Abstract
The whitefly, Bemisia tabaci, is a complex of cryptic species that is a significant pest of different legume hosts that inhabits various regions worldwide with diverse climates and characteristics. Its adaptability is often facilitated by the insect’s microbiome, which can contribute to both the metabolism of host plant secondary compounds and insecticide resistance. The most relevant biotypes in Brazil are Middle East-Asia Minor 1 (MEAM1) and Mediterranean (MED), because of their ability to damage different hosts. Although MEAM1 is the prevalent species in Brazil, MED has great potential to spread, and there is little current knowledge about the biology of this biotype in the country. Therefore, the objective of this study was to evaluate the development and viability of MED on two legumes, soybean and common bean, alongside cotton, bell pepper, and tomato, at temperatures of 20 °C, 23 °C, 26 °C, 29 °C, 32 °C, and 35 °C and characterize the composition of its endosymbionts. Temperatures between 23 °C and 32 °C were the most suitable for B. tabaci MED development and viability across all tested host plants, whereas 35 °C proved harmful for insects reared on legumes. We observed a temperature threshold (°C) and thermal constant (degree-days) that varied according to the host plant, ranging from 9.81 °C and 384.62 for soybean to 11.17 °C and 333.33 for bell pepper, respectively. The main endosymbionts were in a ratio of 80% Hamiltonella and 20% Cardinium. These results allow the future mapping of risk for the MED biotype on different host plants in Brazil and elsewhere in South America.
1. Introduction
The whitefly, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae), is a species complex recognized as a major global pest, with its wide host range and broad geographical distribution. It has been reported on over 700 plant species, including numerous economically important legumes [1,2]. Currently, B. tabaci is recognized as a complex of 45 cryptic species that are morphologically indistinguishable and can only be differentiated through molecular analysis [3,4,5,6,7,8]. Among these, the most prominent biotypes worldwide are Middle East-Asia Minor 1 (MEAM1) and Mediterranean (MED), which are also the most economically significant members of the B. tabaci complex [4,9].
Brazil stands as a very important global producer of legumes such as soybean (Glycine max) and common bean (Phaseolus vulgaris). According to the USDA Foreign Agricultural Service (FAS), the planted area for soybeans in Brazil during the 2024/2025 season was 47.4 million hectares, with an annual production of 169 million tons [10]. The common bean is a staple food in Brazil, playing a crucial role in the country’s food security and agricultural economy. In the 2024/25 season, the planted area was 2784.8 thousand hectares, with a total production of 3172 thousand tons [11]. The large areas of legumes planted, combined with climatic conditions and agronomic practices, contribute to the rapid increase in whitefly population sizes and dispersal [12,13,14].
The B. tabaci management strategies in Brazil—particularly for soybean (G. max), common bean (P. vulgaris), and cotton (Gossypium hirsutum)—have largely relied on insecticides as the primary method of control [15,16,17]. This approach may contribute to the competitive advantage of the MED species over MEAM1 in soybean fields, as MED possesses a natural resistance to several insecticides [18]. Additionally, Brazil’s tropical climate supports year-round whitefly populations. The country’s agricultural landscape, characterized by the proximity of soybean, common bean, cotton, and vegetable crops, further facilitates the continuous presence and spread of whiteflies under field conditions.
For the whitefly biotypes, temperature also plays an important role in influencing distribution [19,20,21]. Although MEAM1 remains the dominant biotype in Brazil, the presence of MED has also been reported, including in soybean fields [15,22], and in some regions of the world MED has already become the prevailing biotype [23]. Previous studies have identified MED as an efficient vector of cowpea mild mottle virus (CPMMV) in both soybean and common bean [22]. This highlights potential future challenges in managing MED whiteflies and suggests a likely increase in CPMMV incidence in soybean and common bean crops.
Numerous studies have examined the impact of temperature on the performance of MEAM1 and/or MED whiteflies [19,20,21,24,25,26,27,28]. Both whitefly biotypes typically complete their development from egg to adult within a temperature range of 15–35 °C, although survival rates are often significantly reduced at temperatures below 20 °C or above 30 °C [27,28,29]. However, the two biotypes exhibit significant differences, which may have important implications for competitive interactions [30]. The MED biotype generally shows greater tolerance to high temperatures than the MEAM1 biotype, particularly during the adult stage [25]. In areas where both biotypes have invaded, MED has been reported to displace MEAM1, indicating that thermal tolerance may influence inter-biotype competition [30,31].
Recent studies have increasingly emphasized the importance of characterizing bacterial endosymbionts in whitefly populations [32,33,34,35]. The primary endosymbiont Portiera aleyrodidarum is consistently present across all whitefly species, while secondary endosymbionts—such as Arsenophonus, Cardinium, Fritschea, Hamiltonella, Hemipteriphilus, Rickettsia, and Wolbachia—occur in varying combinations [34,35]. Wild-type populations may host over 60 bacterial genera, some of which remain undescribed [36,37]. Because the presence of these endosymbionts can provide adaptive plasticity to whiteflies [35], assessing their influence on thermal susceptibility and host plant suitability is essential for a comprehensive understanding.
Given the limited number of studies in Brazil examining the factors influencing the establishment of B. tabaci MED, and the growing significance of this biotype in legume crops, this study aimed to assess the performance of MED on key legumes crops in Brazil—soybean and common bean—under controlled constant temperature regimes, in comparison with three additional host plants, and characterization of the whitefly microbiome. Understanding these dynamics is essential for predicting the potential establishment and spread of MED across Brazil’s diverse climatic regions—particularly considering the country’s continental scale and substantial thermal variability. Additionally, preliminary assessment of the microbiome was conducted as part of this study as previous research has shown the microbiome to influence insecticide resistance [32], but less is known about its importance with host plant use.
2. Materials and Methods
2.1. Whitefly Colony
Samples of B. tabaci previously maintained in the laboratory were identified as MED through molecular analysis. MED adults were placed in cages made of polyester mesh (45 × 45 × 55 cm) and reared on bell pepper (Capsicum annuum) and common bean (P. vulgaris). The rearing was conducted in a climate-controlled environment set at 26 ± 2 °C, with 70 ± 10% relative humidity and a 14 h photoperiod.
2.2. Identification of the Biotype of B. tabaci and Presence of Endosymbiont Group
The whitefly biotype was identified by analysis of the mitochondrial gene cytochrome oxidase I (mtCOI). Initially, individual extraction of the DNA of 20 individuals was performed for each sample collected, using the Chelex (Bio-Rad, Richmond, CA, USA) protocol [38]. The samples were submitted to a PCR reaction with the genetic primers C1-J-2195 and TL2-N-3014 [39]. The PCR reaction was performed in a final volume of 50 µL, containing 50 mM MgCl2, 2.5 mM dNTPs, and 1 µM oligonucleotides, using 0.5 units of Taq polymerase. The reaction cycle consisted of an initial denaturation at 94 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, 45 °C for 45 s, and 72 °C for 1 min, with a final extension at 72 °C for 10 min. Next, polymorphism analysis of the amplicons was conducted using Restriction Fragment Length Polymorphism (RFLP) [40]. From each PCR product (880 bp), 5 µL were used and digested with one unit of TaqI at 65 °C for 2 h in a final volume of 15 µL. The digested DNA was evaluated on a 1% agarose gel stained with ethidium bromide.
The same samples were also subjected to the detection of the primary endosymbiont, Portiera aleyrodidarum, and six secondary endosymbionts: Hamiltonella, Rickettsia, Wolbachia, Cardinium, Arsenophonus, and Fritschea [21,32,34,41]. Specific primers for these genera were used to amplify regions of the 16S and 23S genes.
2.3. Determination of Thermal Requirements in Different Hosts
The period of development of B. tabaci MED was evaluated at constant temperatures of 20 °C, 23 °C, 26 °C, 29 °C, 32 °C, and 35 °C on five different hosts: “TAA Dama” common bean P. vulgaris, “NEO 580” soybean (G. max), “Taurus” bell pepper (C. annuum), “FM 985 GLTP” cotton (Gossypium hirsutum), and “IPA 6” tomato (Solanum lycopersicum), in a BOD (Biochemical Oxygen Demand) incubator chamber (ELETROlab, model EL 202/4, São Paulo, Brazil) with a relative humidity of 70 ± 10% and photophase of 14:10 h L:D. Plant hosts aged 4 to 6 weeks after germination were used in the experiment. The experiment was conducted in September 2022. In this experiment, we tested whiteflies originating from a colony reared on bell pepper. However, to avoid using a pre-adapted host, we reared the whiteflies on common beans for about ten generations before testing bell pepper as the host plant.
For each temperature, the five hosts were tested, with six replications of 20 individuals per sample unit. Approximately 30 adult whiteflies with a maximum age of 72 h were collected from the colony using a Pasteur-type glass pipette and a flexible hose and placed in a ‘clip-cage’ on their respective hosts. Whiteflies remained in the cage for an oviposition period of 48 h. After this period, the adults were removed from the clip-cages. The leaves were examined using a magnifying glass and 20 eggs were selected for monitoring. The area for observation was marked with non-toxic paint glue. Subsequently, the host plants containing the eggs were placed at their respective pre-established temperatures. The development of individuals was monitored daily until they reached their adult stage.
2.4. Analysis
From the data collected, the temperature thresholds (Tb) and the value of the thermal constant (K) in degree-days (GD) were calculated using the Hyperbole method [42]. The development time for each temperature and host plant was established by Kaplan–Meier curves [43]. For the comparison of curves, the non-parametric analysis was used by the log-rank test at 5% of significance [44].
The number of B. tabaci MED individuals that completed egg–adult stage development was counted and viability (%) was calculated. The number of individuals completing development was obtained for each host at each temperature and analyzed using a two-way ANOVA, with post hoc comparisons performed using Tukey’s tests. The assumptions of normality and homogeneity of variances were tested before proceeding with the tests. The statistical analyses were performed in the R computing environment, utilizing the “AgroR” (version 1.3.6), “survival” (version 3.8-3), “survminer” (version 0.5.0), “ggsurvplot” (version 0.4.9) and “ggplot2” (version 3.5.2) packages.
3. Results
The analysis of the mitochondrial gene cytochrome oxidase I (mtCOI) revealed that the colony whiteflies were all the MED biotype. The corresponding nucleotide sequence was deposited in GenBank under accession number MK900733.1. The presence of the primary endosymbiont Portiera aleyrodidarum was detected in 100% of our population. The composition of secondary endosymbionts in the tested population was 80% Hamiltonella and 20% Cardinium, while Rickettsia, Wolbachia, Arsenophonus, and Fristchea were not detected (Table 1).

Table 1.
Infection frequencies (%) of secondary endosymbionts in the Bemisia tabaci MED population.
Significant interactions were observed in the number of viable individuals at the egg-to-adult stage among temperature and host plants tested (F [4,5] = 1.79; p = 0.026) (Table 2). The highest viability of individuals on soybean was recorded at temperatures between 23 °C and 32 °C, with mean values ranging from 9.33 ± 2.28 to 13.17 ± 1.89 (mean ± 1 SE). In contrast, the lowest viability occurred at 35 °C, with only 2.00 ± 0.52 individuals surviving.

Table 2.
Number of viable Bemisia tabaci MED individuals in the egg–adult stage development (mean ± SE) and viability (%) in different hosts across different temperatures 1.
In common bean, the temperature range supporting viability was broader than in soybean. No significant differences were observed in the number of viable individuals reared at temperatures between 20 °C and 32 °C, ranging between 7.67 ± 2.42 and 12.67 ± 1.65. However, no individuals completed development at 35 °C (Table 2).
In cotton, the highest number of viable individuals was observed between 23 °C and 32 °C, with the number of individuals varying between 14.67 ± 2.01 and 17.67 ± 1.23. For bell pepper and tomato, the greatest number of viable individuals was found between 23 °C and 35 °C. For these plants, 20 °C resulted in the lowest viability, with only 4.50 ± 0.72 and 7.67 ± 2.42 viable individuals in bell pepper and tomato, respectively (Table 2).
At temperatures of 20 °C, 23 °C, and 26 °C, no difference in the number of viable individuals was observed among host plants. However, at 29 °C, the lowest viability was observed in soybean, while at 32 °C and 35 °C, the lowest viability was recorded in common bean.
The lowest temperature threshold (Tb) differed slightly among host plants, with the lowest observed in soybean (9.81 °C), followed by tomato (9.89 °C), common bean (10.00 °C), cotton (10.37 °C), and bell pepper (11.17 °C; Table 3). The lowest thermal constant (K) was observed in bell pepper (333.33 degree-days), while the highest was observed in soybean (384.62 degree-days). Common bean, cotton, and tomato showed similar values of K (370.37 degree-days). Somewhat surprisingly, the development of whiteflies reared at 35 °C was longer than that of those reared at 32 °C, which deviates from the expected thermal performance curve. This anomaly suggests that 35 °C may exceed the optimal thermal range for development, potentially inducing heat stress. Therefore, to calculate the regression, the values at 35 °C were discarded. All the curves fit the equation well, with R2 values greater than 0.90 for all host plants (Figure 1).

Table 3.
Temperature threshold (Tb) and thermal constant (K) of white fly Bemisia tabaci (Gennadius) MED egg–adult stage.
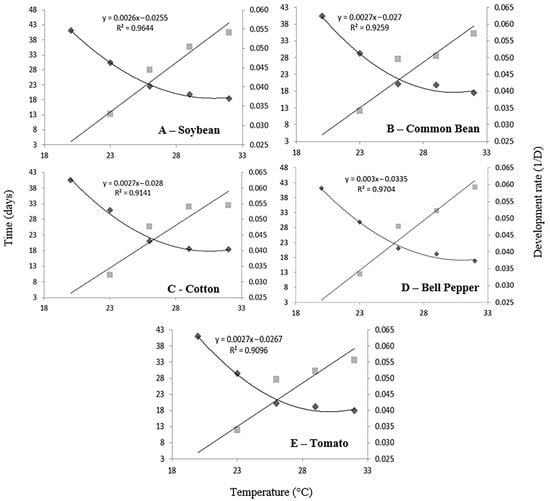
Figure 1.
Temperature-dependent development time and rate of Bemisia tabaci MED on five host plants.
Based on the Kaplan–Meier curves, the fastest development times in soybean were observed at temperatures of 32 °C and 29 °C, where insects completed their development in approximately 18.5 to 20 days. At the highest tested temperature, 35 °C, the development period increased by about 5 days compared to 32 °C and 29 °C. The slowest development occurred at 20 °C, where insects took an average of 41.17 days to complete their development (χ2 = 53.767, df = 5, p < 0.001; Figure 2A).
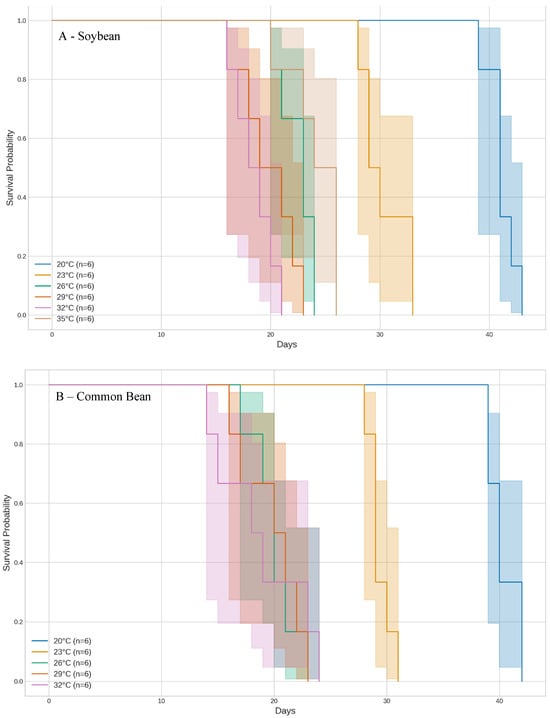
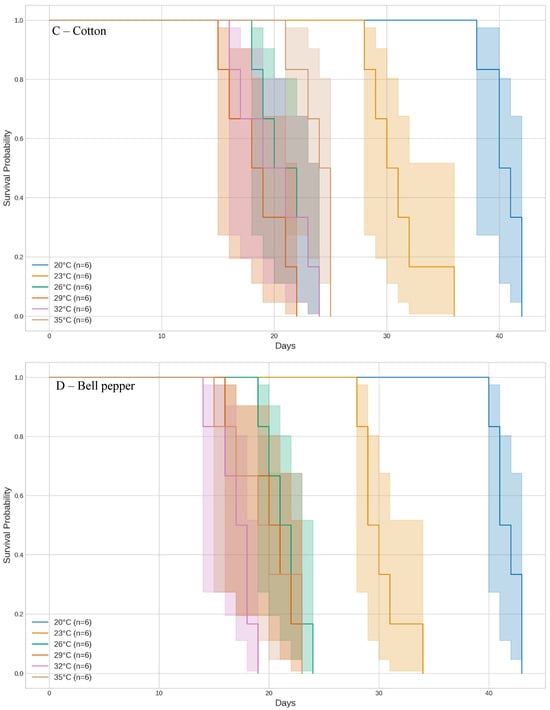
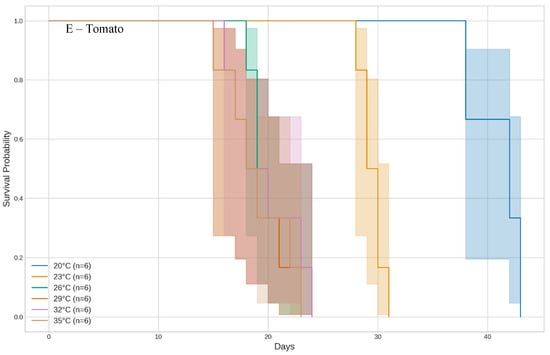
Figure 2.
Development time of whitefly Bemisia tabaci (Gennadius) MED across different temperature regimes on five host plants, analyzed using Kaplan–Meier curves.
Similarly, when common bean was used as the host plant, 20 °C resulted in the longest development time, with insects taking about 40.33 days. No significant differences were observed among the temperatures of 26 °C, 29 °C, and 32 °C, with development durations ranging from 18.83 to 20.17 days (χ2 = 0.172, df = 4, p = 0.967). No whiteflies survived at 35 °C (Figure 2B).
In cotton, the shortest development times were observed at 26 °C, 29 °C, and 32 °C, with insects completing development in approximately 18.5 to 21 days, with no significant differences among these temperatures (χ2 = 5.298, df = 5, p = 0.102). At 20 °C, development was significantly slower, with insects taking more than twice as long (Figure 2C).
For bell pepper and tomato, the fastest development times were observed at the highest tested temperatures (Figure 2D,E). At 32 °C and 35 °C, insects reared on bell pepper completed development in 17 to 19.5 days (χ2 = 46.885, df = 5, p < 0.001). On tomato, development at 29 °C, 32 °C, and 35 °C took between 19 and 19.67 days (χ2 = 34.593, df = 5, p < 0.001). For both host plants, the lowest temperature (20 °C) significantly extended the development period, with durations of 41.67 days for bell pepper and 41.0 days for tomato.
4. Discussion
Although MED is present in Brazil, it has not yet become widespread [15,22]. Studies on the biotic and abiotic factors affecting MED’s development and distribution warrant further investigation, particularly given the economic significance of legume crop production in the country.
Temperature and host plant interactions are critical factors influencing B. tabaci MED development [15,25,27,45,46]. Our study revealed that high temperatures of 35 °C were nearly unsuitable for MED development on soybean and common bean, with minimal survival to adulthood. In contrast, on tomato, over 50% of nymphs successfully reached adulthood at 35 °C, demonstrating significant host-effects in terms of thermal range. Similar results were reported by Bonato et al. [27], where the optimum temperate for immature development was estimated to be 32.5 °C, and temperatures higher than 35 °C were detrimental to B. tabaci MED survivorship, reducing the survival of immatures and longevity. Additionally, these authors reported that the population found on tomato was more tolerant to high temperatures (>33 °C). The optimal development observed here, between 26–32 °C across most host plants, indicates that these temperatures represent the greatest risk for rapid population growth and potential crop damage. This information can assist monitoring and management strategies, particularly in greenhouse settings, where temperature can be manipulated.
Furthermore, the differences in temperature thresholds (Tb) and thermal constants (K) among host plants suggest that development models should be host-specific for more accurate predictions of whitefly phenology. Similar results have been reported in the literature for whitefly MED, with temperature thresholds ranging from 8 °C in tomato [27] to 10.2 °C in poinsettia [46]. Thermal constants have also been reported to vary, from 327 in poinsettia [47], to as high as 400 in tomato [46]. These differences among host plants may be explained by the host’s leaf surface morphology—particularly trichome density, type, and length—as well as its metabolites [48,49], in addition to the plant’s stomatal morphology and distribution [50], which can alter the microclimate at the leaf surface, thereby affecting the thermal biology of whitefly development [51].
Our experimental data indicate that temperatures between 23 °C and 32 °C are most favorable for MED development on soybean, and between 20 °C and 32 °C for common bean—the main legumes cultivated in Brazil. When considering Brazil’s climatological normal, ranging from 27 °C in the northern region to 18.4 °C in the south [52], most regions of Brazil, including the central-west, where 50% of the soybean production is concentrated [10], and the south-central region, where more than 70% of common beans are produced [11], are climatically suitable for MED colonization (Figure 3).
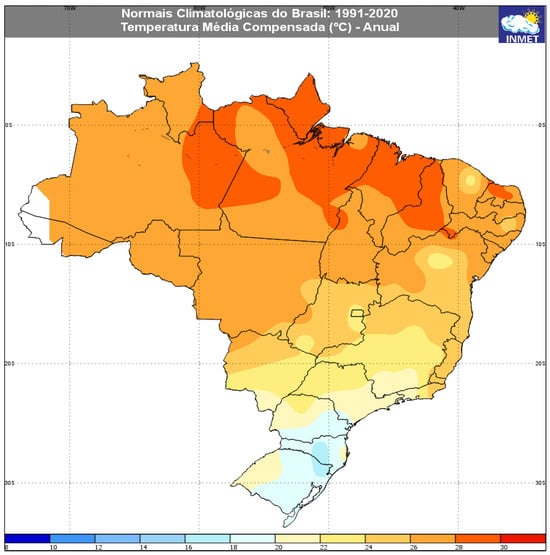
Figure 3.
Climatological thermal averages in Brazil: 1991–2020 (https://clima.inmet.gov.br/temp) (Accessed on 20 April 2023).
While the lethal high temperature for MED has been reported at 45–46 °C [25], our data showed minimal survival on legumes at 35 °C. Notably, endosymbionts play a significant role in this thermal adaptation. Infection with Rickettsia has been shown to induce the expression of thermotolerance-associated genes [53], and Cardinium (present in 20% of our sampled individuals) has been linked to increased thermal tolerance [21]. Cardinium also influences protein levels associated with growth and energy metabolism [35], potentially explaining some of our observed variation in differential development across host plants. Additionally, the presence of Hamiltonella (found in 80% of our samples) is often associated with increased whitefly growth rates, especially on nutritionally poor host plants [54], while Cardinium decreases the whitefly detoxification metabolism ability and decrease the defense response of the host plant [55]. Considering that the association of these two endosymbionts impacts performance and reproduction on whitefly MED [56] and play important role in the host plant–whitefly interaction, future studies should examine endosymbionts after feeding trials at different temperatures and with different host plants to discover if the insects that survived warmer temperatures had these bacteria.
These findings gain greater significance when considered alongside climate change projections for Brazil, which indicate annual mean temperature increases of up to 2.2 °C by 2050 [57]. Projections also estimate that the impacts of climate change on biodiversity in Brazil will vary spatially, with central and northern regions expected to experience the most severe temperature increases [58]. While these areas are already within the optimal temperature range for whitefly development, the projected changes could push temperatures during extreme heat events beyond the upper thermal limits for whitefly survival—particularly for the MED species on certain hosts. In such cases, extreme heat could occasionally serve as a natural population control mechanism in the hottest regions, especially in legume-producing areas. This is particularly significant when contrasted with the MEAM1 biotype, which tends to exhibit reduced fitness at higher temperatures [25,59]. Therefore, MED’s greater tolerance to high temperatures compared to MEAM1 may confer a competitive advantage in terms of distribution and population growth, particularly under field or greenhouse conditions where elevated temperatures are common [25].
It is also crucial to consider the strategies growers adopt when producing legumes in Brazil. Soybean growers frequently practice crop succession with cotton, especially in the central-west region [60], while common bean growers often diversify with vegetables including tomatoes and bell peppers [61,62]. In regions where temperatures increasingly exceed 32 °C due to climate change, we might expect shifts in host plant preference or performance. Crops like tomatoes, which supported better whitefly MED survival at high temperatures in our study, could face increased pest pressure. This potential shift in host plant suitability could have significant implications for agricultural practices, particularly in regions where farmers practice crop succession or rotation involving both legumes and solanaceous crops.
These practices also create complex landscapes that influence whitefly population dynamics. Previous research has shown that, in competitive scenarios without insecticide exposure, MED and MEAM1 remain in equilibrium [14]. However, intensive insecticide use (particularly in cotton cultivation where applications can exceed 20 per season [63]) favors MED. This pattern has been observed in Israel, where MED surpassed MEAM1 during periods of intensive cotton cultivation and insecticide use, while MEAM1 regained dominance when these practices were reduced [23,64].
In the context of integrated pest management, these findings are also important for understanding and exploring the interactions between whitefly MED and its natural enemies [65]. For example, temperature plays a significant role in the development and fecundity of Encarsia acaudaleyrodis Hayat, an important biological control agent of whiteflies. Temperatures of 32 °C shorten the development period from egg to adult; however, they also reduce oviposition [66]. The highest intrinsic rate of population increase was observed at around 25 °C, indicating that this moderate temperature is favorable for the biological control activity of E. acaudaleyrodi [65,66]. Information about the optimal temperatures for the parasitoid, combined with the favorable temperature range for MED development observed in this study (26–32 °C), can be valuable for laboratory mass-rearing programs, helping to optimize production efficiency.
Despite recent surveys indicating that MED is not yet the predominant species in Brazil [15], our findings highlight the need for continuous monitoring. The complex interactions between temperature, host plants, endosymbionts, and agricultural practices could rapidly shift the competitive balance between whitefly biotypes. Effectively managing whitefly populations requires a comprehensive understanding of these symbiotic relationships and their influence on insect biology and stress tolerance [2]. Given Brazil’s dimensions and cropping diversity, continuous sampling efforts are necessary to accurately describe MED’s distribution and potential spread.
5. Conclusions
In conclusion, the potential for MED to expand its range in Brazil depends on a complex interaction of factors, including temperature tolerance, host plant suitability, endosymbiont associations, and agricultural practices. Our findings suggest that the conditions favorable to MED exist across much of Brazil, particularly in cropping systems with intensive insecticide use. Future research should focus on mapping the varying compositions of endosymbiont distributions in field populations and modeling how climate change might further alter the competitive dynamics between these economically important pest biotypes on legumes.
Author Contributions
Conceptualization, D.d.L.A., R.C.d.O. and C.M.; methodology, D.d.L.A., R.C.d.O., C.M., D.M.S., F.B.d.S. and R.K.-S.; software, D.d.L.A., D.M.S. and R.H.; validation, D.d.L.A. and R.H.; formal analysis, D.d.L.A. and R.H.; investigation, D.d.L.A., D.M.S. and F.B.d.S.; resources, R.C.d.O. and R.K.-S.; data curation, D.d.L.A. and R.H.; writing—original draft preparation D.d.L.A., R.H., R.C.d.O. and W.W.H.; writing—review and editing, D.d.L.A., R.H., R.C.d.O. and W.W.H.; visualization, D.d.L.A., R.H., R.C.d.O. and W.W.H.; supervision, R.C.d.O., R.K.-S., C.M. and W.W.H.; project administration, D.d.L.A. and R.C.d.O.; funding acquisition, R.C.d.O. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001; by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; process numbers 2017/21588-7, 2018/02317-5, 2019/10736-0, and 2018/19782-2); and by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 304126/2019-5). Regiane C. de Oliveira holds a CNPq fellowship.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to acknowledge the Department of Entomology and Plant Pathology at Oklahoma State University for all the support given to this research and the financial support provided by the following agencies: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq. During the preparation of this manuscript, the author(s) used Microsoft 365 Copilot to assist with grammar and language editing. The author(s) reviewed and edited the content as needed and take full responsibility for the final version of the manuscript.
Conflicts of Interest
The author Cristiane Müller was employed by Corteva Agriscience. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Navas-Castillo, J.; Fiallo-Olivé, E.; Sánchez-Campos, S. Emerging Virus Diseases Transmitted by Whiteflies. Annu. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef] [PubMed]
- Kavallieratos, N.G.; Wakil, W.; Eleftheriadou, N.; Ghazanfar, M.U.; El-Shafie, H.; Simmons, A.M.; Dimase, M.; Smith, H.A.; Chandler, D. Integrated Management System of the Whitefly Bemisia tabaci: A Review. Entomologia 2024, 44, 1117–1133. [Google Scholar] [CrossRef]
- Boykin, L.M.; Bell, C.D.; Evans, G.; Small, I.; De Barro, P.J. Is Agriculture Driving the Diversification of the Bemisia tabaci Species Complex (Hemiptera: Sternorrhyncha: Aleyrodidae)?: Dating, Diversification and Biogeographic Evidence Revealed. BMC Evol. Biol. 2013, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- De Barro, P.J.; Liu, S.-S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A Statement of Species Status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef]
- Jiu, M.; Hu, J.; Wang, L.-J.; Dong, J.-F.; Song, Y.-Q.; Sun, H.-Z. Cryptic Species Identification and Composition of Bemisia tabaci (Hemiptera: Aleyrodidae) Complex in Henan Province, China. J. Insect Sci. 2017, 17, 78. [Google Scholar] [CrossRef]
- Lee, W.; Park, J.; Lee, G.-S.; Lee, S.; Akimoto, S. Taxonomic Status of the Bemisia tabaci Complex (Hemiptera: Aleyrodidae) and Reassessment of the Number of Its Constituent Species. PLoS ONE 2013, 8, e63817. [Google Scholar] [CrossRef]
- MacLeod, N.; Canty, R.J.; Polaszek, A. Morphology-Based Identification of Bemisia tabaci Cryptic Species Puparia via Embedded Group-Contrast Convolution Neural Network Analysis. Syst. Biol. 2022, 71, 1095–1109. [Google Scholar] [CrossRef]
- Mugerwa, H.; Seal, S.; Wang, H.-L.; Patel, M.V.; Kabaalu, R.; Omongo, C.A.; Alicai, T.; Tairo, F.; Ndunguru, J.; Sseruwagi, P.; et al. African Ancestry of New World, Bemisia tabaci-Whitefly Species. Sci. Rep. 2018, 8, 2734. [Google Scholar] [CrossRef]
- Wan, F.-H.; Yang, N.-W. Invasion and Management of Agricultural Alien Insects in China. Annu. Rev. Entomol. 2016, 61, 77–98. [Google Scholar] [CrossRef]
- Brazil Soybean Area, Yield and Production. Available online: https://ipad.fas.usda.gov/countrysummary/Default.aspx?id=BR&crop=Soybean (accessed on 24 June 2025).
- Companhia Nacional de Abastecimento (CONAB). Boletim da Safra de Grãos—9o Levantamento—Safra 2024/25; Grãos; Conab: Brasília, Brazil, 2025; p. 135. [Google Scholar]
- Filho, A.B.; Inoue-Nagata, A.K.; Bassanezi, R.B.; Belasque, J.; Amorim, L.; Macedo, M.A.; Barbosa, J.C.; Willocquet, L.; Savary, S. The Importance of Primary Inoculum and Area-Wide Disease Management to Crop Health and Food Security. Food Sec. 2016, 8, 221–238. [Google Scholar] [CrossRef]
- Ferreira Rodrigues, R.H.; Silva, L.B.; Silva, M.C.F.; Lopes, J.W.B.; Araujo Lima, E.; Sobreira Barbosa, R.; Oliveira Almeida, L.F. Population Fluctuation and Distribution of Bemisia tabaci MEAM1 (Hemiptera: Aleyrodidae) in Soybean Crops. Front. Agron. 2022, 4, 958498. [Google Scholar] [CrossRef]
- Watanabe, L.F.M.; Bello, V.H.; De Marchi, B.R.; Silva, F.B.d.; Fusco, L.M.; Sartori, M.M.P.; Pavan, M.A.; Krause-Sakate, R. Performance and Competitive Displacement of Bemisia tabaci MEAM1 and MED Cryptic Species on Different Host Plants. Crop Prot. 2019, 124, 104860. [Google Scholar] [CrossRef]
- Fernandes, D.S.; Okuma, D.; Pantoja-Gomez, L.M.; Cuenca, A.; Corrêa, A.S. Bemisia tabaci MEAM1 Still Remains the Dominant Species in Open Field Crops in Brazil. Braz. J. Biol. 2022, 84, e256949. [Google Scholar] [CrossRef]
- Ferreira, A.L.; Ghanim, M.; Xu, Y.; Pinheiro, P.V. Interactions between Common Bean Viruses and Their Whitefly Vector. Viruses 2024, 16, 1567. [Google Scholar] [CrossRef]
- Bevilaqua, J.G.; Padilha, G.; Pozebon, H.; Marques, R.P.; Cargnelutti Filho, A.; Ramon, P.C.; Boeni, L.; Castilhos, L.B.; Da Luz, G.R.; Brum, A.L.S.D.S.; et al. A Sustainable Approach to Control Whitefly on Soybean: Integrating Entomopathogenic Fungi with Insecticides. Crop Prot. 2023, 164, 106145. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Ghanim, M.; Roditakis, E.; Nauen, R.; Ishaaya, I. Insecticide Resistance and Its Management in Bemisia tabaci Species. J. Pest Sci. 2020, 93, 893–910. [Google Scholar] [CrossRef]
- Dai, T.M.; Wang, Y.S.; Liu, W.X.; Lü, Z.C.; Wan, F.H. Thermal Discrimination and Transgenerational Temperature Response in Bemisia tabaci Mediterranean (Hemiptera: Aleyrodidae): Putative Involvement of the Thermo-Sensitive Receptor BtTRPA. Environ. Entomol. 2018, 47, 204–209. [Google Scholar] [CrossRef]
- Pan, H.; Preisser, E.L.; Chu, D.; Wang, S.; Wu, Q.; Carrière, Y.; Zhou, X.; Zhang, Y. Insecticides Promote Viral Outbreaks by Altering Herbivore Competition. Ecol. Appl. 2015, 25, 1585–1595. [Google Scholar] [CrossRef]
- Yang, K.; Yuan, M.-Y.; Liu, Y.; Guo, C.-L.; Liu, T.-X.; Zhang, Y.-J.; Chu, D. First Evidence for Thermal Tolerance Benefits of the Bacterial Symbiont Cardinium in an Invasive Whitefly, Bemisia tabaci. Pest Manag. Sci. 2021, 77, 5021–5031. [Google Scholar] [CrossRef]
- Bello, V.H.; da Silva, F.B.; Watanabe, L.F.M.; Vicentin, E.; Muller, C.; de Freitas Bueno, R.C.O.; Santos, J.C.; De Marchi, B.R.; Nogueira, A.M.; Yuki, V.A.; et al. Detection of Bemisia tabaci Mediterranean Cryptic Species on Soybean in São Paulo and Paraná States (Brazil) and Interaction of Cowpea Mild Mottle Virus with Whiteflies. Plant Pathol. 2021, 70, 1508–1520. [Google Scholar] [CrossRef]
- Tang, X.-T.; Cai, L.; Shen, Y.; Xu, L.-L.; Du, Y.-Z. Competitive Displacement between Bemisia tabaci MEAM1 and MED and Evidence for Multiple Invasions of MED. Insects 2019, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, X.-N.; Lu, M.-X.; Du, Y.-Z. Transcriptional Profiling of MED Exposed to Thermal Stress and Verification of HSP70 Expression. Entomol. Res. 2021, 51, 251–262. [Google Scholar] [CrossRef]
- Xiao, N.; Pan, L.-L.; Zhang, C.-R.; Shan, H.-W.; Liu, S.-S. Differential Tolerance Capacity to Unfavourable Low and High Temperatures between Two Invasive Whiteflies. Sci. Rep. 2016, 6, 24306. [Google Scholar] [CrossRef]
- Chu, D.; Tao, Y.; Zhang, Y.; Wan, F.; Brown, J.K. Effects of Host, Temperature and Relative Humidity on Competitive Displacement of Two Invasive Bemisia tabaci Biotypes [Q and B]. Insect Sci. 2012, 19, 595–603. [Google Scholar] [CrossRef]
- Bonato, O.; Lurette, A.; Vidal, C.; Fargues, J. Modelling Temperature-Dependent Bionomics of Bemisia tabaci (Q-Biotype). Physiol. Entomol. 2007, 32, 50–55. [Google Scholar] [CrossRef]
- Muñiz, M.; Nombela, G. Differential Variation in Development of the B- and Q-Biotypes of Bemisia tabaci (Homoptera: Aleyrodidae) on Sweet Pepper at Constant Temperatures. Environ. Entomol. 2001, 30, 720–727. [Google Scholar] [CrossRef]
- Nuno, M.M.S.A.; Cividanes, F.J. Exigências Térmicas de Bemisia tabaci (Genn.) Biótipo B (Hemiptera: Aleyrodidae). Neotrop. Entomol. 2002, 31, 359–363. [Google Scholar] [CrossRef]
- Xue, Y.; Lin, C.; Wang, Y.; Liu, W.; Wan, F.; Zhang, Y.; Ji, L. Predicting Climate Change Effects on the Potential Distribution of Two Invasive Cryptic Species of the Bemisia tabaci Species Complex in China. Insects 2022, 13, 1081. [Google Scholar] [CrossRef]
- Guo, C.; Zhu, Y.; Zhang, Y.; Keller, M.A.; Liu, T.-X.; Chu, D. Invasion Biology and Management of Sweetpotato Whitefly (Hemiptera: Aleyrodidae) in China. J. Integr. Pest Manag. 2021, 12, 2. [Google Scholar] [CrossRef]
- Alvarez, D.d.L.; Hayashida, R.; Cavallaro, M.C.; Santos, D.M.; Santos, L.M.; Müller, C.; Watanabe, L.F.M.; Bello, V.H.; Krause-Sakate, R.; Hoback, W.W.; et al. Susceptibility of Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) Mediterranean Populations Found in São Paulo, Brazil to 11 Insecticides and Characterization of Their Endosymbionts. Insects 2024, 15, 670. [Google Scholar] [CrossRef]
- Ghanim, M.; Kontsedalov, S. Susceptibility to Insecticides in the Q Biotype of Bemisia tabaci Is Correlated with Bacterial Symbiont Densities. Pest Manag. Sci. 2009, 65, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Moraes, L.A.d.; Muller, C.; Bueno, R.C.O.d.F.; Santos, A.; Bello, V.H.; De Marchi, B.R.; Watanabe, L.F.M.; Marubayashi, J.M.; Santos, B.R.; Yuki, V.A.; et al. Distribution and Phylogenetics of Whiteflies and Their Endosymbiont Relationships after the Mediterranean Species Invasion in Brazil. Sci. Rep. 2018, 8, 14589. [Google Scholar] [CrossRef] [PubMed]
- Milenovic, M.; Ghanim, M.; Hoffmann, L.; Rapisarda, C. Whitefly Endosymbionts: IPM Opportunity or Tilting at Windmills? J. Pest Sci. 2022, 95, 543–566. [Google Scholar] [CrossRef]
- Shah, S.H.J.; Malik, A.H.; Zhang, B.; Bao, Y.; Qazi, J. Metagenomic Analysis of Relative Abundance and Diversity of Bacterial Microbiota in Bemisia tabaci Infesting Cotton Crop in Pakistan. Infect. Genet. Evol. 2020, 84, 104381. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Pérez, D.; Hernández-Zepeda, C.; Chaidez-Quiroz, C.; Pérez-Brito, D.d.l.C.; González-Gómez, J.-P.; Minero-García, Y.; Rosiles-González, G.; Carrillo-Jovel, V.H.; Moreno-Valenzuela, O.A. Composition of the Whiteflies Microbiome in Populations with and without Insecticide Applications in Yucatan Mexico. Biologia 2024, 79, 2569–2579. [Google Scholar] [CrossRef]
- Walsh, P.S.; Metzger, D.A.; Higuchi, R. Chelex 100 as a Medium for Simple Extraction of DNA for PCR-Based Typing from Forensic Material. Biotechniques 1991, 10, 506–513. [Google Scholar] [CrossRef]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, Weighting, and Phylogenetic Utility of Mitochondrial Gene Sequences and a Compilation of Conserved Polymerase Chain Reaction Primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Bosco, D.; Loria, A.; Sartor, C.; Cenis, J.L. PCR-RFLP Identification of Bemisia tabaci Biotypes in the Mediterranean Basin. Phytoparasitica 2006, 34, 243–251. [Google Scholar] [CrossRef]
- Marubayashi, J.M.; Kliot, A.; Yuki, V.A.; Rezende, J.A.M.; Krause-Sakate, R.; Pavan, M.A.; Ghanim, M. Diversity and Localization of Bacterial Endosymbionts from Whitefly Species Collected in Brazil. PLoS ONE 2014, 9, e108363. [Google Scholar] [CrossRef]
- Haddad, M.L.; Parra, J.R.P. Métodos para Estimar os Limites Térmicos e a Faixa Ótima de Desenvolvimento das Diferentes Fases do Ciclo Evolutivo dos Insetos; Agricultura e Desenvolvimento; Escola Superior de Agricultura “Luiz de Queiroz”: Piracicaba, Brazil, 1984; 14p. [Google Scholar]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Mantel, N. Propriety of the Mantel—Haenszel Variance for the Log Rank Test. Biometrika 1985, 72, 471–472. [Google Scholar] [CrossRef]
- Tsagkarakou, A.; Tsigenopoulos, C.S.; Gorman, K.; Lagnel, J.; Bedford, I.D. Biotype Status and Genetic Polymorphism of the Whitefly Bemisia Tabaci (Hemiptera: Aleyrodidae) in Greece: Mitochondrial DNA and Microsatellites. Bull. Entomol. Res. 2007, 97, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, C.D.; Hemming, D.; Baker, R.; Everatt, M.; Eyre, D.; Korycinska, A. A Novel Approach for Exploring Climatic Factors Limiting Current Pest Distributions: A Case Study of Bemisia tabaci in North-West Europe and Assessment of Potential Future Establishment in the United Kingdom under Climate Change. PLoS ONE 2019, 14, e0221057. [Google Scholar] [CrossRef] [PubMed]
- Enkegaard, A. The Poinsettia Strain of the Cotton Whitefly, Bemisia tabaci (Homoptera: Aleyrodidae), Biological and Demographic Parameters on Poinsettia (Euphorbia Pulcherrima) in Relation to Temperature. Bull. Entomol. Res. 1993, 83, 535–546. [Google Scholar] [CrossRef]
- Chen, G.; Klinkhamer, P.G.L.; Escobar-Bravo, R.; Leiss, K.A. Type VI Glandular Trichome Density and Their Derived Volatiles Are Differently Induced by Jasmonic Acid in Developing and Fully Developed Tomato Leaves: Implications for Thrips Resistance. Plant Sci. 2018, 276, 87–98. [Google Scholar] [CrossRef]
- Glas, J.J.; Schimmel, B.C.J.; Alba, J.M.; Escobar-Bravo, R.; Schuurink, R.C.; Kant, M.R. Plant Glandular Trichomes as Targets for Breeding or Engineering of Resistance to Herbivores. Int. J. Mol. Sci. 2012, 13, 17077–17103. [Google Scholar] [CrossRef]
- Harrison, E.L.; Arce Cubas, L.; Gray, J.E.; Hepworth, C. The Influence of Stomatal Morphology and Distribution on Photosynthetic Gas Exchange. Plant J. 2020, 101, 768–779. [Google Scholar] [CrossRef]
- Lin, P.-A.; Chen, Y.; Ponce, G.; Acevedo, F.E.; Lynch, J.P.; Anderson, C.T.; Ali, J.G.; Felton, G.W. Stomata-Mediated Interactions between Plants, Herbivores, and the Environment. Trends Plant Sci. 2022, 27, 287–300. [Google Scholar] [CrossRef]
- Brazilian Climate Data. Available online: https://clima.inmet.gov.br (accessed on 20 April 2023).
- Brumin, M.; Kontsedalov, S.; Ghanim, M. Rickettsia Influences Thermotolerance in the Whitefly Bemisia tabaci B Biotype: Rickettsia Influence on Thermotolerance. Insect Sci. 2011, 18, 57–66. [Google Scholar] [CrossRef]
- Su, Q.; Xie, W.; Wang, S.; Wu, Q.; Liu, B.; Fang, Y.; Xu, B.; Zhang, Y. The Endosymbiont Hamiltonella Increases the Growth Rate of Its Host Bemisia tabaci during Periods of Nutritional Stress. PLoS ONE 2014, 9, e89002. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, K.; Wang, J.; Chu, D. Cardinium Infection Alters Cotton Defense and Detoxification Metabolism of Its Whitefly Host. Insect Sci. 2023, 30, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.-W.; Liu, S.-S. The Costs and Benefits of Two Secondary Symbionts in a Whitefly Host Shape Their Differential Prevalence in the Field. Front. Microbiol. 2021, 12, 739521. [Google Scholar] [CrossRef] [PubMed]
- Spano, D.; Armiento, M.; Aslam, M.F.; Bacciu, V.; Bigano, A.; Bosello, F.; Breil, M.; Buonocore, M.; Butenschön, M.; Cadau, M.; et al. G20 Climate Risk Atlas. Impacts, Policy and Economics in the G20 2021. Available online: https://www.cmcc.it/g20. (accessed on 22 May 2025).
- Malecha, A.; Manes, S.; Vale, M.M. Climate Change and Biodiversity in Brazil: What We Know, What We Don’t, and Paris Agreement’s Risk Reduction Potential. Perspect. Ecol. Conserv. 2025, 23, 77–84. [Google Scholar] [CrossRef]
- Elbaz, M.; Weiser, M.; Morin, S. Asymmetry in Thermal Tolerance Trade-offs between the B and Q Sibling Species of Bemisia tabaci (Hemiptera: Aleyrodidae). J. Evol. Biol. 2011, 24, 1099–1109. [Google Scholar] [CrossRef]
- Lima, F.F.D.; Alves, L.R.A. Portfolio Theory Approach to Plan Areas for Growing Cotton, Soybean, and Corn in Mato Grosso, Brazil. Rev. Econ. Sociol. Rural 2023, 61, e258224. [Google Scholar] [CrossRef]
- Stratton, A.E.; Wittman, H.; Blesh, J. Diversification Supports Farm Income and Improved Working Conditions during Agroecological Transitions in Southern Brazil. Agron. Sustain. Dev. 2021, 41, 35. [Google Scholar] [CrossRef]
- Rivas, M.; Vidal, R.; Neitzke, R.S.; Priori, D.; Almeida, N.; Antunes, I.F.; Galván, G.A.; Barbieri, R.L. Diversity of Vegetable Landraces in the Pampa Biome of Brazil and Uruguay: Utilization and Conservation Strategies. Front. Plant Sci. 2023, 14, 1232589. [Google Scholar] [CrossRef]
- Quintão, F.C.S.; Dias Da Silva Furtado, J.; Mendes Diniz Tripode, B.; Miranda, J.E. Inseticidas para controle do bicudo do algodoeiro—Eficiência, período residual e perdas por escorrimento. In Pesquisa e Inovação nas Ciências que Alimentam o Mundo; Agrárias; Editora Artemis: Curitiba, Brazil, 2020; Volume IV, pp. 55–65. ISBN 978-65-87396-25-5. [Google Scholar]
- Horowitz, A.R.; Ishaaya, I. Dynamics of Biotypes B and Q of the Whitefly Bemisia tabaci and Its Impact on Insecticide Resistance. Pest Manag. Sci. 2014, 70, 1568–1572. [Google Scholar] [CrossRef]
- Abubakar, M.; Koul, B.; Chandrashekar, K.; Raut, A.; Yadav, D. Whitefly (Bemisia tabaci) Management (WFM) Strategies for Sustainable Agriculture: A Review. Agriculture 2022, 12, 1317. [Google Scholar] [CrossRef]
- Zandi-Sohani, N.; Shishehbor, P. Temperature Effects on the Development and Fecundity of Encarsia Acaudaleyrodis (Hymenoptera: Aphelinidae), a Parasitoid of Bemisia tabaci (Homoptera: Aleyrodidae) on Cucumber. BioControl 2011, 56, 257–263. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).