Abstract
Background: The regulation of oil and protein contents in cottonseed is governed by a complex genetic network. Gaining insight into the mechanisms controlling these traits is necessary for dissecting the formation patterns of cottonseed quality. Method: In this study, Xinluzhong 37 (P1) and Xinluzhong 51 (P2) were selected as parental lines for two reciprocal crosses: P1 × P2 (F1) and its reciprocal P2 × P1 (F1′). Each F1 was selfed and backcrossed to both parents to generate the F2 (F2′), B1 (B1′), and B2 (B2′) generations. To assess nutritional traits in hairy (non-delinted) and lint-free (delinted) seeds, two indicators, oil content and protein content, were measured in both seed types. Joint segregation analysis was employed to analyze the inheritance of these traits, based on a major gene plus polygene model. Results: In the orthogonal crosses, the CVs for the four nutritional traits ranged at 2.710–7.879%, 4.086–11.070%, 2.724–6.727%, and 3.717–9.602%. In the reciprocal crosses, CVs ranged at 2.710–8.053%, 4.086–9.572%, 2.724–6.376%, and 3.717–8.845%. All traits exhibited normal or skewed-normal distributions. For oil content in undelinted/delinted seeds, polygenic heritabilities in the orthogonal cross were 0.64/0.52, and 0.40/0.36 in the reciprocal cross. For protein content, major-gene heritabilities in the orthogonal cross were 0.79 (undelinted) and 0.78 (delinted), while those in the reciprocal cross were both 0.62. Conclusions: Oil and protein contents in cottonseeds are quantitative traits. In both orthogonal and reciprocal crosses, oil content is controlled by multiple genes and is shaped by additive, dominance, and epistatic effects. Protein content, in contrast, is largely controlled by two major genes along with minor genes. In the P1 × P2 combination, major genes act through additive, dominance, and epistatic effects, while in the P2 × P1 combination, their effects are additive only. In both combinations, minor genes contribute through additive and dominance effects. In summary, the oil content in cottonseed is mainly regulated by polygenes, whereas the protein content is primarily determined by major genes. These genetic features in both linted, and lint-free seeds may offer a theoretical foundation for molecular breeding aimed at improving cottonseed oil and protein quality.
1. Introduction
As a major source of edible vegetable oil, the fatty acid composition of cottonseed oil is closely linked to its nutritional value, a feature that has drawn increasing attention in recent years [1]. Cottonseed oil also plays a significant role across various industrial sectors [2,3], with demand rising steadily. For instance, in the bioenergy field, cottonseed oil can be used as a highly efficient biofuel feedstock [4]. In addition to oil, cottonseed protein offers a well-balanced amino acid profile, and research has confirmed several health-related benefits, including physiological functions such as slowing the aging process and helping regulate blood pressure [5]. Notably, as the global population expands and living standards continue to improve, the need for protein and oil in human diets and animal feeds is growing rapidly [6]. In this context, identifying new sources and increasing the availability of such resources has become increasingly pressing [7,8].
Owing to differences in genotype and the estimation methods used, the genetic characteristics of cottonseed oil and protein content show considerable variation. Yuan et al. [9] showed that the oil content was mainly controlled by the additive gene effect. Other studies have also demonstrated that both additive and dominant gene effects are key factors influencing oil content [10,11]. Singh S et al. [12] analyzed combining ability using eight parental lines and 56 derived F1 hybrids. Due to differences in the parental genotypes, the genetic behavior of oil content showed a variation in both additive and non-additive effects, suggesting that the cultivar type has a significant impact on the genetic profile governing cottonseed oil. Du et al. [13] conducted a genome-wide association study (GWAS) on protein, oil, and five fatty acids using 316 germplasm lines and approximately 390K SNPs. The study applied a genome-wide model incorporating fixed gene effects and random gene–environment interactions. The results showed that the protein content was mainly regulated by epistatic effects, while oil content and the levels of most fatty acids (excluding palmitic acid) were largely shaped by the main gene effects. This highlights a clear difference in the genetic control mechanisms of oil and protein traits. In addition, the environment also influences the accumulation of oil and protein content. Gong et al. [14] identified “positive–negative differentiation” effects of daily average and cumulative rainfall on oil content in cotton kernels. A moderate and evenly distributed water supply supported the conversion of photosynthetic products into oil, whereas excessive water disrupted oil synthesis through mechanisms such as dilution and metabolic interference. In addition, Yang Hongkun et al. [15] explored the relationship between soil fertility and cottonseed oil and protein content by designing a study with two fertility gradients, and they found evidence of a correlation. Rochester [16] observed that seeds produced with high nitrogen fertilizer showed reduced seed vigor, an increased protein content, and a decreased oil content. In a separate study, Hu et al. [17] examined the effects of the potassium concentration on oil and protein contents using low potassium-tolerant and low potassium-sensitive cotton varieties. Three potassium levels were tested. The results indicated that potassium application significantly raised oil and non-structural carbohydrate levels but did not change the protein content. Altogether, these studies suggest that both genetic factors and environmental influences contribute to the variation in cottonseed oil and protein content. Continued investigation into these traits will support breeding programs aimed at developing improved cultivars.
The determination of cottonseed protein and oil contents involves multiple technical approaches. The commonly used methods for assessing the oil content include near-infrared spectroscopy [18] and Soxhlet extraction [19]. For the protein content, both the Kjeldahl method [20] and near-infrared spectroscopy are employed. In this study, the oil content in cottonseeds was determined using the Soxhlet extraction method, and the protein content was measured by the Kjeldahl nitrogen determination method.
Quantitative traits are shaped by numerous genes [21], with some exerting major effects and others contributing minor effects [22]. To address this complexity, a model known as the major gene plus polygene model was proposed [23] and has gained wide acceptance in the academic community. For instance, Professor Gai’s research team [24] systematically demonstrated the broad applicability of this mixed genetic model in analyzing quantitative traits. Within this framework, researchers can estimate the number and effects of major genes, assess polygenic influence, and calculate components of genetic variance. This model has been applied to crops including rice [25,26], wheat [27], maize [28,29], and soybean [30,31]. In cotton, previous genetic studies have primarily focused on yield-related traits [32], resistance to verticillium wilt [33], and morphological characteristics [34,35]. Although some progress has been made in analyzing oil and protein traits in cottonseed, significant knowledge gaps remain. These traits are generally recognized as quantitative and controlled by multiple genes, likely following a mixed model involving both major genes and polygenes. However, detailed studies on the genetic structure underlying these traits remain limited. As such, the objective of this study was to systematically analyze the genetic mechanism of cottonseed oil and protein traits in linted seeds and lint-free seeds, and to provide a theoretical basis for molecular breeding of cotton oil and protein quality improvement.
2. Materials and Methods
2.1. Test Materials
In this study, the long fruiting-branch upland cotton variety Xinluzhong 37 was used as the female parent (P1), while the short fruiting-branch upland cotton variety Xinluzhong 51 served as the male parent (P2). During the process of combinatorial configuration, as shown in Figure 1, the P1 and P2 lines were crossed to produce the F1 generation, which was then self-pollinated to generate the F2 population. The F1 plants were used as female parents also backcrossed to both P1 and P2, producing the B1 and B2 generations, respectively. These six generations (P1, P2, F1, F2, B1, B2) form the basis for joint segregation analysis. To establish a reciprocal cross population, the parental roles of Xinluzhong 37 and Xinluzhong 51 were reversed, producing six additional generations: P1, P2, F1′, F2′, B1′, and B2′. The same steps of hybridization, self-pollination, and backcrossing were followed to develop these generations (in the reverse cross combinations, the F1′ lines were also used as female parents to backcross with P1 and P2, respectively, generating B1′ and B2′). Both Xinluzhong 37 [36] and Xinluzhong 51 [37], used in the construction of these genetic populations, have been approved by the Crop Variety Appraisal Committee of the Xinjiang Uygur Autonomous Region.
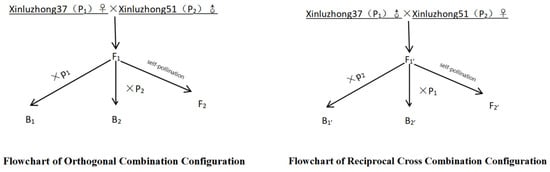
Figure 1.
Combination configuration flow chart.
2.2. Field Trials
On 7 April 2023, seeds from six generations of upland cotton were sown at the experimental site of the 12th Regiment of Tarim University, located in Alar City, Xinjiang. Planting areas were arranged according to generation, and each generation was grown in a separate experimental plot. A randomized block design was employed. The row–column planting layout included three replicates per generation. Each replicate consisted of three planting areas: the border row, middle row 1, and middle row 2, which helped control edge effects and improve the comparability of data. During the cottonseed quality assessment phase, each sample underwent two independent measurements of oil and protein contents. These repeated measurements were analyzed using statistical methods to ensure accuracy and reliability. The soil at the site is predominantly saline-alkali, and cotton had been grown as the previous crop. Each test plot was 5 m in length. The row spacing configuration was set as (10 + 66 + 10 + 66 + 10) cm, with a plant spacing of 10 cm. Seeds were sown manually, using a six-row, one-mold planting method. During the growth and development of cotton, we watered 11 times and used urea and other fertilizers according to the needs of the trial. Field management practices followed standard field production procedures.
2.3. Material Selection
In both the orthogonal and reciprocal crosses, 25 cotton plants with uniform growth were selected from each of the P1, P2, and F1 generations. For the B1, B2, and F2 generations, 150 plants were initially selected. During the evaluation process, individuals exhibiting clear signs of dwarfism, weak growth, yellowing or curling leaves, or visible disease and insect damage were identified and excluded, as these abnormalities could compromise the accuracy of the results. Plants showing unrepresentative traits, such as bent or twisted stems, irregular leaf morphology, or malformed flowers and buds, were also eliminated. In total, 1000 plants were sampled. After removing 175 diseased or abnormal individuals, 825 remained. Final sample sizes were as follows: in the orthogonal cross, 25 plants for P1, 22 for P2, and 23 for F1; 97 for F2, 123 for B1, and 142 for B2. In the reciprocal cross, 22 plants for P1, 25 for P2, 22 for F1′, 97 for F2′, and 137 each for B1′ and B2′.
2.4. Cotton Growth and Development Period
Table 1 shows the timeline and duration of the cotton growth stages, from sowing to harvest.

Table 1.
Cotton growth stages and time periods.
2.5. Crop Management
In this study, drip fertigation technology was used for a total of 11 applications. The fertilizers used included urea (N ≥ 46.4%) as the nitrogen source, monoammonium phosphate (N-P2O5-K2O ≥ 58%) for phosphorus, and potassium sulfate (K2O ≥ 50%) for potassium. The irrigation timing, along with the quantity of water and fertilizer used in each application, is detailed in Table 2.

Table 2.
Calendar of fertilizer applications.
2.6. Temperature Changes and Precipitation at the Test Site
Figure 2 presents the meteorological profile of the experimental site, illustrating diurnal patterns of precipitation, humidity, and temperature. Precipitation levels varied by the hour, with occasional peaks reaching 1.60 mm/h, though most time periods recorded no rainfall. Humidity showed broad fluctuations over the course of the day, ranging from 2.01% to 92.45%, reflecting shifts in environmental conditions. Temperature followed a distinct diurnal cycle, ranging from 1.09 °C to 40.09 °C, typically increasing during daylight hours and decreasing at night. These daily changes in weather conditions shaped the broader climatic environment experienced at the cotton trial site.
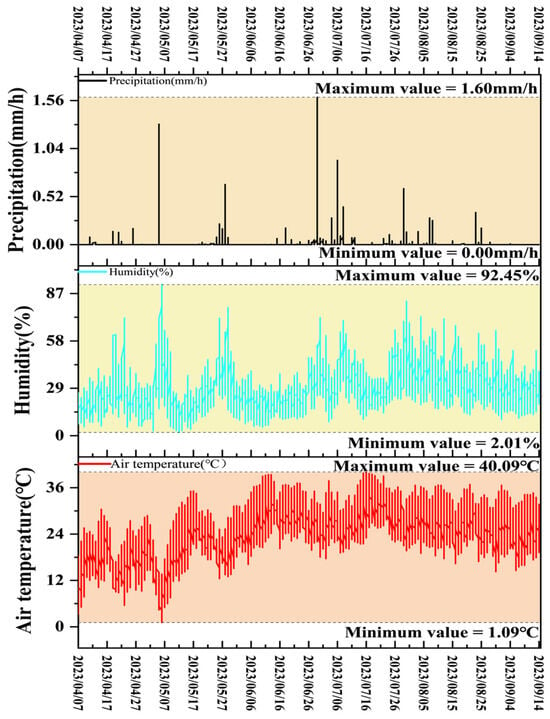
Figure 2.
Weather change diagram of the test site. Note: The temperature and precipitation data referenced here were obtained from the Xihe Energy Meteorological Big Data Platform (https://xihe-energy.com/). Precipitation refers to the total liquid or melted solid water falling to the ground and accumulating on a horizontal surface without being lost to evaporation, infiltration, or runoff. Air temperature is measured within a louvered box located approximately 1.5–2 m above the ground. Humidity refers to the relative humidity measured at a height of 1.25–2 m above the ground.
2.7. Trait Determination
2.7.1. Sampling of Undelinted and Delinted Cotton Seeds
After harvesting, mature seed cotton was dried and processed using a portable cotton lint tester (Model: MX-20, Hebei, China) to separate fiber from seed, yielding undelinted (hairy) cottonseeds. These seeds were then screened to remove debris, aborted seeds, and other impurities. The intact seeds were split into two groups: One was used to determine the nutritional traits of hairy seeds. The other group was delinted with sulfuric acid, followed by repeated cleaning and drying. Afterward, impurities and desiccated seeds were removed once more, and only full, healthy lint-free seeds were retained. For each plant, 10–15 g of cottonseed was prepared based on single-plant sampling.
2.7.2. Determination of Oil Content of Undelinted and Delinted Seeds
In this experiment, the Soxhlet extraction method was used to determine the oil content of cottonseed. The procedure was as follows: A weighing bottle and filter paper were placed in an oven at 105 °C and dried for 2 h. After drying, they were removed, cooled to room temperature, and weighed. This mass was recorded as m1. One gram of cottonseed powder (prepared by grinding the seed into a fine powder capable of passing through a 40-mesh sieve) was weighed and wrapped in filter paper. The wrapped sample was sealed, placed in the weighing bottle, and dried in the oven at 105 °C for 3 h to eliminate excess moisture. After drying, the sample was cooled to room temperature and weighed again, recorded as m2. The net mass of cottonseed powder was calculated as (m2 − m1) grams. Then it was placed in the extraction cylinder and injected with anhydrous ether to cover the sample. After a 12 h soak, the ether was drained into the extraction flask. An additional amount of anhydrous ether was added to ensure complete immersion of the sample. The apparatus was then assembled, and the condenser was connected to flowing water. The water bath was heated, maintaining a bath temperature between 65 °C and 80 °C. Extraction was carried out for 8 h, during which the condensed ether formed beads beneath the condenser. After extraction, the sample package was removed and placed in a well-ventilated area to allow the solvent to evaporate. It was then returned to the original weighing bottle and dried again in the oven at 105 °C for 2 h. Once dried, it was cooled to room temperature and weighed. This final mass was recorded as m3. The oil content (%) was then calculated using the following formula:
Cottonseed oil content (%) = (m2 − m3)/(m2 − m1) × 100
The method for determining the oil content of lint-free seeds is the same as above.
2.7.3. Determination of Protein Content of Undelinted and Delinted Seeds
Protein content in cottonseed was determined using the Kjeldahl method. The analysis was performed with the Autokjeldahl Unit K-370, manufactured by BUCHI (Shanghai, China). Cottonseeds were ground into a fine, uniform powder. All samples were sealed and subjected to a water balance treatment for more than 48 h. From each sample, 5 g was weighed and dried at 130 °C for one hour, then weighed again to determine the moisture content. Next, 0.5 g of the sample (accurate to 0.0002 g) was precisely weighed and transferred into a digestion tube along with two catalyst tablets (copper sulfate and potassium sulfate in a 1:5 ratio). Then, 12 mL of concentrated sulfuric acid was added. The tube was gently shaken to ensure the sample was fully soaked with acid. The digestion tube was placed in a digestion furnace and occasionally shaken until carbonization occurred and foaming ceased. The temperature was then increased to 400 °C and maintained until the digest turned a clear blue–green. Digestion was continued for one additional hour. After digestion, the tube was cooled to room temperature for 15 min. Then, 50–100 mL of distilled water was added and shaken to fully dissolve the sulfates. After cooling, two zeolite chips were added. A receiving bottle was prepared with 50 mL of 4% boric acid solution and 5 drops of a mixed indicator (methyl red 0.1% ethanol solution and bromocresol green 0.5% ethanol solution, mixed in equal volumes). This bottle was positioned beneath the condenser, ensuring that the condenser outlet was submerged below the liquid level. Next, 80 mL of 40% NaOH solution was carefully poured down the side of the digestion flask. The distillation unit was then connected, and distillation was started. Steam passed through the condenser into the collection bottle until approximately 150 mL of distillate had been collected. At that point, the bottle was lowered to allow the condenser outlet to rise above the liquid level, and distillation was continued for an additional minute. The condenser outlet was rinsed with distilled water, and the rinse was also collected in the absorption bottle. Distillation was then stopped. Immediately afterward, the distillate was titrated with 0.1 mol/L hydrochloric acid. The endpoint of titration was reached when the solution changed from blue–green to light purple, and the volume of acid consumed was recorded. A blank control was performed using 0.5 g of sucrose instead of a seed sample, following the same procedure. The blank value for hydrochloric acid consumption did not exceed 0.2 mL. The protein content was then calculated using the following formula:
Cottonseed protein content (%) = (T − B) × N × 14.007 × 100/sample (mg) × F
Here, T represents the volume of hydrochloric acid used for titrating the sample; B is the volume of hydrochloric acid used in the blank test; N refers to the number of moles of hydrochloric acid; and F is the protein conversion factor, with a value of 6.25 applied for hairy seed samples.
The method used to determine the protein content of lint-free seeds was identical to that used for hairy seeds. The procedures for determining cottonseed oil and protein content were mainly based on the experimental protocols described by Guo Tingting [38] and Meng Fanqi [39].
2.8. Statistical Analysis of Data
Data were first categorized and organized using EXCEL software. The processed data were then analyzed using IBM SPSS Statistics 25 [40]. For each of the four nutritional quality traits, we calculated the mean, standard deviation, coefficient of variation, and skewness. Origin 2025 software [41] was used to generate box plots and normal distribution curves. These graphical outputs were combined with the results from IBM SPSS Statistics 25 to assess whether the data followed a normal distribution. To further examine the genetic structure, prior studies have shown that Mather and Jinks [42] reinterpreted the theoretical model originally proposed by Cavalli [43], drawing on earlier research. Building on this, researchers such as Gale [44] conducted comparative analyses of Cavalli’s “joint scaling tests”, which aim to determine whether genetic variation is governed by simpler or more complex regulatory patterns through a comparison of generational means. Based on this theoretical foundation, the present study applies a mixed major gene–polygene model and uses SEA 2.0 R software, developed by Wang Jingtian et al. [45], for analysis. A joint segregation analysis was conducted on six generations from the orthogonal cross, P1, P2, F1, B1, B2, and F2, covering four nutritional traits: oil content in undelinted seeds, protein content in undelinted seeds, oil content in delinted seeds, and protein content in delinted seeds. A corresponding analysis was also performed on the six generations from the reciprocal cross: P1, P2, F1′, F2′, B1′, and B2′. Through computational analysis, 24 AIC (Akaike Information Criterion) values were generated for different genetic models, which were grouped into five categories: no major gene, one pair of major genes, two pairs of major genes, one pair of major genes plus polygenes, and two pairs of major genes plus polygenes. In parallel, maximum likelihood function values (MLVs) were calculated using the maximum likelihood method. Following the principle of minimum AIC [46], three candidate models were selected from the 24 possibilities. The selection aimed to balance model fit with biological plausibility to improve the reliability of the outcomes. These three models were then subjected to further goodness-of-fit tests, including uniformity tests U12, U22, and U32, along with the Smirnov test (nW2) and Kolmogorov test (Dn), using relevant statistical measures [22]. Following this multi-step evaluation process, the optimal genetic model was identified. Genetic parameters corresponding to the selected model were then calculated, including the additive effects of major genes (da, db), dominant effects (ha, hb), additive × additive interaction (i), additive × dominant interaction (jab), dominant × additive interaction (jba), and dominant × dominant interaction (l). In addition, the variances due to major genes (σ2mg) and polygenes (σ2pg), as well as the heritabilities of major genes (h2mg) and polygenes (h2pg), were estimated. These parameters provide a data-driven foundation for subsequent research efforts.
3. Results
3.1. Phenotypic Data Analysis of Nutritional Traits of Orthogonal and Reverse Cross Cottonseed
The data were categorized into orthogonal and reciprocal crosses (Table 3) and included statistics across multiple generations [P1, P2, F1 (F1′), B1 (B1′), B2 (B2′), F2 (F2′)]. The traits analyzed were the mean, standard deviation (SD), coefficient of variation (CV%), and p values from the Kolmogorov–Smirnov (K–S) test. The results showed that the oil content in hairy seeds differed between the orthogonal F1 generation (17.744%) and reciprocal F1′ generation (18.474%). Similar trends were observed across generations, suggesting that inheritance of the oil content may be affected by the direction of the cross. The protein content in hairy seeds exhibited significant generational fluctuations, with values rising from 17.993% in F1 to 19.921% in F2 (orthogonal cross). Some generations had CVs greater than 10%, indicating considerable individual variation. For lint-free seeds, the oil content ranged from 20.786% to 22.707% in the orthogonal cross and 20.786% to 22.070% in the reciprocal cross, showing both generational and directional differences that reflect the complexity of the trait’s genetic basis. The protein content in lint-free seeds followed different trends in each cross type: in the orthogonal group, values increased from F1 (21.511%) to F2 (23.522%), while in the reciprocal group, values decreased from F1 (22.886%) to F2 (21.296%). These contrasting trends further suggest that the cross direction influences trait expression.

Table 3.
Analysis of phenotypic data distribution.
The Kolmogorov–Smirnov (K–S) test was used to examine the normality of the four traits. As shown in Table 3, all traits had p values > 0.05, indicating no significant deviation from normality. To further assess this, box plots and normal distribution curves were generated (Figure 3 and Figure 4). Together with the K–S results, the data show that the traits follow normal or skewed normal distributions in both orthogonal and reciprocal crosses. In quantitative genetics, traits controlled by multiple genes with cumulative effects typically display continuous variation and often conform to normal or near-normal distributions. The four traits examined in this study exhibit such patterns, consistent with quantitative inheritance. Therefore, a major gene + polygene model can be appropriately used to analyze the genetic architecture of cottonseed nutritional traits.
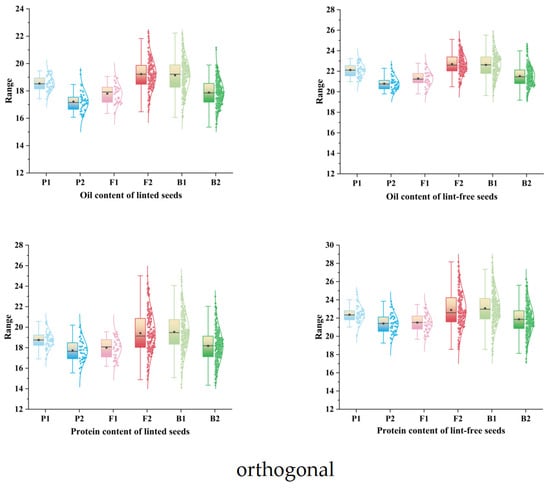
Figure 3.
Box line plots and normal curves for the four nutritional traits of cotton seed (orthogonal).
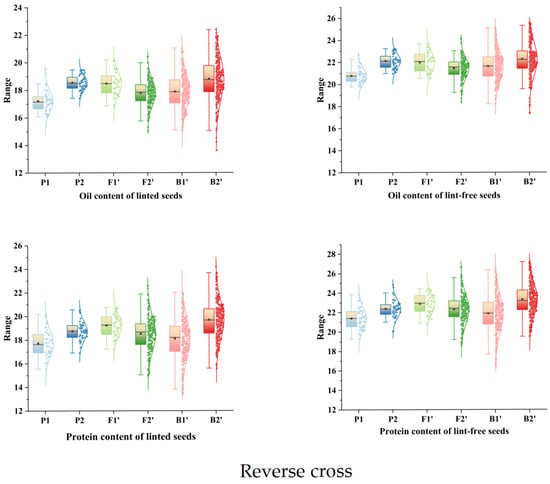
Figure 4.
Box line plots and normal curves for the four nutritional traits of cotton seed (reverse cross).
3.2. Selection of Genetic Model
To investigate the genetic characteristics of cottonseed nutritional traits, the SEA 2.0 software was used to analyze the mixed genetic model of “major gene + polygene” across six generations from both orthogonal and reciprocal crosses. A total of 24 genetic models were generated, falling into five categories (Table 4 and Table 5). The maximum likelihood values (MLVs) and AIC values were calculated for each model, and the three models with the lowest AIC values were selected as candidates for further analysis.

Table 4.
Maximum likelihood values (MLVs) and AIC values of 24 genetic models for 4 orthogonal cottonseed nutritional traits.

Table 5.
Maximum likelihood values (MLVs) and AIC values of 24 genetic models for 4 cottonseed nutritional traits in reverse cross.
For the orthogonal cross (Table 4), among the four traits studied, we found the following: For the oil content of linted seeds, the selected models were PG-ADI, MX1-AD-ADI, and MX2-ADI-ADI, with AIC values of 2458.531, 2462.531, and 2470.933, respectively. For the protein content of linted seeds, PG-ADI, MX2-ADI-AD, and MX2-A-AD were selected, with AIC values of 3324.050, 3314.668, and 3322.650. For the oil content of lint-free seeds, PG-ADI, MX1-AD-ADI, and MX2-ADI-ADI were identified as candidates, with AIC values of 2404.175, 2408.175, and 2406.940. For the protein content of lint-free seeds, PG-ADI, MX2-ADI-AD, and MX2-A-AD were selected, showing AIC values of 3349.196, 3343.842, and 3345.980.
For the reciprocal cross (Table 5), the model selection for the four traits was as follows: For the oil content of linted seeds, the selected models were PG-ADI, MX1-AD-ADI, and MX2-ADI-AD, with AIC values of 2731.657, 2735.657, and 2744.731. For the protein content of linted seeds, PG-ADI, MX2-ADI-AD, and MX2-A-AD were chosen, with AIC values of 3218.053, 3217.306, and 3208.990. For the oil content of lint-free seeds, PG-ADI, MX1-AD-ADI, and MX2-ADI-ADI were selected, with AIC values of 2786.914, 2790.914, and 2797.376. For the protein content of lint-free seeds, the selected models were PG-ADI, MX2-ADI-AD, and MX2-A-AD, with AIC values of 3301.460, 3298.802, and 3291.363.
3.3. Suitability Test of Orthogonal Alternative Model and Determination of Final Model
To determine the optimal genetic model for the four cottonseed nutritional traits, each candidate model was subjected to a fitness test, with the results summarized in Table 6. The model with the fewest significant results in the fitness tests was selected as the final model, as it more effectively fits the observed data and more accurately reflects the underlying genetic patterns of these traits.

Table 6.
Suitability test of orthogonal alternative models.
In the orthogonal cross, none of the three candidate models for the oil content of linted seeds or oil content of lint-free seeds showed significance in the fitness test. Based on the lowest AIC value, the PG-ADI model was selected as the optimal model for both traits. These results suggest that the oil content in both hairy and lint-free cottonseeds is governed by multiple genes, and the genetic effects involved include additive, dominance, and epistasis. For the protein content of linted seeds, the numbers of candidate models reaching a significant level in the fitness test were 1, 0, and 0, respectively. Following the minimum AIC criterion, the MX2-ADI-AD model was selected as the optimal model. This indicates that the protein content of linted seeds is controlled by two pairs of major genes along with polygenes. The major genes exhibit additive, dominance, and epistatic effects, while the polygenes contribute additive and dominance effects. In the case of the protein content of lint-free seeds, the numbers of significant results among the three alternative models were 1, 0, and 1. Thus, the MX2-ADI-AD model was again chosen as the best fit. This outcome shows that the protein content of lint-free seeds is regulated by two pairs of major genes and polygenes. The major genes act through additive, dominance, and epistatic effects, whereas the polygenes show additive and dominance effects (Table 6).
3.4. Suitability Test of Reverse Cross Alternative Model and Determination of Final Model
As shown in Table 7, for the reciprocal cross, none of the three candidate models for the oil content in linted seeds reached significance in the fitness test. Based on the lowest AIC value, the PG-ADI model was selected as the final model. This indicates that the oil content of linted seeds is controlled by multiple genes, with additive, dominance, and epistatic effects involved. For the protein content of linted seeds, none of the three models reached a significant level in the fitness test. According to the minimum AIC value, the MX2-A-AD model was chosen as the optimal model. These results suggest that the protein content of linted seeds is governed by two pairs of major genes and polygenes. The major genes display additive effects, while the polygenes contribute both additive and dominance effects. Similarly, for the oil content of lint-free seeds, none of the three candidate models showed significant results in the fitness test. The PG-ADI model was selected based on the lowest AIC value, indicating that this trait is also influenced by multiple genes, with additive–dominant–epistatic effects. For the protein content of lint-free seeds, all three candidate models yielded non-significant results in the fitness test. Following the principle of minimum AIC value, the MX2-A-AD model was selected as the best fit. This outcome indicates that the protein content of lint-free seeds is regulated by two pairs of major genes along with polygenes. The major genes show additive effects, while polygenes exhibit both additive and dominance effects.

Table 7.
Suitability test of reverse cross alternative model.
3.5. Estimation of Genetic Parameters for Optimal Genetic Models
3.5.1. Estimation of Genetic Parameters of Orthogonal Optimal Genetic Model
As shown in Table 8, for the optimal four orthogonal genetic models, the population mean oil content of linted seeds was 18.54%, with a polygenic heritability of 64.05%. The average oil content of lint-free seeds was 22.12%, and polygenic heritability reached 52.31%. These values indicate that polygenes play a primary role in regulating the oil content in both seed types.

Table 8.
Genetic parameters of optimal orthogonal genetic model.
For the protein content of linted seeds, the population mean was 20.94%. Major gene analysis showed that the additive effects of the two major gene pairs (da and db) were both −0.69, indicating negative additive contributions. Dominant effects (ha = −0.90, hb = −0.80) were also negative, with the first gene pair showing a stronger negative dominance effect. For gene interactions, the additive × additive interaction (i) was −2.71, the additive × dominant effect (jab) was positive at 1.91, while the dominant × additive (jba = −0.44) and dominant × dominant (I = −0.31) effects were negative. Notably, major-gene heritability reached 79.18%, significantly higher than the polygenic contribution. This suggests that the genetic control of protein in hairy cottonseed is primarily maintained by major genes. The negative additive and dominant effects imply that breeding strategies can aim to reduce undesirable traits by selecting appropriate allelic combinations. The average protein content of lint-free seeds was 24.45%. Additive effects of major genes (da and db = −0.71) were more negative than those observed in hairy seeds. Dominant effects (ha = −0.77, hb = −0.84) were also negative, with the second gene pair exerting a stronger effect (|hb| > |ha|). The additive × additive interaction (i = −2.57) was negative, the additive × dominant interaction (jab = 1.76) was positive, while the dominant × additive (jba = −0.54) and dominant × dominant (I = −0.37) effects were negative. The major-gene heritability for this trait was 77.71%, again indicating a dominant influence of major genes over polygenes in determining protein levels in lint-free seeds. These findings offer a theoretical basis for cotton breeding. Since the protein content in both linted and lint-free seeds is primarily regulated by major genes with strong negative additive and dominance effects, selecting parent lines that exploit these effects and account for interaction patterns can guide the design of effective cross combinations for an improved seed protein content.
In summary, the optimal genetic model derived from the orthogonal cross clarifies the genetic structure underlying cottonseed nutritional traits by analyzing how major genes and polygenes contribute and interact. Oil traits are mainly influenced by additive effects from polygenes, suggesting that improvement requires the accumulation of multiple minor-effect genes. In contrast, protein traits are largely controlled by major genes, which allows for targeted regulation through marker-assisted selection. These results offer a clear genetic foundation for breeding cotton varieties with higher oil and protein contents.
3.5.2. Estimation of Genetic Parameters of the Optimal Genetic Model for Reverse Cross
As shown in Table 9, the first- and second-order genetic parameters from the optimal models for the four traits in the reciprocal cross population indicate the following: the average oil content of hairy cotton seeds was 17.21%, with polygenic heritability at 40.19%. For lint-free seeds, the average oil content was 20.79%, with polygenic heritability at 35.55%. These results suggest that in the reciprocal cross population, the oil content in both seed types is primarily influenced by polygenes, which are the main contributors to trait variation.

Table 9.
Genetic parameters of the optimal genetic model of reverse cross.
For the protein content, the average in hairy seeds was 18.26%. The additive effects of the major genes were da = −0.98 and db = 0.92, reflecting a negative contribution from the first gene pair and a positive contribution from the second. Major gene variance was 1.32, and major-gene heritability reached 62.29%. In lint-free seeds, the average protein content was 21.94%. Additive effect values were da = −1.03 and db = 0.98, again showing a negative effect from the first gene pair and a positive effect from the second. Major gene variance was 1.42, and heritability reached 61.64%. These values indicate that the protein content in both hairy and lint-free seeds is mainly regulated by major genes.
Taken together, these results outline the genetic structure of oil and protein traits in the reciprocal cross population: The oil content shows low heritability and is mainly shaped by polygenes. The protein content shows high heritability (above 60%) and is governed by major genes. The opposing additive effects of the two major gene pairs highlight a pattern of positive–negative differentiation. These findings support targeted breeding strategies: by selecting for the second gene pair’s positive additive effect through marker-assisted selection, directional improvement of the protein content can be achieved.
4. Discussion
For a long time, breeders have concentrated primarily on fiber traits, while cottonseed, an important by-product, has often been neglected [47]. However, cottonseed constitutes a significant share of total cotton output and contains valuable oil and protein. Traits related to oil and protein contents are essential components of cottonseed quality. Investigating the genetic control of these traits may contribute to improving the seed composition of modern cotton varieties.
The findings from this study indicate that the oil content in both linted and lint-free seeds from orthogonal and reciprocal crosses is largely influenced by additive, dominant, and epistatic effects. This result is in line with observations reported by Ye [48] and consistent with earlier research by Singh [49] and Zhao Yongguo [50]. In contrast, other studies have reached different conclusions. Dani and Kohel [51] suggested that the oil content is heavily affected by maternal effects. In some hybrids, the oil content was found to be shaped mainly by embryonic additive effects, along with notable embryonic dominance. Similarly, Chen Chen et al. [52] reported that the oil content is regulated by a combination of embryonic, cytoplasmic, and maternal influences. These inconsistencies may stem from differences in genetic backgrounds or environmental conditions during experiments. However, the results of the present reciprocal cross suggest that both seed types, linted and lint-free, exhibit stable genetic patterns for oil content. This consistency points to nuclear genes as the primary drivers of variation in cottonseed oil content. The observed additive and dominant effects may reflect the action of alleles associated with oil biosynthesis and appear unaffected by whether the fuzz-controlling gene is dominant or recessive. For the protein content, the orthogonal cross results indicate that both hairy and lint-free seeds are influenced by two pairs of major genes in combination with polygenes. The major genes show additive, dominant, and epistatic effects, while polygenes contribute additive and dominant effects. The reciprocal cross findings reinforce this pattern, with the same genetic structure, two pairs of major genes and polygenes. In this case, the major genes act through additive effects, while polygenes again show a mix of additive and dominance. In a related study, Zhang Yumei [53] reported that protein and oil contents in four hybrid populations (P1, P2, F1, and F2) followed a mixed inheritance model involving two pairs of additive–dominant–epistatic major genes in combination with polygenes. In the present study, however, the genetic effects identified for the protein content in hairy seeds and lint-free seeds differed between orthogonal and reciprocal crosses. This variation may be due to cytoplasmic influences. Cytoplasmic regulation of the protein content has been documented in other crops such as barley, soybean, wheat, and rapeseed. Bao Haizhu et al. [54] and Ren et al. [55] found that the barley protein content was primarily regulated by cytoplasmic genetic factors. Similarly, Zhang [56] concluded that cytoplasmic effects played a significant role in the inheritance of the protein content. Wei Dahai [57] observed significant differences in protein levels between direct and reciprocal F1 hybrids, based on t-test results, suggesting that cytoplasmic factors could impact the grain protein content. El-Bok et al. [58] also reported that cytoplasmic and epigenetic influences were both strong and stable in the inheritance of protein traits in hard wheat, indicating a clear role of cytoplasmic control in protein expression. Lanhai [59,60], using 13 common maize inbred lines in an NCII (7 × 6) mating design, analyzed the genetic components of key nutritional traits. Results indicated that these traits were shaped not only by the diploid embryo and triploid endosperm but also by four genetic systems, including the cytoplasm and diploid maternal plant. Li Jinrong [61] further pointed out that the protein content is predominantly governed by maternal additive effects and also influenced by interactions with the cytoplasm. These findings have steadily expanded the understanding of genetic and cytoplasmic contributions across various crop species. While studies focusing on cottonseed protein are still limited, seed protein plays a key role in plant physiological functions across species. Prior research in soybean, barley, wheat, and rapeseed has demonstrated that cytoplasmic influences are important in determining protein levels. Therefore, the use of different female parents in reciprocal crosses results in distinct cytoplasmic compositions in the F1 generation, which may explain the differences observed in protein inheritance patterns. In this experiment, when the F1 generation was used as the female parent in backcrossing, its cytoplasmic background directly shaped the cytoplasmic environment of the resulting progeny, which may have influenced the genetic model of seed protein. From the perspective of cytoplasmic inheritance, genes encoded by organellar DNA, such as those in the mitochondrial and chloroplast genomes, may participate in protein metabolism. These cytoplasmic factors add an additional layer of genetic control and open up a new avenue for improving cottonseed protein through breeding. Future breeding strategies should take into account not only nuclear gene combinations but also interactions between nuclear and cytoplasmic genomes. This study also found that oil content traits in both hairy and lint-free seeds followed consistent genetic models across reciprocal crosses, characterized by additive, dominant, and epistatic effects. This consistency indicates that oil content expression is largely unaffected by the direction of the cross. In contrast, protein traits displayed different genetic models between orthogonal and reciprocal crosses. Since the biosynthesis of oil and protein involves interconnected metabolic pathways, these results offer insight into the genetic regulation of nutritional traits in cottonseed through shared physiological processes. In the major gene–polygene analysis of oil content, the results showed significant polygene heritability and variance but no detectable major gene effect, suggesting that oil the content is predominantly regulated by polygenes. On the other hand, the analysis of the protein content revealed strong major-gene heritability and variance with no contribution from polygenes, pointing to regulation mainly by major genes. These contrasting results may be influenced by the assumptions embedded in the genetic model. Although the major gene–polygene framework allows for combined regulation by both types of genetic components, the estimation algorithm may converge on models where only one component appears significant, due to correlations among parameters or the complexity of the model itself. The underlying reasons for this tendency will require further investigation in future studies.
In conclusion, this study clarifies the genetic differences observed in reciprocal crosses by analyzing the inheritance patterns of oil and protein traits in cottonseed. The results provide a theoretical foundation for advancing molecular breeding efforts aimed at improving cottonseed nutritional quality.
5. Conclusions
This study aimed to systematically analyze the genetic mechanisms governing cottonseed oil and protein traits in linted and lint-free seeds, with the goal of providing theoretical support for molecular breeding efforts focused on improving cottonseed oil and protein quality. Xinluzhong 37 and Xinluzhong 51 were selected as parental lines for orthogonal and reciprocal crosses to examine the genetic structure of key nutritional traits in cottonseed. The findings revealed that oil content in both hairy and lint-free seeds followed the same genetic model in both orthogonal and reciprocal crosses. These traits were influenced by additive, dominant, and epistatic effects. The consistency across cross directions indicates that the inheritance of oil content is stable, with all three genetic effects contributing to trait expression regardless of the hybrid combination. For protein content, orthogonal crosses involving both seed types followed a model involving two pairs of major genes along with polygenes. In these crosses, major genes exhibited additive, dominance, and epistatic effects, while polygenes showed additive and dominance effects. This suggests that the protein content in orthogonal crosses is mainly controlled by major genes, with an additional influence from polygenes. In reciprocal crosses, the protein content in linted, and lint-free seeds also followed the same genetic structure. However, in this case, the major genes contributed only additive effects, while polygenes continued to exhibit both additive and dominance effects. In summary, the regulation of the cottonseed oil content involves a complex interplay of multiple genes, while the protein content is primarily controlled by major genes, with polygenic contributions varying by cross direction. These findings provide a scientific foundation for the targeted regulation of oil biosynthesis in cottonseed molecular breeding and offer solid theoretical support for genetic approaches aimed at improving protein quality. This research contributes to advancing the broader goals of cotton quality breeding.
Author Contributions
Conceptualization, X.C.; methodology, Y.L.; software, Y.L.; validation, W.G. and L.H.; formal analysis, Y.L.; investigation, Y.L., W.G., L.H. and X.C.; resources, X.C.; data curation, Y.L.; writing—original draft preparation, Y.L.; writing—review and editing, X.C.; visualization, Y.L.; supervision, X.C.; project administration, Y.L.; funding acquisition, X.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by BTNYGG (NYHXGG, 2023AA102) and the National Key R&D Program of China (2023YFD2301200).
Data Availability Statement
The original contributions presented in this study are included in the article and further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xin, Y.; Ma, J.; Song, J.; Jia, B.; Yang, S.; Wu, L.; Huang, L.; Pei, W.; Wang, L.; Yu, J.; et al. Genomewide association study identifies candidate genes related to fatty acid components in upland cotton (Gossypium hirsutum L.). Ind. Crop. Prod. 2022, 183, 114999. [Google Scholar] [CrossRef]
- Kumar, M.; Zhang, B.; Potkule, J.; Sharma, K.; Radha; Hano, C.; Sheri, V.; Chandran, D.; Dhumal, S.; Dey, A.; et al. Cottonseed Oil: Extraction, Characterization, Health Benefits, Safety Profile, and Application. Food Anal. Methods 2022, 16, 266–280. [Google Scholar] [CrossRef]
- Vonsul, M.; Webster, D. Investigation of cottonseed oil as renewable source for the development of highly functional UV-curable materials. Prog. Org. Coatings 2023, 185, 107883. [Google Scholar] [CrossRef]
- Zhu, D.; Le, Y.; Zhang, R.; Li, X.; Lin, Z. A global survey of the gene network and key genes for oil accumulation in cultivated tetraploid cottons. Plant Biotechnol. J. 2020, 19, 1170–1182. [Google Scholar] [CrossRef]
- Gao, D.; Cao, Y.; Li, H. Antioxidant activity of peptide fractions derived from cottonseed protein hydrolysate. J. Sci. Food Agric. 2010, 90, 1855–1860. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Grasso, S.; Arrutia, F.; Choudhary, J.; Singh, S.; Verma, P.; Mahapatra, A.; Patil, S.; et al. Cottonseed: A sustainable contributor to global protein requirements. Trends Food Sci. Technol. 2021, 111, 100–113. [Google Scholar] [CrossRef]
- Wu, M.; Pel, W.F.; Wedegaertner, T.; Zhang, J.f.; Yu, J.w. Genetics, Breeding and Genetic Engineering to Improve Cottonseed Oil and Protein: A Review. Front. Plant Sci. 2022, 13, 864850. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Liu, J.; Ren, M.; Li, F. Utilising cottonseed in animal feeding: A dialectical perspective. Mod. Agric. 2023, 1, 112–121. [Google Scholar] [CrossRef]
- Yuan, Y.L.; Zhang, T.Z.; Jing, S.R.; Pan, J.J.; Xing, C.Z.; Guo, L.P.; Tang, C.M. Studies of the inheritance of seed qualities and the exploitation of F2 heterosis in low gossypol strains in upland cotton. Yi Chuan Xue Bao Acta Genet. Sin. 2001, 28, 471–481. [Google Scholar]
- Wu, J.X.; Jenkins, J.N.; McCarty, J.C.; Thaxton, P. Seed trait evaluation of Gossypium barbadense L. chromosomes/arms in a G. hirsutum L. background. Euphytica 2009, 167, 371–380. [Google Scholar] [CrossRef]
- Ji, D.F.; Zhu, J. Genetic analysis of seed oil and amino acid composition in land cotton hybrids. Acta Agron. Sin. 1988, 1, 1–6. [Google Scholar]
- Singh, S.; Singh, V.V.; Choudhary, A.D. Combining ability estimates for oil content, yield components and fibre quality traits in cotton (G. hirsutum) using an 8 × 8 diallel mating design. Trop. Subtrop. Agroecosystems 2010, 12, 161–166. [Google Scholar]
- Du, X.; Liu, S.; Sun, J.; Zhang, G.; Jia, Y.; Pan, Z.; Xiang, H.; He, S.; Xia, Q.; Xiao, S.; et al. Dissection of complicate genetic architecture and breeding perspective of cottonseed traits by genome-wide association study. BMC Genom. 2018, 19, 451. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Kong, D.; Liu, C.; Li, P.; Liu, P.; Xiao, X.; Liu, R.; Lu, Q.; Shang, H.; Shi, Y.; et al. Multi-environment evaluations across ecological regions reveal that the kernel oil content of cottonseed is equally determined by genotype and environment. J. Agric. Food Chem. 2022, 70, 2529–2544. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, X.; Chen, B.; Meng, Y.l.; Wang, Y.H.; Zhao, W.Q.; Zhou, Z.G. Integrated Management Strategies Increase Cottonseed, Oil and Protein Production: The Key Role of Carbohydrate Metabolism. Front. Plant Sci. 2017, 8, 48. [Google Scholar] [CrossRef]
- Rochester, I.J.; Constable, G.A. Nitrogen-fertiliser application effects on cotton lint percentage, seed size, and seed oil and protein concentrations. Crop Pasture Sci. 2020, 71, 831–836. [Google Scholar] [CrossRef]
- Hu, W.; Dai, Z.; Yang, J.; Snider, J.; Wang, S.; Meng, Y.; Wang, Y.; Chen, B.; Zhao, W.; Zhou, Z. Cultivar sensitivity of cotton seed yield to potassium availability is associated with differences in carbohydrate metabolism in the developing embryo. Field Crops Res. 2017, 214, 301–309. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.; Zhou, W.; Liu, R.; Dong, X.; Jin, G. Rapid and non-destructive determination of oil content in fuzzy cottonseeds via near-infrared reflectance spectroscopy. Microchem. J. 2024, 207, 112005. [Google Scholar] [CrossRef]
- Govindhan, P. Phytochemical screening, elemental analysis and physicochemical properties of the oil from Bauhinia variegata seeds. Nat. Prod. Res. 2025, 1–11. [Google Scholar] [CrossRef]
- Rizvi, N.B.; Aleem, S.; Khan, M.R.; Ashraf, S.; Busquets, R. Quantitative Estimation of Protein in Sprouts of Vigna radiate (Mung Beans), Lens culinaris (Lentils), and Cicer arietinum (Chickpeas) by Kjeldahl and Lowry Methods. Molecules 2022, 27, 814. [Google Scholar] [CrossRef]
- Wang, J.; Gai, J. A hybrid F2 generation was used to identify the main gene-multiplexed inheritance model of quantitative traits and estimate its genetic effects. Acta Genet. 1997, 24, 432–440. [Google Scholar]
- Gai, J.Y.; Zhang, Y.M.; Wang, J.K. Genetic System of Quantitative Traits in Plants; Science Press: Bejing, China, 2003; pp. 30–150. ISBN 7-03-010596-6. (In Chinese) [Google Scholar]
- Fan, Z.; Gao, Y.; Liu, R.; Wang, X.; Guo, Y.; Zhang, Q. The major gene and polygene effects of ornamental traits in bearded iris (Iris germanica) using joint segregation analysis. Sci. Hortic. 2020, 260, 108882. [Google Scholar] [CrossRef]
- Gai, J.Y. Study on separation and analysis methods of plant quantitative trait genetic system. Hered. Beijing 2005, 1, 130–136. [Google Scholar]
- Huang, R.; Jiang, L.; Zheng, J.; Wang, T.; Wang, H.; Huang, Y.; Hong, Z. Genetic bases of rice grain shape: So many genes, so little known. Trends Plant Sci. 2013, 18, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, J.; Wang, F.; Bao, Y.; Wu, Y.; Zhang, H. Segregation analysis of rice seed germination under cold stress using major gene plus polygene mixed inheritance model. Seed Sci. Technol. 2010, 38, 104–113. [Google Scholar] [CrossRef]
- Wu, B.; Wang, S.; Li, T.; Pang, Y.; Ma, Z.; Li, J.; Wang, L.; Dong, P. Genetic Analysis of Major Genes + Polygenes for the Restoration of Wheat T—type Male Sterility. J. Cereals Crops 2025, 45, 1011–1016. [Google Scholar]
- Irfan, M.; Sun, J.X.; Liu, Y.B.; Li, X.; Yang, S. Genetic analysis of chlorophyll content in maize by mixed major and polygene models. Genetika 2014, 46, 1037–1046. [Google Scholar] [CrossRef]
- Zheng, R.; Zhou, Y.; Lv, D.; Tong, B.; Luo, H. Genetic analysis of stay green related traits in maize with major gene plus polygenes mixed model. PLoS ONE 2024, 19, e0303602. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, D.; Ma, J.; Fu, Y.P.; Qu, J.; Wang, P.W. Genetic analysis of the major gene plus polygene model in soybean resistance to Leguminivora glycinivorella. Genet. Mol. Res. GMR 2014, 13, 4983–4989. [Google Scholar] [CrossRef]
- Yao, D.; Zhao, Q.; Li, T.; Wang, J.; Wang, L.; Liu, Y.; Hao, W.; Liu, H. Genetic analysis and quantitative trait locus mapping using the major gene plus polygene model for soybean [Glycine max (L.) merr.] main quality traits. Legume Res. Int. J. 2023, 46, 18–24. [Google Scholar] [CrossRef]
- Ma, X.; Guo, W.; He, L.; Cao, X. Polygenic Genetic Analysis of Principal Genes for Yield Traits in Land Cotton. Agronomy 2024, 14, 2749. [Google Scholar] [CrossRef]
- Zhang, H.C.; Zhang, W.W.; Jian, G.L.; Qi, F.J.; Si, N. Analysis of the main gene and polygenic genetic characteristics of resistance to Verticillium wilt in Zhongzhi Cotton No.2. Cotton Sci. 2016, 28, 513–518. [Google Scholar]
- Ma, L.; Li, J.; Xu, S.; Chen, H.; Liu, W.; Ning, X.; Lin, H. Analysis of the main gene and multi-gene mixed inheritance model of the branching angle trait of land cotton fruit branches. Biotechnol. Bull. 2022, 38, 148–158. [Google Scholar]
- Li, C.; Wang, Q.; Dong, N.; Fu, Y.; Guo, T. Genetic analysis of cotton plant type traits. J. Jiangsu Agric. Sci. 2011, 27, 25–30. [Google Scholar]
- Cao, J.; Li, T.Y. Exploration into the Successful Experience of Promoting Xinlu No.37. China Cotton 2017, 7, 40–42. [Google Scholar]
- Li, Y.J.; Liu, Y.J.; Sun, J.; Zhang, X.Y. Early maturing cotton variety-Xinluzhong 51. China Cotton. 2012, 12, 31. [Google Scholar]
- Guo, T.T. Establishment of Near Infrared Analysis Model for Protein and Oil Content of land Cotton Seeds and Screening and Mapping of QTL. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, June 2011. [Google Scholar]
- Meng, F.Q. Multi Generational Genetic Analysis of Oil Content in Cotton Seeds. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, June 2013. [Google Scholar]
- Stehlik, K.; Babinec, A.J. Data Analysis with IBM SPSS Statistics: Implementing Data Modeling, Descriptive Statistics and ANOVA; Packt Publishing Limited: Birmingham, UK, 2017. [Google Scholar]
- Xiao, H.; Liu, H.; Li, Y. Experimental Data Processing and Experimental Design Methods; Chemical Industry Press: Beijing, China, 2024; Volume 4, p. 242. ISBN 978-7-122-43954-3. [Google Scholar]
- Mather, K.; Jinks, J.L. Biometrical Genetics. The Study of Continuous Variation; Springer: Berlin/Heidelberg, Germany, 1971. [Google Scholar]
- Cavalli, L.L.; Maccacaro, G.A. Polygenic inheritance of drug-resistance in the bacterium. Heredity 1952, 6, 311. [Google Scholar] [CrossRef][Green Version]
- Walters, D.E.; Gale, J.S. A note on the hayman analysis of variance for a full diallel table. Heredity 1977, 38, 401. [Google Scholar] [CrossRef]
- Wang, J.T.; Zhang, Y.W.; Du, Y.W.; Ren, W.L.; Li, H.F.; Sun, W.X.; Ge, C.; Zhang, Y.Y. Quantitative trait major gene + polygene mixed genetic analysis R software package SEA v2.0. Acta Agron. Sin. 2021, 48, 1416–1424. [Google Scholar] [CrossRef]
- Gai, J.Y.; Wang, J.K. Identification of major gene-polygene mixed inheritance model for quantitative traits using backcross generations. Genetic 1998, 112–113. [Google Scholar]
- Rathore, K.S.; Pandeya, D.; Campbell, L.M.; Wedegaertner, T.C.; Puckhaber, L.; Stipanovic, R.D.; Thenell, J.S.; Hague, S.; Hake, K. Ultra-Low Gossypol Cottonseed: Selective Gene Silencing Opens Up a Vast Resource of Plant-Based Protein to Improve Human Nutrition. Crit. Rev. Plant Sci. 2020, 39, 1–29. [Google Scholar] [CrossRef]
- Ye, Z.; Lu, Z.; Zhu, J. Genetic analysis for developmental behavior of some seed quality traits in Upland cotton (Gossypum hirsutum L.). Euphytica 2003, 129, 183–191. [Google Scholar] [CrossRef]
- Singh, M.; Singh, T.H.; Chahal, G.S. Genetic analysis of some seed quality characters in upland cotton (Gossypium hirsutum L.). Theor. Appl. Genet. 1985, 71, 126–128. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Lu, G.Y. Analysis of the Genetic Characteristics of the Oil Content in Cotton Seeds. Hubei Agric. Sci. 2019, 58, 14–17. [Google Scholar]
- Dani, R.G.; Kohel, R.J. Maternal effects and generation mean analysis of seed-oil content in cotton (Gossypium hirsutum L.). Theor. Appl. Genet. 1989, 77, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chao, X.; Guo, W.; Hu, S.; He, L. Genetic analysis of physical traits and oil traits of land cotton seeds. Acta Agric. Boreali-Occident. Sin. 2021, 30, 1167–1174. [Google Scholar]
- Zhang, Y. Genetic Variation of Nutritional Quality Traits in Vegetable Soybeans. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, June 2006. [Google Scholar]
- Bao, H.; Zhang, F.; Xu, S.; Liu, Z.; Lv, E. Analysis of quality traits, embryo, endosperm, cytoplasm and maternal genetic effects of beer barley grains. Chin. J. Grain Oil 2018, 33, 22–26. [Google Scholar]
- Ren, X.; Sun, D. Genetic analysis of protein and some agronomic traits in barley hybrid combinations. Hubei Agric. Sci. 2008, 4, 397–400. [Google Scholar]
- Zhang, H. Analysis of Embryo, Cytoplasmic, and Maternal Genetic Effects on Quality Characteristics of Rapeseed. Doctoral Dissertation, Zhejiang University, Hangzhou, China, May 2004. [Google Scholar]
- Wei, D. Genetic Analysis of Protein Content in Barley Grains and its Correlation with Some Traits. Master’s Thesis, Yangzhou University, Yangzhou, China, May 2005. [Google Scholar]
- El-Bok, S.; Bnejdi, F.; El-Gazzah, M. Evidence of cytoplasmic and epistatic effects in inheritance of grain protein content in durum wheat. J. Food Agric. Environ. 2013, 11, 804–806. [Google Scholar]
- Lan, H.; Tan, D.; Gao, S.; Tang, Q.; Cao, M.; Pan, G.; Rong, Y. Genetic effect analysis of main nutritional quality traits in common maize. Acta Agron. Sin. 2006, 5, 716–722. [Google Scholar]
- Lan, H. Genetic Study on Main Nutritional Quality and Seed Dormancy of Common Maize. Doctoral Dissertation, Sichuan Agricultural University, Yaan, China, June 2006. [Google Scholar]
- Li, J. Genetic Effects and QTL Mapping of Embryo and Maternal Plant Quality Traits in Cottonseed. Doctoral Dissertation, Zhejiang University, Hangzhou, China, December 2014. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).