 | Post |
| pelargonic acid | 7 | Post |
| pyraflufen-ethyl (plum, apple, cherry, grape) | 7 | Post |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knolmajer, B.; Jócsák, I.; Taller, J.; Keszthelyi, S.; Kazinczi, G. Common Ragweed—Ambrosia artemisiifolia L.: A Review with Special Regards to the Latest Results in Protection Methods, Herbicide Resistance, New Tools and Methods. Agronomy 2025, 15, 1765. https://doi.org/10.3390/agronomy15081765
Knolmajer B, Jócsák I, Taller J, Keszthelyi S, Kazinczi G. Common Ragweed—Ambrosia artemisiifolia L.: A Review with Special Regards to the Latest Results in Protection Methods, Herbicide Resistance, New Tools and Methods. Agronomy. 2025; 15(8):1765. https://doi.org/10.3390/agronomy15081765
Chicago/Turabian StyleKnolmajer, Bence, Ildikó Jócsák, János Taller, Sándor Keszthelyi, and Gabriella Kazinczi. 2025. "Common Ragweed—Ambrosia artemisiifolia L.: A Review with Special Regards to the Latest Results in Protection Methods, Herbicide Resistance, New Tools and Methods" Agronomy 15, no. 8: 1765. https://doi.org/10.3390/agronomy15081765
APA StyleKnolmajer, B., Jócsák, I., Taller, J., Keszthelyi, S., & Kazinczi, G. (2025). Common Ragweed—Ambrosia artemisiifolia L.: A Review with Special Regards to the Latest Results in Protection Methods, Herbicide Resistance, New Tools and Methods. Agronomy, 15(8), 1765. https://doi.org/10.3390/agronomy15081765









