Root Phenotyping: A Contribution to Understanding Drought Stress Resilience in Grain Legumes
Abstract
1. Introduction
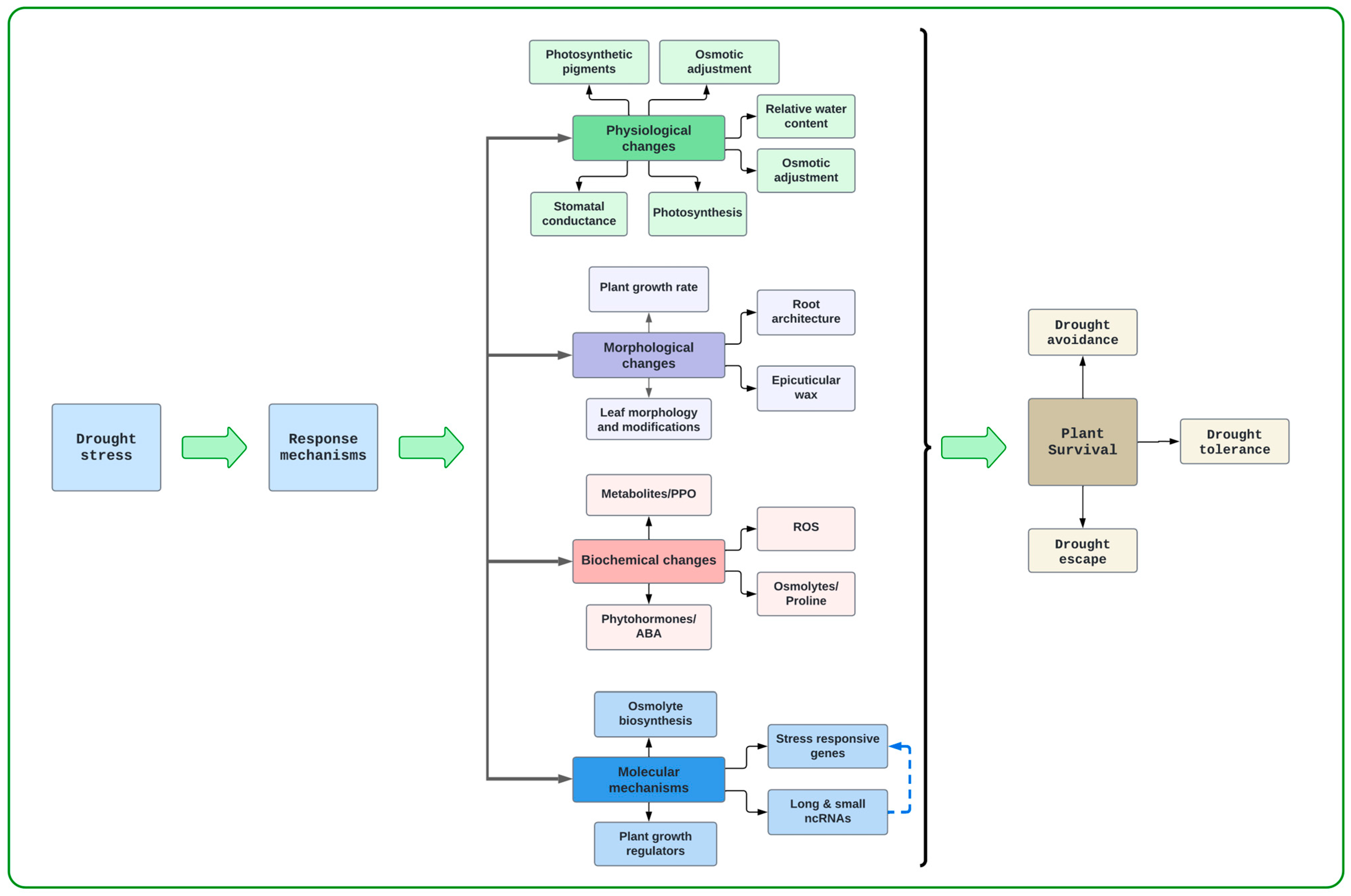
2. Drought Stress Responses in Grain Legumes
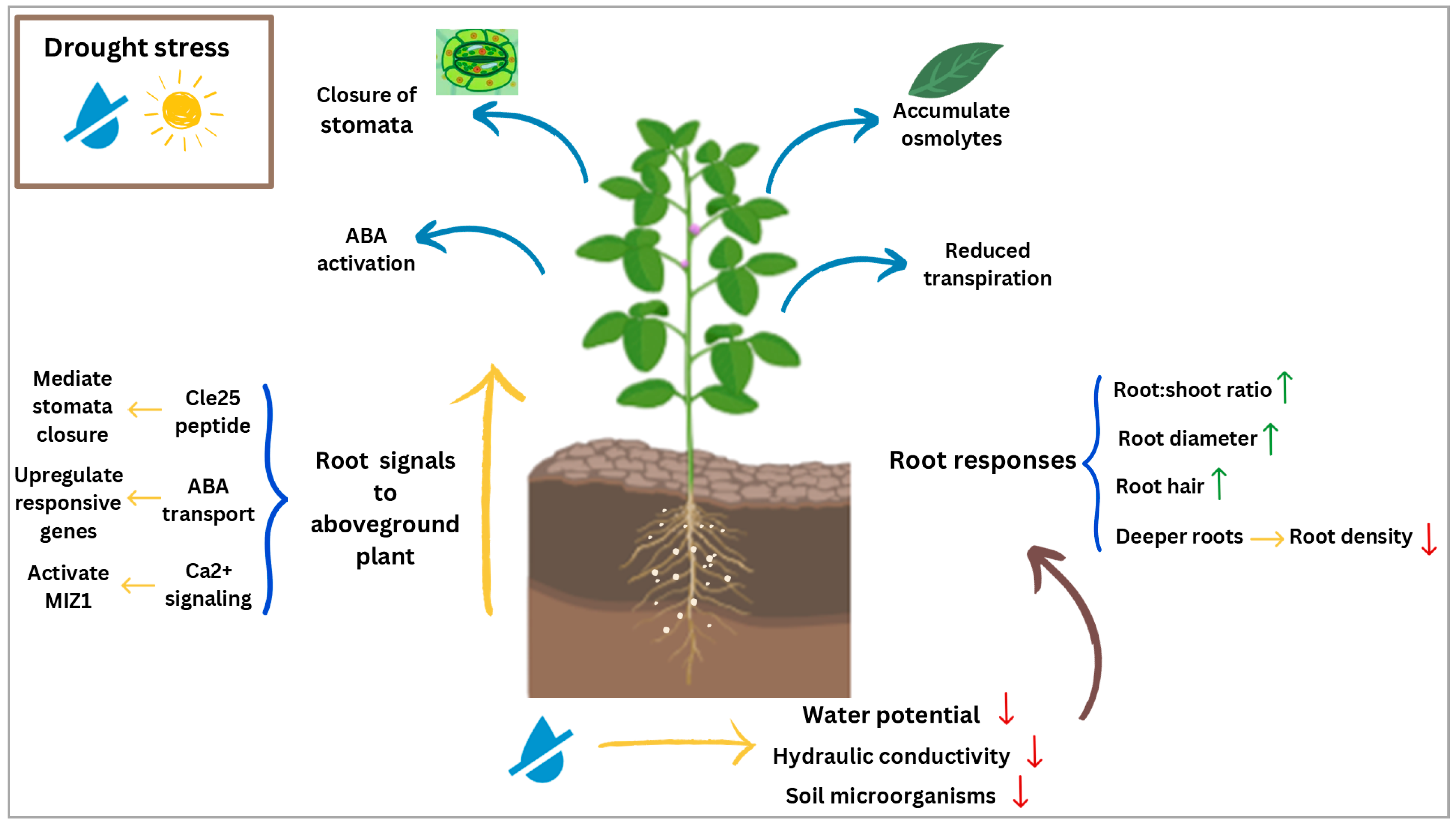
3. Roots and Their Role in Drought Stress Resilience
3.1. Morphological and Physiological Changes in Roots Under Drought Stress
3.2. Biochemical and Molecular Changes in Roots Under Drought Stress
4. Different Techniques for Root Phenotyping
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| BNF | biological nitrogen fixation |
| CTX | X-ray computed tomography |
| DIRT | digital imaging of root traits |
| H2O2 | hydrogen peroxide |
| MRI | magnetic resonance imaging |
| NIPs | NOD26-type intrinsic proteins |
| PEG | polyethylene glycol |
| PIPs | plasma membrane intrinsic proteins |
| RSA | root system architecture |
| ROS | reactive oxygen species |
| SIPs | small basic intrinsic proteins |
| TIPs | tonoplast intrinsic proteins |
| WUE | water-use efficiency |
| XIPs | uncharacterized intrinsic proteins |
References
- Smýkal, P.; Coyne, C.J.; Ambrose, M.J.; Maxted, N.; Schaefer, H.; Blair, M.W.; Berger, J.; Greene, S.L.; Nelson, M.N.; Besharat, N.; et al. Legume Crops Phylogeny and Genetic Diversity for Science and Breeding. CRC Crit. Rev. Plant Sci. 2015, 34, 43–104. [Google Scholar] [CrossRef]
- Carvalho, M.; Carnide, V.; Sobreira, C.; Castro, I.; Coutinho, J.; Barros, A.; Rosa, E. Cowpea Immature Pods and Grains Evaluation: An Opportunity for Different Food Sources. Plants 2022, 11, 2079. [Google Scholar] [CrossRef] [PubMed]
- Tharanathan, R.N.; Mahadevamma, S. Grain Legumes—A Boon to Human Nutrition. Trends Food Sci. Technol. 2003, 14, 507–518. [Google Scholar] [CrossRef]
- Zhu, H.; Choi, H.K.; Cook, D.R.; Shoemaker, R.C. Bridging Model and Crop Legumes through Comparative Genomics. Plant Physiol. 2005, 137, 1189–1196. [Google Scholar] [CrossRef]
- Mousavi-Derazmahalleh, M.; Bayer, P.E.; Hane, J.K.; Valliyodan, B.; Nguyen, H.T.; Nelson, M.N.; Erskine, W.; Varshney, R.K.; Papa, R.; Edwards, D. Adapting Legume Crops to Climate Change Using Genomic Approaches. Plant Cell Environ. 2019, 42, 6–19. [Google Scholar] [CrossRef]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple Benefits of Legumes for Agriculture Sustainability: An Overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef]
- Gogoi, N.; Baruah, K.K.; Meena, R.S. Grain Legumes: Impact on Soil Health and Agroecosystem. In Legumes for Soil Health and Sustainable Management; Springer: Singapore, 2018; pp. 511–539. [Google Scholar] [CrossRef]
- Okumu, O.; Otieno, H.M.O.; Okeyo, G.O. Production Systems and Contributions of Grain Legumes to Soil Health and Sustainable Agriculture: A Review. Arch. Agric. Environ. Sci. 2023, 8, 259–267. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Merwad, A.R.M.A.; Desoky, E.S.M.; Rady, M.M. Response of Water Deficit-Stressed Vigna unguiculata Performances to Silicon, Proline or Methionine Foliar Application. Sci. Hortic. 2018, 228, 132–144. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant Adaptation to Drought Stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research Progress and Perspective on Drought Stress in Legumes: A Review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M. Inducing Drought Tolerance in Plants: Recent Advances. Biotechnol. Adv. 2010, 28, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.A.; Pound, M.P.; Bennett, M.J.; Wells, D.M. Uncovering the Hidden Half of Plants Using New Advances in Root Phenotyping. Curr. Opin. Biotechnol. 2019, 55, 1–8. [Google Scholar] [CrossRef]
- Kalra, A.; Goel, S.; Elias, A.A. Understanding Role of Roots in Plant Response to Drought: Way Forward to Climate-Resilient Crops. Plant Genome 2024, 17, e20395. [Google Scholar] [CrossRef]
- Kumar, M. Crop Plants and Abiotic Stresses. J. Biomol. Res. Ther. 2014, 03, 7956. [Google Scholar] [CrossRef]
- Gelaw, T.A.; Sanan-Mishra, N. Non-Coding RNAs in Response to Drought Stress. Int. J. Mol. Sci. 2021, 22, 12519. [Google Scholar] [CrossRef]
- Carvalho, M.; Lino-Neto, T.; Rosa, E.; Carnide, V. Cowpea: A Legume Crop for a Challenging Environment. J. Sci. Food Agric. 2017, 97, 4273–4284. [Google Scholar] [CrossRef]
- Yang, H.; Cui, Y.; Feng, Y.; Hu, Y.; Liu, L.; Duan, L. Long Non-Coding RNAs of Plants in Response to Abiotic Stresses and Their Regulating Roles in Promoting Environmental Adaption. Cells 2023, 12, 729. [Google Scholar] [CrossRef]
- Petrushin, I.S.; Vasilev, I.A.; Markova, Y.A. Drought Tolerance of Legumes: Physiology and the Role of the Microbiome. Curr. Issues Mol. Biol. 2023, 45, 6311–6324. [Google Scholar] [CrossRef]
- Karlova, R.; Boer, D.; Hayes, S.; Testerink, C. Root Plasticity under Abiotic Stress. Plant Physiol. 2021, 187, 1057–1070. [Google Scholar] [CrossRef]
- Li, G.; Wang, K.; Qin, Q.; Li, Q.; Mo, F.; Nangia, V.; Liu, Y. Integrated Microbiome and Metabolomic Analysis Reveal Responses of Rhizosphere Bacterial Communities and Root Exudate Composition to Drought and Genotype in Rice (Oryza sativa L.). Rice 2023, 16, 1–17. [Google Scholar] [CrossRef]
- Kou, X.; Han, W.; Kang, J. Responses of Root System Architecture to Water Stress at Multiple Levels: A Meta-Analysis of Trials under Controlled Conditions. Front. Plant Sci. 2022, 13, 1085409. [Google Scholar] [CrossRef]
- Sofi, P.A.; Rehman, K.; Gull, M.; Kumari, J.; Djanaguiraman, M.; Prasad, P.V.V. Integrating Root Architecture and Physiological Approaches for Improving Drought Tolerance in Common Bean (Phaseolus vulgaris L.). Plant Physiol. Rep. 2021, 26, 4–22. [Google Scholar] [CrossRef]
- Xiong, R.; Liu, S.; Considine, M.J.; Siddique, K.H.M.; Lam, H.M.; Chen, Y. Root System Architecture, Physiological and Transcriptional Traits of Soybean (Glycine max L.) in Response to Water Deficit: A Review. Physiol. Plant. 2021, 172, 405–418. [Google Scholar] [CrossRef]
- Wang, Z.; Yung, W.S.; Gao, Y.; Huang, C.; Zhao, X.; Chen, Y.; Li, M.W.; Lam, H.M. From Phenotyping to Genetic Mapping: Identifying Water-Stress Adaptations in Legume Root Traits. BMC Plant Biol. 2024, 24, 749. [Google Scholar] [CrossRef]
- Gonzalez-Rizzo, S.; Laporte, P.; Crespi, M.; Frugier, F. Legume Root Architecture: A Peculiar Root System; Wiley: Hoboken, NJ, USA, 2018; Volume 37, ISBN 9781119312994. [Google Scholar]
- Zhang, Y.; Wu, X.; Wang, X.; Dai, M.; Peng, Y. Crop Root System Architecture in Drought Response. J. Genet. Genom. 2024, 52, 4–13. [Google Scholar] [CrossRef]
- Shelden, M.C.; Munns, R. Crop Root System Plasticity for Improved Yields in Saline Soils. Front. Plant Sci. 2023, 14, 1120583. [Google Scholar] [CrossRef]
- Burridge, J.; Jochua, C.N.; Bucksch, A.; Lynch, J.P. Legume Shovelomics: High-Throughput Phenotyping of Common Bean (Phaseolus vulgaris L.) and Cowpea (Vigna unguiculata subsp. unguiculata) Root Architecture in the Field. Field Crop. Res. 2016, 192, 21–32. [Google Scholar] [CrossRef]
- Chen, Y.; Ghanem, M.E.; Siddique, K.H.M. Characterising Root Trait Variability in Chickpea (Cicer arietinum L.) Germplasm. J. Exp. Bot. 2017, 68, 1987–1999. [Google Scholar] [CrossRef]
- Ye, H.; Roorkiwal, M.; Valliyodan, B.; Zhou, L.; Chen, P.; Varshney, R.K.; Nguyen, H.T. Genetic Diversity of Root System Architecture in Response to Drought Stress in Grain Legumes. J. Exp. Bot. 2018, 69, 3267–3277. [Google Scholar] [CrossRef]
- Kang, B.H.; Kim, W.J.; Chowdhury, S.; Moon, C.Y.; Kang, S.; Kim, S.H.; Jo, S.H.; Jun, T.H.; Do Kim, K.; Ha, B.K. Transcriptome Analysis of Differentially Expressed Genes Associated with Salt Stress in Cowpea (Vigna unguiculata L.) during the Early Vegetative Stage. Int. J. Mol. Sci. 2023, 24, 4267. [Google Scholar] [CrossRef] [PubMed]
- Wasaya, A.; Zhang, X.; Fang, Q.; Yan, Z. Root Phenotyping for Drought Tolerance: A Review. Agronomy 2018, 8, 241. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Review Article Plant Drought Stress: E Ff Ects, Mechanisms and Management. Agron. Sustain. Dev 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Cajiao, C.; Grajales, M. Selection for Drought Resistance in Common Bean Also Improves Yield in Phosphorus Limited and Favorable Environments. Crop Sci. 2008, 48, 582–592. [Google Scholar] [CrossRef]
- Mohammed, S.B.; Burridge, J.D.; Ishiyaku, M.F.; Boukar, O.; Lynch, J.P. Phenotyping Cowpea for Seedling Root Architecture Reveals Root Phenes Important for Breeding Phosphorus Efficient Varieties. Crop Sci. 2022, 62, 326–345. [Google Scholar] [CrossRef]
- Purushothaman, R.; Krishnamurthy, L.; Upadhyaya, H.D.; Vadez, V.; Varshney, R.K. Genotypic Variation in Soil Water Use and Root Distribution and Their Implications for Drought Tolerance in Chickpea. Funct. Plant Biol. 2017, 44, 235–252. [Google Scholar] [CrossRef]
- Kumar, J.; Sen Gupta, D.; Djalovic, I.; Kumar, S.; Siddique, K.H.M. Root-Omics for Drought Tolerance in Cool-Season Grain Legumes. Physiol. Plant. 2021, 172, 629–644. [Google Scholar] [CrossRef]
- Lobet, G.; Couvreur, V.; Meunier, F.; Javaux, M.; Draye, X. Plant Water Uptake in Drying Soils. Plant Physiol. 2014, 164, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root Traits Contributing to Plant Productivity under Drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- Charng, Y.Y.; Mitra, S.; Yu, S.J. Maintenance of Abiotic Stress Memory in Plants: Lessons Learned from Heat Acclimation. Plant Cell 2023, 35, 187–200. [Google Scholar] [CrossRef]
- Yadav, S.S.; Redden, R.; McNeil, D.L.; Patil, S.A. Climate Change and Management of Cool Season Grain Legume Crops; Springer Nature: Dordrecht, The Netherlands, 2010; pp. 1–460. [Google Scholar] [CrossRef]
- Khatun, M.; Sarkar, S.; Era, F.M.; Islam, A.K.M.M.; Anwar, M.P.; Fahad, S.; Datta, R.; Islam, A.K.M.A. Drought Stress in Grain Legumes: Effects, Tolerance Mechanisms and Management. Agronomy 2021, 11, 2374. [Google Scholar] [CrossRef]
- Santos, R.; Carvalho, M.; Rosa, E.; Carnide, V.; Castro, I. Root and Agro-Morphological Traits Performance in Cowpea under Drought Stress. Agronomy 2020, 10, 1604. [Google Scholar] [CrossRef]
- Liu, F.; Andersen, M.N.; Jacobsen, S.E.; Jensen, C.R. Stomatal Control and Water Use Efficiency of Soybean (Glycine max L. Merr.) during Progressive Soil Drying. Environ. Exp. Bot. 2005, 54, 33–40. [Google Scholar] [CrossRef]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones Enhanced Drought Tolerance in Plants: A Coping Strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef] [PubMed]
- Ariani, A.; Gepts, P. Genome—Wide Identification and Characterization of Aquaporin Gene Family in Common Bean (Phaseolus vulgaris L.). Mol. Genet. Genomics 2015, 290, 1771–1785. [Google Scholar] [CrossRef]
- Aroca, R.; Porcel, R.; Ruiz-lozano, J.M. Regulation of Root Water Uptake under Abiotic Stress Conditions. J. Exp. Bot. 2012, 63, 43–57. [Google Scholar] [CrossRef]
- Tayade, R.; Rana, V.; Shafiqul, M.; Begum, R.; Nabi, S.; Raturi, G. Genome-Wide Identification of Aquaporin Genes in Adzuki Bean (Vigna angularis) and Expression Analysis under Drought Stress. Int. J. Mol. Sci. 2022, 23, 16189. [Google Scholar] [CrossRef]
- Wu, L.; Chang, Y.; Wang, L.; Wang, S.; Wu, J. The Aquaporin Gene PvXIP1; 2 Conferring Drought Resistance Identified by GWAS at Seedling Stage in Common Bean. Theor. Appl. Genet. 2022, 135, 485–500. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Gupta, K.; Lopato, S.; Agarwal, P. Dehydration Responsive Element Binding Transcription Factors and Their Applications for the Engineering of Stress Tolerance. J. Exp. Bot. 2017, 68, 2135–2148. [Google Scholar] [CrossRef]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF Family Transcription Factors in Plant Abiotic Stress Responses. Biochim. Biophys. Acta—Gene Regul. Mech. 2012, 1819, 86–96. [Google Scholar] [CrossRef]
- Cortés, A.J.; This, D.; Chavarro, C.; Madriñán, S.; Blair, M.W. Nucleotide Diversity Patterns at the Drought-Related DREB2 Encoding Genes in Wild and Cultivated Common Bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2012, 125, 1069–1085. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.N.; Balaji, J.; Upadhyaya, H.D.; Hash, C.T.; Kishor, P.B.K.; Chattopadhyay, D.; Rodriquez, L.M.; Blair, M.W.; Baum, M.; McNally, K.; et al. Isolation and Sequence Analysis of DREB2A Homologues in Three Cereal and Two Legume Species. Plant Sci. 2009, 177, 460–467. [Google Scholar] [CrossRef]
- Carvalho, M.; Castro, I.; Moutinho-Pereira, J.; Correia, C.; Egea-Cortines, M.; Matos, M.; Rosa, E.; Carnide, V.; Lino-Neto, T. Evaluating Stress Responses in Cowpea under Drought Stress. J. Plant Physiol. 2019, 241, 153001. [Google Scholar] [CrossRef]
- Yang, Z.; Du, H.; Sun, J.; Xing, X.; Kong, Y.; Li, W.; Li, X.; Zhang, C. A Nodule-Localized Small Heat Shock Protein GmHSP17.1 Confers Nodule Development and Nitrogen Fixation in Soybean. Front. Plant Sci. 2022, 13, 838718. [Google Scholar] [CrossRef]
- Afonso, P.; Castro, I.; Carvalho, M. Salt-Resilient Cowpeas: Early Identification Through Growth Parameters and Gene Expression at Germination Stage. Int. J. Mol. Sci. 2025, 26, 1892. [Google Scholar] [CrossRef]
- Eisenhardt, B.D. Small Heat Shock Proteins: Recent Developments. Biomol. Concepts 2013, 4, 583–595. [Google Scholar] [CrossRef]
- Adu, M.O.; Asare, P.A.; Yawson, D.O.; Dzidzienyo, D.K.; Nyadanu, D.; Asare-Bediako, E.; Afutu, E.; Tachie-Menson, J.W.; Amoah, M.N. Identifying Key Contributing Root System Traits to Genetic Diversity in Field-Grown Cowpea (Vigna unguiculata L. Walp.) Genotypes. Field Crop. Res. 2019, 232, 106–118. [Google Scholar] [CrossRef]
- Shoaib, M.; Banerjee, B.P.; Hayden, M.; Kant, S. Roots’ Drought Adaptive Traits in Crop Improvement. Plants 2022, 11, 2256. [Google Scholar] [CrossRef]
- Tayade, R.; Kim, S.H.; Tripathi, P.; Choi, Y.D.; Yoon, J.B.; Kim, Y.H. High-Throughput Root Imaging Analysis Reveals Wide Variation in Root Morphology of Wild Adzuki Bean (Vigna angularis) Accessions. Plants 2022, 11, 405. [Google Scholar] [CrossRef]
- Singh, V.; Bell, M. Genotypic Variability in Architectural Development of Mungbean (Vigna radiata L.) Root Systems and Physiological Relationships With Shoot Growth Dynamics. Front. Plant Sci. 2021, 12, 725915. [Google Scholar] [CrossRef]
- Chiteri, K.O.; Jubery, T.Z.; Dutta, S.; Ganapathysubramanian, B.; Cannon, S.; Singh, A. Dissecting the Root Phenotypic and Genotypic Variability of the Iowa Mung Bean Diversity Panel. Front. Plant Sci. 2022, 12, 808001. [Google Scholar] [CrossRef]
- Zhao, J.; Bodner, G.; Rewald, B.; Leitner, D.; Nagel, K.A.; Nakhforoosh, A. Root Architecture Simulation Improves the Inference from Seedling Root Phenotyping towards Mature Root Systems. J. Exp. Bot. 2017, 68, 965–982. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.A.; Lim, K.B.; Rahman, E.K.A.; Nurmawati, M.H.; Zuruzi, A.S. Agar with Embedded Channels to Study Root Growth. Sci. Rep. 2020, 10, 14231. [Google Scholar] [CrossRef]
- Chen, Y.L.; Dunbabin, V.M.; Diggle, A.J.; Siddique, K.H.M.; Rengel, Z. Assessing Variability in Root Traits of Wild Lupinus angustifolius Germplasm: Basis for Modelling Root System Structure. Plant Soil 2012, 354, 141–155. [Google Scholar] [CrossRef]
- Liu, S.; Begum, N.; An, T.; Zhao, T.; Xu, B.; Zhang, S.; Deng, X.; Lam, H.M.; Nguyen, H.T.; Siddique, K.H.M.; et al. Characterization of Root System Architecture Traits in Diverse Soybean Genotypes Using a Semi-Hydroponic System. Plants 2021, 10, 2781. [Google Scholar] [CrossRef]
- Salim, M.; Chen, Y.; Ye, H.; Nguyen, H.T.; Solaiman, Z.M.; Siddique, K.H.M. Screening of Soybean Genotypes Based on Root Morphology and Shoot Traits Using the Semi-Hydroponic Phenotyping Platform and Rhizobox Technique. Agronomy 2022, 12, 56. [Google Scholar] [CrossRef]
- Belachew, K.Y.; Nagel, K.A.; Fiorani, F.; Stoddard, F.L. Diversity in Root Growth Responses to Moisture Deficit in Young Faba Bean (Vicia faba L.) Plants. PeerJ 2018, 2018, e4401. [Google Scholar] [CrossRef]
- Gerth, S.; Claußen, J.; Eggert, A.; Wörlein, N.; Waininger, M.; Wittenberg, T.; Uhlmann, N. Semiautomated 3D Root Segmentation and Evaluation Based on X-Ray CT Imagery. Plant Phenomics 2021, 2021, 8747930. [Google Scholar] [CrossRef]
- Rascher, U.; Blossfeld, S.; Fiorani, F.; Jahnke, S.; Jansen, M.; Kuhn, A.J.; Matsubara, S.; Mrtin, L.L.A.; Merchant, A.; Metzner, R.; et al. Non-Invasive Approaches for Phenotyping of Enhanced Performance Traits in Bean. Funct. Plant Biol. 2011, 38, 968–983. [Google Scholar] [CrossRef]
- Van Dusschoten, D.; Metzner, R.; Kochs, J.; Postma, J.A.; Pflugfelder, D.; Bühler, J.; Schurr, U.; Jahnke, S. Quantitative 3D Analysis of Plant Roots Growing in Soil Using Magnetic Resonance Imaging. Plant Physiol. 2016, 170, 1176–1188. [Google Scholar] [CrossRef]
- Metzner, R.; Chlubek, A.; Bühler, J.; Pflugfelder, D.; Schurr, U.; Huber, G.; Koller, R.; Jahnke, S. In Vivo Imaging and Quantification of Carbon Tracer Dynamics in Nodulated Root Systems of Pea Plants. Plants 2022, 11, 632. [Google Scholar] [CrossRef] [PubMed]
- Salter, W.T.; Shrestha, A.; Barbour, M.M. Open Source 3D Phenotyping of Chickpea Plant Architecture across Plant Development. Plant Methods 2021, 17, 95. [Google Scholar] [CrossRef] [PubMed]
- Yazdanbakhsh, N.; Fisahn, J. Analysis of Arabidopsis thaliana Root Growth Kinetics with High Temporal and Spatial Resolution. Ann. Bot. 2010, 105, 783–791. [Google Scholar] [CrossRef]
- Galkovskyi, T.; Mileyko, Y.; Bucksch, A.; Moore, B.; Symonova, O.; Price, C.A.; Topp, C.N.; Iyer-Pascuzzi, A.S.; Zurek, P.R.; Fang, S.; et al. GiA Roots: Software for the High Throughput Analysis of Plant Root System Architecture. BMC Plant Biol. 2012, 12, 116. [Google Scholar] [CrossRef]
- Hund, A.; Trachsel, S.; Stamp, P. Growth of Axile and Lateral Roots of Maize: I Development of a Phenotying Platform. Plant Soil 2009, 325, 335–349. [Google Scholar] [CrossRef]
- Pound, M.P.; French, A.P.; Atkinson, J.A.; Wells, D.M.; Bennett, M.J.; Pridmore, T. RootNav: Navigating Images of Complex Root Architectures. Plant Physiol. 2013, 162, 1802–1814. [Google Scholar] [CrossRef]
- Leonova, T.; Shumillina, J.; Kim, A.; Frolova, N.; Wessjohann, L.; Bilova, T.; Frolov, A. Agar-Based Polyethylene Glycol (PEG) Infusion Model for Pea (Pisum sativum L.)—Perspectives of Translation to Legume Crop Plants. Bio. Comm. 2022, 67, 236–244. [Google Scholar]
- Huang, X.; Zheng, S.; Zhu, N. High-Throughput Legume Seed Phenotyping Using a Handheld 3D Laser Scanner. Remote Sens. 2022, 14, 431. [Google Scholar] [CrossRef]
- Mooney, S.J.; Pridmore, T.P.; Helliwell, J.; Bennett, M.J. Developing X-Ray Computed Tomography to Non-Invasively Image 3-D Root Systems Architecture in Soil. Plant Soil 2012, 352, 1–22. [Google Scholar] [CrossRef]
- Tabb, A.; Duncan, K.E.; Topp, C.N. Segmenting Root Systems in X-Ray Computed Tomography Images Using Level Sets. In Proceedings of the 2018 IEEE Winter Conference on Applications of Computer Vision (WACV), Lake Tahoe, NV, USA, 12–15 March 2018; pp. 586–595. [Google Scholar] [CrossRef]
- Pohlmeier, A.; Haber-Pohlmeier, S.; Javaux, M.; Vereecken, H. Magnetic Resonance Imaging Techniques for Visualization of Root Growth and Root Water Uptake Processes. Soil-Water-Root Process. Adv. Tomogr. Imaging 2015, 61, 137–156. [Google Scholar] [CrossRef]
- Das, A.; Schneider, H.; Burridge, J.; Ascanio, A.K.M.; Wojciechowski, T.; Topp, C.N.; Lycnch, J.P.; Weitz, J.S.; Bucksch, A. Digital imaging of root traits (DIRT): A high-throughput computing and collaboration platform for field-based root phenomics. Plant Methods 2015, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sherard Barrow, C.; Hanlon, M.; Lynch, J.P.; Bucksch, A. DIRT/3D: 3D Root Phenotyping for Field-Grown Maize (Zea mays). Plant Physiol. 2021, 187, 739–757. [Google Scholar] [CrossRef]
- Smith, D.T.; Potgieter, A.B.; Chapman, S.C. Scaling up High-Throughput Phenotyping for Abiotic Stress Selection in the Field. Theor. Appl. Genet. 2021, 134, 1845–1866. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Bai, X.; Zhang, C.; He, Y. Advanced High-Throughput Plant Phenotyping Techniques for Genome-Wide Association Studies: A Review. J. Adv. Res. 2022, 35, 215–230. [Google Scholar] [CrossRef] [PubMed]
| Root Phenotyping Techniques | Software | Species | References | |
|---|---|---|---|---|
| 2D approach | Soil | WinRhizo™ Pro 2019 software | Adzuki bean (Vigna angularis L.) | [62] |
| Perspex sheets | WinRhizo™ Pro 2019 software | Mung bean (Vigna radiata L.) | [63] | |
| Paper pouch | SmartShooter software version 3.0 | Mung bean (Vigna radiata L.) | [64] | |
| Agar medium | GrowScreen-Root platform | Pea (Pisum sativum L.) | [65] | |
| ImageJ software version 1.53 | Mung bean (Vigna radiata L.) | [66] | ||
| Semi-hydroponic system | WinRhizo Pro software (version 2009) | Lupin (Lupinus angustifolius L.) | [67] | |
| WinRhizo Pro software (version 2009) | Soybean (Glycine max L.) | [68] | ||
| Rhizobox | WinRhizo Pro software (version 2009) | Soybean (Glycine max L.) | [69] | |
| Rhizotron | GrowScreen-Root platform | Faba bean (Vicia faba L.) | [70] | |
| 3D approach | Computed X-ray tomography (CTX) | RootForce approach | Common bean (Phaseolus vulgaris L.) | [71] |
| Magnetic resonance imaging (MRI) | GrowScreen-Root platform | Common bean (Phaseolus vulgaris L.) | [72,73] | |
| Positron emission tomography | MeVisLab version 2.8.2 | Pea (Pisum sativum L.) | [74] | |
| Root imaging with a photogrammetric camera | ImageJ version 1.52p | Chickpea (Cicer arietinum L.) | [75] | |
| Soil | Digital Imaging of Root Traits (DIRT) platform (https://quantitative-plant.org/software/dirt, accessed on 19 March 2025) | Common bean (Phaseolus vulgaris L.) Cowpea (Vigna unguiculata L. Walp.) | [30] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonso, P.; Castro, I.; Couto, P.; Leal, F.; Carnide, V.; Rosa, E.; Carvalho, M. Root Phenotyping: A Contribution to Understanding Drought Stress Resilience in Grain Legumes. Agronomy 2025, 15, 798. https://doi.org/10.3390/agronomy15040798
Afonso P, Castro I, Couto P, Leal F, Carnide V, Rosa E, Carvalho M. Root Phenotyping: A Contribution to Understanding Drought Stress Resilience in Grain Legumes. Agronomy. 2025; 15(4):798. https://doi.org/10.3390/agronomy15040798
Chicago/Turabian StyleAfonso, Patrícia, Isaura Castro, Pedro Couto, Fernanda Leal, Valdemar Carnide, Eduardo Rosa, and Márcia Carvalho. 2025. "Root Phenotyping: A Contribution to Understanding Drought Stress Resilience in Grain Legumes" Agronomy 15, no. 4: 798. https://doi.org/10.3390/agronomy15040798
APA StyleAfonso, P., Castro, I., Couto, P., Leal, F., Carnide, V., Rosa, E., & Carvalho, M. (2025). Root Phenotyping: A Contribution to Understanding Drought Stress Resilience in Grain Legumes. Agronomy, 15(4), 798. https://doi.org/10.3390/agronomy15040798









