The Causative Agent of Soft Rot in Plants, the Phytopathogenic Bacterium Pectobacterium carotovorum subsp. carotovorum: A Brief Description and an Overview of Methods to Control It
Abstract
1. Introduction
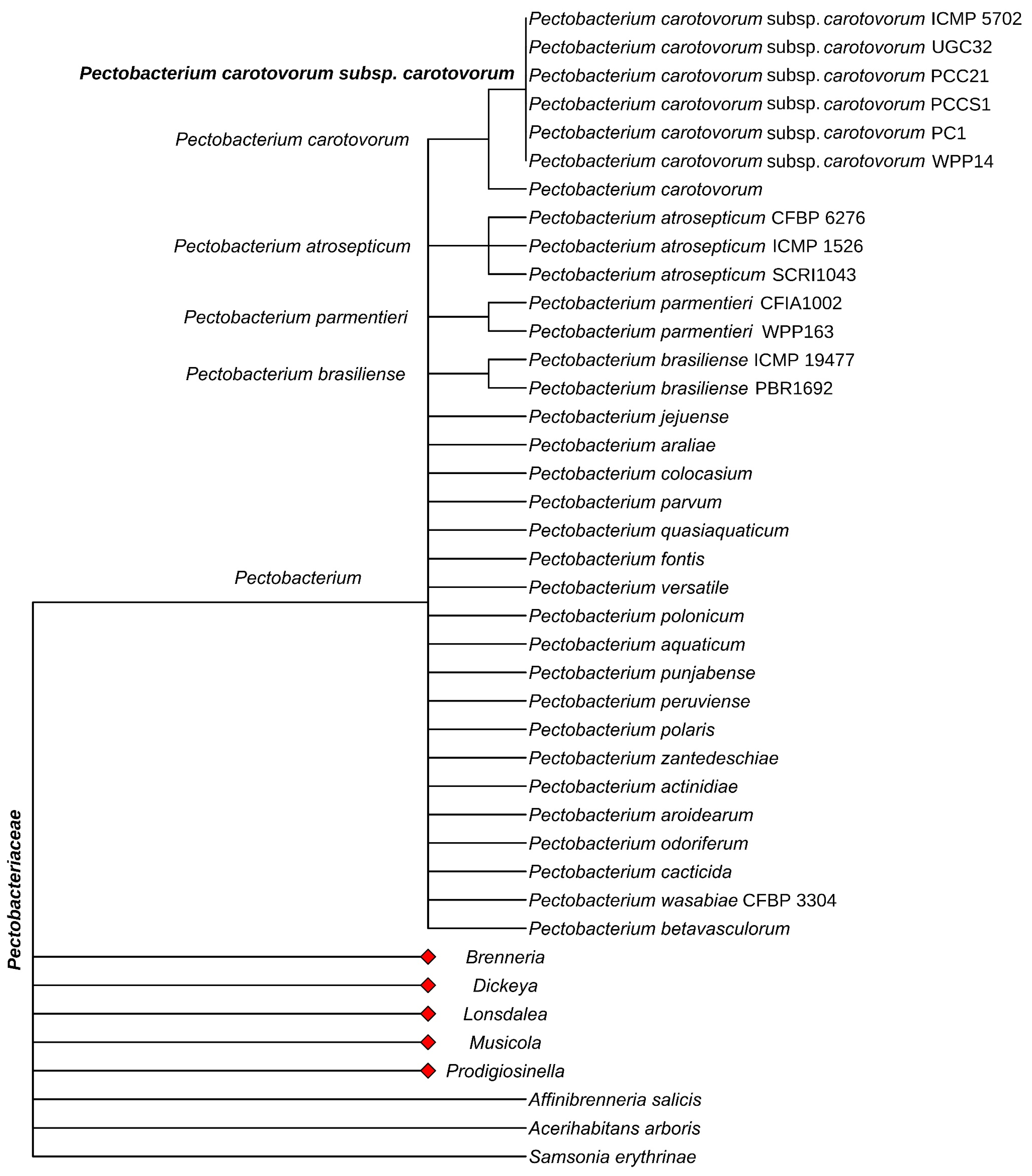
2. Systematic Position, Morphological, and Physiological–Biochemical Characteristics of Pectobacterium carotovorum
Bacteriocins
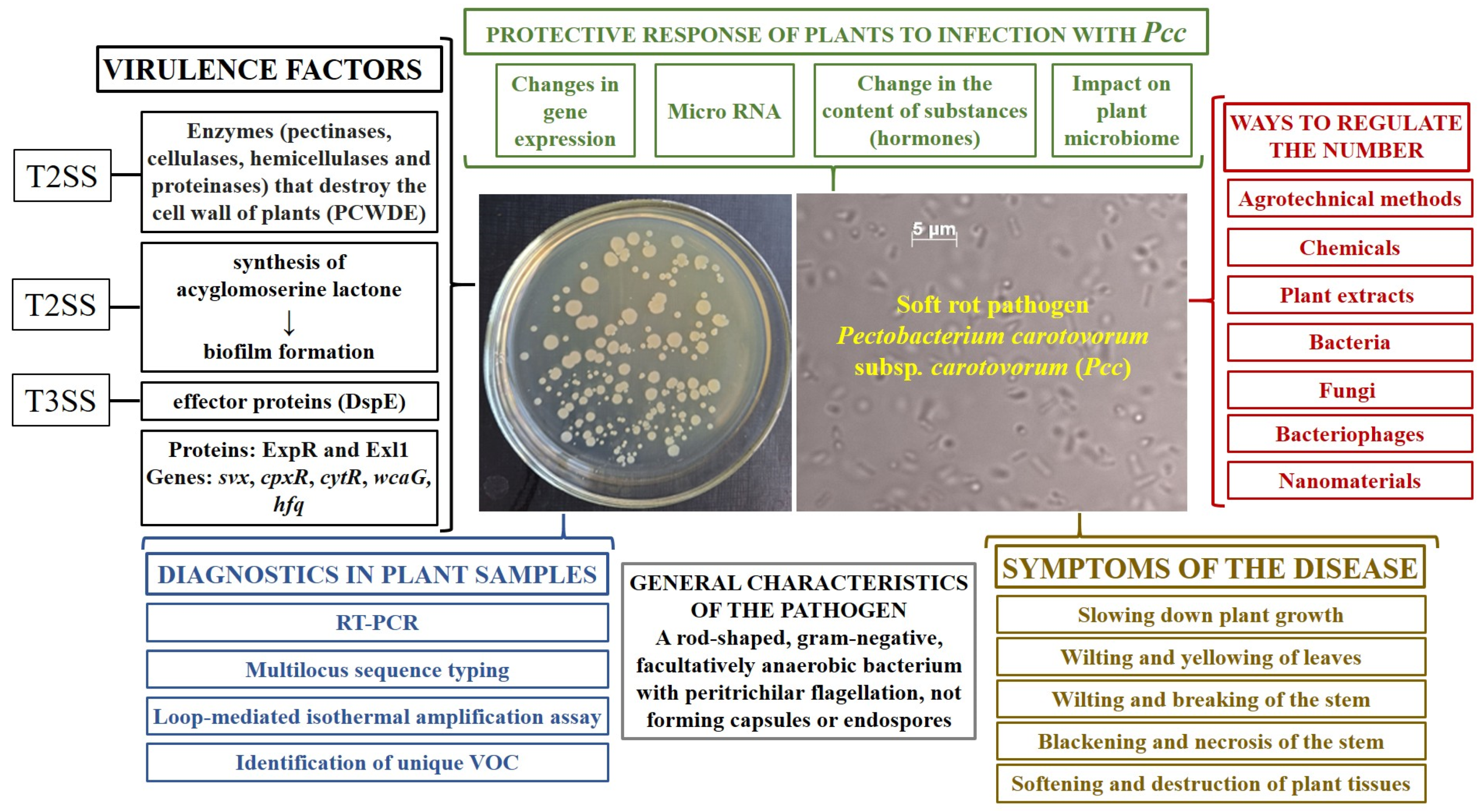
3. Plant Damage Symptoms, Virulence Factors and Effect of Temperature on Pathogenesis
3.1. Symptoms of the Disease
3.2. Methods of Plant Infection
3.3. Virulence Factors
3.4. Effect of Temperature on Virulence
4. Diagnosis of the Disease
5. Plant Defense Reaction
5.1. Gene Expression in Plant Tissues During Pcc Infection
5.2. Small RNAs
5.3. Impact of Pcc Infection on Plant Microbiome
5.4. Resistance of Different Plant Varieties and Hybrids
6. Methods to Control Pcc
6.1. Agrotechnical Methods
6.2. Chemical Substance
6.3. Plant Extracts
6.3.1. Herbaceous Plants
6.3.2. Shrubs and Trees
6.3.3. Seaweed
7. Competition and Interaction of Pcc with Bacteria, Fungi, Bacteriophages
7.1. Bacillus spp.
7.2. Pseudomonas
7.3. Lactic Acid Bacteria
7.4. Streptomycetes
7.5. Other Types of Bacteria
7.6. Fungi
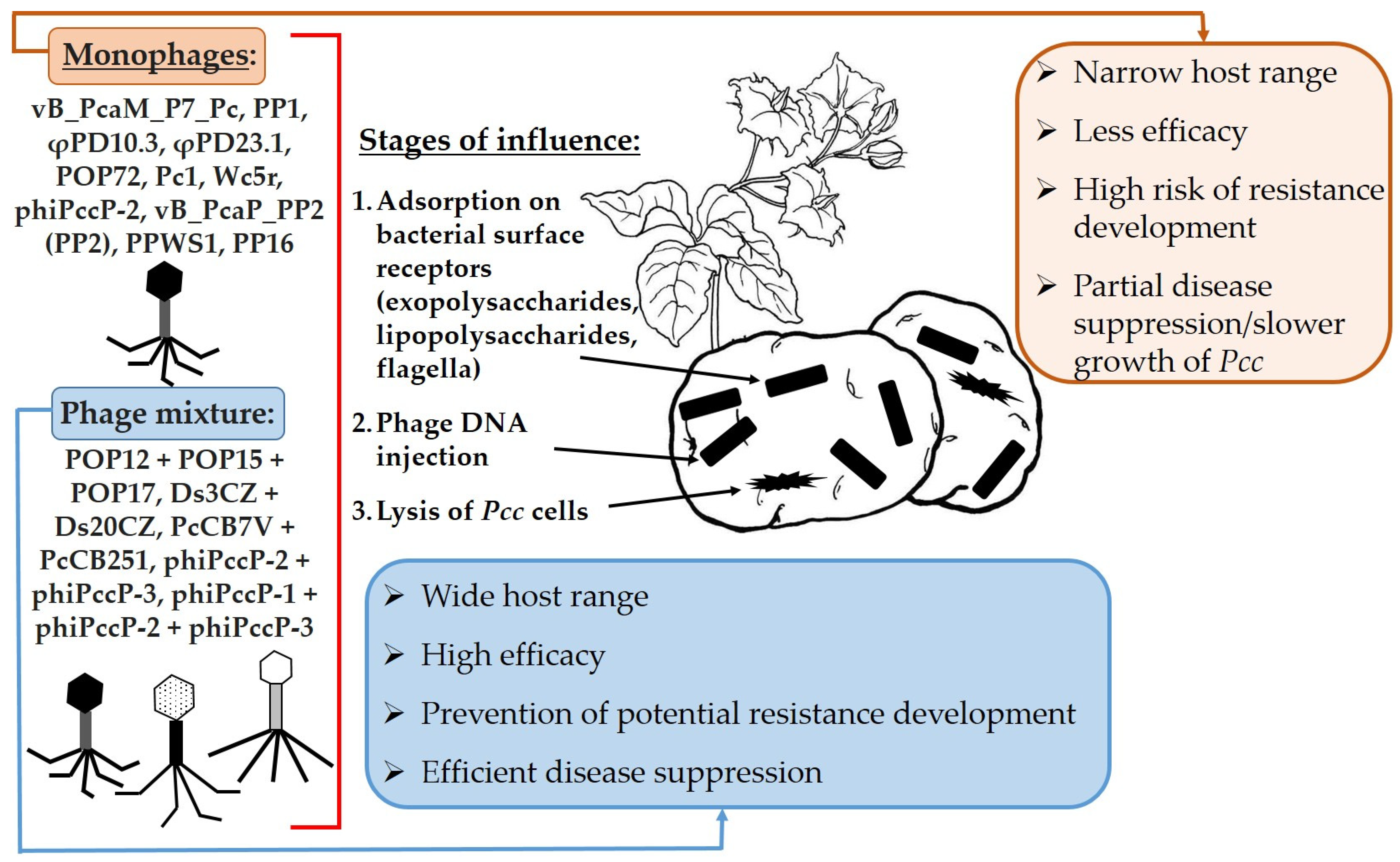
7.7. Bacteriophages
8. Using Nanomaterials to Control Pcc
8.1. Chitosan Nanoparticles (NPs)
8.2. Chalcogen-Containing NPs
8.3. Metal-Containing NPs
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AHL | N-acyl-homoserine lactone |
| AiiA L | acyl homoserine lactonase |
| DEGs | differentially expressed genes |
| EPS | exopolysaccharides |
| LAMP | loop-mediated isothermal amplification |
| LPS | lipopolysaccharides |
| MLST | multilocus sequence typing |
| OHH | N-(3-oxohexanoyl)-L-homoserine lactone |
| Pcc | Pectobacterium carotovorum subsp. carotovorum |
| PCWDEs | plant cell wall-degrading enzymes (pectinases, polygalacturonases, cellulases, and proteases) |
| QS | quorum sensing |
| ROS | reactive oxygen species |
| TEM | transmission electron microscope |
| TSS | type secretion system |
| VOCs | volatile organic compounds |
References
- Kang, M.; Kim, S.-J.; Lee, J.Y.; Yoon, S.-R.; Kim, S.H.; Ha, J.-H. Inactivation of Pectobacterium carotovorum subsp. carotovorum on Chinese cabbage (Brassica rapa L. subsp. pekinensis) by wash treatments with phenolic compounds. LWT 2018, 93, 229–236. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Park, N.I.; Park, Y.; Kim, B.S.; Eum, H.L. Effect of disinfecting harvesting knives with sodium hypochlorite on soft rot infection of Kimchi cabbage. Food Sci. Biotechnol. 2021, 30, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Huang, J.; Yi, Y.; Liu, S.; Liu, R.; Xiao, Z.; Li, C. Effects of rhapontigenin as a novel quorum-sensing inhibitor on exoenzymes and biofilm formation of Pectobacterium carotovorum subsp. carotovorum and its application in vegetables. Molecules 2022, 27, 8878. [Google Scholar] [CrossRef]
- Teoh, S.H.; Wong, G.R.; Teo, W.F.A.; Mazumdar, P. First report of Pectobacterium carotovorum and Pectobacterium aroidearum causing bacterial soft rot on curly dwarf pak choy (Brassica rapa var. chinensis) in Malaysia. Plant Dis. 2023, 107, 3631. [Google Scholar] [CrossRef]
- Cao, X.; Liu, Y.; Luo, X.; Wang, C.; Yue, L.; Elmer, W.; Dhankher, O.P.; White, J.C.; Wang, Z.; Xing, B. Mechanistic investigation of enhanced bacterial soft rot resistance in lettuce (Lactuca sativa L.) with elemental sulfur nanomaterials. Sci. Total Environ. 2023, 884, 163793. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, J.J.; Kim, H.; Choi, G.J. Differential resistance of radish cultivars against bacterial soft rot caused by Pectobacterium carotovorum subsp. carotovorum. Plant Pathol. J. 2024, 40, 151–159. [Google Scholar] [CrossRef]
- He, W.; Luo, W.; Zhou, J.; Zhu, X.; Xu, J. Pectobacterium carotovorum subsp. brasiliense causing soft rot in eggplant in Xinjiang, China. Microorganisms 2023, 11, 2662. [Google Scholar] [CrossRef]
- Marković, S.; Milić Komić, S.; Jelušić, A.; Iličić, R.; Bagi, F.; Stanković, S.; Popović, T. First report of Pectobacterium versatile causing blackleg of potato in Serbia. Plant Dis. 2022, 106, 312. [Google Scholar] [CrossRef]
- Bhatta, B.P.; Khanal, M.; Malla, S. Whole genome and 16S rRNA dataset of Pectobacterium carotovorum strain 21TX0081 isolated from a symptomatic onion foliage in Texas. Data Brief 2022, 46, 108823. [Google Scholar] [CrossRef]
- Ghuffar, S.; Cheema, K.L.; Abbass, W.; Sabtain, U.; Yasin, M.U.; Mehmood, N.; Rashid, K.; Qayyum, A.; Hassan, Z.; Rauf, M. Characterization of Pectobacterium carotovoratum subsp. carotovorum causing bacterial soft rot disease of turnip in Pakistan. Pak. J. Biochem. Mol. Biol. 2023, 4, 1–11. [Google Scholar] [CrossRef]
- Djami-Tchatchou, A.T.; Matsaunyane, L.B.T.; Kalu, C.M.; Ntushelo, K. Gene expression and evidence of coregulation of the production of some metabolites of chilli pepper inoculated with Pectobacterium carotovorum ssp. carotovorum. Funct. Plant Biol. 2019, 46, 1114–1122. [Google Scholar] [CrossRef]
- Yang, M.; Gao, P.; Guo, J.; Qi, Y.; Li, L.; Yang, S.; Zhao, Y.; Liu, J.; Yu, L. The endophytic fungal community plays a crucial role in the resistance of host plants to necrotic bacterial pathogens. Physiol. Plant. 2024, 176, e14284. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiao, M.; Yan, C.; Hu, F.C.; Zhang, S.; Feng, X.; Fan, H.Y. First report of bark split disease caused by Pectobacterium carotovorum on Artocarpus heterophyllus (Jackfruit) in China. Plant Dis. 2023, 107, 2509. [Google Scholar] [CrossRef]
- Buitenhuis, R.; Poleatewich, A.; Jandricic, M.; Brownbridge, M. Risk of spreading soft rot through cutting dips against whiteflies in greenhouse-grown poinsettia. Plant Dis. 2020, 104, 2262–2268. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, M.; Yang, L.; Yao, P.; Ma, L.; Wang, C.; Wang, H.; Qian, G.; Hu, B.; Fan, J. The ribosomal protein RplY is required for Pectobacterium carotovorum virulence and is induced by Zantedeschia elliotiana extract. Phytopathology 2017, 107, 1322–1330. [Google Scholar] [CrossRef]
- Lipsky, A.; Joshi, J.R.; Carmi, N.; Yedidia, I. Expression levels of antimicrobial peptide tachyplesin I in transgenic Ornithogalum lines affect the resistance to Pectobacterium infection. J. Biotechnol. 2016, 238, 22–29. [Google Scholar] [CrossRef]
- Yedidia, I.; Schultz, K.; Golan, A.; Gottlieb, H.E.; Kerem, Z. Structural elucidation of three novel kaempferol O-tri-glycosides that are involved in the defense response of hybrid Ornithogalum to Pectobacterium carotovorum. Molecules 2019, 24, 2910. [Google Scholar] [CrossRef]
- Smoktunowicz, M.; Jonca, J.; Stachowska, A.; May, M.; Waleron, M.M.; Waleron, M.; Waleron, K. The international trade of ware vegetables and orna-mental plants-an underestimated risk of accelerated spreading of phytopathogenic bacteria in the era of globalisation and ongoing climatic changes. Pathogens 2022, 11, 728. [Google Scholar] [CrossRef]
- Luo, M.; Li, X.; Zhang, J.; Miao, Y.; Liu, D. The C3H gene PtZFP2-like in Pinellia ternata acts as a positive regulator of the resistance to soft rot caused by Pectobacterium carotovorum. Physiol. Plant. 2025, 177, e70121. [Google Scholar] [CrossRef]
- Osdaghi, E. Pectobacterium carotovorum (bacterial soft rot). CABI Compend. 2023, 7, 21913. [Google Scholar] [CrossRef]
- Perombelon, M.C.M.; Kelman, A. Ecology of the soft rot Erwinias. Annu. Rev. Phytopathol. 1980, 18, 361–388. [Google Scholar] [CrossRef]
- Perombelon, M.C.M. Potato diseases caused by soft rot erwinias: An overview of pathogenesis. Plant Pathol. 2002, 51, 1–12. [Google Scholar] [CrossRef]
- Szulta, S.; Kornicka, A. Pectobacterium and Dickeya genus—A review on structural variations of O-polysaccharides and their role in the pathogenic process of plants. Plant Pathol. 2023, 72, 998–1010. [Google Scholar] [CrossRef]
- Czajkowski, R.; Pérombelon, M.; Jafra, S.; Lojkowska, E.; Potrykus, M.; van der Wolf, J.; Sledz, W. Detection, identification and differentiation of Pectobacterium and Dickeya species causing potato blackleg and tuber soft rot: A review. Ann. Appl. Biol. 2015, 166, 18–38. [Google Scholar] [CrossRef]
- Van Gijsegem, F.; Toth, I.K.; van der Wolf, J.M. Soft rot Pectobacteriaceae: A brief overview. In Plant Diseases Caused by Dickeya and Pectobacterium Species; Van Gijsegem, F., van der Wolf, J.M., Toth, I.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–11. [Google Scholar] [CrossRef]
- Gardan, L.; Gouy, C.; Christen, R.; Samson, R. Elevation of three subspecies of Pectobacterium carotovorum to species level: Pectobacterium atrosepticum sp. nov., Pectobacterium betavasculorum sp. nov. and Pectobacterium wasabiae sp. nov. Int. J. Syst. Evol. Microbiol. 2003, 53, 381–391. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, Q.; Loria, R. A re-evaluation of the taxonomy of phytopathogenic genera Dickeya and Pectobacterium using whole-genome sequencing data. Syst. Appl. Microbiol. 2016, 39, 252–259. [Google Scholar] [CrossRef]
- Portier, P.; Pédron, J.; Taghouti, G.; Fischer-Le Saux, M.; Caullireau, E.; Bertrand, C.; Laurent, A.; Chawki, K.; Oulgazi, S.; Moumni, M.; et al. Elevation of Pectobacterium carotovorum subsp. odoriferum to species level as Pectobacterium odoriferum sp. nov., proposal of Pectobacterium brasiliense sp. nov. and Pectobacterium actinidiae sp. nov., emended description of Pectobacterium carotovorum and description of Pectobacterium versatile sp. nov., isolated from streams and symptoms on diverse plants. Int. J. Syst. Evol. Microbiol. 2019, 69, 3207–3216. [Google Scholar] [CrossRef]
- Voronina, M.V.; Lukianova, A.A.; Shneider, M.M.; Korzhenkov, A.A.; Toschakov, S.V.; Miroshnikov, K.A.; Vasiliev, D.M.; Ignatov, A. First report of Pectobacterium polaris causing soft rot and black leg of potato in Russia. Plant Dis. 2021, 105, 1851. [Google Scholar] [CrossRef]
- Portier, P.; Pédron, J.; Taghouti, G.; Dutrieux, C.; Barny, M.-A. Updated Taxonomy of Pectobacterium Genus in the CIRM-CFBP Bacterial Collection: When Newly Described Species Reveal “Old” Endemic Population. Microorganisms 2020, 8, 1441. [Google Scholar] [CrossRef] [PubMed]
- Oulghazi, S.; Ed-Dra, A.; Ali, F.; Sarfraz, S. Pectobacterium. In Compendium of Phytopathogenic Microbes in Agro-Ecology; Amaresan, N., Kumar, K., Eds.; Springer: Cham, Switzerland, 2025; pp. 93–114. [Google Scholar] [CrossRef]
- Barış, Ö. Erzurum Ilindeki Mağaralarda Damlataşı Oluşumunda Etkili Bakterilerin Izolasyonu, Karakterizasyonu ve Tanısı. Doktora Tezi. Ph.D. Thesis, Fen Bilimleri Enstitüsü, Atatürk Üniversitesi, Erzurum, Türkiye, 2009. [Google Scholar]
- Ryskaliyeva, B.Z.; Vasilyev, D.A.; Feoktistova, N.A.; Lyashenko, E.A. Development and testing of the bacteriological scheme of identification of bacteria Pectobacterium carotovorum. Taurida Herald Agrar. Sci. 2020, 2, 134–142, (In Russian with English Abstract). [Google Scholar] [CrossRef]
- Ryskalieva, B.Z.; Feoktistova, N.A.; Vasiliev, D.A. Studying of biological properties of the bacterium of the type of Pectobacterium carotovorum. Universum Chem. Biol. 2017, 7, 18–20. Available online: https://7universum.com/pdf/nature/7(37)/Ryskalieva.pdf (accessed on 15 March 2025). (In Russian with English Abstract).
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinate Bacteriology, 9th ed.; Williams & Wilkins: Baltimore, MD, USA, 1994; p. 484. ISBN 978-0683006032. [Google Scholar]
- Hélias, V.; Hamon, P.; Huchet, E.; Wolf, J.V.D.; Andrivon, D. Two new effective semiselective crystal violet pectate media for isolation of Pectobacterium and Dickeya. Plant Pathol. 2011, 61, 339–345. [Google Scholar] [CrossRef]
- Baek, J.H.; Lee, S.Y.; Bae, J.Y.; Oh, S.W. Development of a selective and differential medium for effective isolation of Pectobacterium carotovorum from soft rot-infected agricultural products. Food Sci. Biotechnol. 2024, 34, 1517–1524. [Google Scholar] [CrossRef]
- Moretti, C.; Fakhr, R.; Cortese, C.; De Vos, P.; Cerri, M.; Geagea, L.; Cleenwerck, I.; Buonaurio, R. Pectobacterium aroidearum and Pectobacterium carotovorum subsp. carotovorum as causal agents of potato soft rot in Lebanon. Eur. J. Plant Pathol. 2016, 144, 205–211. [Google Scholar] [CrossRef]
- Cinisli, K.T.; Kiliç, S.M.; Uçar, S.; Canca, E.; Dikbaş, N. Isolation of Pectobacterium carotovorum, identification with 16S rRNA, phytase activity and characterization of the bacteria. Res. Sq. 2019, 1, 1–19. [Google Scholar] [CrossRef]
- Grinter, R.; Josts, I.; Zeth, K.; Roszak, A.W.; McCaughey, L.C.; Cogdell, R.J.; Milner, J.J.; Kelly, S.M.; Byron, O.; Walker, D. Structure of the atypical bacteriocin pectocin M2 implies a novel mechanism of protein uptake. Mol. Microbiol. 2014, 93, 234–246. [Google Scholar] [CrossRef]
- Grinter, R.; Milner, J.; Walker, D. Beware of proteins bearing gifts: Protein antibiotics that use iron as a Trojan horse. FEMS Microbiol. Lett. 2013, 338, 1–9. [Google Scholar] [CrossRef]
- Jantarit, N.; Tanaka, H.; Lin, Y.; Lee, Y.H.; Kurisu, G. Crystal structure of pectocin M1 reveals diverse conformations and interactions during its initial step via the ferredoxin uptake system. FEBS Open Bio 2024, 14, 1731–1745. [Google Scholar] [CrossRef]
- Grinter, R.; Josts, I.; Mosbahi, K.; Roszak, A.W.; Cogdell, R.J.; Bonvin, A.M.J.J.; Milner, J.J.; Kelly, S.M.; Byron, O.; Smith, B.O.; et al. Structure of the bacterial plant-ferredoxin receptor FusA. Nat. Commun. 2016, 7, 13308. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Derilo, R.C.; Lagitnay, R.B.J.S.; Wu, H.P.; Chen, K.I.; Chuang, D.Y. Identification and characterization of the bacteriocin Carocin S3 from the multiple bacteriocin producing strain of Pectobacterium carotovorum subsp. carotovorum. BMC Microbiol. 2020, 20, 273. [Google Scholar] [CrossRef]
- Chung, P.C.; Lagitnay, R.B.J.S.; Derilo, R.C.; Wu, J.L.; Chuang, Y.; Lin, J.D.; Chuang, D.Y. Unraveling the uncharacterized domain of Carocin S2: A ribonuclease Pectobacterium carotovorum subsp. carotovorum bacteriocin. Microorganisms 2022, 10, 359. [Google Scholar] [CrossRef]
- Lagitnay, R.B.J.S.; Chen, H.L.; Chen, Y.C.; Chuang, D.Y. Diguanylate cyclase (DGC) implicated in the synthesis of multiple bacteriocins via the flagellar-type III secretion system produced by Pectobacterium carotovorum subsp. carotovorum. Int. J. Mol. Sci. 2022, 23, 5649. [Google Scholar] [CrossRef]
- Wu, H.P.; Derilo, R.C.; Chen, H.L.; Li, T.R.; Lagitnay, R.B.J.S.; Chan, Y.C.; Chuang, Y.; Chuang, D.Y. Injectisome T3SS subunits as potential chaperones in the extracellular export of Pectobacterium carotovorum subsp. carotovorum bacteriocins Carocin S1 and Carocin S3 secreted via flagellar T3SS. BMC Microbiol. 2021, 21, 345. [Google Scholar] [CrossRef]
- Chuang, D.Y.; Chien, Y.C.; Wu, H.P. Cloning and expression of the Erwinia carotovora subsp. carotovora gene encoding the low-molecular-weight bacteriocin carocin S1. J. Bacteriol. 2007, 189, 620–626. [Google Scholar] [CrossRef]
- Chan, Y.C.; Wu, J.L.; Wu, H.P.; Tzeng, K.C.; Chuang, D.Y. Cloning, purification, and functional characterization of Carocin S2, a ribonuclease bacteriocin produced by Pectobacterium carotovorum. BMC Microbiol. 2011, 11, 99. [Google Scholar] [CrossRef]
- Wu, H.P.; Derilo, R.C.; Hsu, S.H.; Hu, J.M.; Chuang, D.Y. A novel deoxyribonuclease low-molecular-weight bacteriocin, Carocin S4, from Pectobacterium carotovorum subsp. carotovorum. Microorganisms 2023, 11, 1854. [Google Scholar] [CrossRef]
- Roh, E.; Park, T.H.; Kim, M.I.; Lee, S.; Ryu, S.; Oh, C.S.; Rhee, S.; Kim, D.H.; Park, B.S.; Heu, S. Characterization of a new bacteriocin, Carocin D, from Pectobacterium carotovorum subsp. carotovorum Pcc21. Appl. Environ. Microbiol. 2010, 76, 7541–7549. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Tomita, T.; Hirota, M.; Sato, T.; Kamio, Y. A simple purification method and morphology and component analyses for carotovoricin Er, a phage-tail-like bacteriocin from the plant pathogen Erwinia carotovora Er. Biosci. Biotechnol. Biochem. 1999, 63, 1360–1369. [Google Scholar] [CrossRef]
- Yamada, K.; Hirota, M.; Niimi, Y.; Nguyen, H.A.; Takahara, Y.; Kamio, Y.; Kaneko, J. Nucleotide sequences and organization of the genes for carotovoricin (Ctv) from Erwinia carotovora indicate that Ctv evolved from the same ancestor as Salmonella typhi prophage. Biosci. Biotechnol. Biochem. 2006, 70, 2236–2247. [Google Scholar] [CrossRef]
- Krylova, E.D.; Tovkach, F.I. The characteristic of the colispecific bacteriocins of Erwinia carotovora subsp. carotovora Ec153. Mikrobiolohichnyi Zhurnal 2009, 71, 25–30, (In Russian with English Abstract). [Google Scholar] [PubMed]
- Yamada, K.; Kaneko, J.; Kamio, Y.; Itoh, Y. Binding sequences for RdgB, a DNA damage-responsive transcriptional activator, and temperature-dependent expression of bacteriocin and pectin lyase genes in Pectobacterium carotovorum subsp. carotovorum. Appl. Environ. Microbiol. 2008, 74, 6017–6025. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Davidsson, P.R.; Kariola, T.; Niemi, O.; Palva, E.T. Pathogenicity of and plant immunity to soft rot pectobacteria. Front. Plant Sci. 2013, 4, 191. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, S.J.; Ha, J.H. Quantification of Pectobacterium carotovorum subsp. carotovorum in kimchi cabbage using a surface-enhanced Raman scattering platform with silver nanostructures. Biosens. Bioelectron. 2025, 267, 116766. [Google Scholar] [CrossRef]
- Anandan, K.; Vittal, R.R. Quorum quenching strategies of endophytic Bacillus thuringiensis KMCL07 against soft rot pathogen Pectobacterium carotovorum subsp. carotovorum. Microb. Pathog. 2025, 200, 107356. [Google Scholar] [CrossRef]
- Borodin, S.G.; Kotlyarova, I.A.; Tereshchenko, G.A.; Pashayan, N.V. Bacterial diseases of sunflower. Oil Crops (Sci. Tech. Bull. All-Russ. Sci. Res. Inst. Oil Crops) 2012, 150, 1–13, (In Russian with English Abstract). [Google Scholar]
- Li, N.-P.; Tang, W.-Q.; Lee, S.; Wang, C.-L.; Chu, C.-C. First report of Pectobacterium carotovorum and Pectobacterium brasiliense causing bacterial soft rot of bok choy in Taiwan. Plant Dis. 2023, 107, 2216. [Google Scholar] [CrossRef]
- Aizawa, S.-I. Chapter 18—Pectobacterium carotovorum—Subpolar hyper-flagellation. In The Flagellar World: Electron Microscopic Images of Bacterial Flagella and Related Surface Structures; Aizawa, S.-I., Ed.; Academic Press: Oxford, UK, 2014; pp. 58–59. [Google Scholar] [CrossRef]
- Sledz, W.; Zoledowska, S.; Motyka, A.; Kadziński, L.; Banecki, B. Growth of bacterial phytopathogens in animal manures. Acta Biochim. Pol. 2017, 64, 151–159. [Google Scholar] [CrossRef]
- Kang, J.; Yoon, H.M.; Jung, J.; Yu, S.; Choi, S.Y.; Bae, H.W.; Cho, Y.H.; Chung, E.H.; Lee, Y. Pleiotropic effects of N-acylhomoserine lactone synthase ExpI on virulence, competition, and transmission in Pectobacterium carotovorum subsp. carotovorum Pcc21. Pest. Manag. Sci. 2024, 80, 687–697. [Google Scholar] [CrossRef]
- Toth, I.K.; Bell, K.S.; Holeva, M.C.; Birch, P.R.J. Soft rot erwiniae: From genes to genomes. Mol. Plant Pathol. 2003, 4, 17–30. [Google Scholar] [CrossRef]
- Singh, A.A.; Singh, A.K.; Nerurkar, A. Disrupting the quorum sensing mediated virulence in soft rot causing Pectobacterium carotovorum by marine sponge associated Bacillus sp. OA10. World J. Microbiol. Biotechnol. 2021, 37, 5. [Google Scholar] [CrossRef] [PubMed]
- Mallick, T.; Mishra, R.; Mohanty, S.; Joshi, R.K. Genome wide analysis of the potato soft rot pathogen Pectobacterium carotovorum strain ICMP 5702 to predict novel insights into its genetic features. Plant Pathol. J. 2022, 38, 102–114. [Google Scholar] [CrossRef]
- Fan, J.; Ma, L.; Zhao, C.; Yan, J.; Che, S.; Zhou, Z.; Wang, H.; Yang, L.; Hu, B. Transcriptome of Pectobacterium carotovorum subsp. carotovorum PccS1 infected in calla plants in vivo highlights a spatiotemporal expression pattern of genes related to virulence, adaptation, and host response. Mol. Plant Pathol. 2020, 21, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yuan, L.; Shi, Y.; Xie, X.; Chai, A.; Wang, Q.; Li, B. Comparative genomic analysis of Pectobacterium carotovorum subsp. brasiliense SX309 provides novel insights into its genetic and phenotypic features. BMC Genom. 2019, 20, 486. [Google Scholar] [CrossRef]
- Pineau, C.; Guschinskaya, N.; Gonçalves, I.R.; Ruaudel, F.; Robert, X.; Gouet, P.; Ballut, L.; Shevchik, V.E. Structure-function analysis of pectate lyase Pel3 reveals essential facets of protein recognition by the bacterial type 2 secretion system. J. Biol. Chem. 2021, 296, 100305. [Google Scholar] [CrossRef] [PubMed]
- Marín-Rodríguez, M.C.; Orchard, J.; Seymour, G.B. Pectate lyases, cell wall degradation and fruit softening. J. Exp. Bot. 2002, 53, 2115–2119. [Google Scholar] [CrossRef]
- Maisuria, V.B.; Nerurkar, A.S. Biochemical properties and thermal behavior of pectate lyase produced by Pectobacterium carotovorum subsp. carotovorum BR1 with industrial potentials. Biochem. Eng. J. 2012, 63, 22–30. [Google Scholar] [CrossRef]
- Ni, L.; Guo, L.; Custers, J.B.M.; Zhang, L. Characterization of calla Lily soft rot caused by Pectobacterium carotovorum subsp. carotovorum ZT0505 bacterial growth and pectate lyase activity under different conditions. J. Plant Pathol. 2010, 92, 421–428. [Google Scholar]
- Hogan, C.S.; Mole, B.M.; Grant, S.R.; Willis, D.K.; Charkowski, A.O. The type III secreted effector DspE is required early in solanum tuberosum leaf infection by Pectobacterium carotovorum to cause cell death, and requires Wx(3-6)D/E motifs. PLoS ONE 2013, 8, e65534. [Google Scholar] [CrossRef]
- Gutierrez-Pacheco, M.M.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Lizardi-Mendoza, J.; Madera-Santana, T.J.; Bernal-Mercado, A.T.; Ayala-Zavala, J.F. Carvacrol inhibits biofilm formation and production of extracellular polymeric substances of Pectobacterium carotovorum subsp. carotovorum. Food Control 2018, 89, 210–218. [Google Scholar] [CrossRef]
- Morohoshi, T.; Ogasawara, Y.; Xie, X.; Hamamoto, H.; Someya, N. Genetic and biochemical diversity for N-acylhomoserine lactone biosynthesis in the plant pathogen Pectobacterium carotovorum subsp. carotovorum. Microbes Environ. 2019, 34, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Schikora, A.; Schenk, S.T.; Hartmann, A. Beneficial effects of bacteria-plant communication based on quorum sensing molecules of the N-acyl homoserine lactone group. Plant Mol. Biol. 2016, 90, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Põllumaa, L.; Alamäe, T.; Mäe, A. Quorum sensing and expression of virulence in pectobacteria. Sensors 2012, 12, 3327–3349. [Google Scholar] [CrossRef]
- Saha, N.D.; Chaudhary, A.; Singh, S.D.; Singh, D.; Walia, S.; Das, T.K. Plant pathogenic microbial communication affected by elevated temperature in Pectobacterium carotovorum subsp. carotovorum. Curr. Microbiol. 2015, 71, 585–593. [Google Scholar] [CrossRef]
- Wang, C.; Pu, T.; Lou, W.; Wang, Y.; Gao, Z.; Hu, B.; Fan, J. Hfq, a RNA chaperone, contributes to virulence by regulating plant cell wall-degrading enzyme production, type VI secretion system expression, bacterial competition, and suppressing host defense response in Pectobacterium carotovorum. Mol. Plant Microbe Interact. 2018, 31, 1166–1178. [Google Scholar] [CrossRef]
- Islam, R.; Brown, S.; Taheri, A.; Dumenyo, C.K. The gene encoding NAD-dependent epimerase/dehydratase, wcaG, affects cell surface properties, virulence, and extracellular enzyme production in the soft rot phytopathogen, Pectobacterium carotovorum. Microorganisms 2019, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, J.; Wang, S.; Yang, Z.; Zhang, H.; Zhou, X. Cloning, expression, purification, and biochemical characterization of CpxR protein from Pectobacterium carotovorum. Biotechnol. Appl. Biochem. 2022, 69, 898–905. [Google Scholar] [CrossRef]
- Tendiuk, N.; Diakonova, A.; Petrova, O.; Mukhametzyanov, T.; Makshakova, O.; Gorshkov, V. Svx Peptidases of Phytopathogenic Pectolytic Bacteria: Structural, Catalytic and Phytoimmune Properties. Int. J. Mol. Sci. 2024, 25, 756. [Google Scholar] [CrossRef]
- Mattinen, L.; Nissinen, R.; Riipi, T.; Kalkkinen, N.; Pirhonen, M. Host-extract induced changes in the secretome of the plant pathogenic bacterium Pectobacterium atrosepticum. Proteomics 2007, 7, 3527–3537. [Google Scholar] [CrossRef]
- Haque, M.M.; Oliver, M.M.H.; Nahar, K.; Alam, M.Z.; Hirata, H.; Tsuyumu, S. CytR homolog of Pectobacterium carotovorum subsp. carotovorum controls air-liquid biofilm formation by regulating multiple genes involved in cellulose production, c-di-GMP signaling, motility, and type III secretion system in response to nutritional and environmental signals. Front. Microbiol. 2017, 8, 972. [Google Scholar] [CrossRef]
- Grant, W.D.; Sutherland, I.W.; Wilkinson, J.F. Exopolysaccharide colanic acid and its occurrence in the Enterobacteriaceae. J. Bacteriol. 1969, 100, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Herrera, O.E.; Rodríguez, M.; Olarte-Lozano, M.; Sampedro-Guerrero, J.A.; Guerrero, A.; Pinto-Cámara, R.; Alvarado-Affantranger, X.; Wood, C.D.; Moran-Mirabal, J.M.; Pastor, N.; et al. Analysis of the binding of expansin Exl1, from Pectobacterium carotovorum, to plant xylem and comparison to EXLX1 from Bacillus subtilis. ACS Omega 2018, 3, 7008–7018. [Google Scholar] [CrossRef]
- Su, Z.; Liu, X.; Guo, Q.; Xuan, L.; Lu, X.; Dong, L.; Zhang, X.; Wang, P.; Zhao, W.; Qu, Y.; et al. Insights into complex infection by two Pectobacterium species causing potato blackleg and soft rot. Microbiol. Res. 2022, 261, 127072. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.; Choi, J.G.; Lee, Y.G.; Kwon, M.; Hwang, I.; Heu, S. Distribution of Pectobacterium species isolated in south Korea and comparison of temperature effects on pathogenicity. Plant. Pathol. J. 2020, 36, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Skelsey, P.; Humphris, S.N.; Campbell, E.J.; Toth, I.K. Threat of establishment of non-indigenous potato blackleg and tuber soft rot pathogens in Great Britain under climate change. PLoS ONE 2018, 13, e0205711. [Google Scholar] [CrossRef]
- Nikitin, M.M.; Statsyuk, N.V.; Frantsuzov, P.A.; Dzhavakhiya, V.G.; Golikov, A.G. Matrix approach to the simultaneous detection of multiple potato pathogens by real-time PCR. J. Appl. Microbiol. 2018, 124, 797–809. [Google Scholar] [CrossRef]
- Suárez, M.B.; Diego, M.; Feria, F.J.; Martín-Robles, M.J.; Moreno, S.; Palomo, J.L. New PCR-based assay for the identification of Pectobacterium carotovorum causing potato soft rot. Plant Dis. 2022, 106, 676–684. [Google Scholar] [CrossRef]
- Ranjan, R.K.; Singh, D.; Baranwal, V.K. Simultaneous detection of brown rot- and soft rot-causing bacterial pathogens from potato tubers through multiplex PCR. Curr. Microbiol. 2016, 73, 652–659. [Google Scholar] [CrossRef]
- Baek, J.H.; Lee, S.Y.; Oh, S.W. Rapid and sensitive detection of Pectobacterium carotovorum subsp. carotovorum in fresh produce using silica-coated magnetic nanoparticles combined with filtration-based real-time PCR. Crop Prot. 2023, 166, 106178. [Google Scholar] [CrossRef]
- Yasuhara-Bell, J.; Marrero, G.; De Silva, A.; Alvarez, A.M. Specific detection of Pectobacterium carotovorum by loop-mediated isothermal amplification. Mol. Plant Pathol. 2016, 17, 1499–1505. [Google Scholar] [CrossRef]
- Moon, Y.J.; Lee, S.Y.; Kim, U.; Oh, S.W. Naked-eye detection with loop-mediated isothermal amplification for P. carotovorum subsp. carotovorum in agricultural products. Food Sci. Biotechnol. 2023, 33, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Jelušić, A.; Mitrović, P.; Marković, S.; Iličić, R.; Milovanović, P.; Stanković, S.; Popović Milovanović, T. Diversity of bacterial soft rot-causing Pectobacterium species affecting cabbage in Serbia. Microorganisms 2023, 11, 335. [Google Scholar] [CrossRef] [PubMed]
- Lazazzara, V.; Avesani, S.; Robatscher, P.; Oberhuber, M.; Pertot, I.; Schuhmacher, R.; Perazzolli, M. Biogenic volatile organic compounds in the grapevine response to pathogens, beneficial microorganisms, resistance inducers, and abiotic factors. J. Exp. Bot. 2022, 73, 529–554. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.; Singh, S.S.; Sircar, D. Early asymptomatic prediction of potato soft rot disease using phytohormone-induced volatile biomarkers. Physiol. Plant. 2024, 176, e14481. [Google Scholar] [CrossRef]
- Yang, J.S.; Lee, H.W.; Song, H.; Ha, J.H. Volatile metabolic markers for monitoring Pectobacterium carotovorum subsp. carotovorum using headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry. J. Microbiol. Biotechnol. 2021, 31, 70–78. [Google Scholar] [CrossRef]
- Steglińska, A.; Pielech-Przybylska, K.; Janas, R.; Grzesik, M.; Borowski, S.; Kręgiel, D.; Gutarowska, B. Volatile organic compounds and physiological parameters as markers of potato (Solanum tuberosum L.) infection with phytopathogens. Molecules 2022, 27, 3708. [Google Scholar] [CrossRef]
- Kate, A.; Tiwari, S.; Gujar, J.P.; Modhera, B.; Tripathi, M.K.; Ray, H.; Ghosh, A.; Mohapatra, D. Spotting of volatile signatures through GC-MS analysis of bacterial and fungal infections in stored potatoes (Solanum tuberosum L.). Foods 2023, 12, 2083. [Google Scholar] [CrossRef]
- Djami-Tchatchou, A.T.; Matsaunyane, L.B.T.; Ntushelo, K. Gene expression responses of tomato inoculated with Pectobacterium carotovorum subsp. carotovorum. Microbiol. Open 2019, 8, e911. [Google Scholar] [CrossRef]
- Hong, C.Y.; Zheng, J.L.; Chen, T.Y.; Chao, H.R.; Lin, Y.H. PFLP-intensified disease resistance against bacterial soft rot through the MAPK Pathway in PAMP-triggered immunity. Phytopathology 2018, 108, 1467–1474. [Google Scholar] [CrossRef]
- Catinot, J.; Huang, J.-B.; Huang, P.-Y.; Tseng, M.-Y.; Chen, Y.-L.; Gu, S.-Y.; Lo, W.-S.; Wang, L.-C.; Chen, Y.-R.; Zimmerli, L. ETHYLENE RESPONSE FACTOR 96 positively regulates Arabidopsis resistance to necrotrophic pathogens by direct binding to GCC elements of jasmonate- and ethylene-responsive defence genes. Plant Cell Environ. 2015, 38, 2721–2734. [Google Scholar] [CrossRef]
- Alvarez, A.; Montesano, M.; Schmelz, E.; Ponce de León, I. Activation of shikimate, phenylpropanoid, oxylipins, and auxin pathways in Pectobacterium carotovorum elicitors-treated moss. Front. Plant Sci. 2016, 7, 328. [Google Scholar] [CrossRef] [PubMed]
- Shu, F.; Han, J.; Ndayambaje, J.P.; Jia, Q.; Sarsaiya, S.; Jain, A.; Huang, M.; Liu, M.; Chen, J. Transcriptomic analysis of Pinellia ternata (Thunb.) Breit T2 plus line provides insights in host responses resist Pectobacterium carotovorum infection. Bioengineered 2021, 12, 1173–1188. [Google Scholar] [CrossRef]
- Yu, S.; Kang, J.; Chung, E.H.; Lee, Y. Disruption of the metC gene affects methionine biosynthesis in Pectobacterium carotovorum subsp. carotovorum Pcc21 and reduces soft-rot disease. Plant Pathol. J. 2023, 39, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhao, Q.; Jia, Z.; Zhang, S.; Wang, J.; Song, S.; Jia, Y. N-3-oxo-octanoyl homoserine lactone primes plant resistance against necrotrophic pathogen Pectobacterium carotovorum by coordinating jasmonic acid and auxin-signaling pathways. Front. Plant Sci. 2022, 13, 886268. [Google Scholar] [CrossRef]
- Hua, D.; Duan, J.; Ma, M.; Li, Z.; Li, H. Reactive oxygen species induce cyanide-resistant respiration in potato infected by Erwinia carotovora subsp. carotovora. J. Plant Physiol. 2020, 246–247, 153132. [Google Scholar] [CrossRef]
- Ma, M.; Muhammad, S.; Duan, J.; Bai, L.; Li, H. Impairment of respiratory chain function and involvement of alternative respiratory pathway in mitochondria of potato tubers infected by Pectobacterium carotovorum subsp. carotovorum. Foods 2022, 11, 1574. [Google Scholar] [CrossRef]
- Song, G.C.; Im, H.; Jung, J.; Lee, S.; Jung, M.Y.; Rhee, S.K.; Ryu, C.M. Plant growth-promoting archaea trigger induced systemic resistance in Arabidopsis thaliana against Pectobacterium carotovorum and Pseudomonas syringae. Environ. Microbiol. 2019, 21, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, P.Y.; Cheng, C.P.; Koh, K.W.; Chan, M.T. The Arabidopsis defensin gene, AtPDF1.1, mediates defence against Pectobacterium carotovorum subsp. carotovorum via an iron-withholding defence system. Sci. Rep. 2017, 7, 9175. [Google Scholar] [CrossRef]
- Zhan, J.; Meyers, B.C. Plant small RNAs: Their biogenesis, regulatory roles, and functions. Annu. Rev. Plant Biol. 2023, 74, 21–51. [Google Scholar] [CrossRef]
- Djami-Tchatchou, A.T.; Ntushelo, K. Expression profile of stress-responsive Arabidopsis thaliana miRNAs and their target genes in response to inoculation with Pectobacterium carotovorum subsp. carotovorum. Pak. J. Biol. Sci. 2017, 20, 147–153. [Google Scholar] [CrossRef]
- Yang, M.; Qi, Y.; Liu, J.; Gao, P.; Huang, F.; Yu, L.; Chen, H. Different response mechanisms of rhizosphere microbial communities in two species of Amorphophallus to Pectobacterium carotovorum subsp. carotovorum infection. Plant Pathol. J. 2023, 39, 207–219. [Google Scholar] [CrossRef] [PubMed]
- He, F. Response of root-associated bacterial communities to different degrees of soft rot damage in Amorphophallus konjac under a Robinia pseudoacacia plantation. Front. Microbiol. 2021, 12, 652758. [Google Scholar] [CrossRef] [PubMed]
- George, A.S.; Cox, C.E.; Desai, P.; Porwollik, S.; Chu, W.; de Moraes, M.H.; McClelland, M.; Brandl, M.T.; Teplitski, M. Interactions of Salmonella enterica serovar typhimurium and Pectobacterium carotovorum within a tomato soft rot. Appl. Environ. Microbiol. 2018, 84, e01913–e01917. [Google Scholar] [CrossRef] [PubMed]
- Cowles, K.N.; Groves, R.L.; Barak, J.D. Leafhopper-induced activation of the jasmonic acid response benefits Salmonella enterica in a flagellum-dependent manner. Front. Microbiol. 2018, 9, 1987. [Google Scholar] [CrossRef]
- Gao, P.; Qi, Y.; Li, L.; Yang, S.; Guo, J.; Liu, J.; Wei, H.; Huang, F.; Yu, L. Phenylpropane biosynthesis and alkaloid metabolism pathways involved in resistance of Amorphophallus spp. against soft rot disease. Front. Plant Sci. 2024, 15, 1334996. [Google Scholar] [CrossRef]
- Rai, K.K.; Pandey, N.; Rai, S.P. Salicylic acid and nitric oxide signaling in plant heat stress. Physiol. Plant. 2020, 168, 241–255. [Google Scholar] [CrossRef]
- Ntushelo, K. Effect of salicylic acid on the growth and chemical responses of Pectobacterium carotovorum subsp. carotovorum. Pak. J. Biol. Sci. 2017, 20, 278–288. [Google Scholar] [CrossRef][Green Version]
- Xu, X.H.; Jiang, Z.L.; Feng, F.Q.; Lu, R.R. Mechanisms of Nα-lauroyl arginate ethyl ester against Penicillium digitatum and Pectobacterium carotovorum subsp. carotovorum. J. Food Sci. Technol. 2018, 55, 3675–3682. [Google Scholar] [CrossRef]
- Sumayo, M.S.; Son, J.S.; Ghim, S.Y. Exogenous application of phenylacetic acid promotes root hair growth and induces the systemic resistance of tobacco against bacterial soft-rot pathogen Pectobacterium carotovorum subsp. carotovorum. Funct. Plant Biol. 2018, 45, 1119–1127. [Google Scholar] [CrossRef]
- Víchová, J.; Jílková, B.; Michutová, M.; Kmoch, M. In vitro and in vivo antibacterial activity of selected essential oil components against Pectobacterium carotovorum subsp. carotovorum and Pectobacterium atrosepticum causing bacterial soft rot of potato tubers. Heliyon 2024, 10, e32081. [Google Scholar] [CrossRef]
- Ahmed, F.A.; Arif, M.; Alvarez, A.M. Antibacterial effect of potassium tetraborate tetrahydrate against soft rot disease agent Pectobacterium carotovorum in tomato. Front. Microbiol. 2017, 8, 1728. [Google Scholar] [CrossRef]
- Morales-Irigoyen, E.E.; de Las Mercedes Gómez-Y-Gómez, Y.; Flores-Moreno, J.L.; Franco-Hernández, M.O. A bionanohybrid ZnAl-NADS ecological pesticide as a treatment for soft rot disease in potato (Solanum tuberosum L.). Environ. Sci. Pollut. Res. Int. 2018, 25, 21430–21439. [Google Scholar] [CrossRef] [PubMed]
- Çetinkaya, N.; Pazarlar, S.; Paylan, İ.C. Ozone treatment inactivates common bacteria and fungi associated with selected crop seeds and ornamental bulbs. Saudi J. Biol. Sci. 2022, 29, 103480. [Google Scholar] [CrossRef] [PubMed]
- Estelle, D.; Laurence, L.; Marc, O.; Caroline, C.; Magali, D.; Marie-Laure, F. Linolenic fatty acid hydroperoxide acts as biocide on plant pathogenic bacteria: Biophysical investigation of the mode of action. Bioorg. Chem. 2020, 100, 103877. [Google Scholar] [CrossRef]
- Bartz, J.A.; Huber, D.J.; Stahl, S.L.; Lee, J.H.; Spiceland, D.; Elkahky, M.T. Susceptibility of lenticels within the stem depression of tomato fruit to bacterial soft rot. Plant Dis. 2016, 100, 1906–1909. [Google Scholar] [CrossRef] [PubMed]
- Brazda, G.; Pett, B. Einfluss von chloramphenicol und streptomycinsulfat auf das wachstum von Pectobacterium carotovorum var. atrosepticum (von Hall) Dowson (The influence of choramphenicol and streptomycinsulphate on the growth of Pectobacterium carotovorum var. atrosepticum (van Hall) Dowson). Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. 1976, 131, 751–756, (In German with English Abstract). [Google Scholar] [CrossRef]
- Balaraju, K.; Kim, C.J.; Park, D.J.; Nam, K.W.; Zhang, K.; Sang, M.K.; Park, K. Paromomycin derived from Streptomyces sp. AG-P 1441 induces resistance against two major pathogens of chili pepper. J. Microbiol. Biotechnol. 2016, 26, 1542–1550. [Google Scholar] [CrossRef]
- Shen, Y.; Li, Y.; Wang, L.; Wu, C.; Su, X.; Tian, Y. Carvacrol and Streptomycin in combination weaken streptomycin resistance in Pectobacterium carotovorum subsp. carotovorum. Plants 2025, 14, 908. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, M.; Zhang, B.; Liu, Y.; Fan, J.; Wang, Z.; Song, L.; Mohamed Abdul, P.; Zhang, H. Effects of natural Rheum. tanguticum on the cell wall integrity of resistant phytopathogenic Pectobacterium carotovorum subsp. carotovorum. Molecules 2022, 27, 5291. [Google Scholar] [CrossRef]
- Cai, J.; Wang, S.; Gao, Y.; Wang, Q. Antibacterial activity and mechanism of Polygonum orientale L. essential oil against Pectobacterium carotovorum subsp. carotovorum. Foods 2022, 11, 1585. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.; Chang, Y.; Imm, J.Y. Synergistic antimicrobial effect and mode of action of palmarosa oil-loaded nanoemulsion and citric acid against Pectobacterium carotovorum. Food Sci. Biotechnol. 2022, 32, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Behiry, S.; Al-Askar, A.A.; Kowalczewski, P.Ł.; Emaish, H.H.; Abdelkhalek, A. Antibacterial, antifungal, and phytochemical properties of Salsola kali ethanolic extract. Open Life Sci. 2024, 19, 20220962. [Google Scholar] [CrossRef] [PubMed]
- Jílková, B.; Víchová, J.; Holková, L.; Pluháčková, H.; Michutová, M.; Kmoch, M. Laboratory efficacy of essential oils against Pectobacterium carotovorum subsp. carotovorum and Pectobacterium atrosepticum causing soft rot of potato tubers. Potato Res. 2025, 68, 641–660. [Google Scholar] [CrossRef]
- Bhat, K.A.; Viswanath, H.S.; Bhat, N.A.; Wani, T.A. Bioactivity of various ethanolic plant extracts against Pectobacterium carotovorum subsp. carotovorum causing soft rot of potato tubers. Indian Phytopathol. 2017, 70, 463–470. [Google Scholar] [CrossRef]
- Ashmawy, N.A.; Salem, M.Z.M.; El-Hefny, M.; Abd El-Kareem, M.S.M.; El-Shanhorey, N.A.; Mohamed, A.A.; Salem, A.Z.M. Antibacterial activity of the bioactive compounds identified in three woody plants against some pathogenic bacteria. Microb. Pathog. 2018, 121, 331–340. [Google Scholar] [CrossRef]
- Kobisi, A.N.A.; Balah, M.A.; Hassan, A.R. Bioactivity of silverleaf nightshade (Solanum elaeagnifolium Cav.) berries parts against Galleria mellonella and Erwinia carotovora and LC-MS chemical profile of its potential extract. Sci. Rep. 2024, 14, 18747. [Google Scholar] [CrossRef]
- Chen, D.; Liu, J.R.; Cheng, Y.; Cheng, H.; He, P.; Sun, Y. Metabolism of rhaponticin and activities of its metabolite, rhapontigenin: A review. Curr. Med. Chem. 2020, 27, 3168–3186. [Google Scholar] [CrossRef]
- Alymanesh, M.R.; Solhjoo, A.; Pishgar, E.; Akhlaghi, M. Falcaria vulgaris extract: A mixture of quorum sensing inhibitors for controlling Pectobacterium carotovorum subsp. carotovorum. Food Microbiol. 2024, 122, 104535. [Google Scholar] [CrossRef]
- Castrosanto, M.A.; Alvarez, M.R.; Salamanez, K.C.; Nacario, R.C.; Completo, G.C. Barnyard grass [Echinochloa crus-galli (L.) Beauv] leaves extract against tomato pests. J. Sci. Food Agric. 2021, 101, 6289–6299. [Google Scholar] [CrossRef]
- Iobbi, V.; Donadio, G.; Lanteri, A.P.; Maggi, N.; Kirchmair, J.; Parisi, V.; Minuto, G.; Copetta, A.; Giacomini, M.; Bisio, A.; et al. Targeted metabolite profiling of Salvia rosmarinus Italian local ecotypes and cultivars and inhibitory activity against Pectobacterium carotovorum subsp. carotovorum. Front. Plant Sci. 2024, 15, 1164859. [Google Scholar] [CrossRef]
- Aamer, H.A.; Elalem, S.F.; Al-Askar, A.A.; Sharaf, O.A.; Gaber, M.A.; Kowalczewski, P.; Behiry, S.; Abdelkhalek, A. Antioxidant and antimicrobial activities of Salsola imbricata methanolic extract and its phytochemical characterization. Open Life Sci. 2024, 19, 20221011. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Galindo, E.P.; Nesic, A.; Cabrera-Barjas, G.; Dublan-García, O.; Ventura-Aguilar, R.I.; Vázquez-Armenta, F.J.; Aguilar-Montes de Oca, S.; Mardones, C.; Ayala-Zavala, J.F. Physico-chemical and antiadhesive properties of poly(lactic acid)/grapevine cane extract films against food pathogenic microorganisms. Polymers 2020, 12, 2967. [Google Scholar] [CrossRef] [PubMed]
- Bouchekouk, C.; Kara, F.Z.; Tail, G.; Saidi, F.; Benabdelkader, T. Essential oil composition and antibacterial activity of Pteridium aquilinum (L.) Kuhn. Biol. Futur. 2019, 70, 56–61. [Google Scholar] [CrossRef]
- Jung, J.; Jo, D.; Kim, S.J. Transcriptional response of Pectobacterium carotovorum to cinnamaldehyde treatment. J. Microbiol. Biotechnol. 2024, 34, 538–546. [Google Scholar] [CrossRef]
- Imm, S.; Kim, Y.; Imm, J.Y.; Chang, Y. Inhibition of Pectobacterium carotovorum-mediated potato soft rot by carboxymethyl cellulose-based antibacterial edible coating containing green tea extract. Food Sci. Biotechnol. 2024, 33, 2789–2796. [Google Scholar] [CrossRef]
- Vasilchenko, A.S.; Poshvina, D.V.; Sidorov, R.Y.; Iashnikov, A.V.; Rogozhin, E.A.; Vasilchenko, A.V. Oak bark (Quercus sp. cortex) protects plants through the inhibition of quorum sensing mediated virulence of Pectobacterium carotovorum. World J. Microbiol. Biotechnol. 2022, 38, 184. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; Cozzolino, A.; De Feo, V.; Coppola, R.; Ombra, M.N.; Nazzaro, F. Polyphenols, antioxidant, antibacterial, and biofilm inhibitory activities of peel and pulp of Citrus medica L., Citrus bergamia, and Citrus medica cv. Salò cultivated in southern Italy. Molecules 2019, 24, 4577. [Google Scholar] [CrossRef]
- El-Hefny, M.; Ashmawy, N.A.; Salem, M.Z.M.; Salem, A.Z.M. Antibacterial activities of the phytochemicals-characterized extracts of Callistemon viminalis, Eucalyptus camaldulensis and Conyza dioscoridis against the growth of some phytopathogenic bacteria. Microb. Pathog. 2017, 113, 348–356. [Google Scholar] [CrossRef]
- Almasoudi, N.M.; Al-Qurashi, A.D.; Abo-Elyousr, K.A.M. Assessment of certain plant extracts for controlling potato tuber soft rot disease caused by Pectobacterium carotovorum subsp. carotovorum. J. Plant Pathol. 2024, 106, 1591–1602. [Google Scholar] [CrossRef]
- Ambrico, A.; Trupo, M.; Magarelli, R.; Balducchi, R.; Ferraro, A.; Hristoforou, E.; Marino, T.; Musmarra, D.; Casella, P.; Molino, A. Effectiveness of Dunaliella salina extracts against Bacillus subtilis and bacterial plant pathogens. Pathogens 2020, 9, 613. [Google Scholar] [CrossRef]
- Htwe Maung, C.E.; Choub, V.; Cho, J.Y.; Kim, K.Y. Control of the bacterial soft rot pathogen, Pectobacterium carotovorum by Bacillus velezensis CE 100 in cucumber. Microb. Pathog. 2022, 173, 105807. [Google Scholar] [CrossRef]
- Yamchi, A.; Rahimi, M.; Akbari, R.; Ghobadi, C.; Aryapour, H. Effects of Bacillus in Pectobacterium quorum quenching: A survey of two different acyl-homoserine lactonases. Folia Microbiol. 2024, 69, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; He, P.; Munir, S.; He, P.; He, Y.; Li, X.; Yang, L.; Wang, B.; Wu, Y.; He, P. Biocontrol of soft rot of chinese cabbage using an endophytic bacterial strain. Front. Microbiol. 2019, 10, 1471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xin, Y.; Wang, J.; Dhanasekaran, S.; Yue, Q.; Feng, F.; Gu, X.; Li, B.; Zhao, L.; Zhang, H. Characterization of a Bacillus velezensis strain as a potential biocontrol agent against soft rot of eggplant fruits. Int. J. Food Microbiol. 2024, 410, 110480. [Google Scholar] [CrossRef]
- Paul, E.; Sharma, C.; Chaturvedi, P.; Bhatnagar, P. Quorum quenching activity of endophytic Bacillus sp. EBS9 from Tecomella undulata and its biocontrol applications. Curr. Res. Microb. Sci. 2024, 7, 100307. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.J.; Yoo, S.J.; Hong, J.K.; Weon, H.Y.; Song, J.; Sang, M.K. Effect of Bacillus aryabhattai H26-2 and B. siamensis H30-3 on growth promotion and alleviation of heat and drought stresses in chinese cabbage. Plant Pathol. J. 2019, 35, 178–187. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Feng, T.; Du, R.; Tian, X.; Wang, Y.; Zhang, X.H. Heterologous expression of the marine-derived quorum quenching enzyme MomL can expand the antibacterial spectrum of Bacillus brevis. Mar. Drugs 2019, 17, 128. [Google Scholar] [CrossRef]
- Zeriouh, H.; Romero, D.; Garcia-Gutierrez, L.; Cazorla, F.M.; de Vicente, A.; Perez-Garcia, A. The iturin-like lipopeptides are essential components in the biological control arsenal of Bacillus subtilis against bacterial diseases of cucurbits. Mol. Plant Microbe Interact. 2011, 24, 1540–1552. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, X.; Yan, Z.; Liu, H.; Zhang, L.; Chen, W.; Chen, S. The isolate Pseudomonas multiresinivorans QL-9a quenches the quorum sensing signal and suppresses plant soft rot disease. Plants 2023, 12, 3037. [Google Scholar] [CrossRef]
- Julian, W.T.; Vasilchenko, A.V.; Shpindyuk, D.D.; Poshvina, D.V.; Vasilchenko, A.S. Bacterial-derived plant protection metabolite 2,4-diacetylphloroglucinol: Effects on bacterial cells at inhibitory and subinhibitory concentrations. Biomolecules 2020, 11, 13. [Google Scholar] [CrossRef]
- Rodríguez, M.; Torres, M.; Blanco, L.; Béjar, V.; Sampedro, I.; Llamas, I. Plant growth-promoting activity and quorum quenching-mediated biocontrol of bacterial phytopathogens by Pseudomonas segetis strain P6. Sci. Rep. 2020, 10, 4121. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chung, K.R.; Huang, J.W. A synergistic effect of chitosan and lactic acid bacteria on the control of cruciferous vegetable diseases. Plant. Pathol. J. 2020, 36, 157–169. [Google Scholar] [CrossRef]
- Yi, L.; Qi, T.; Li, X.; Zeng, K. Controlling soft rot of green pepper by bacteriocin paracin wx3 and its effect on storage quality of green pepper. Food Chem. 2024, 447, 138962. [Google Scholar] [CrossRef]
- Garmasheva, I.; Tomila, T.; Kharkhota, M.; Oleschenko, L. Exopolysaccharides of lactic acid bacteria as protective agents against bacterial and viral plant pathogens. Int. J. Biol. Macromol. 2024, 276, 133851. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Gálvez, N.; Luengo-Uribe, P.; Mancilla, S.; Maurin, A.; Torres, C.; Ruiz, P.; France, A.; Acuña, I.; Urrutia, H. Antagonistic activity of endophytic actinobacteria from native potatoes (Solanum tuberosum subsp. tuberosum L.) against Pectobacterium carotovorum subsp. carotovorum and Pectobacterium atrosepticum. BMC Microbiol. 2021, 21, 335. [Google Scholar] [CrossRef]
- Le, K.D.; Yu, N.H.; Park, A.R.; Park, D.J.; Kim, C.J.; Kim, J.C. Streptomyces sp. AN090126 as a biocontrol agent against bacterial and fungal plant diseases. Microorganisms 2022, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Promnuan, Y.; Promsai, S.; Meelai, S. Antimicrobial activity of Streptomyces spp. isolated from Apis dorsata combs against some phytopathogenic bacteria. Peer J. 2020, 8, e10512. [Google Scholar] [CrossRef]
- Al-Zubairy, M.A.; Hussein, K.; Alkhyat, S.H.; Al-Mahdi, A.Y.; Alghalibi, S.M.; Al-Gheethi, A.A.; Al-Shaibani, M.M.; El Enshasy, H.A.; Sidik, N.M. Antibacterial activity of a novel oligosaccharide from Streptomyces californics against Erwinia carotovora subsp. carotovora. Molecules 2022, 27, 2384. [Google Scholar] [CrossRef]
- Fan, X.; Ye, T.; Li, Q.; Bhatt, P.; Zhang, L.; Chen, S. Potential of a quorum quenching bacteria isolate Ochrobactrum intermedium D-2 against soft rot pathogen Pectobacterium carotovorum subsp. carotovorum. Front. Microbiol. 2020, 11, 898. [Google Scholar] [CrossRef]
- Hao, L.; Liang, J.; Chen, S.; Zhang, J.; Zhang, Y.; Xu, Y. MzmL, a novel marine derived N-acyl homoserine lactonase from Mesoflavibacter zeaxanthinifaciens that attenuates Pectobacterium carotovorum subsp. carotovorum virulence. Front. Microbiol. 2024, 15, 1353711. [Google Scholar] [CrossRef]
- Garge, S.S.; Nerurkar, A.S. Attenuation of quorum sensing regulated virulence of Pectobacterium carotovorum subsp. carotovorum through an AHL lactonase produced by Lysinibacillus sp. Gs50. PLoS ONE 2016, 11, e0167344. [Google Scholar] [CrossRef] [PubMed]
- Reina, J.C.; Torres, M.; Llamas, I. Stenotrophomonas maltophilia AHL-degrading strains isolated from marine invertebrate microbiota attenuate the virulence of Pectobacterium carotovorum and Vibrio coralliilyticus. Mar. Biotechnol. 2019, 21, 276–290. [Google Scholar] [CrossRef]
- Hug, S.; Heiniger, B.; Bolli, K.; Paszti, S.; Eberl, L.; Ahrens, C.H.; Pessi, G. Paraburkholderia sabiae uses one type VI secretion system (T6SS-1) as a powerful weapon against notorious plant pathogens. Microbiol. Spectr. 2023, 11, e0162223. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Kwon, Y.M.; Park, A.R.; Yu, N.H.; Choi, G.; Kim, J.C. Exploring Pediococcus sp. M21F004 for biocontrol of bacterial and fungal phytopathogens. Mar. Drugs 2024, 22, 534. [Google Scholar] [CrossRef] [PubMed]
- Kusada, H.; Tamaki, H.; Kamagata, Y.; Hanada, S.; Kimura, N. A novel quorum-quenching N-acylhomoserine lactone acylase from Acidovorax sp. strain MR-S7 mediates antibiotic resistance. Appl. Environ. Microbiol. 2017, 83, e00080-17. [Google Scholar] [CrossRef]
- Morohoshi, T.; Hirose, K.; Someya, N. Identification and characterization of novel N-acylhomoserine lactonase from nonpathogenic Allorhizobium vitis, a candidate for biocontrol agent. J. Biosci. Bioeng. 2024, 137, 437–444. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, S.B. Quorum quenching potential of Reyranella sp. isolated from riverside soil and description of Reyranella humidisoli sp. nov. J. Microbiol. 2024, 62, 449–461. [Google Scholar] [CrossRef]
- Maisuria, V.B.; Nerurkar, A.S. Interference of quorum sensing by Delftia sp. VM4 depends on the activity of a novel N-acylhomoserine lactone-acylase. PLoS ONE 2015, 10, e0138034. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, Q.; Zhang, Y.; Fan, X.; Ye, T.; Mishra, S.; Bhatt, P.; Zhang, L.; Chen, S. Quorum quenching in a novel Acinetobacter sp. XN-10 bacterial strain against Pectobacterium carotovorum subsp. carotovorum. Microorganisms 2020, 8, 1100. [Google Scholar] [CrossRef]
- Dou, L.; Liu, W.; Hu, J.; Zhang, S.; Kong, X.; Qu, X.; Jiang, W. Separation and purification of antimicrobial substances from Paenibacillus polymyxa KH-19 and analysis of its physicochemical characterization. Antonie Van Leeuwenhoek 2024, 118, 23. [Google Scholar] [CrossRef]
- Qingwei, Z.; Lushi, T.; Yu, Z.; Yu, S.; Wanting, W.; Jiangchuan, W.; Xiaolei, D.; Xuejiao, H.; Bilal, M. Isolation and characterization of phosphate-solubilizing bacteria from rhizosphere of poplar on road verge and their antagonistic potential against various phytopathogens. BMC Microbiol. 2023, 23, 221. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Yi, Y.; Kang, G.H.; Choi, Y.H.; Kim, P.; Baek, N.I.; Kim, Y. Identification of an antibacterial compound, benzylideneacetone, from Xenorhabdus nematophila against major plant-pathogenic bacteria. FEMS Microbiol. Lett. 2004, 239, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.A.; Lee, D.H.; Kim, B.Y.; Heu, S. Draft genome sequence of Pantoea agglomerans R190, a producer of antibiotics against phytopathogens and foodborne pathogens. J. Biotechnol. 2014, 188, 7–8. [Google Scholar] [CrossRef]
- Morohoshi, T.; Arai, W.; Someya, N. N-acylhomoserine lactone-degrading activity of Trichoderma species and its application in the inhibition of bacterial quorum sensing. J. Microorg. Control 2023, 28, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Khair, H.; Abdel-Gaied, T.G.; Mikhail, M.S.; Abdel-Alim, A.I.; Seif El-Nasr, H.I. Biological control of Pectobacterium carotovorum subsp. carotovorum, the causal agent of bacterial soft rot in vegetables, in vitro and in vivo tests. Bull. Natl. Res. Cent. 2021, 45, 37. [Google Scholar] [CrossRef]
- Kwak, A.M.; Min, K.J.; Lee, S.Y.; Kang, H.W. Water extract from spent mushroom substrate of Hericium erinaceus suppresses bacterial wilt disease of tomato. Mycobiology 2015, 43, 311–318. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Tsivileva, O.M.; Ibragimova, D.N.; Koftin, O.V.; Fedotova, O.V. Effect of selenium-containing biocomposites based on Ganoderma mushroom isolates grown in the presence of oxopropyl-4-hydroxycoumarins on bacterial phytopathogens. Mikrobiologiia 2017, 86, 172–181, (In Russian with English Abstract). [Google Scholar] [CrossRef]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G., Jr. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef]
- Muturi, P.; Yu, J.; Maina, A.N.; Kariuki, S.; Mwaura, F.B.; Wei, H. Bacteriophages iolated in China for the control of Pectobacterium carotovorum causing potato soft rot in Kenya. Virol. Sin. 2019, 34, 287–294. [Google Scholar] [CrossRef]
- Fister, S.; Robben, C.; Witte, A.K.; Schoder, D.; Wagner, M.; Rossmanith, P. Influence of environmental factors on phage–bacteria interaction and on the efficacy and infectivity of phage P100. Front. Microbiol. 2016, 7, 1152. [Google Scholar] [CrossRef]
- Vu, N.T.; Kim, H.; Hwang, I.S.; Oh, C.S. Colanic acid and lipopolysaccharide in Pectobacterium carotovorum Pcc21 serve as receptors for the bacteriophage phiPccP-2. Microbiol. Res. 2025, 290, 127939. [Google Scholar] [CrossRef] [PubMed]
- Naligama, K.N.; Halmillawewa, A.P. Pectobacterium carotovorum phage vB_PcaM_P7_Pc is a new member of the genus certrevirus. Microbiol. Spectr. 2022, 10, e0312622. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.A.; Jee, S.; Lee, D.H.; Roh, E.; Jung, K.; Oh, C.; Heu, S. Biocontrol of Pectobacterium carotovorum subsp. carotovorum using bacteriophage PP1. J. Microbiol. Biotechnol. 2013, 23, 1147–1153. [Google Scholar] [CrossRef]
- Czajkowski, R.; Ozymko, Z.; De Jager, V.; Siwinska, J.; Smolarska, A.; Ossowicki, A.; Lojkowska, E. Genomic, proteomic and morphological characterization of two novel broad host lytic bacteriophages φPD10. 3 and φPD23. 1 infecting pectinolytic Pectobacterium spp. and Dickeya spp. PLoS ONE 2015, 10, e0119812. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Bai, J.; Lim, J.A.; Heu, S.; Ryu, S. Colanic acid is a novel phage receptor of Pectobacterium carotovorum subsp. carotovorum Phage POP72. Front. Microbiol. 2019, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Marei, E.M.; El-Afifi, S.I.; Hammad, A.M. Biochemical and molecular characteristics of Pc1 virulent phage isolate infecting Pectobacterium carotovorum. Pak. J. Biol. Sci. 2020, 23, 1481–1486. [Google Scholar] [CrossRef]
- Lim, J.A.; Heu, S.; Park, J.; Roh, E. Genomic characterization of bacteriophage vB_PcaP_PP2 infecting Pectobacterium carotovorum subsp. carotovorum, a new member of a proposed genus in the subfamily Autographivirinae. Arch. Virol. 2017, 162, 2441–2444. [Google Scholar] [CrossRef]
- Hirata, H.; Kashihara, M.; Horiike, T.; Suzuki, T.; Dohra, H.; Netsu, O.; Tsuyumu, S. Genome sequence of Pectobacterium carotovorum phage PPWS1, isolated from japanese horseradish [Eutrema japonicum (Miq.) Koidz] showing soft-rot symptoms. Genome Announc. 2016, 4, e01625-15. [Google Scholar] [CrossRef]
- Voronina, M.V.; Bugaeva, E.N.; Vasiliev, D.M.; Kabanova, A.P.; Barannik, A.P.; Shneider, M.M.; Kulikov, E.E.; Korzhenkov, A.A.; Toschakov, S.V.; Ignatov, A.N.; et al. Characterization of Pectobacterium carotovorum subsp. carotovorum bacteriophage PP16 prospective for biocontrol of potato soft rot. Microbiology 2019, 88, 451–460. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef]
- Abedon, S.T.; Danis-Wlodarczyk, K.M.; Wozniak, D.J. Phage cocktail development for bacteriophage therapy: Toward improving spectrum of activity breadth and depth. Pharmaceuticals 2021, 14, 1019. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Jee, S.N.; Heu, S.; Ryu, S. Development of a bacteriophage cocktail against Pectobacterium carotovorum subsp. carotovorum and its effects on Pectobacterium virulence. Appl. Environ. Microbiol. 2022, 88, e0076122. [Google Scholar] [CrossRef]
- Beňo, F.; Horsáková, I.; Kmoch, M.; Petrzik, K.; Krátká, G.; Ševčík, R. Bacteriophages as a strategy to protect potato tubers against Dickeya dianthicola and Pectobacterium carotovorum soft rot. Microorganisms 2022, 10, 2369. [Google Scholar] [CrossRef] [PubMed]
- Vu, N.T.; Kim, H.; Lee, S.; Hwang, I.S.; Kwon, C.T.; Oh, C.S. Bacteriophage cocktail for biocontrol of soft rot disease caused by Pectobacterium species in Chinese cabbage. Appl. Microbiol. Biotechnol. 2024, 108, 11. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Su, X.; Yan, S.; Shen, J. Multifunctional nanoparticles and nanopesticides in agricultural application. Nanomaterials 2023, 13, 1255. [Google Scholar] [CrossRef] [PubMed]
- Román-Doval, R.; Torres-Arellanes, S.P.; Tenorio-Barajas, A.Y.; Gómez-Sánchez, A.; Valencia-Lazcano, A.A. Chitosan: Properties and Its application in agriculture in context of molecular weight. Polymers 2023, 15, 2867. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.E.; Correa-Pacheco, Z.; Corona-Rangel, M.L.; Villanueva-Arce, R.; Bautista-Baños, S. Cellular alterations in Pectobacterium carotovorum treated with nanostructured formulations during the incubation time. Arch. Microbiol. 2019, 201, 615–622. [Google Scholar] [CrossRef]
- Akdaşçi, E.; Duman, H.; Eker, F.; Bechelany, M.; Karav, S. Chitosan and its nanoparticles: A multifaceted approach to antibacterial applications. Nanomaterials 2025, 15, 126. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Corona-Rangel, M.L.; Bautista-Baños, S.; Ventura-Aguilar, R.I. Application of natural-based nanocoatings for extending the shelf life of green bell pepper fruit. J. Food Sci. 2021, 86, 95–102. [Google Scholar] [CrossRef]
- Cai, J.; Yang, D.; Wang, Q. Preparation and characterization of chitosan nanoparticles loaded with Athyrium sinense essential oil with antibacterial properties against Pectobacterium carotovorum subsp. carotovorum. Ind. Crops Prod. 2023, 195, 116382. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Bashir, S.I.; Rabei, N.H.; Saber, W.I. Innovative biosynthesis, artificial intelligence-based optimization, and characterization of chitosan nanoparticles by Streptomyces microflavus and their inhibitory potential against Pectobacterium carotovorum. Sci. Rep. 2022, 12, 21851. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Kharasova, A.R.; Nozhkina, O.A.; Sidorov, A.V.; Graskova, I.A.; Krutovsky, K.V. Effect of nanopriming with selenium nanocomposites on potato productivity in a field experiment, soybean germination and viability of Pectobacterium carotovorum. Horticulturae 2023, 9, 458. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Zakharova, O.V.; Graskova, I.A.; Krutovsky, K.V. Effect of selenium, copper and manganese nanocomposites in arabinogalactan matrix on potato colonization by phytopathogens Clavibacter sepedonicus and Pectobacterium carotovorum. Plants 2024, 13, 3496. [Google Scholar] [CrossRef]
- Zaki, S.A.E.; Kamal, A.; Ashmawy, N.A.; Shoeib, A.A. Nano-metals forming bacteria in Egypt. I. Synthesis, characterization and effect on some phytopathogenic bacteria in vitro. Sci. Rep. 2021, 11, 12876. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, E.; Al-Askar, A.A.; El-Gendi, H.; Khamis, W.M.; Behiry, S.I.; Valentini, F.; Abd-Elsalam, K.A.; Abdelkhalek, A. Zinc oxide nanoparticles biosynthesized by Eriobotrya japonica leaf extract: Characterization, insecticidal and antibacterial properties. Plants 2023, 12, 2826. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Fu, X.; Gao, Y.; Shi, L.; Liu, Q.; Yang, W.; Feng, J. Synthesis, antibacterial evaluation, and safety assessment of CuS NPs against Pectobacterium carotovorum subsp. carotovorum. Pest. Manag. Sci. 2022, 78, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Usman, O.; Mohsin Baig, M.M.; Ikram, M.; Iqbal, T.; Islam, S.; Syed, W.; Al-Rawi, M.B.A.; Naseem, M. Green synthesis of metal nanoparticles and study their anti-pathogenic properties against pathogens effect on plants and animals. Sci. Rep. 2024, 14, 11354. [Google Scholar] [CrossRef]
- Abdelghany, W.A.; Mohamedin, A.H.; Abo-Elyousr, K.A.M.; Hussein, M.A.M. Control of bacterial soft rot disease of potato caused by Pectobacterium carotovorum subsp. carotovorum using different nanoparticles. Arch. Phytopathol. Plant Prot. 2022, 55, 1638–1660. [Google Scholar] [CrossRef]
- Wei, Z.; Xu, S.; Jia, H.; Zhang, H. Green synthesis of silver nanoparticles from Mahonia fortunei extracts and characterization of its inhibitory effect on Chinese cabbage soft rot pathogen. Front. Microbiol. 2022, 13, 1030261. [Google Scholar] [CrossRef]
- Beltrán Pineda, M.E.; Lizarazo Forero, L.M.; Sierra, C.A. Antibacterial fibers impregnated with mycosynthetized AgNPs for control of Pectobacterium carotovorum. Heliyon 2023, 10, e23108. [Google Scholar] [CrossRef]
- Soleimani, P.; Mehrvar, A.; Michaud, J.P.; Vaez, N. Optimization of silver nanoparticle biosynthesis by entomopathogenic fungi and assays of their antimicrobial and antifungal properties. J. Invertebr. Pathol. 2022, 190, 107749. [Google Scholar] [CrossRef] [PubMed]
- Trzcińska-Wencel, J.; Wypij, M.; Rai, M.; Golińska, P. Biogenic nanosilver bearing antimicrobial and antibiofilm activities and its potential for application in agriculture and industry. Front. Microbiol. 2023, 14, 1125685. [Google Scholar] [CrossRef]
- Elkobrosy, D.; Al-Askar, A.A.; El-Gendi, H.; Su, Y.; Nabil, R.; Abdelkhalek, A.; Behiry, S. Nematocidal and bactericidal activities of green synthesized silver nanoparticles mediated by Ficus sycomorus leaf extract. Life 2023, 13, 1083. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M. A Review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Warrer, E.A.K.; Payne, C.K. Cellular binding of nanoparticles disrupts the membrane potential. RSC Adv. 2015, 5, 13660–13666. [Google Scholar] [CrossRef] [PubMed]
- Ayisigi, M.; Cokislerel, A.; Kucukcobanoglu, Y.; Yalcin, T.; Aktas, L.Y. Green synthesized silver nanoparticles for an effective control on soft rot disease pathogen Pectobacterium carotovorum and growth stimulation in pepper. Bulg. J. Agric. Sci. 2020, 26, 574–584. [Google Scholar]
- Pineda, M.E.B.; Lizarazo Forero, L.M.; Sierra Avila, C.A. Antibacterial activity of biosynthesized silver nanoparticles (AgNps) against Pectobacterium carotovorum. Braz. J. Microbiol. 2022, 53, 1175–1186. [Google Scholar] [CrossRef]
- Soltani Nejad, M.; Samandari Najafabadi, N.; Aghighi, S.; Zargar, M.; Stybayev, G.; Baitelenova, A.; Kipshakbayeva, G. Application of biosynthesized silver nanoparticles from oak fruit exudates against Pectobacterium carotovorum subsp. carotovorum causing postharvest soft rot disease in vegetables. Agronomy 2023, 13, 1624. [Google Scholar] [CrossRef]
| Substance | Effects | Reference |
|---|---|---|
| salicylic acid | inhibit the growth of Pcc bacteria | [122] |
| ethyl Nα-lauroyl arginate ester (LAE) | increased membrane permeability, decreased membrane potential of Pcc and damaged organelles in bacteria | [123] |
| phenylacetic acid | induced systemic resistance in tobacco against Pcc | [124] |
| cinnamaldehyde, l-menthone and carvacrol | blocking disease development symptoms on potato slices | [125] |
| NaOCl | delayed the development of disease symptoms of soft rot and also affected gene expression in plant tissues | [3] |
| potassium tetraborate tetrahydrate | destruction of bacterial membrane, inhibition of bacterial growth | [126] |
| ZnAl-NADS | bacteriostatic and biocidal effects | [127] |
| O3, ozonated water | recovering plant bulbs from soft rot | [128] |
| linolenic fatty acid hydroperoxide | rearrangements in the bacterial membrane | [129] |
| Cl | improving tomato resistance to soft rot infection | [130] |
| antibiotics | antibacterial action | [131,132,133] |
| Species | Substance | Antimicrobial Effects on Pcc | Reference |
|---|---|---|---|
| Herbaceous plants | |||
| Rheum tanguticum | extract | decreased motility of Pcc bacterial flagella | [134] |
| inhibition of QS Pcc | [142] | ||
| Polygonum orientale | essential oil | increase in the surface potential of the Pcc cell and its hydrophobicity, damage to the cell wall, destruction of the integrity and permeability of the cell membrane, suppression of the activity of bacterial enzymes pyruvate kinase, succinate dehydrogenase and adenosine triphosphatase | [135] |
| Cymbopogon martini | nanoemulsions and citric acid | effects on membrane integrity and intracellular ATP depletion | [136] |
| Salsola kali | ethanol extract | antibacterial activity (well method) | [137] |
| Mentha piperita | essential oil | antibacterial activity (well method) | [138] |
| Datura stramonium Urtica dioica | seed extract leaf extract | antibacterial activity (well method), reduction of disease symptoms development after potato tubers treatment | [139] |
| Falcaria vulgaris | extract | inhibition of QS Pcc | [143] |
| Echinochloa crus-galli | crude extract | antibacterial activity (well method) | [144] |
| Solanum elaeagnifolium | ethanol extract | antibacterial activity (well method) | [141] |
| Shrubs and Trees | |||
| Salvia rosmarinus | methanol extract | inactivation of pectate lyase 1 and endopolygalacturonase | [145] |
| Salsola imbricata | methanol extract | antibacterial activity (well method) | [146] |
| Vitis vinifera | extract | reduced the adhesive properties of Pcc | [147] |
| Duranta plumieri Lantana camara | leaf extract | antibacterial activity (well method) | [140] |
| Pteridium aquilinum | essential oil | antibacterial activity (well method) | [148] |
| Cinnamomum cassia | bark | impaired aerobic respiration of Pc, effects on nitrate reductase activity and regulation of the citrate cycle | [149] |
| Camellia sinensis | film of carboxymethylcellulose with extract of leaves | reducing tuber infection during storage | [150] |
| Quercus sp. cortex | bark extract | decreased acyl-HSL Pc synthesis, decreased bacterial cellulolytic and protease activity, inhibition of QS-related genes | [151] |
| Callistemon viminalis Eucalyptus camaldulensis | butanol extract from flowers; bark | antibacterial activity (well method) | [153] |
| Punica granatum | fruit peel extract | antibacterial activity (well method) | [154] |
| Species | Effects on Pcc and Plants | Reference |
|---|---|---|
| Bacteria | ||
| Bacillus | ||
| B. velezensis CE 100 | Pc growth inhibition; cytoprotective effect on cucumber plants | [156] |
| B. thuringiensis KMCL07 | suppression of QS Pcc; inhibition of extracellular Pcc enzymes cellulase, pectate lyase and proteinase | [59] |
| Bacillus sp. OA10 | suppression of QS Pc (inhibition of acylserine lactone synthesis); reduction of potato tissue maceration | [66] |
| Bacillus sp. A24 Bacillus sp. DMS133 | inhibition of QS Pc by acylhomoserine lactonase | [157] |
| B. amyloliquefaciens KC-1 | reducing colonization of Chinese cabbage plants by Pcc | [158] |
| B. velezensis | inhibition of Pcc growth; phytoprotective effect on eggplant plants due to increased activity of antioxidant enzymes | [159] |
| Bacillus sp. EBS9 | suppression of QS Pcc by synthesis of N-acyl homoserine lactone antagonists; phytoprotective effect on radish plants | [160] |
| B. aryabhattai H26-2 B. siamensis H30-3 | phytoprotective effect on Chinese cabbage plants by reducing the colonization of the rhizosphere by Pcc and increasing the content of abscisic acid in leaves | [161] |
| B. brevis | suppression of QS Pcc due to the synthesis of the enzyme BbMomL, which degrades N-acyl homoserine lactones; reduction of secretion of virulence factors Pcc | [162] |
| B. subtilis UMAF6614, UMAF6639 | destruction of the bacterial plasma membrane Pcc under the influence of lipopeptide antibiotics Bacillus spp.: surfactins, iturins, and fengycins | [163] |
| Pseudomonas | ||
| Ps. multiresinivorans QL-9a | suppression of QS Pc by N-(-3-oxohexanoyl)-L-homoserine lactone acylase cleavage | [164] |
| Pseudomonas spp. | increased permeability of the bacterial membrane Pc; suppression of biosynthesis of acyl homoserine lactones Pc | [165] |
| P. segetis P6 | suppression of QS Pc by cleavage of N-acyl homoserine lactones | [166] |
| Lactic acid bacteria | ||
| Lactobacillus pentosus, Leuconostoc fallax | inhibition of disease symptom development on radish | [167] |
| Lactobacillus paracasei WX322 | phytoprotective effect on pepper plants due to the synthesis of bacteriocin parocin | [168] |
| exopolysaccharides of bacteria of the genera Leuconostoc, Pediococcus, and Lactobacillus | bacteriostatic and antibiofilm effect on Pc | [169] |
| Streptomyces | ||
| Streptomyces spp. | bacteriostatic effect on Pcc; inhibition of potato tuber tissue maceration | [170] |
| Streptomyces sp. AN090126 | antimicrobial effect against Pcc due to the synthesis of volatile organic compounds (VOCs) | [171] |
| Streptomyces sp. | antibacterial activity (well method) | [172] |
| S. californics | antibacterial activity (well method) | [173] |
| Streptomyces sp. AG-P 1441 | reduction of soft rot symptoms development on the plant due to the antibiotic paromomycin; phytoprotective effect due to the increased expression of PR genes | [132] |
| Other types of bacteria | ||
| Cedecea sp., Cellulosimicrobium sp., Delftia sp., Ensifer sp., Paenibacillus sp., Pantoea sp., Phyllobacterium sp., Pseudomonas sp., Rhizobium sp., Sinorhizobium sp., Staphylococcus sp. | inhibition of Pc growth | [186] |
| Paenibacillus polymyxa KH-19 | inhibited the growth of Pc by synthesizing the enzyme lysophosphatidyl esterase | [185] |
| Ochrobactrum intermedium D-2 | suppression of QS Pcc by AHL-lactonase synthesis; reduction of maceration on radish and potato slices | [174] |
| Mesoflavibacter zeaxanthinifaciens XY-85 | reduction of Pcc virulence, inhibition of biofilm formation, and synthesis of cellulolytic enzymes | [175] |
| Lysinibacillus sp. Gs50 | suppression of QS Pcc by lactonase synthesis | [176] |
| Stenotrophomonas maltophilia | suppression of QS Pc by cleavage of N-acyl homoserine lactones | [177] |
| Paraburkholderia sabiae | cytoprotective effect on potato plants | [178] |
| Pediococcus sp. M21F004 | antimicrobial effect on Pcc through oleic acid synthesis; phytoprotective effect on kim chi and tomato plants | [179] |
| Acidovorax sp. MR-S7 | suppression of QS Pc by AHL-lactonase synthesis | [180] |
| Allorhizobium vitis | suppression of QS Pcc by cleavage of N-acyl homoserine lactones | [181] |
| Reyranella sp. | suppression of QS Pc by lactonase synthesis | [182] |
| Delftia sp. VM4 | suppression of QS Pcc by lactonase synthesis | [183] |
| Acinetobacter sp. XN-10 | suppression of QS Pcc by cleaving N-acyl homoserine lactones and inhibition of tissue maceration in carrot, potato, and Chinese cabbage | [184] |
| Xenorhabdus nematophila | inhibition of Pcc viability by the antibiotic benzylideneacetone (trans-4-phenyl-3-buten-2-one) | [187] |
| Pantoea agglomerans | antibacterial activity (well method) | [188] |
| Fungi | ||
| Trichoderma asperellum | suppression of PCWDE and QS Pcc enzyme synthesis by emodin synthesis; phytoprotective effect on cabbage, carrot and cherry tomato plants | [64] |
| Trichoderma sp. | suppression of QS Pcc by lactonase synthesis | [189] |
| T. viride, T. virens | suppression of maceration of potato tuber tissues | [190] |
| Hericium erinaceus, Lentinula edodes, Grifola frondosa, Hypsizygus marmoreus | antimicrobial effect against Pcc; phytoprotective effect on tomato plants due to the development of induced systemic resistance | [191] |
| Ganoderma colossus | antibacterial activity (well method) and bacteriostatic effect against Pc of selenium biocomposites obtained on the basis of fungal culture medium | [192] |
| Bacteriophages | ||
| PP1 | lytic activity against Pcc in liquid medium | [198] |
| φPD10.3; φPD23.1 | reduction by >80% of maceration of potato tuber tissues | [199] |
| vB_PcaM_P7_P | Pc cell lysis | [197] |
| POP72 | protection of Chinese cabbage from Pcc infection | [200] |
| Wc5r | suppression of phage-resistant strains | [194] |
| phiPccP-2 | lytic activity against Pc/Pcc | [196] |
| vB_PcaP_PP2 (PP2) | [202] | |
| PPWS1 | [203] | |
| PP16 | suppression of infection caused by Pcc in vitro and in planta | [204] |
| POP12 + POP15 + POP17 | in vitro suppression of phage-resistant Pcc isolates and development of soft rot symptoms on Chinese cabbage | [207] |
| Ds3CZ + Ds20CZ; PcCB7V + PcCB251 | slowing down the development of the disease both in whole tubers and on potato slices | [208] |
| phiPccP-2 + phiPccP-3 | suppression of phage-resistant strains, prevention of development of soft rot symptoms in mature leaves of Chinese cabbage | [209] |
| phiPccP-1 + phiPccP-2 + phiPccP-3 | suppression of a mixture of Pectobacterium strains on Chinese cabbage seedlings | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perfileva, A.I.; Strekalovskaya, E.I.; Klushina, N.V.; Gorbenko, I.V.; Krutovsky, K.V. The Causative Agent of Soft Rot in Plants, the Phytopathogenic Bacterium Pectobacterium carotovorum subsp. carotovorum: A Brief Description and an Overview of Methods to Control It. Agronomy 2025, 15, 1578. https://doi.org/10.3390/agronomy15071578
Perfileva AI, Strekalovskaya EI, Klushina NV, Gorbenko IV, Krutovsky KV. The Causative Agent of Soft Rot in Plants, the Phytopathogenic Bacterium Pectobacterium carotovorum subsp. carotovorum: A Brief Description and an Overview of Methods to Control It. Agronomy. 2025; 15(7):1578. https://doi.org/10.3390/agronomy15071578
Chicago/Turabian StylePerfileva, Alla I., Elena I. Strekalovskaya, Nadezhda V. Klushina, Igor V. Gorbenko, and Konstantin V. Krutovsky. 2025. "The Causative Agent of Soft Rot in Plants, the Phytopathogenic Bacterium Pectobacterium carotovorum subsp. carotovorum: A Brief Description and an Overview of Methods to Control It" Agronomy 15, no. 7: 1578. https://doi.org/10.3390/agronomy15071578
APA StylePerfileva, A. I., Strekalovskaya, E. I., Klushina, N. V., Gorbenko, I. V., & Krutovsky, K. V. (2025). The Causative Agent of Soft Rot in Plants, the Phytopathogenic Bacterium Pectobacterium carotovorum subsp. carotovorum: A Brief Description and an Overview of Methods to Control It. Agronomy, 15(7), 1578. https://doi.org/10.3390/agronomy15071578








