Maize and Pea Root Interactions Promote Symbiotic Nitrogen Fixation, Thereby Accelerating Nitrogen Assimilation and Partitioning in Intercropped Pea
Abstract
1. Introduction
2. Materials and Methods
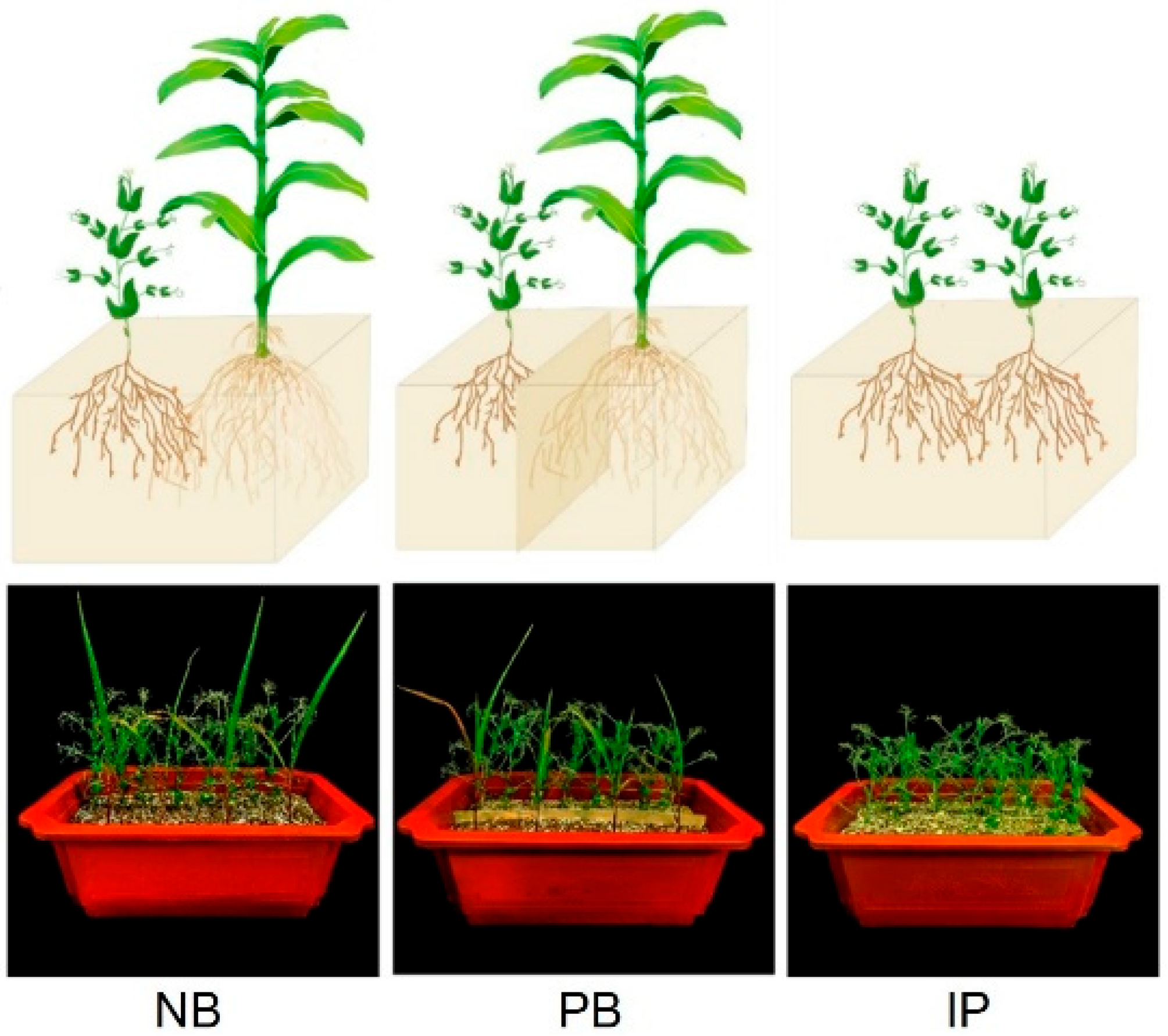
2.1. Experiment Design and Plant Cultivation
2.2. Root Characteristics, Chlorophyll Concentration, and Nodule Performance
2.3. N Metabolic Enzyme Activities
2.4. Ammonium and AA Concentrations
2.5. RNA Extraction and qRT-PCR Analysis
2.6. The Seed Number, Seed Yield, N Contents, and NUE in Pea
3. Results
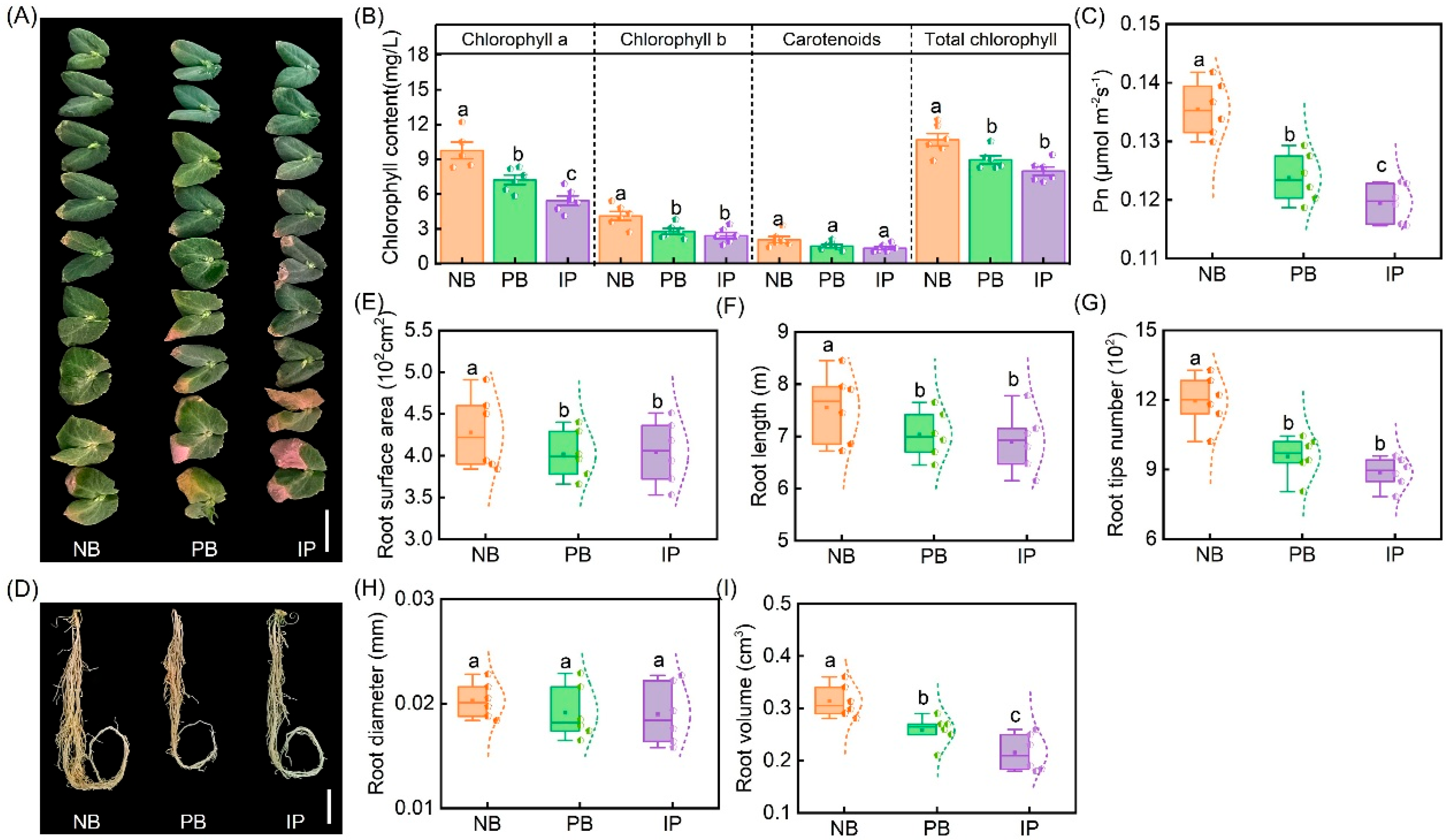
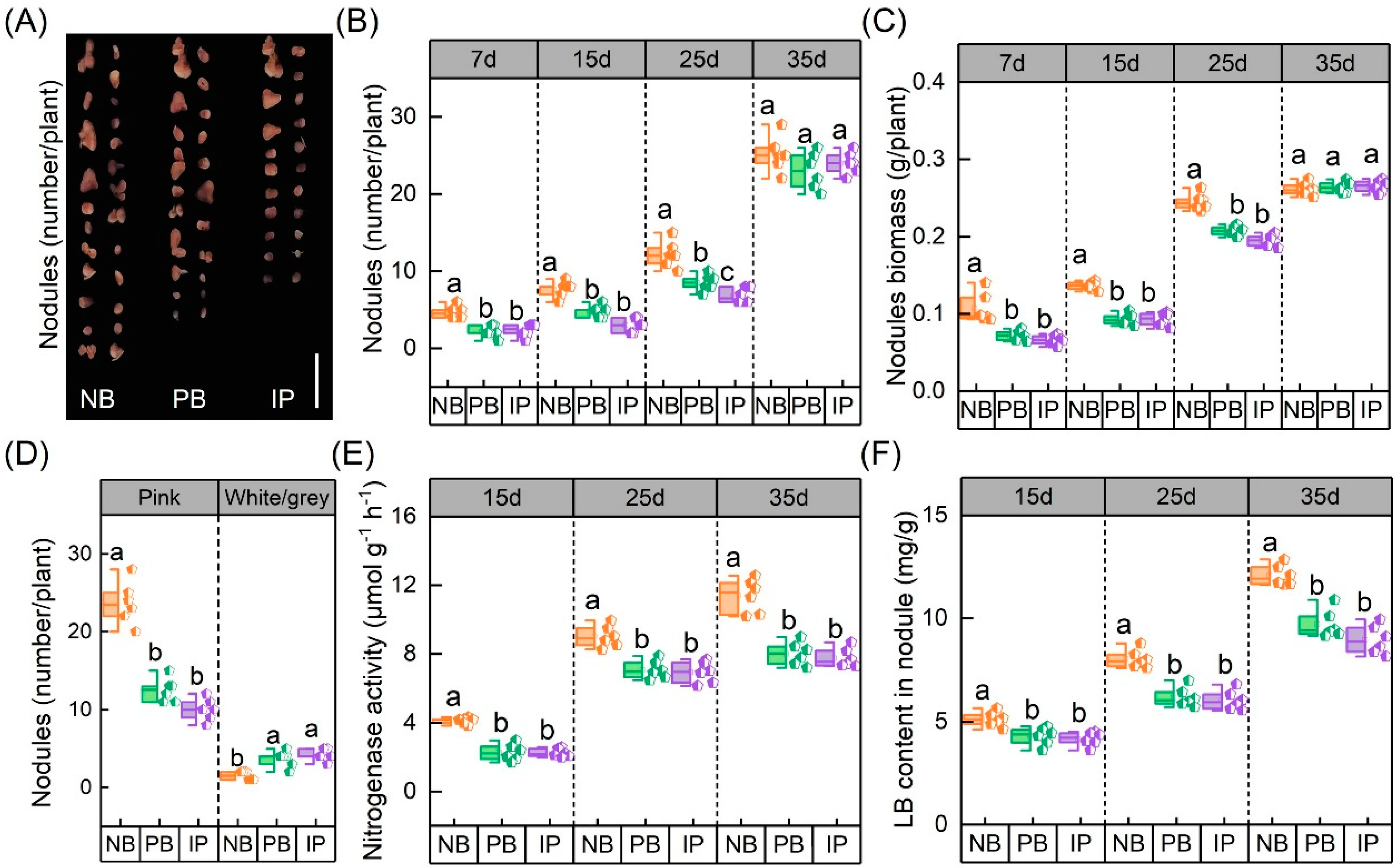
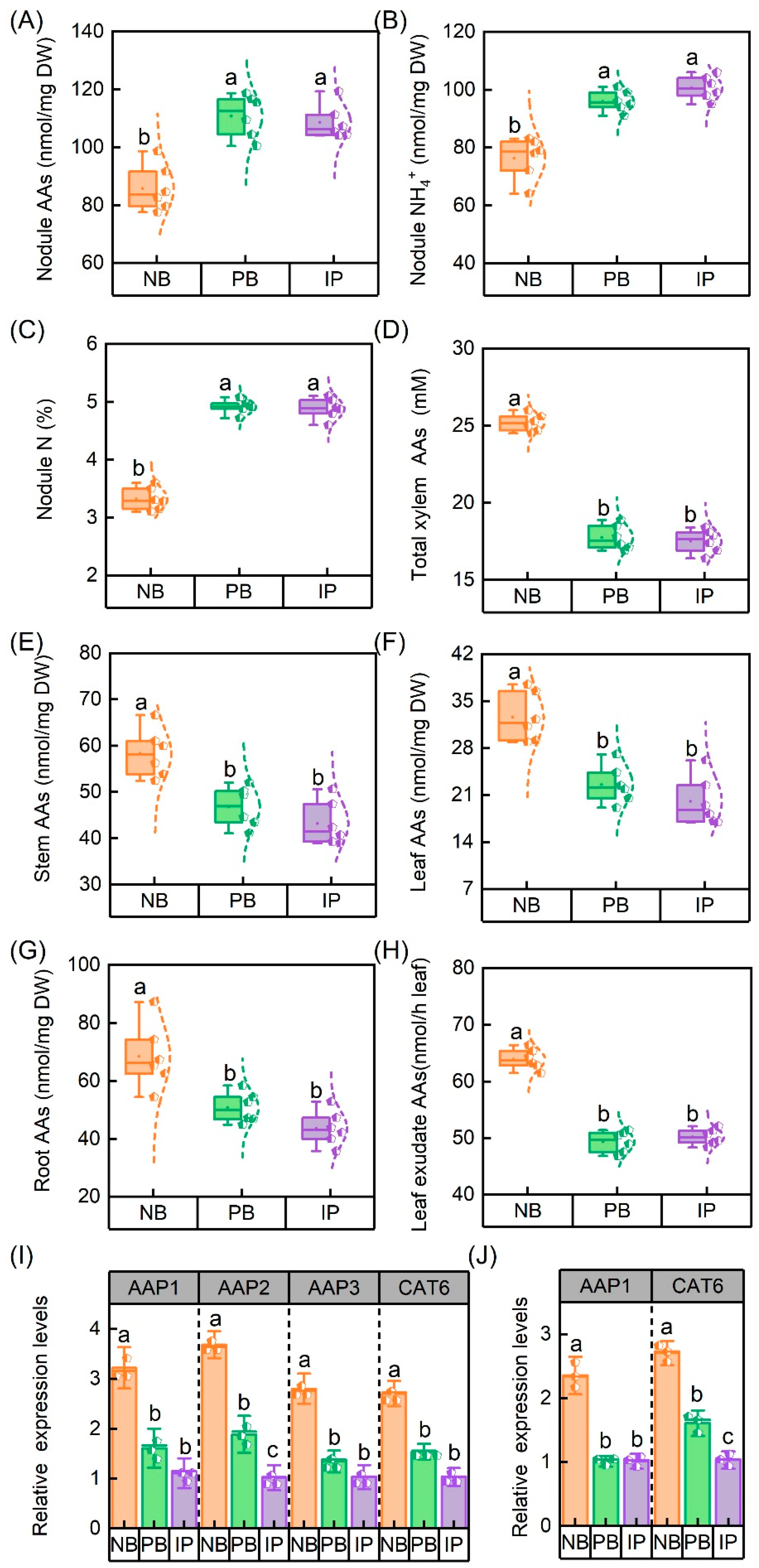
3.1. Effects of IRIs on Chlorophyll Concentrations, Root Properties, and Nodule N-Fixing Performances
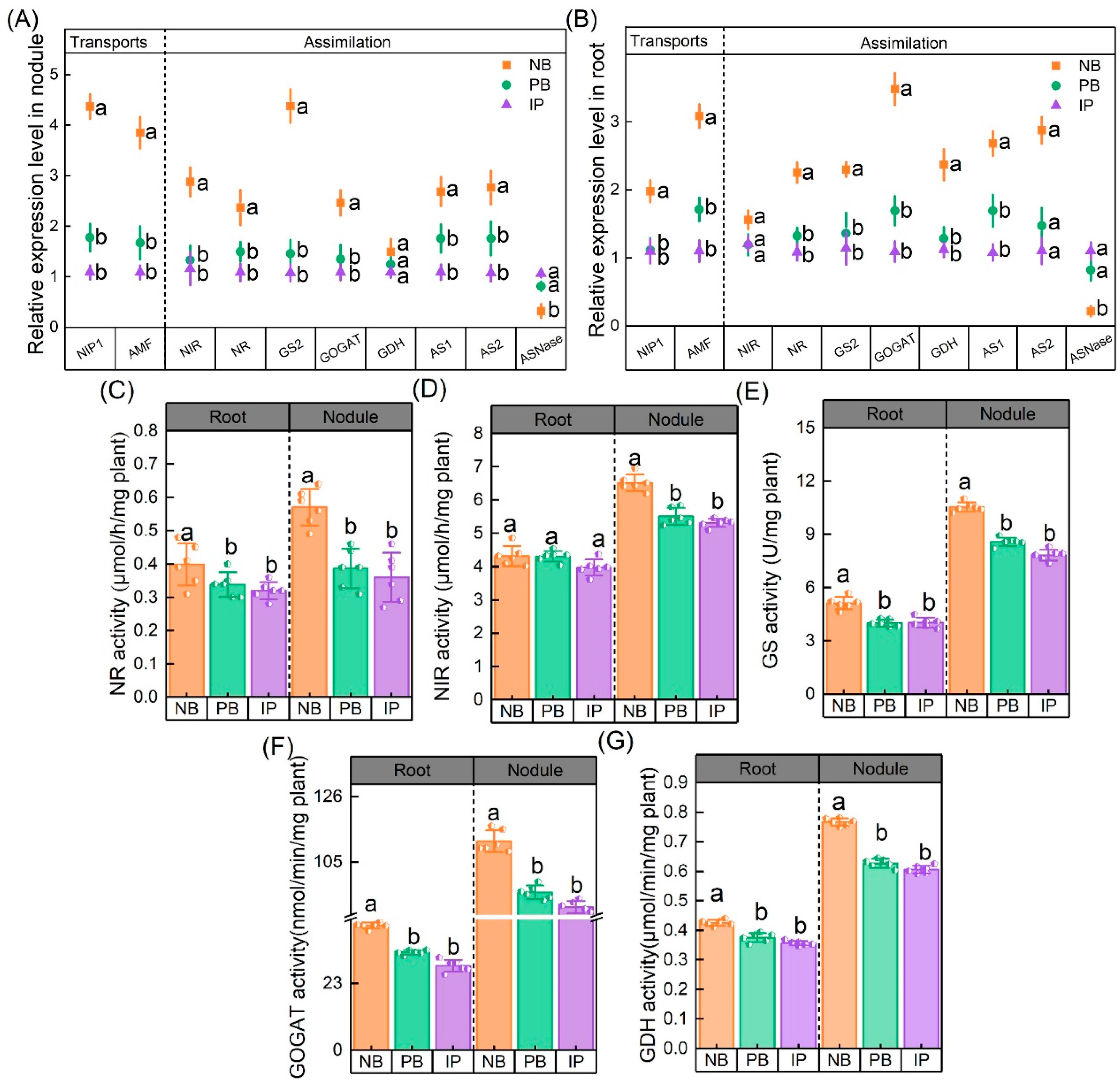
3.2. N Metabolism Is Regulated Following Increases in Enzyme Activity and Gene Expression
3.3. IRIs Accelerate AA Delivery from Nodules to Roots and Shoots
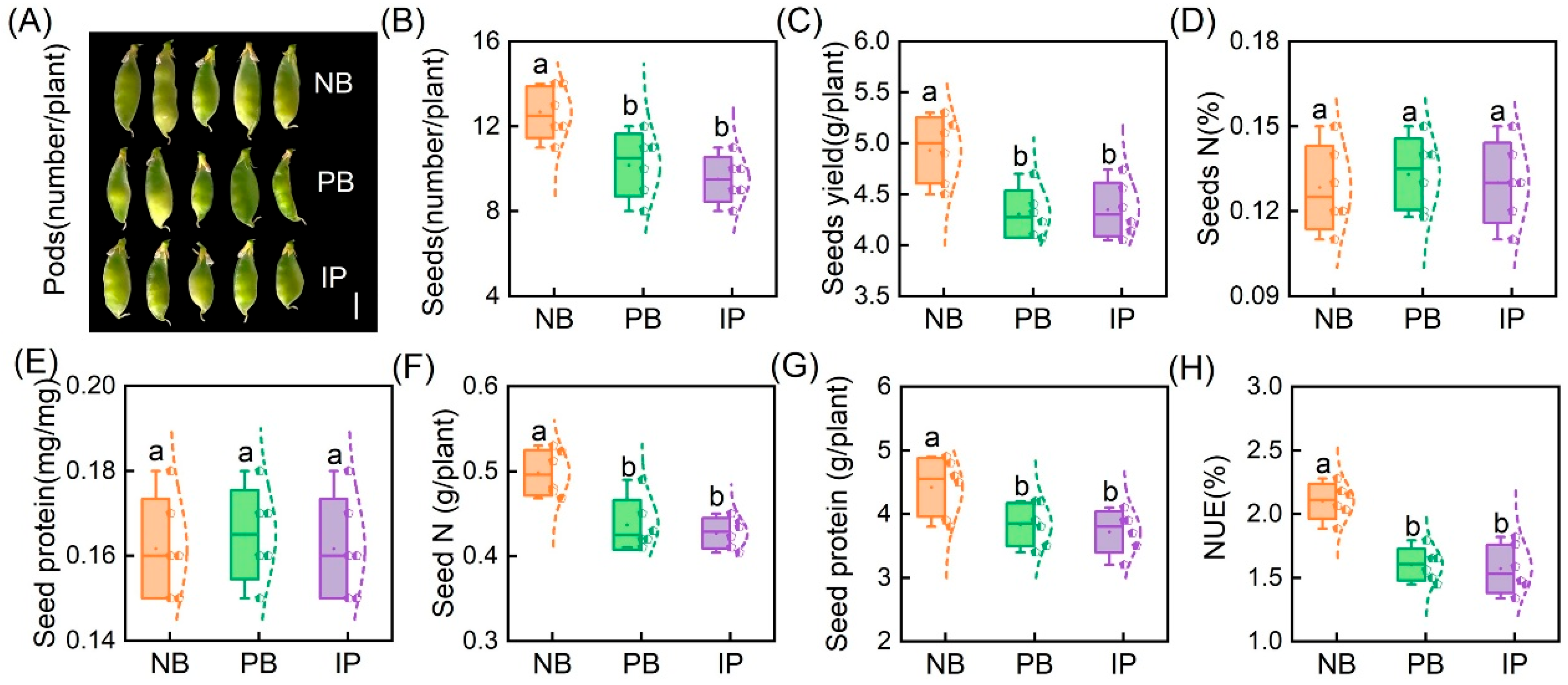
3.4. IRIs Improve Pea NUE and Crop Quality by Increasing the Number of Seeds
4. Discussions
4.1. IRIs Optimize Intercropped Pea Phenotypes
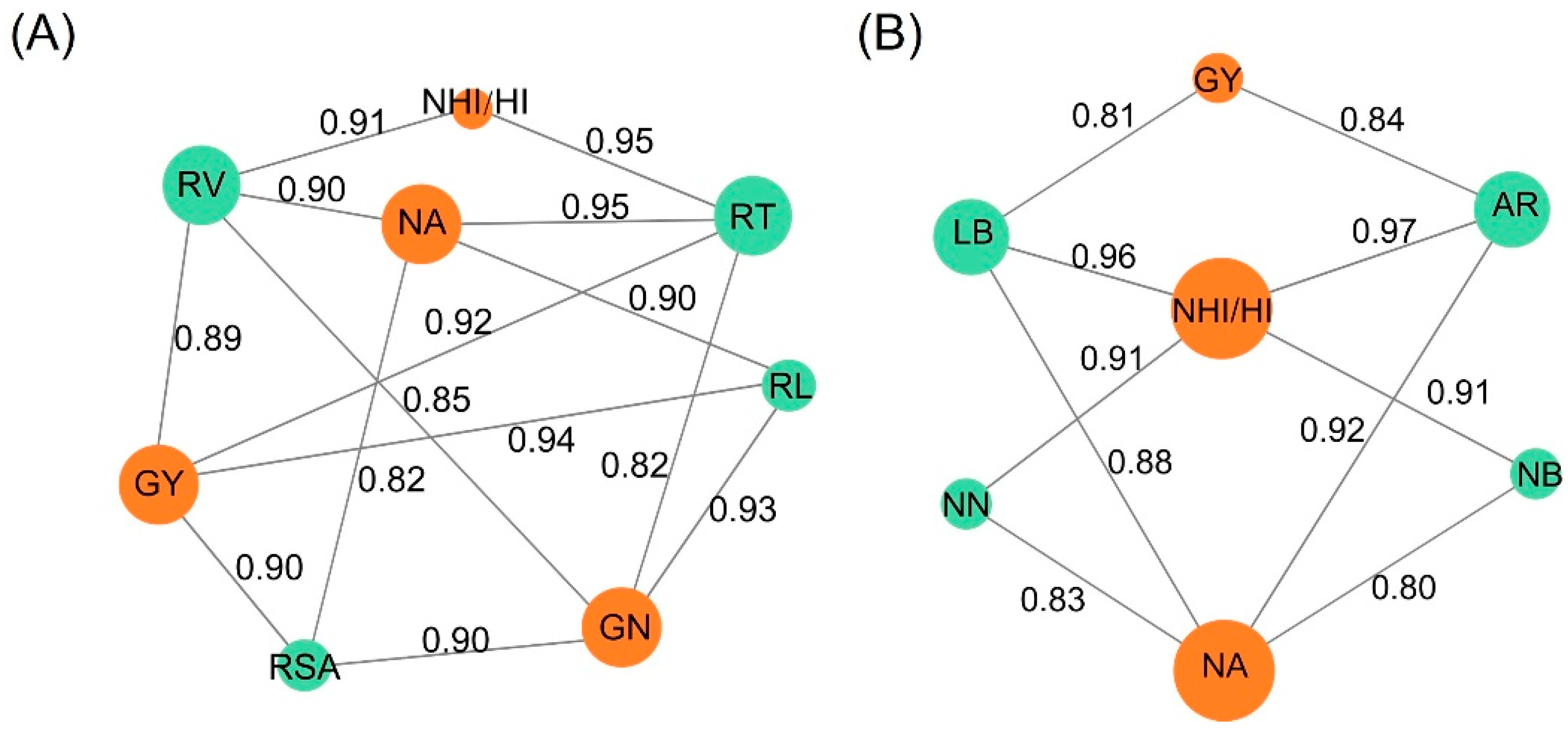
4.2. SNF-Driven N Metabolism and AAs Transport Accelerates Source-to-Sink N Partitioning, Thereby Increasing NUE and Crop Quality
4.3. Mechanisms of SNF Enhancement in Pea via IRIs During Intercropping
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, Z.L.; Zhang, H.Y.; Chen, X.P.; Zhang, C.C.; Ma, W.Q.; Huang, C.D.; Zhang, W.F.; Mi, G.H.; Miao, Y.X.; Li, X.L.; et al. Pursuing sustainable productivity with millions of smallholder farmers. Nature 2018, 555, 363–366. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Ross, J.J.; Reid, J.B. Nodulation phenotypes of gibberellin and brassinosteroid mutants of pea. Plant Physiol. 2005, 138, 2396–2405. [Google Scholar] [CrossRef] [PubMed]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The evolution and future of earth’s Nitrogen cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, Y.Q.; Li, X.B.; Song, M.H.; Fang, X.X.; Jiang, Y.; Xu, X.L. Nitrogen fixation and transfer between legumes and cereals under various cropping regimes. Rhizosphere 2022, 22, 100546. [Google Scholar] [CrossRef]
- Jensen, E.S.; Carlsson, G.; Hauggaard-Nielsen, H. Intercropping of grain legumes and cereals improves the use of soil N resources and reduces the requirement for synthetic fertilizer N: A global-scale analysis. Agron. Sustain. Dev. 2020, 40, 9. [Google Scholar] [CrossRef]
- Chamkhi, I.; Cheto, S.; Geistlinger, J.; Zeroual, Y.; Kouisni, L.; Bargaz, A.; Ghoulam, C. Legume-based intercropping systems promote beneficial rhizobacterial community and crop yield under stressing conditions. Ind. Crops Prod. 2022, 183, 114958. [Google Scholar] [CrossRef]
- Shao, Z.Q.; Zheng, C.C.; Postma, J.A.; Lu, W.L.; Gao, Q.; Gao, Y.Z.; Zhang, J.J. Nitrogen acquisition, fixation and transfer in maize/alfalfa intercrops are increased through root contact and morphological responses to interspecies competition. J. Integr. Agric. 2021, 20, 2240–2254. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, G.P.; Chang, D.N.; Gao, S.J.; Han, M.; Zhang, J.D.; Sun, X.F.; Cao, W.D. Transfer characteristics of nitrogen fixed by leguminous green manure crops when intercropped with maize in northwestern China. J. Integr. Agric. 2022, 21, 1177–1187. [Google Scholar] [CrossRef]
- Zheng, B.; Zhou, Y.; Chen, P.; Zhang, X.; Du, Q.; Yang, H.; Wang, X.; Yang, F.; Xiao, T.; Li, L.; et al. Maize–legume intercropping promote N uptake through changing the root spatial distribution, legume nodulation capacity, and soil N availability. J. Integr. Agric. 2022, 21, 1755–1771. [Google Scholar]
- Duchene, O.; Vian, J.F.; Celette, F. Intercropping with legume for agroecological cropping systems: Complementarity and facilitation processes and the importance of soil microorganisms. Agric. Ecosyst. Environ. 2017, 240, 148–161. [Google Scholar] [CrossRef]
- Glaze-Corcoran, S.; Hashemi, M.; Sadeghpour, A.; Jahanzad, E.; Afshar, R.K.; Liu, X.B.; Herbert, S.J. Understanding intercropping to improve agricultural resiliency and environmental sustainability. Adv. Agron. 2020, 162, 199–256. [Google Scholar]
- Lv, Y.; Francis, C.; Wu, P.; Chen, X.; Zhao, X. Maize–Soybean Intercropping Interactions Above and Below Ground. Crop Sci. 2014, 54, 914–922. [Google Scholar] [CrossRef]
- Mommer, L.; Kirkegaard, J.; van Ruijven, J. Root–Root interactions: Towards a rhizosphere framework. Trends Plant Sci. 2016, 21, 209–217. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.-X.; Li, X.-F. Intercropping to maximize root–root interactions in agricultural plants. In The Root Systems in Sustainable Agricultural Intensification; Rengel, Z., Djalovic, I., Eds.; Wiley: New York, NY, USA, 2021; pp. 309–328. [Google Scholar]
- Zhou, X.; Zhang, J.; Khashi u Rahman, M.; Gao, D.; Wei, Z.; Wu, F.; Dini-Andreote, F. Interspecific plant interaction via root exudates structures the disease suppressiveness of rhizosphere microbiomes. Mol. Plant 2023, 16, 849–864. [Google Scholar] [CrossRef]
- Hu, F.; Tan, Y.; Yu, A.; Zhao, C.; Fan, Z.; Yin, W.; Chai, Q.; Coulter, J.A.; Cao, W. Optimizing the split of N fertilizer application over time increases grain yield of maize-pea intercropping in arid areas. Eur. J. Agron. 2020, 119, 126117. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.-Y.; Wu, H.-M.; Zhang, F.-F.; Li, C.-J.; Li, X.-X.; Lambers, H.; Li, L. Root exudates drive interspecific facilitation by enhancing nodulation and N2 fixation. Proc. Natl. Acad. Sci. USA 2016, 113, 6496–6501. [Google Scholar] [CrossRef]
- Liu, Y.C.; Yin, X.H.; Xiao, J.X.; Tang, L.; Zheng, Y. Interactive influences of intercropping by nitrogen on flavonoid exudation and nodulation in faba bean. Sci. Rep. 2019, 9, 4818. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.J.; Liu, X.J.; Tong, C.C.; Wu, Y. Effect of root interaction on nodulation and nitrogen fixation ability of alfalfa in the simulated alfalfa/triticale intercropping in pots. Sci. Rep. 2020, 10, 4269. [Google Scholar] [CrossRef]
- White, J.; Prell, J.; James, E.K.; Poole, P.S. Nutrient sharing between symbionts. Plant Physiol. 2007, 144, 604–614. [Google Scholar] [CrossRef]
- Tian, C.J.; Kasiborski, B.; Koul, R.; Lammers, P.J.; Bücking, H.; Shachar-Hill, Y. Regulation of the nitrogen transfer pathway in the arbuscular mycorrhizal symbiosis: Gene characterization and the coordination of expression with nitrogen flux. Plant Physiol. 2010, 153, 1175–1187. [Google Scholar] [CrossRef]
- Tegeder, M. Transporters involved in source to sink partitioning of amino acids and ureides: Opportunities for crop improvement. J. Exp. Bot. 2014, 65, 1865–1878. [Google Scholar] [CrossRef]
- Santiago, J.P.; Tegeder, M. Connecting source with sink: The role of Arabidopsis AAP8 in phloem loading of amino acids. Plant Physiol. 2016, 171, 508–521. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, Q.; Lee, R.; Trethewy, A.; Lee, Y.-H.; Tegeder, M. Altered xylem-phloem transfer of amino acids affects metabolism and leads to increased seed yield and oil content in Arabidopsis. Plant Cell 2010, 22, 3603–3620. [Google Scholar] [CrossRef] [PubMed]
- Schiltz, S.; Munier-Jolain, N.; Jeudy, C.; Burstin, J.; Salon, C. Dynamics of exogenous Nitrogen partitioning and Nitrogen remobilization from vegetative organs in Pea revealed by 15N in vivo labeling throughout seed filling. Plant Physiol. 2005, 137, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.; Collier, R.; Trethewy, A.; Gould, G.; Sieker, R.; Tegeder, M. AAP1 regulates import of amino acids into developing Arabidopsis embryos. Plant J. 2009, 59, 540–552. [Google Scholar] [CrossRef]
- Zhang, L.; Garneau, M.G.; Majumdar, R.; Grant, J.; Tegeder, M. Improvement of pea biomass and seed productivity by simultaneous increase of phloem and embryo loading with amino acids. Plant J. 2015, 81, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Zhang, L.; Grant, J.; Cooper, P.; Tegeder, M. Increased phloem transport of S-Methylmethionine positively affects sulfur and Nitrogen metabolism and seed development in pea plants. Plant Physiol. 2010, 154, 1886–1896. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Perchlik, M.; Tegeder, M. Improving plant Nitrogen use efficiency through alteration of amino acid transport processes. Plant Physiol. 2017, 175, 235–247. [Google Scholar] [CrossRef]
- Mao, L.L.; Zhang, L.Z.; Li, W.Q.; van der Werf, W.; Sun, J.H.; Spiertz, H.; Li, L. Yield advantage and water saving in maize/pea intercrop. Field Crops Res. 2012, 138, 11–20. [Google Scholar] [CrossRef]
- Yin, W.; Chai, Q.; Zhao, C.; Yu, A.Z.; Fan, Z.L.; Hu, F.L.; Fan, H.; Guo, Y.; Coulter, J.A. Water utilization in intercropping: A review. Agric. Water Manag. 2020, 241, 11–20. [Google Scholar] [CrossRef]
- Chai, Q.; Nemecek, T.; Liang, C.; Zhao, C.; Yu, A.Z.; Coulter, J.A.; Wang, Y.F.; Hu, F.L.; Wang, L.; Siddique, K.H.M.; et al. Integrated farming with intercropping increases food production while reducing environmental footprint. Proc. Natl. Acad. Sci. USA 2021, 118, e2106382118. [Google Scholar] [CrossRef] [PubMed]
- Fåhraeus, G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. Microbiology 1957, 16, 374–381. [Google Scholar] [CrossRef]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and program to determine total carotenoids and chlorophylls A and B of leaf extracts in different solvents. In Advances in Photosynthesis Research, Proceedings of the VIth International Congress on Photosynthesis, Brussels, Belgium, 1–6 August 1983; Sybesma, C., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; Volume 2, pp. 9–12. [Google Scholar]
- Oke, V.; Long, S.R. Bacterial genes induced within the nodule during the Rhizobium-legume symbiosis. Mol. Microbiol. 1999, 32, 837–849. [Google Scholar] [CrossRef]
- Riley, I.T.; Dilworth, M.J. Cobalt requirement for nodule development and function in Lupinus angustifolius L. New Phytol. 1985, 100, 347–359. [Google Scholar] [CrossRef]
- Aoyama, C.; Santa, T.; Tsunoda, M.; Fukushima, T.; Kitada, C.; Imai, K. A fully automated amino acid analyzer using NBD-F as a fluorescent derivatization reagent. Biomed. Chromatogr. 2004, 18, 630–636. [Google Scholar] [CrossRef]
- Bongers, F.J.; Evers, J.B.; Anten, N.P.R. Plastic responses for intercrop functioning. NPJ Sustain. Agric. 2025, 3, 8. [Google Scholar] [CrossRef]
- Yin, W.; Chai, Q.; Fan, Z.L.; Hu, F.L.; Zhao, L.H.; Fan, H.; He, W.; Zhao, C.; Yu, A.Z.; Sun, Y.L.; et al. Review on physiological and ecological characteristics and agronomic regulatory pathways of intercropping to delay root and canopy senescence of crops. J. Integr. Agric. 2025, 24, 1–22. [Google Scholar] [CrossRef]
- Liu, Y.C.; Qin, X.M.; Xiao, J.X.; Tang, L.; Wei, C.Z.; Wei, J.J.; Zheng, Y. Intercropping influences component and content change of flavonoids in root exudates and nodulation of Faba bean. J. Plant Interact. 2017, 12, 187–192. [Google Scholar] [CrossRef]
- Kant, S.; Bi, Y.M.; Rothstein, S.J. Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 2011, 62, 1499–1509. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.-A.; Dong, Y.; Tang, L.; Zheng, Y.; Xiao, J. Interspecies interaction for nitrogen use efficiency via up-regulated glutamine and glutamate synthase under wheat-faba bean intercropping. Field Crops Res. 2021, 274, 108324. [Google Scholar] [CrossRef]
- Andrews, M.; Lea, P.J.; Raven, J.A.; Lindsey, K. Can genetic manipulation of plant nitrogen assimilation enzymes result in increased crop yield and greater N-use efficiency? An assessment. Ann. Appl. Biol. 2004, 145, 25–40. [Google Scholar] [CrossRef]
- Atkins, C.A.; Smith, P.M.C. Translocation in Legumes: Assimilates, Nutrients, and Signaling Molecules. Plant Physiol. 2007, 144, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Fan, Z.L.; Hu, F.L.; Yin, W.; Zhao, C.; Yu, A.Z.; Chai, Q. Source-to-Sink Translocation of Carbon and Nitrogen Is Regulated by Fertilization and Plant Population in Maize-Pea Intercropping. Front. Plant Sci. 2019, 10, 891. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.Y.; Zhao, J.H.; Sun, J.J.; Xue, Y.F.; Eagling, T.; Bao, X.X.; Zhang, F.S.; Li, L. Maize grain concentrations and above-ground shoot acquisition of micronutrients as affected by intercropping with turnip, faba bean, chickpea, and soybean. Sci. China Life Sci. 2013, 56, 823–834. [Google Scholar] [CrossRef]
- Ladrera, R.; Marino, D.; Larrainzar, E.; González, E.; Arrese-Igor, C. Reduced carbon availability to bacteroids and elevated ureides in nodules, but not in shoots, are involved in the Nitrogen fixation response to early drought in Soybean. Plant Physiol. 2007, 145, 539–546. [Google Scholar] [CrossRef]
- Gil-Quintana, E.; Larrainzar, E.; Arrese-Igor, C.; González, E. Is N-feedback involved in the inhibition of nitrogen fixation in drought-stressed Medicago truncatula? J. Exp. Bot. 2013, 64, 281–292. [Google Scholar] [CrossRef]
- Sulieman, S.; Tran, L.-S.P. Asparagine: An amide of particular distinction in the regulation of symbiotic nitrogen fixation of legumes. Crit. Rev. Biotechnol. 2013, 33, 309–327. [Google Scholar] [CrossRef]
- Collier, R.; Tegeder, M. Soybean ureide transporters play a critical role in nodule development, function and nitrogen export. Plant J. 2012, 72, 355–367. [Google Scholar] [CrossRef]
- Schulze, J. Source-sink manipulations suggest an N-feedback mechanism for the drop in N2 fixation during pod-filling in pea and broad bean. J. Plant Physiol. 2003, 160, 531–537. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Chardon, F. Exploring nitrogen remobilization for seed filling using natural variation in Arabidopsis thaliana. J. Exp. Bot. 2011, 62, 2131–2142. [Google Scholar] [CrossRef] [PubMed]
- Salon, C.; Munier-Jolain, N.; Duc, G.; Voisin, A.-S.; Grandgirard, D.; Larmure, A.; Emery, R.J.; Ney, B. Grain legume seed filling in relation to nitrogen acquisition: A review and prospects with particular reference to Pea. Agronomy 2001, 21, 539–552. [Google Scholar] [CrossRef]
- Burstin, J.; Marget, P.; Huart, M.; Moessner, A.; Mangin, B.; Duchene, C.; Desprez, B.; Munier-Jolain, N.; Duc, G. Developmental genes have pleiotropic effects on plant morphology and source capacity, eventually impacting on seed protein content and productivity in pea. Plant Physiol. 2007, 144, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Garneau, M.G.; Lu, M.-Z.; Grant, J.; Tegeder, M. Role of source-to-sink transport of methionine in establishing seed protein quantity and quality in legumes. Plant Physiol. 2021, 187, 2134–2155. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, K.; Küster, H.; Radchuk, R.; Müller, M.; Weichert, H.; Fait, A.; Fernie, A.R.; Saalbach, I.; Weber, H. Increasing amino acid supply in pea embryos reveals specific interactions of N and C metabolism, and highlights the importance of mitochondrial metabolism. Plant J. 2008, 55, 909–926. [Google Scholar] [CrossRef]
- Rolletschek, H.; Hosein, F.; Miranda, M.; Heim, U.; Götz, K.-P.; Schlereth, A.; Borisjuk, L.; Saalbach, I.; Wobus, U.; Weber, H. Ectopic expression of an amino acid transporter (VfAAP1) in seeds of Vicia narbonensis and Pea increases storage proteins. Plant Physiol. 2005, 137, 1236–1249. [Google Scholar] [CrossRef]
- Lepetit, M.; Brouquisse, R. Control of the rhizobium-legume symbiosis by the plant nitrogen demand is tightly integrated at the whole plant level and requires inter-organ systemic signaling. Front. Plant Sci. 2023, 14, 1114840. [Google Scholar] [CrossRef]
- Parsons, R.; Stanforth, A.; Raven, J.A.; Sprent, J.I. Nodule growth and activity may be regulated by a feedback mechanism involving phloem nitrogen. Plant Cell Environ. 2010, 16, 125–136. [Google Scholar] [CrossRef]
- Sulieman, S.; Schulze, J. Phloem-derived γ-aminobutyric acid (GABA) is involved in upregulating nodule N2 fixation efficiency in the model legume Medicago truncatula. Plant Cell Environ. 2010, 33, 2162–2172. [Google Scholar] [CrossRef]
| Genes | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| Nitrogen transport and assimilation | ||
| Ammonium facilitator 3 (AMF) | CTCGCCTCTTGTCGGAGTTGTCC | CGGTGGTTGTACTTGTCTCCTCTAC |
| Nodulin26-like protein 1(NIP1) | CACCGATAACAGAGCGATTGG | TCCATATTCCTCGATATTCATTGTG |
| Nitrite reductase (NiR) | TGATACACGACCTTACAACAAC | ACTGAAGAAACCACCAACAA |
| Nitrate reductase (NR) | GAGATTGCGGTGCGTGCTTG | CGGATTGGTTGCCTGGTTGG |
| Glutamine synthetase 2 (GS2) | GAAAATGGCACCATCAATAGGGTAGA | AGGGATGCGAAACAGGCTTTGATAT |
| Glutamate dehydrogenase (GDH) | CGAATAAATGACTGGAACGAGGTG | GTGCTGGGCATACCCTACCG |
| Glutamate synthase-NADH dependent (GOGAT) | CACAGATTGCATWGGAACATCCATT | CCATTTTCATCTCCCAMAAACCTCTT |
| Asparagine synthetase 1 (AS1) | CTGTCACTGCTAGATACCTTGCTGGT | CTGTCACTGCTAGATACCTTGCTGGT |
| Asparagine synthetase 2 (AS2) | CCATCACTTCTCGCTACCTAGCAACC | TCGACATGAGAAACATAGGCGTGCT |
| L-Asparaginase (ASNase) | GTYATGGAHAARTCYCCDCATTCCTA | AMCARGATTGYDTTTGCYTCCTTTG |
| Amino acid phloem loading and transporter | ||
| Amino acid permease 1 (AAP1) | GCTGGAACCATTACTGGAGTAAATGA | GACTCTGACGGTGGTGGTGCTTTTA |
| Amino acid permease 2 (AAP2) | AGCAAGCCACGAGGATAAGTATAGGC | CAGTGAGTAAGTTTCCTGGCGATGTG |
| Amino acid permease 3 (AAP3) | CGTCACAGATTATTGAACATCAAGCA | GGGTCAAAATCACGGGTTGAAATAG |
| Cationic amino acid transporter 6 (CAT6) | GGTTCGGAGTGTTTTCGGCTGTTACG | GGTTCGGAGTGTTTTCGGCTGTTACG |
| Reference genes | ||
| Ubiquitin | GGCAAAAATACAGGACAAGGAGGGA | GCAAMACHAGGTGRAGAGTRGACT |
| Elongation factor 1α (EF1α) | CAGTGGGACGTGTTGAAACTGGTGTT | ATCGAACATTGTCTCCTGGAAGAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wu, Z.; Hu, F.; Fan, H.; He, W.; Zhao, L.; Guo, C.; Bao, X.; Chai, Q.; Zhao, C. Maize and Pea Root Interactions Promote Symbiotic Nitrogen Fixation, Thereby Accelerating Nitrogen Assimilation and Partitioning in Intercropped Pea. Agronomy 2025, 15, 1615. https://doi.org/10.3390/agronomy15071615
Sun Y, Wu Z, Hu F, Fan H, He W, Zhao L, Guo C, Bao X, Chai Q, Zhao C. Maize and Pea Root Interactions Promote Symbiotic Nitrogen Fixation, Thereby Accelerating Nitrogen Assimilation and Partitioning in Intercropped Pea. Agronomy. 2025; 15(7):1615. https://doi.org/10.3390/agronomy15071615
Chicago/Turabian StyleSun, Yali, Zefeng Wu, Falong Hu, Hong Fan, Wei He, Lianhao Zhao, Congcong Guo, Xiaoyuan Bao, Qiang Chai, and Cai Zhao. 2025. "Maize and Pea Root Interactions Promote Symbiotic Nitrogen Fixation, Thereby Accelerating Nitrogen Assimilation and Partitioning in Intercropped Pea" Agronomy 15, no. 7: 1615. https://doi.org/10.3390/agronomy15071615
APA StyleSun, Y., Wu, Z., Hu, F., Fan, H., He, W., Zhao, L., Guo, C., Bao, X., Chai, Q., & Zhao, C. (2025). Maize and Pea Root Interactions Promote Symbiotic Nitrogen Fixation, Thereby Accelerating Nitrogen Assimilation and Partitioning in Intercropped Pea. Agronomy, 15(7), 1615. https://doi.org/10.3390/agronomy15071615







