CRISPR/Cas9-Mediated Knockout of PxPGRP4 Influences Midgut Microbial Homeostasis and Immune Responses in Plutella xylostella
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Preparation of Cry1Ac Protoxin and Bioassays
2.3. Cloning and Sequence Analysis of PxPGRP4
2.4. Spatiotemporal Expression Profile Analysis of PxPGRP4
2.5. PxPGRP4 Fusion Expression and Polyclonal Antibody Preparation
2.6. The Preparation of sgRNA and Cas9 Protein
2.7. sgRNA/Cas9 Protein Microinjection
2.8. Genetic Crosses and PCR-Based Genotyping
2.9. Immunofluorescence Assay
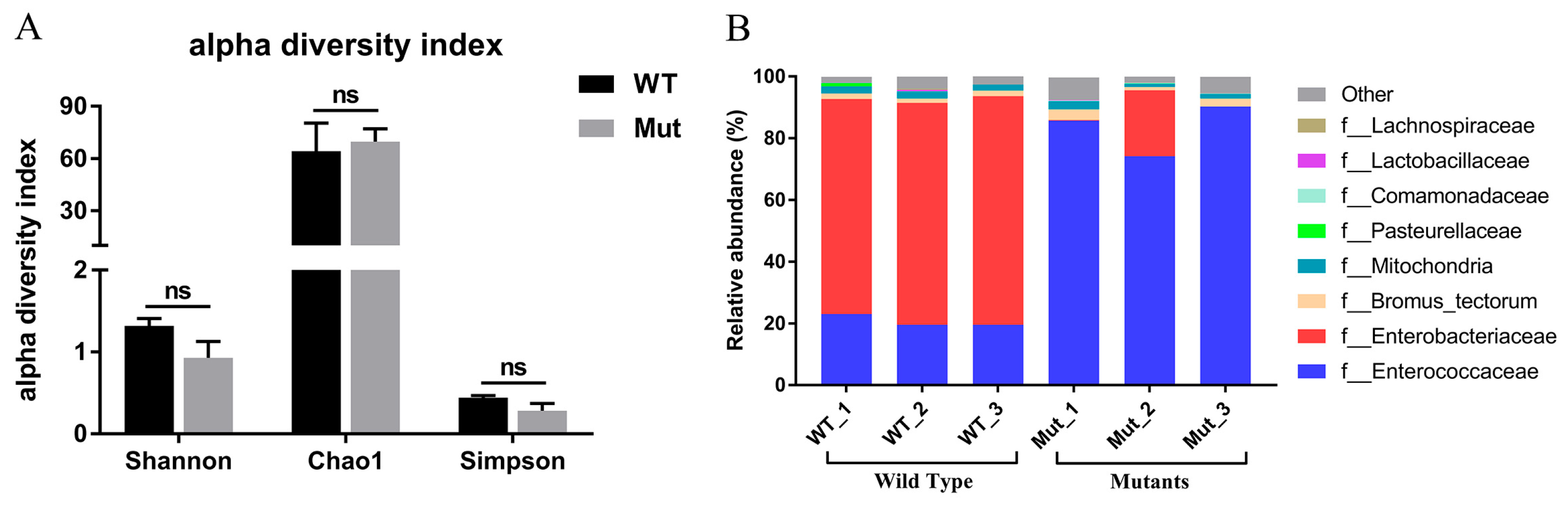
2.10. Midgut Microbiota Analysis
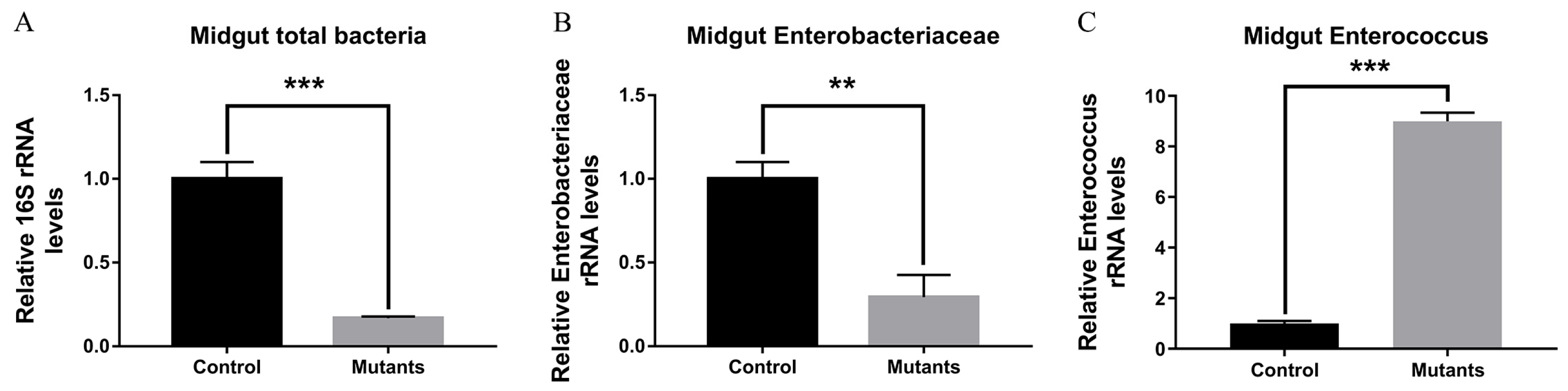
2.11. Bacterial Quantitation and Gene Expression Analysis
2.12. Statistical Analysis
3. Results
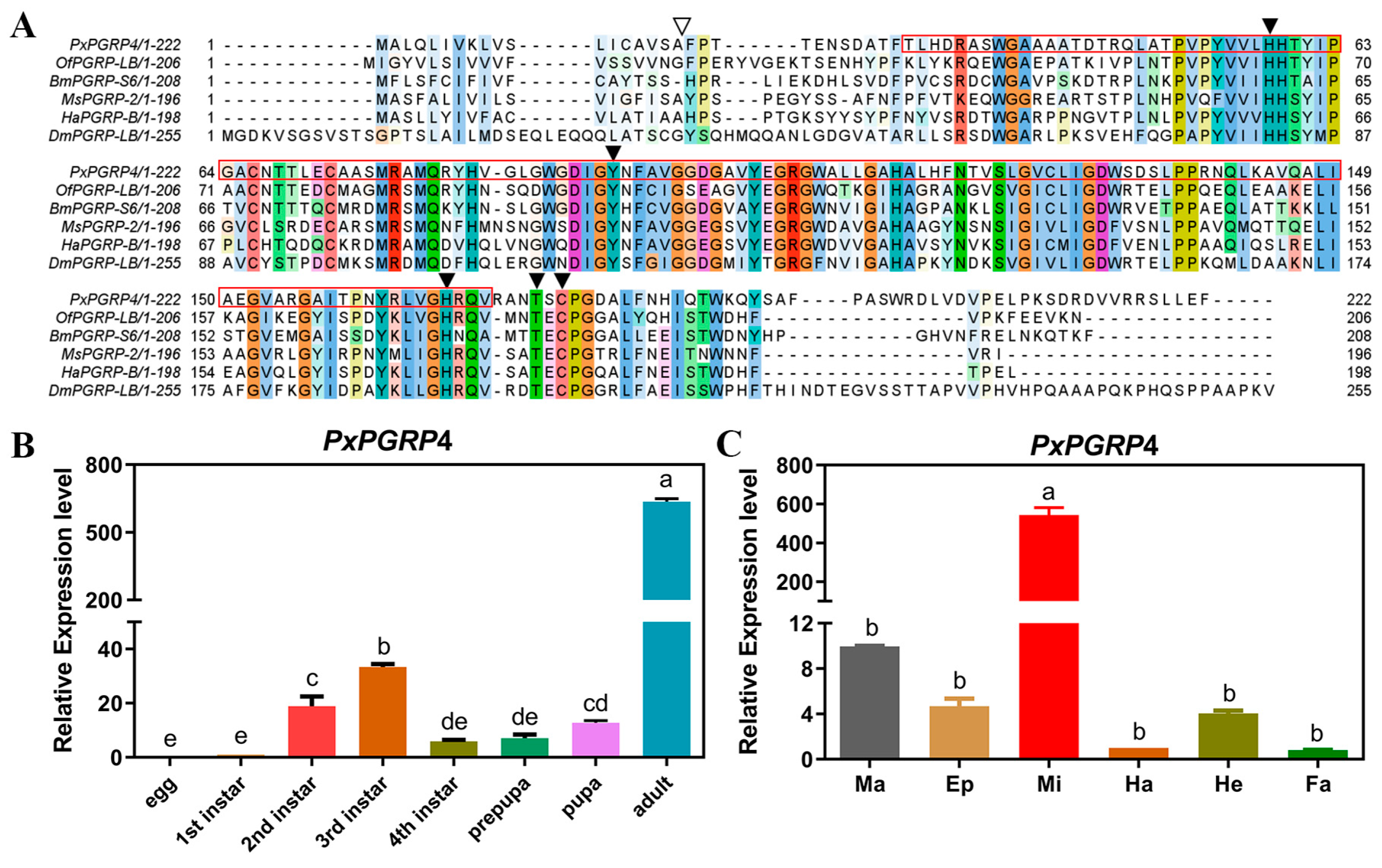
3.1. PxPGRP4 Characterization
3.2. Expression Profile of PxPGRP4
3.3. Recombinant Expression and Purification of PxPGRP4
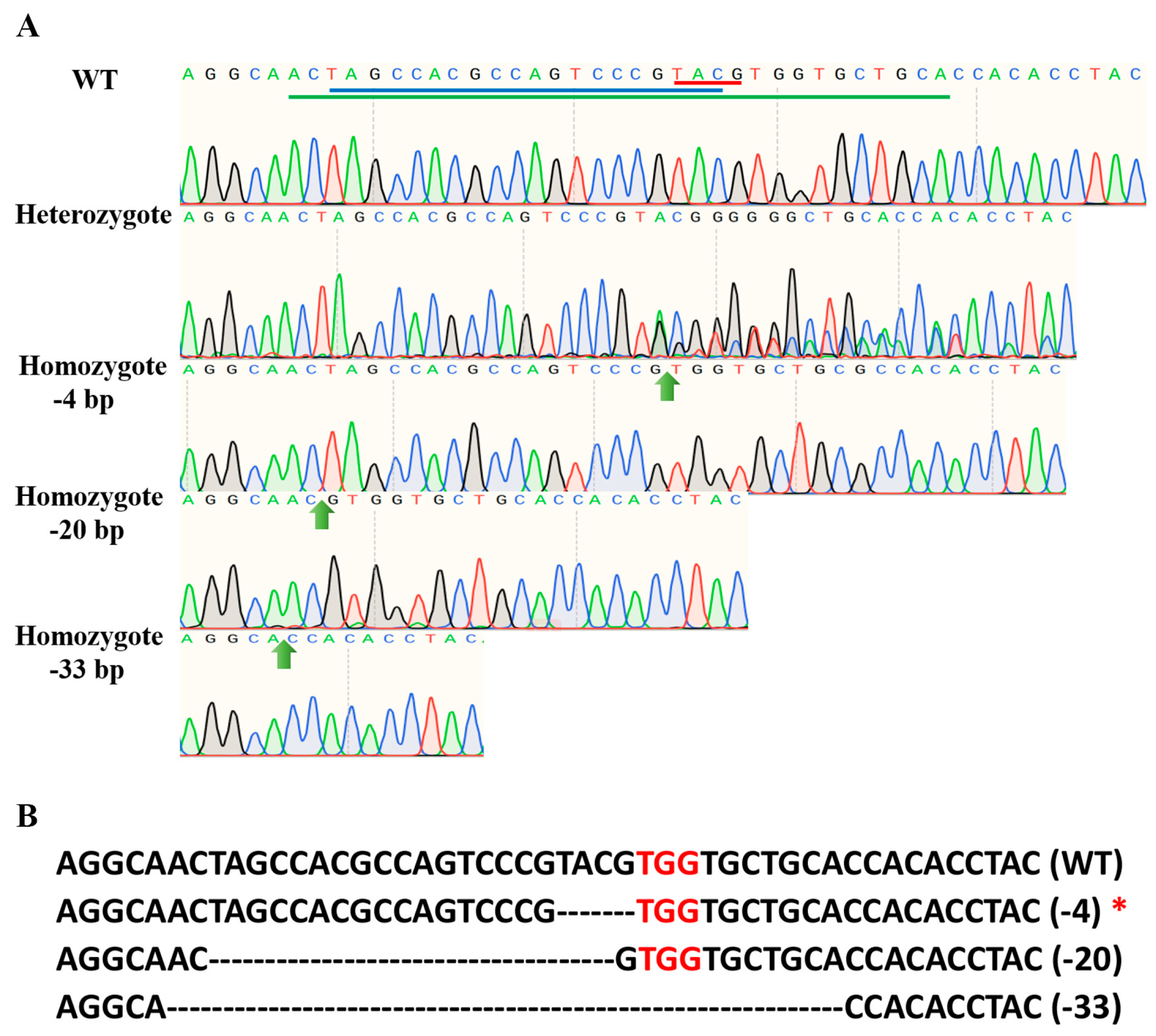
3.4. Establishment of the PxPGRP4 Homozygous Mutant
3.5. PxPGRP4 Knockout Affect Cry1Ac Insecticidal Activity
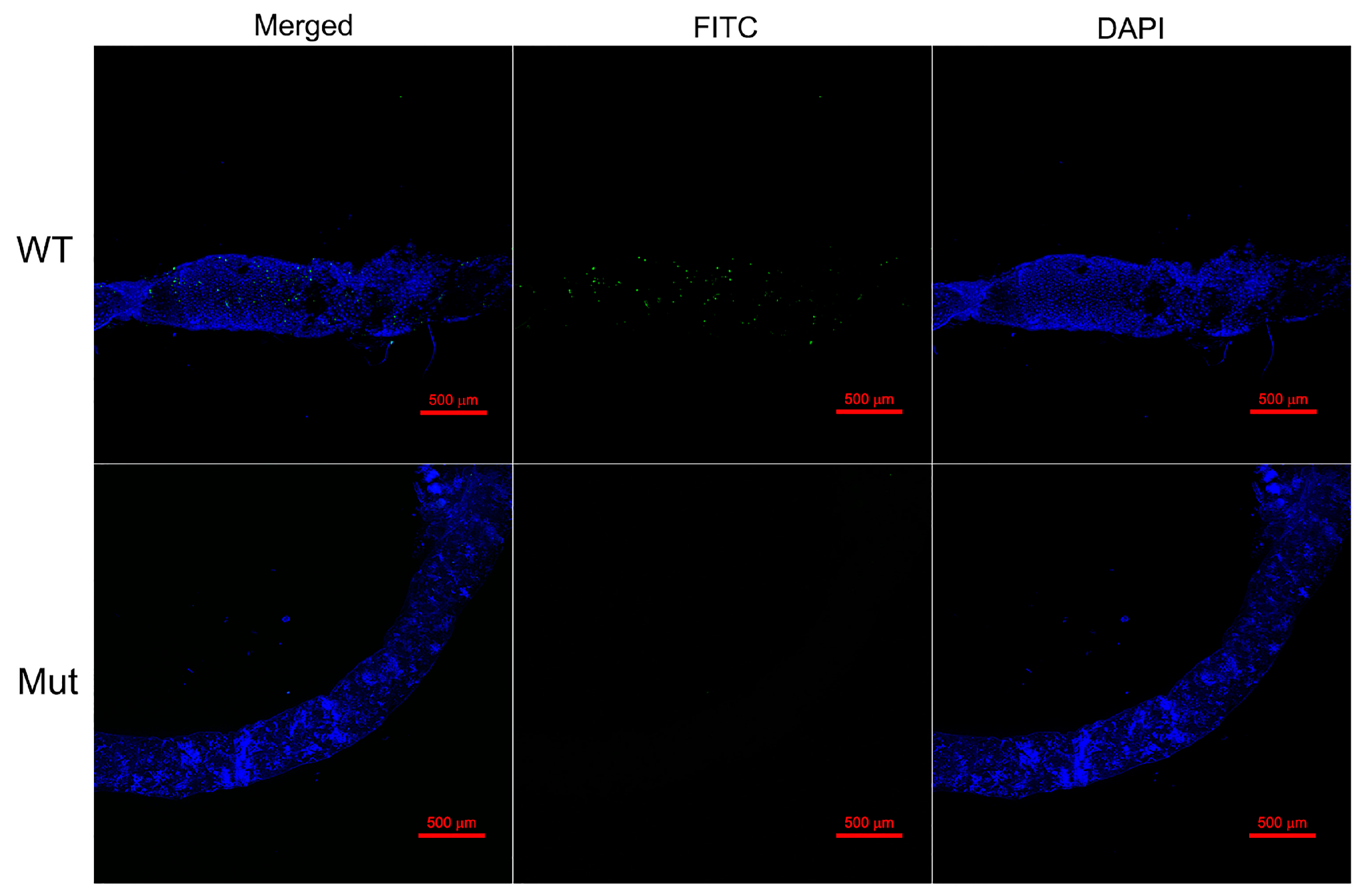
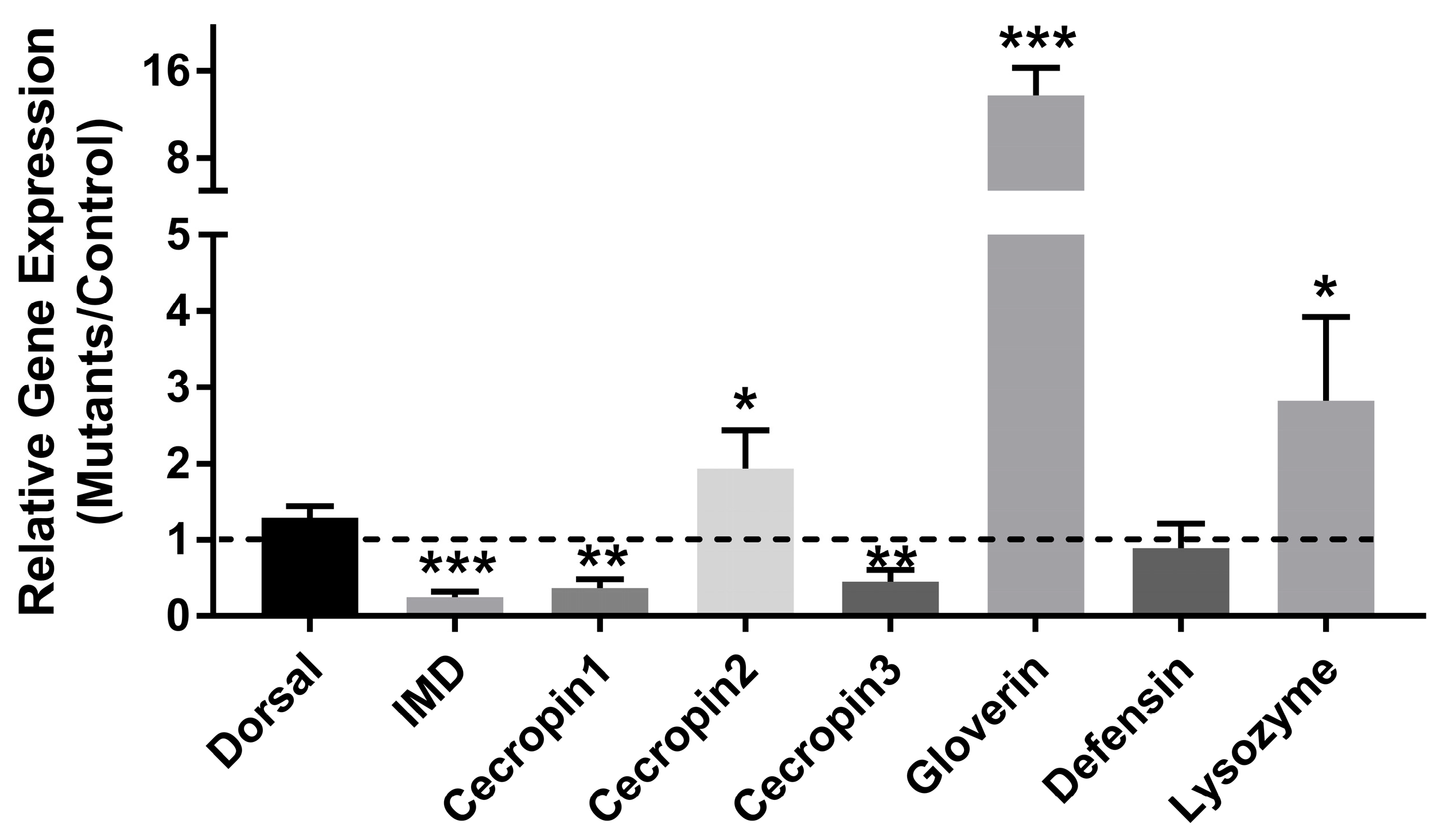
3.6. The Effects of Defective PxPGRP4 on Midgut Homeostasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bravo, A.; Gill, S.S.; Soberon, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Romeis, J.; Meissle, M.; Bigler, F. Transgenic crops expressing Bacillus thuringiensis toxins and biological control. Nat. Biotechnol. 2006, 24, 63–71. [Google Scholar] [CrossRef]
- Bel, Y.; Ferre, J.; Hernandez-Martinez, P. Bacillus thuringiensis toxins: Functional characterization and mechanism of action. Toxins 2020, 12, 785. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chandra, A.; Pandey, K.C. Bacillus thuringiensis (Bt) transgenic crop: An environment friendly insect-pest management strategy. J. Environ. Biol. 2008, 29, 641–653. [Google Scholar]
- Tabashnik, B.E.; Van Rensburg, J.B.; Carriere, Y. Field-evolved insect resistance to Bt crops: Definition, theory, and data. J. Econ. Entomol. 2009, 102, 2011–2025. [Google Scholar] [CrossRef]
- Melo, A.L.; Soccol, V.T.; Soccol, C.R. Bacillus thuringiensis: Mechanism of action, resistance, and new applications: A review. Crit. Rev. Biotechnol. 2016, 36, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; De Mandal, S.; Xu, X.; Jin, F. The tripartite interaction of host immunity-Bacillus thuringiensis infection-gut microbiota. Toxins 2020, 12, 514. [Google Scholar] [CrossRef]
- Caccia, S.; Di Lelio, I.; La Storia, A.; Marinelli, A.; Varricchio, P.; Franzetti, E.; Banyuls, N.; Tettamanti, G.; Casartelli, M.; Giordana, B.; et al. Midgut microbiota and host immunocompetence underlie Bacillus thuringiensis killing mechanism. Proc. Natl. Acad. Sci. USA 2016, 113, 9486–9491. [Google Scholar] [CrossRef]
- Pardo-Lopez, L.; Soberon, M.; Bravo, A. Bacillus thuringiensis insecticidal three-domain Cry toxins: Mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 2013, 37, 3–22. [Google Scholar] [CrossRef]
- Ceja-Navarro, J.A.; Vega, F.E.; Karaoz, U.; Hao, Z.; Jenkins, S.; Lim, H.C.; Kosina, P.; Infante, F.; Northen, T.R.; Brodie, E.L. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat. Commun. 2015, 6, 7618. [Google Scholar] [CrossRef]
- Storelli, G.; Defaye, A.; Erkosar, B.; Hols, P.; Royet, J.; Leulier, F. Lactobacillus plantarum Promotes Drosophila Systemic Growth by Modulating Hormonal Signals through TOR-Dependent Nutrient Sensing. Cell Metab. 2011, 14, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.H.; Yao, Z.C.; Li, Y.S.; Xi, Z.Y.; Bourtzis, K.; Zhao, Z.; Bai, S.; Zhang, H.Y. Intestinal probiotics restore the ecological fitness decline of Bactrocera dorsalis by irradiation. Evol. Appl. 2018, 11, 1946–1963. [Google Scholar] [CrossRef]
- Shin, S.C.; Kim, S.H.; You, H.; Kim, B.; Kim, A.C.; Lee, K.A.; Yoon, J.H.; Ryu, J.H.; Lee, W.J. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 2011, 334, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.F.; Xing, L.S.; Wang, M.L.; Hu, Z.H.; Zou, Z. Microbiota modulates gut immunity and promotes baculovirus infection in Helicoverpa armigera. Insect Sci. 2021, 28, 1766–1779. [Google Scholar] [CrossRef]
- Xia, X.; Sun, B.; Gurr, G.M.; Vasseur, L.; Xue, M.; You, M. Gut microbiota mediate insecticide resistance in the diamondback moth, Plutella xylostella (L.). Front. Microbiol. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Wang, L.Y.; Fan, X.; Yu, C.; Feng, L.; Yi, L. An insight into diversity and functionalities of gut microbiota in insects. Curr. Microbiol. 2020, 77, 1976–1986. [Google Scholar] [CrossRef]
- Yun, J.H.; Roh, S.W.; Whon, T.W.; Jung, M.J.; Kim, M.S.; Park, D.S.; Yoon, C.; Nam, Y.D.; Kim, Y.J.; Choi, J.H.; et al. Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef]
- Hernandez-Martinez, P.; Naseri, B.; Navarro-Cerrillo, G.; Escriche, B.; Ferre, J.; Herrero, S. Increase in midgut microbiota load induces an apparent immune priming and increases tolerance to Bacillus thuringiensis. Environ. Microbiol. 2010, 12, 2730–2737. [Google Scholar] [CrossRef]
- Li, Y.Z.; Zhao, D.; Wu, H.; Ji, Y.J.; Liu, Z.R.; Guo, X.C.; Guo, W.; Bi, Y. Bt GS57 interaction with gut microbiota accelerates Spodoptera exigua mortality. Front. Microbiol. 2022, 13, 835227. [Google Scholar] [CrossRef]
- Li, S.Z.; Xu, X.X.; De Mandal, S.; Shakeel, M.; Hua, Y.Y.; Shoukat, R.F.; Fu, D.R.; Jin, F.L. Gut microbiota mediate Plutella xylostella susceptibility to Bt Cry1Ac protoxin is associated with host immune response. Environ. Pollut. 2021, 271, 116271. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, L.; Wang, Y.; Tang, T.; Liu, F.; Zhang, F. Peptidoglycan recognition protein SC (PGRP-SC) shapes gut microbiota richness, diversity and composition by modulating immunity in the house fly Musca domestica. Insect Mol. Biol. 2023, 32, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Hanson, M.A.; Kondo, S.; Erkosar, B.; Lemaitre, B. Drosophila antimicrobial peptides and lysozymes regulate gut microbiota composition and abundance. mBio 2021, 12, e0082421. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Xiao, X.; Liu, Y.; Zhang, R.; Liu, J.; Liu, Q.; Wang, P.; Cheng, G. Mosquito C-type lectins maintain gut microbiome homeostasis. Nat. Microbiol. 2016, 1, 16023. [Google Scholar] [CrossRef]
- Song, X.; Wang, M.; Dong, L.; Zhu, H.; Wang, J. PGRP-LD mediates A. stephensi vector competency by regulating homeostasis of microbiota-induced peritrophic matrix synthesis. PLoS Pathog. 2018, 14, e1006899. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Xiao, R.; Wang, X.; Xie, E.; Ji, T.; Li, X.; Muhammad, A.; Yin, X.; Hou, Y.; Shi, Z. An IMD-like pathway mediates the intestinal immunity to modulate the homeostasis of gut microbiota in Rhynchophorus ferrugineus Olivier (Coleoptera: Dryophthoridae). Dev. Comp. Immunol. 2019, 97, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Bosco-Drayon, V.; Poidevin, M.; Boneca, I.G.; Narbonne-Reveau, K.; Royet, J.; Charroux, B. Peptidoglycan sensing by the receptor PGRP-LE in the Drosophila gut induces immune responses to infectious bacteria and tolerance to microbiota. Cell Host Microbe 2012, 12, 153–165. [Google Scholar] [CrossRef]
- Li, S.; Xu, X.; Shakeel, M.; Xu, J.; Zheng, Z.; Zheng, J.; Yu, X.; Zhao, Q.; Jin, F. Bacillus thuringiensis suppresses the humoral immune system to overcome defense mechanism of Plutella xylostella. Front. Physiol. 2018, 9, 1478. [Google Scholar] [CrossRef]
- Zhou, Z.S.; Yang, S.J.; Shu, C.L.; Song, F.P.; Zhou, X.P.; Zhang, J. Comparison and optimization of the method for Cry1Ac protoxin preparation in HD73 strain. J. Integr. Agric. 2015, 14, 1598–1603. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. 1925. J. Am. Mosq. Control. Assoc. 1987, 3, 302–303. [Google Scholar]
- Finney, D. Probit Analysis, 3rd ed.; Cambridge University Press: London, UK, 1971. [Google Scholar]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kastrup, J.; Feldt-Rasmussen, U.; Bartram, H.R.; Witten, J.; Sand Hansen, H. An enzyme linked immunosorbent assay (ELISA) for measurement of human serum thyroglobulin. Evaluation of the influence of thyroglobulin auto-antibodies. Scand. J. Clin. Lab. Investig. 1985, 45, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Denman, S.E.; McSweeney, C.S. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. Fems Microbiol. Ecol. 2006, 58, 572–582. [Google Scholar] [CrossRef]
- Royet, J.; Dziarski, R. Peptidoglycan recognition proteins: Pleiotropic sensors and effectors of antimicrobial defences. Nat. Rev. Microbiol. 2007, 5, 264–277. [Google Scholar] [CrossRef]
- Kurata, S. Peptidoglycan recognition proteins in Drosophila immunity. Dev. Comp. Immunol. 2014, 42, 36–41. [Google Scholar] [CrossRef]
- Werner, T.; Liu, G.; Kang, D.; Ekengren, S.; Steiner, H.; Hultmark, D. A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2000, 97, 13772–13777. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, J.; De Mandal, S.; Li, S.; Zheng, Z.; Jin, F.; Xu, X. An immune-responsive PGRP-S1 regulates the expression of antibacterial peptide genes in diamondback moth, Plutella xylostella (L.). Int. J. Biol. Macromol. 2020, 142, 114–124. [Google Scholar] [CrossRef]
- Wang, W.W.; Wang, G.J.; Zhuo, X.R.; Liu, Y.; Tang, L.; Liu, X.S.; Wang, J.L. C-type lectin-mediated microbial homeostasis is critical for Helicoverpa armigera larval growth and development. PLoS Pathog. 2020, 16, e1008901. [Google Scholar] [CrossRef] [PubMed]
- Dawadi, B.; Wang, X.H.; Xiao, R.; Muhammad, A.; Hou, Y.M.; Shi, Z.H. PGRP-LB homolog acts as a negative modulator of immunity in maintaining the gut-microbe symbiosis of red palm weevil, Rhynchophorus ferrugineus Olivier. Dev. Comp. Immunol. 2018, 86, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; Zhou, J.; Zhang, Y.; Ma, Y.; Wang, D.; Jiang, Y.; Shi, S.; Qin, L. Peptidoglycan recognition proteins regulate immune response of Antheraea pernyi in different ways. J. Invertebr. Pathol. 2019, 166, 107204. [Google Scholar] [CrossRef]
- Mason, K.L.; Stepien, T.A.; Blum, J.E.; Holt, J.F.; Labbe, N.H.; Rush, J.S.; Raffa, K.F.; Handelsman, J. From commensal to pathogen: Translocation of Enterococcus faecalis from the midgut to the hemocoel of Manduca sexta. mBio 2011, 2, e00065-11. [Google Scholar] [CrossRef]
- Batool, K.; Alam, I.; Jin, L.; Xu, J.; Wu, C.; Wang, J.; Huang, E.; Guan, X.; Yu, X.Q.; Zhang, L. CTLGA9 interacts with ALP1 and APN receptors to modulate Cry11Aa toxicity in Aedes aegypti. J. Agric. Food Chem. 2019, 67, 8896–8904. [Google Scholar] [CrossRef]
- Gao, M.; Dong, S.; Hu, X.; Zhang, X.; Liu, Y.; Zhong, J.; Lu, L.; Wang, Y.; Chen, L.; Liu, X. Roles of midgut cadherin from two moths in different Bacillus thuringiensis action mechanisms: Correlation among toxin binding, cellular toxicity, and synergism. J. Agric. Food Chem. 2019, 67, 13237–13246. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Kang, S.; Chen, D.; Wu, Q.; Wang, S.; Xie, W.; Zhu, X.; Baxter, S.W.; Zhou, X.; Jurat-Fuentes, J.L.; et al. MAPK signaling pathway alters expression of midgut ALP and ABCC genes and causes resistance to Bacillus thuringiensis Cry1Ac toxin in diamondback moth. PLoS Genet. 2015, 11, e1005124. [Google Scholar] [CrossRef]
- Liao, C.; Jin, M.; Cheng, Y.; Yang, Y.; Soberón, M.; Bravo, A.; Liu, K.; Xiao, Y. Bacillus thuringiensis Cry1Ac protoxin and activated toxin exert differential toxicity due to a synergistic interplay of Cadherin with ABCC transporters in the cotton bollworm. Appl. Environ. Microbiol. 2022, 88, e0250521. [Google Scholar] [CrossRef]
- Qin, J.; Guo, L.; Ye, F.; Kang, S.; Sun, D.; Zhu, L.; Bai, Y.; Cheng, Z.; Xu, L.; Ouyang, C.; et al. MAPK-activated transcription factor PxJun suppresses PxABCB1 expression and confers resistance to Bacillus thuringiensis Cry1Ac toxin in Plutella xylostella (L.). Appl. Environ. Microbiol. 2021, 87, e0046621. [Google Scholar] [CrossRef]
- Guo, L.; Cheng, Z.; Qin, J.; Sun, D.; Wang, S.; Wu, Q.; Crickmore, N.; Zhou, X.; Bravo, A.; Soberon, M.; et al. MAPK-mediated transcription factor GATAd contributes to Cry1Ac resistance in diamondback moth by reducing PxmALP expression. PLoS Genet. 2022, 18, e1010037. [Google Scholar] [CrossRef]
- Xu, L.; Qin, J.; Fu, W.; Wang, S.; Wu, Q.; Zhou, X.; Crickmore, N.; Guo, Z.; Zhang, Y. MAP4K4 controlled transcription factor POUM1 regulates PxABCG1 expression influencing Cry1Ac resistance in Plutella xylostella (L.). Pestic. Biochem. Physiol. 2022, 182, 105053. [Google Scholar] [CrossRef] [PubMed]
- Sarvari, M.; Mikani, A.; Mehrabadi, M. The innate immune gene Relish and Caudal jointly contribute to the gut immune homeostasis by regulating antimicrobial peptides in Galleria mellonella. Dev. Comp. Immunol. 2020, 110, 103732. [Google Scholar] [CrossRef] [PubMed]
| Strains | n | Slope ± SE | LC50 (μg/g) | 95% Fiducial Limits |
|---|---|---|---|---|
| Wild Type | 240 | 1.475 ± 0.178 | 12.153 | 10.024–14.367 |
| Mutant | 240 | 1.767 ± 0.223 | 0.389 | 0.342–0.458 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Xu, X.; Fu, D.; Liu, M.; Feng, C.; Jin, F. CRISPR/Cas9-Mediated Knockout of PxPGRP4 Influences Midgut Microbial Homeostasis and Immune Responses in Plutella xylostella. Agronomy 2025, 15, 1294. https://doi.org/10.3390/agronomy15061294
Li S, Xu X, Fu D, Liu M, Feng C, Jin F. CRISPR/Cas9-Mediated Knockout of PxPGRP4 Influences Midgut Microbial Homeostasis and Immune Responses in Plutella xylostella. Agronomy. 2025; 15(6):1294. https://doi.org/10.3390/agronomy15061294
Chicago/Turabian StyleLi, Shuzhong, Xiaoxia Xu, Dongran Fu, Mingyou Liu, Congjing Feng, and Fengliang Jin. 2025. "CRISPR/Cas9-Mediated Knockout of PxPGRP4 Influences Midgut Microbial Homeostasis and Immune Responses in Plutella xylostella" Agronomy 15, no. 6: 1294. https://doi.org/10.3390/agronomy15061294
APA StyleLi, S., Xu, X., Fu, D., Liu, M., Feng, C., & Jin, F. (2025). CRISPR/Cas9-Mediated Knockout of PxPGRP4 Influences Midgut Microbial Homeostasis and Immune Responses in Plutella xylostella. Agronomy, 15(6), 1294. https://doi.org/10.3390/agronomy15061294







