Morphology and Coating of ZnO Nanoparticles Affect Growth and Gas Exchange Parameters of Bell Pepper Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Location of the Experiment
2.2. Synthesis of ZnO-NPs
2.3. Surface Coating of ZnO-NPs with MD
2.4. Characterization of ZnO-NPs
2.5. Treatments and Seedling Establishment
2.6. Evaluated Parameters
2.7. Experimental Design and Statistical Analysis
3. Results and Discussion
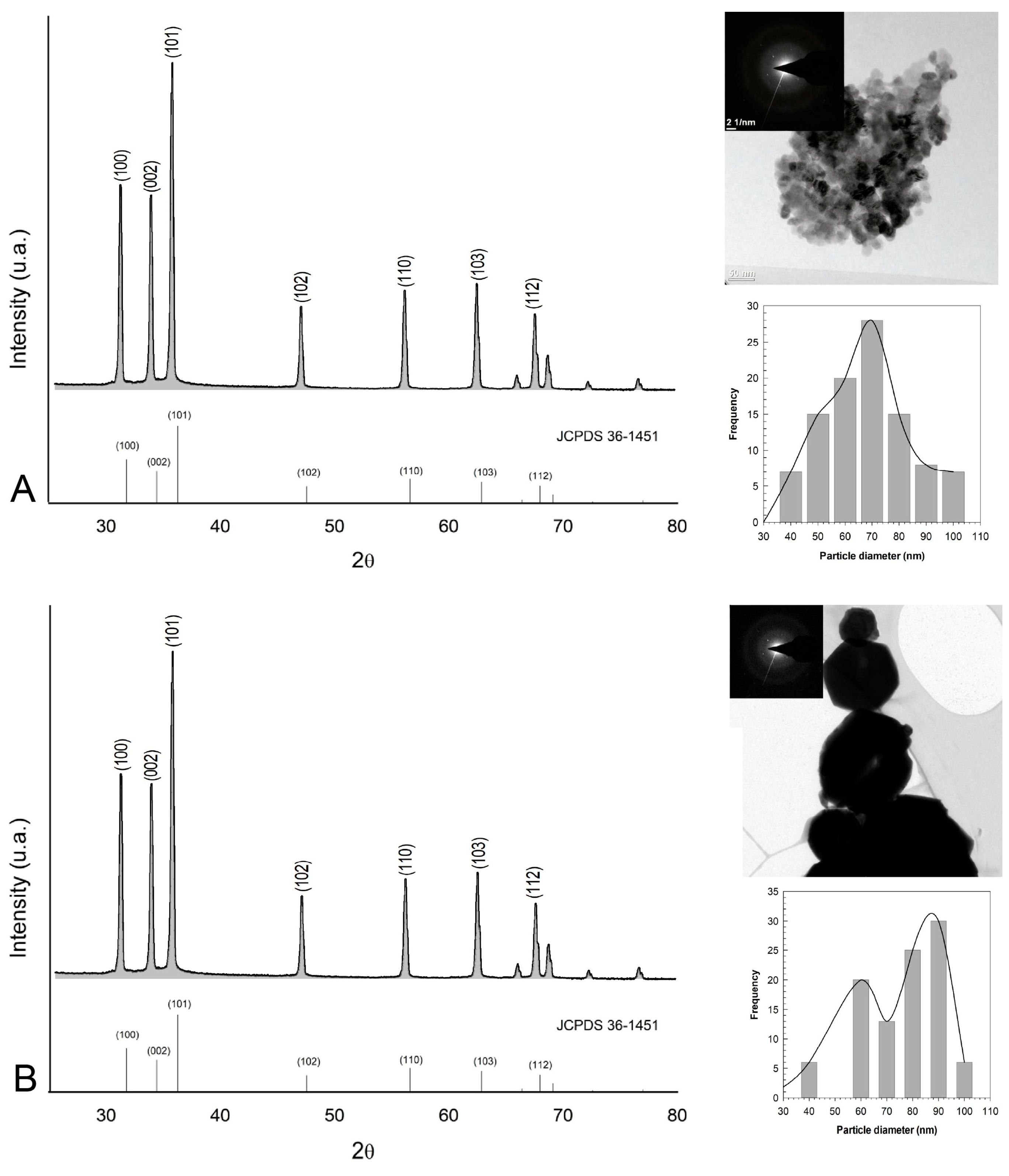
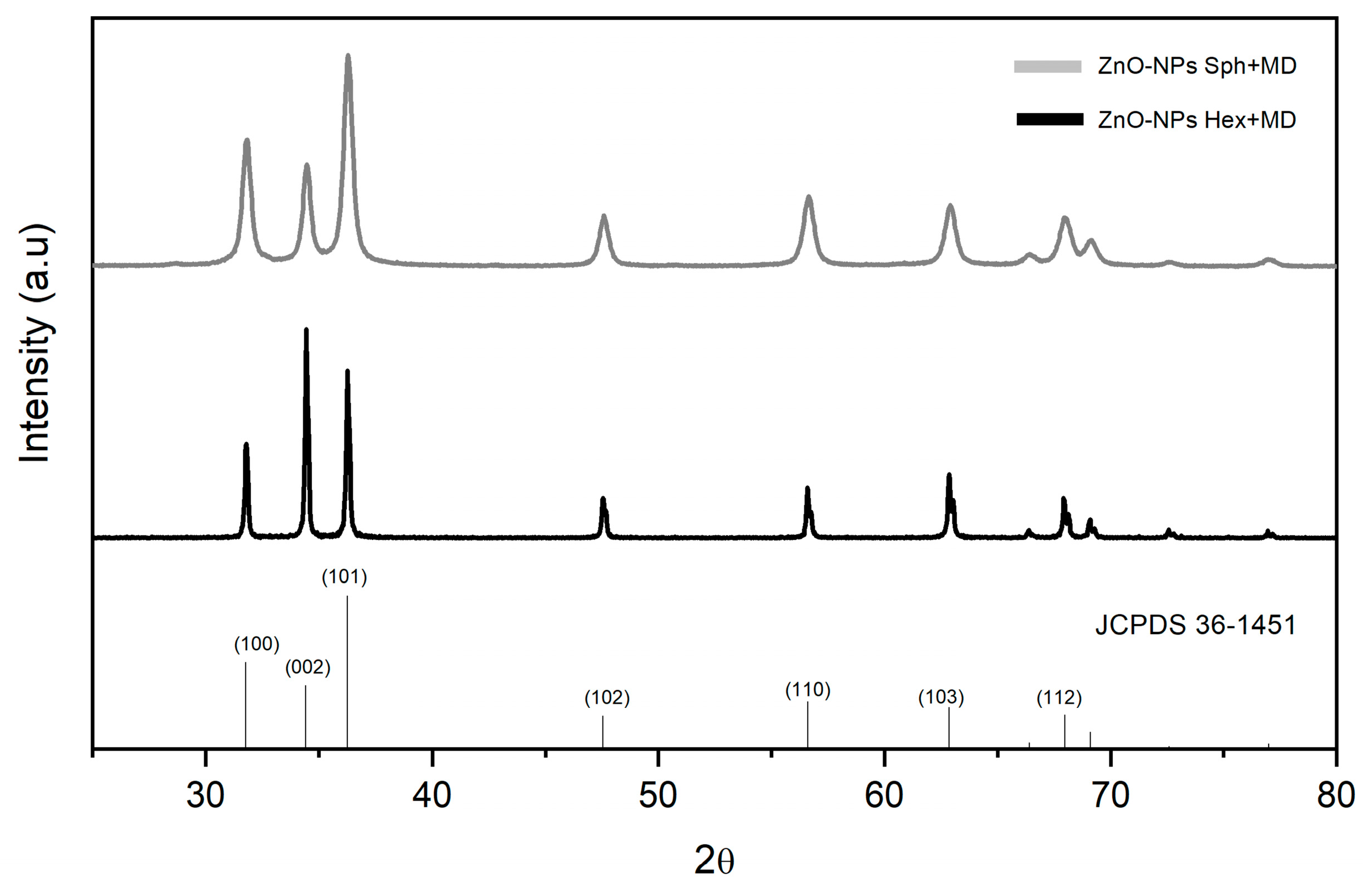
3.1. Structure and Morphology
3.2. Effect of ZnO-NPs on the Growth and Biomass of Bell Pepper Seedlings
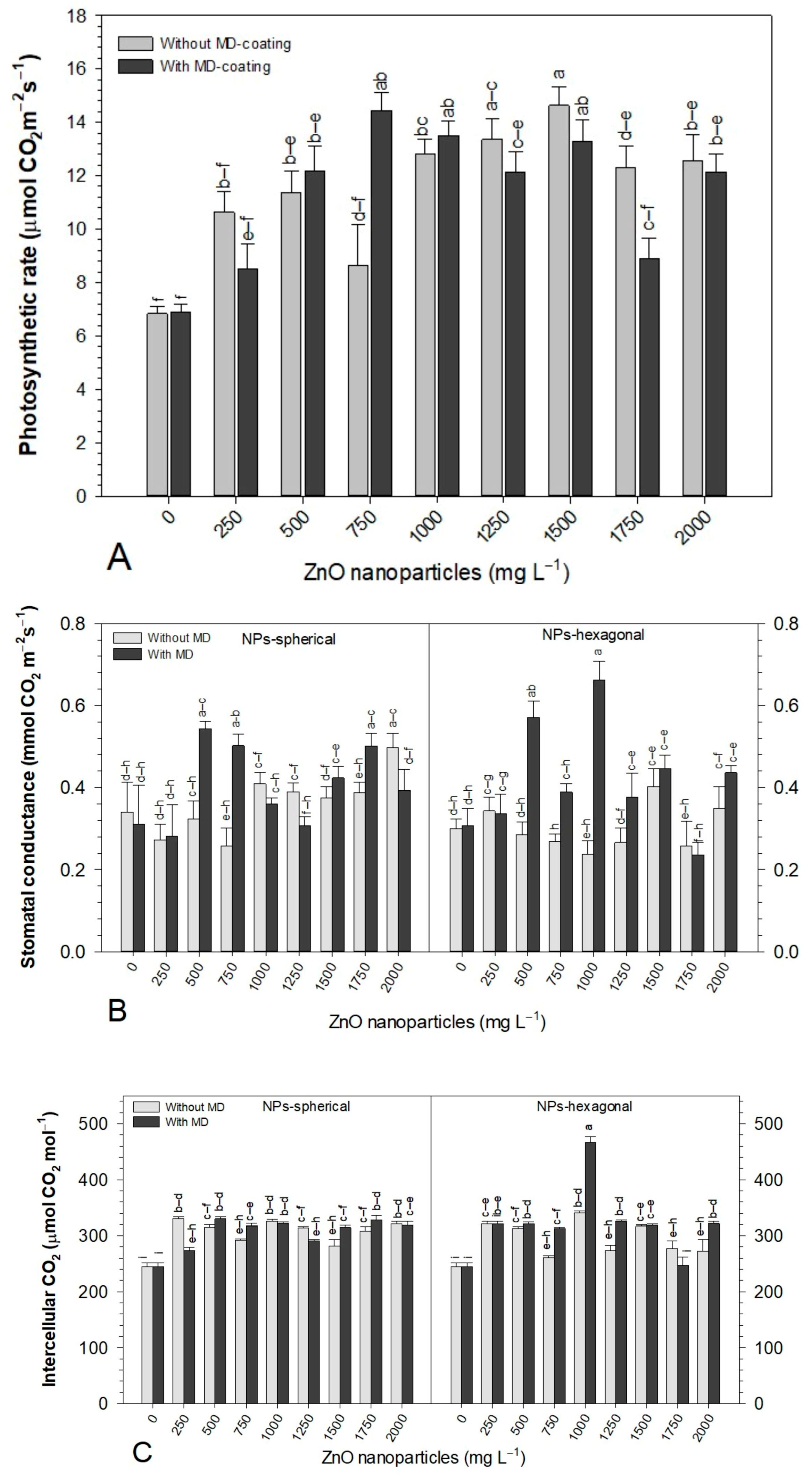
3.3. Effect of ZnO-NPs on Gas Exchange and SPAD Units of Bell Pepper Seedlings
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Selwey, W.A.; Alsadon, A.A.; Ibrahim, A.A.; Labis, J.P.; Seleiman, M.F. Effects of Zinc Oxide and Silicon Dioxide Nanoparticles on Physiological, Yield, and Water Use Efficiency Traits of Potato Grown under Water Deficit. Plants 2023, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; ur Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in Agriculture: Current Status, Challenges and Future Opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Almutairi, K.F.; Alotaibi, M.; Shami, A.; Alhammad, B.A.; Battaglia, M.L. Nano-Fertilization as an Emerging Fertilization Technique: Why Can Modern Agriculture Benefit from Its Use? Plants 2020, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Kour, D.; Khan, S.S.; Kumari, S.; Singh, S.; Khan, R.T.; Kumari, C.; Kumari, S.; Dasila, H.; Kour, H.; Kaur, M.; et al. Microbial Nanotechnology for Agriculture, Food, and Environmental Sustainability: Current Status and Future Perspective. Folia Microbiol. 2024, 69, 491–520. [Google Scholar] [CrossRef]
- Taha, R.A.; Hassan, M.M.; Ibrahim, E.A.; Abou Baker, N.H.; Shaaban, E.A. Carbon Nanotubes Impact on Date Palm in Vitro Cultures. Plant Cell Tissue Organ. Cult. 2016, 127, 525–534. [Google Scholar] [CrossRef]
- Lacerda, J.S.; Martinez, H.E.P.; Pedrosa, A.W.; Clemente, J.M.; Santos, R.H.S.; Oliveira, G.L.; Jifon, J.L. Importance of Zinc for Arabica Coffee and Its Effects on the Chemical Composition of Raw Grain and Beverage Quality. Crop Sci. 2018, 58, 1360–1370. [Google Scholar] [CrossRef]
- Cakmak, I.; Brown, P.; Colmenero-Flores, J.M.; Husted, S.; Kutman, B.Y.; Nikolic, M.; Rengel, Z.; Schmidt, S.B.; Zhao, F.J. Micronutrients. In Marschner’s Mineral Nutrition of Plants; Academic Press: New York, NY, USA, 2023; pp. 283–385. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Alotaibi, M.A.; Alhammad, B.A.; Alharbi, B.M.; Refay, Y.; Badawy, S.A. Effects of ZnO Nanoparticles and Biochar of Rice Straw and Cow Manure on Characteristics of Contaminated Soil and Sunflower Productivity, Oil Quality, and Heavy Metals Uptake. Agronomy 2020, 10, 790. [Google Scholar] [CrossRef]
- Moghaddasi, S.; Fotovat, A.; Khoshgoftarmanesh, A.H.; Karimzadeh, F.; Khazaei, H.R.; Khorassani, R. Bioavailability of Coated and Uncoated ZnO Nanoparticles to Cucumber in Soil with or without Organic Matter. Ecotoxicol. Environ. Saf. 2017, 144, 543–551. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Raliya, R.; Mahawar, H.; Rathore, I. Development of Zinc Nanofertilizer to Enhance Crop Production in Pearl Millet (Pennisetum americanum). Agric. Res. 2014, 3, 257–262. [Google Scholar] [CrossRef]
- Garciá-López, J.I.; Zavala-Garcia, F.; Olivares-Saénz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Ruiz-Torres, N.A.; Ramos-Cortez, E.; Vázquez-Alvarado, R.; Ninõ-Medina, G. Zinc Oxide Nanoparticles Boosts Phenolic Compounds and Antioxidant Activity of Capsicum annuum L. during Germination. Agronomy 2018, 8, 215. [Google Scholar] [CrossRef]
- Modi, S.; Yadav, V.K.; Choudhary, N.; Alswieleh, A.M.; Sharma, A.K.; Bhardwaj, A.; Khan, S.H.; Yadav, K.K.; Cheon, J.K.; Jeon, B.H. Onion Peel Waste Mediated-Green Synthesis of Zinc Oxide Nanoparticles and Their Phytotoxicity on Mung Bean and Wheat Plant Growth. Materials 2022, 15, 2393. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.; Bashir, S.; Bashir, S.; Aslam, Z.; Ahmad, N.; Younas, T.; Asghar, R.M.A.; Alkahtani, J.; Dwiningsih, Y.; Elshikh, M.S. Zinc Oxide Nanoparticles Improved Chlorophyll Contents, Physical Parameters, and Wheat Yield under Salt Stress. Front. Plant Sci. 2022, 13, 932861. [Google Scholar] [CrossRef] [PubMed]
- Reddy Pullagurala, V.L.; Adisa, I.O.; Rawat, S.; Kalagara, S.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. ZnO Nanoparticles Increase Photosynthetic Pigments and Decrease Lipid Peroxidation in Soil Grown Cilantro (Coriandrum sativum). Plant Physiol. Biochem. 2018, 132, 120–127. [Google Scholar] [CrossRef]
- Betancourt, G.R.; Berlanga, D.M.L.; Puente, U.B.; Rodríguez, F.O.; Sánchez-Valdes, S. Surface Modification of ZnO Nanoparticles. In Proceedings of the Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2010; Volume 644, pp. 61–64. [Google Scholar]
- Raveendran, S.; Rochani, A.K.; Maekawa, T.; Kumar, D.S. Smart Carriers and Nanohealers: A Nanomedical Insight on Natural Polymers. Materials 2017, 10, 929. [Google Scholar] [CrossRef]
- Plucinski, A.; Lyu, Z.; Schmidt, B.V.K.J. Polysaccharide Nanoparticles: From Fabrication to Applications. J. Mater. Chem. B 2021, 9, 7030–7062. [Google Scholar] [CrossRef]
- Syu, Y.y; Hung, J.H.; Chen, J.C.; Chuang, H. wen Impacts of Size and Shape of Silver Nanoparticles on Arabidopsis Plant Growth and Gene Expression. Plant Physiol. Biochem. 2014, 83, 57–64. [Google Scholar] [CrossRef]
- Helmlinger, J.; Sengstock, C.; Groß-Heitfeld, C.; Mayer, C.; Schildhauer, T.A.; Köller, M.; Epple, M. Silver Nanoparticles with Different Size and Shape: Equal Cytotoxicity, but Different Antibacterial Effects. RSC Adv. 2016, 6, 18490–18501. [Google Scholar] [CrossRef]
- Taheri, A.; Behnamian, M.; Dezhsetan, S.; Karimirad, R. Shelf Life Extension of Bell Pepper by Application of Chitosan Nanoparticles Containing Heracleum persicum Fruit Essential Oil. Postharvest Biol. Technol. 2020, 170, 111313. [Google Scholar] [CrossRef]
- González-García, Y.; Cárdenas-Álvarez, C.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Cabrera-de-la-Fuente, M.; Sandoval-Rangel, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Effect of Three Nanoparticles (Se, Si and Cu) on the Bioactive Compounds of Bell Pepper Fruits under Saline Stress. Plants 2021, 10, 217. [Google Scholar] [CrossRef]
- Carrera-Castaño, G.; Calleja-Cabrera, J.; Pernas, M.; Gómez, L.; Oñate-Sánchez, L. An Updated Overview on the Regulation of Seed Germination. Plants 2020, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Lopez, R.G. Supplemental Radiation Quality Influences Cucumber, Tomato, and Pepper Transplant Growth and Development. HortScience 2020, 55, 804–811. [Google Scholar] [CrossRef]
- Pavani, K.V.; Beulah, M.; Sai Poojitha, G.U. The Effect of Zinc Oxide Nanoparticles (ZnO NPs) on Vigna mungo L. Seedling Growth and Antioxidant Activity. Nanosci. Nanotechnol. Asia 2020, 10, 117–122. [Google Scholar] [CrossRef]
- Pérez-Velasco, E.A.; Valdez-Aguilar, L.A.; Betancourt-Galindo, R.; Martínez-Juárez, J.; Lozano-Morales, S.A.; González-Fuentes, J.A. Gas Exchange Parameters, Fruit Yield, Quality, and Nutrient Status in Tomato Are Stimulated by ZnO Nanoparticles of Modified Surface and Morphology and Their Application Form. J. Soil. Sci. Plant Nutr. 2021, 21, 991–1003. [Google Scholar] [CrossRef]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef]
- Das, S.; Dutta, K.; Pramanik, A. Morphology Control of ZnO with Citrate: A Time and Concentration Dependent Mechanistic Insight. CrystEngComm 2013, 15, 6349–6358. [Google Scholar] [CrossRef]
- de Peres, M.L.; Delucis, R.d.A.; Amico, S.C.; Gatto, D.A. Zinc Oxide Nanoparticles from Microwave-Assisted Solvothermal Process: Photocatalytic Performance and Use for Wood Protection against Xylophagous Fungus. Nanomater. Nanotechnol. 2019, 9, 1847980419876201. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Abdoli, S. Improving ATPase and PPase Activities, Nutrient Uptake and Growth of Salt Stressed Ajowan Plants by Salicylic Acid and Iron-Oxide Nanoparticles. Plant Cell Rep. 2021, 40, 559–573. [Google Scholar] [CrossRef]
- Gupta, A.; Bharati, R.; Kubes, J.; Popelkova, D.; Praus, L.; Yang, X.; Severova, L.; Skalicky, M.; Brestic, M. Zinc Oxide Nanoparticles Application Alleviates Salinity Stress by Modulating Plant Growth, Biochemical Attributes and Nutrient Homeostasis in Phaseolus vulgaris L. Front. Plant Sci. 2024, 15, 1432258. [Google Scholar] [CrossRef]
- Castro-Cabado, M.; Parra-Ruiz Francisco, J.; Casado, A.L.; Román, J.S. Thermal Crosslinking of Maltodextrin and Citric Acid. Methodology to Control the Polycondensation Reaction under Processing Conditions. Polym. Polym. Compos. 2016, 24, 643–654. [Google Scholar] [CrossRef]
- Sritham, E.; Gunasekaran, S. FTIR Spectroscopic Evaluation of Sucrose-Maltodextrin-Sodium Citrate Bioglass. Food Hydrocoll. 2017, 70, 371–382. [Google Scholar] [CrossRef]
- Aquino, P.; Osorio, A.M.; Ninán, E.; Torres, F. Characterization of ZnO nanoparticles synthesized by precipitation method and its evaluation in the incorporation in enamel paints. Rev. Soc. Quím. Perú 2018, 84, 5–17. [Google Scholar]
- da Trindade, L.G.; Minervino, G.B.; Trench, A.B.; Carvalho, M.H.; Assis, M.; Li, M.S.; de Oliveira, A.J.A.; Pereira, E.C.; Mazzo, T.M.; Longo, E. Influence of Ionic Liquid on the Photoelectrochemical Properties of ZnO Particles. Ceram. Int. 2018, 44, 10393–10401. [Google Scholar] [CrossRef]
- Faizan; Faraz, A.; Hayat, S. Effective Use of Zinc Oxide Nanoparticles through Root Dipping on the Performance of Growth, Quality, Photosynthesis and Antioxidant System in Tomato. J. Plant Biochem. Biotechnol. 2020, 29, 553–567. [Google Scholar] [CrossRef]
- Zafar, H.; Ali, A.; Ali, J.S.; Haq, I.U.; Zia, M. Effect of ZnO Nanoparticles on Brassica nigra Seedlings and Stem Explants: Growth Dynamics and Antioxidative Response. Front. Plant Sci. 2016, 7, 535. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Latta, D.E.; McLean, J.E.; Britt, D.W.; Boyanov, M.I.; Anderson, A.J. Fate of CuO and ZnO Nano- and Microparticles in the Plant Environment. Environ. Sci. Technol. 2013, 47, 4734–4742. [Google Scholar] [CrossRef]
- Wang, X.P.; Li, Q.Q.; Pei, Z.M.; Wang, S.C. Effects of Zinc Oxide Nanoparticles on the Growth, Photosynthetic Traits, and Antioxidative Enzymes in Tomato Plants. Biol. Plant 2018, 62, 801–808. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, S.; Zhong, L.; Zhao, X.; Wang, L. Effects of Zinc Oxide Nanoparticles on Growth, Development, and Flavonoid Synthesis in Ginkgo biloba. Int. J. Mol. Sci. 2023, 24, 15775. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, N.A.; Al-Qurainy, F.; Tarroum, M.; Nadeem, M.; Khan, S.; Salih, A.M.; Shaikhaldein, H.O.; Alfarraj, N.S.; Gaafar, A.-R.Z.; Al-Hashimi, A.; et al. Zinc Oxide Nanoparticles (ZnO NPs), Biosynthesis, Characterization and Evaluation of Their Impact to Improve Shoot Growth and to Reduce Salt Toxicity on Salvia officinalis In Vitro Cultivated. Processes 2022, 10, 1273. [Google Scholar] [CrossRef]
- Zafar, H.; Aziz, T.; Khan, B.; Mannan, A.; Rehman, R.u.; Zia, M. CuO and ZnO Nanoparticle Application in Synthetic Soil Modulates Morphology, Nutritional Contents, and Metal Analysis of Brassica nigra. ACS Omega 2020, 5, 13566–13577. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Mir, A.R.; Hayat, S. Role of Zinc Oxide Nanoparticles in Countering Negative Effects Generated by Cadmium in Lycopersicon esculentum. J. Plant Growth Regul. 2021, 40, 101–115. [Google Scholar] [CrossRef]
- Prasad, T.N.V.K.V.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Raja Reddy, K.; Sreeprasad, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of Nanoscale Zinc Oxide Particles on the Germination, Growth and Yield of Peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of Nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: New York, NY, USA, 2012; pp. 191–248. [Google Scholar] [CrossRef]
- Amer, H.E.A. Green Synthesized ZnO Nanoparticles Improve the Growth and Phytohormones Biosyn-Thesis and Modulate the Expression of Resistance Genes in Phaseolus vulgaris. Egypt. J. Bot. 2023, 64, 145–163. [Google Scholar] [CrossRef]
- Vankova, R.; Landa, P.; Podlipna, R.; Dobrev, P.I.; Prerostova, S.; Langhansova, L.; Gaudinova, A.; Motkova, K.; Knirsch, V.; Vanek, T. ZnO Nanoparticle Effects on Hormonal Pools in Arabidopsis thaliana. Sci. Total Environ. 2017, 593–594, 535–542. [Google Scholar] [CrossRef]
- Restrepo, C.V.; Villa, C.C. Synthesis of Silver Nanoparticles, Influence of Capping Agents, and Dependence on Size and Shape: A Review. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100428. [Google Scholar] [CrossRef]
- Ahangaran, F.; Navarchian, A.H. Recent Advances in Chemical Surface Modification of Metal Oxide Nanoparticles with Silane Coupling Agents: A Review. Adv. Colloid Interface Sci. 2020, 286, 102298. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Huynh Nguyen, T.T.; Tran Pham, B.T.; Van Tran, T.; Bach, L.G.; Bui Thi, P.Q.; Ha Thuc, C.N. Development of Poly (Vinyl Alcohol)/Agar/Maltodextrin Coating Containing Silver Nanoparticles for Banana (Musa acuminate) Preservation. Food Packag. Shelf Life 2021, 29, 100740. [Google Scholar] [CrossRef]
- Medina-Velo, I.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Assessing Plant Uptake and Transport Mechanisms of Engineered Nanomaterials from Soil. MRS Bull. 2017, 42, 379–383. [Google Scholar] [CrossRef]
- Qiu, C.; Qin, Y.; Jiang, S.; Liu, C.; Xiong, L.; Sun, Q. Preparation of Active Polysaccharide-Loaded Maltodextrin Nanoparticles and Their Stability as a Function of Ionic Strength and PH. LWT—Food Sci. Technol. 2017, 76, 164–171. [Google Scholar] [CrossRef]
- Okumuş, E.; Bakkalbaşı, E.; Javidipour, I.; Meral, R.; Ceylan, Z. A Novel Coating Material: Ellagitannins-Loaded Maltodextrin and Lecithin-Based Nanomaterials. Food Biosci. 2021, 42, 101158. [Google Scholar] [CrossRef]
- Martins, N.C.T.; Avellan, A.; Rodrigues, S.; Salvador, D.; Rodrigues, S.M.; Trindade, T. Composites of Biopolymers and ZnO NPs for Controlled Release of Zinc in Agricultural Soils and Timed Delivery for Maize. ACS Appl. Nano Mater. 2020, 3, 2134–2148. [Google Scholar] [CrossRef]
- Joye, I.J.; Nelis, V.A.; McClements, D.J. Gliadin-Based Nanoparticles: Stabilization by Post-Production Polysaccharide Coating. Food Hydrocoll. 2015, 43, 236–242. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Joshi, A.S.; Perumalsamy, H.; Mijakovic, I.; Singh, P. Advancing Sustainable Agriculture: A Critical Review of Smart and Eco-Friendly Nanomaterial Applications. J. Nanobiotechnol. 2023, 21, 372. [Google Scholar] [CrossRef] [PubMed]
- Murgueitio-Herrera, E.; Falconí, C.E.; Cumbal, L.; Gómez, J.; Yanchatipán, K.; Tapia, A.D.; Martínez, K.; Sinde-Gonzalez, I.; Toulkeridis, T. Synthesis of Iron, Zinc, and Manganese Nanofertilizers, Using Andean Blueberry Extract, and Their Effect in the Growth of Cabbage and Lupin Plants. Nanomaterials 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, H.; Singh, S.K.; Mahajan, S.; Kishor, S.; Maurya, D.; Kumar, V. Efficacy of Mineral Nutrients and Nanomaterials on the Productivity of Capsicum (Capsicum annuum L. Cv. Rani) under Polyhouse. J. Appl. Biol. Biotechnol. 2023, 11, 206–212. [Google Scholar] [CrossRef]
- Ridolfo, R.; Tavakoli, S.; Junnuthula, V.; Williams, D.S.; Urtti, A.; Van Hest, J.C.M. Exploring the Impact of Morphology on the Properties of Biodegradable Nanoparticles and Their Diffusion in Complex Biological Medium. Biomacromolecules 2021, 22, 126–133. [Google Scholar] [CrossRef]
- Mazaheri Tirani, M.; Madadkar Haghjou, M.; Ismaili, A. Hydroponic Grown Tobacco Plants Respond to Zinc Oxide Nanoparticles and Bulk Exposures by Morphological, Physiological and Anatomical Adjustments. Funct. Plant Biol. 2019, 46, 360–375. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of Nanoparticles: Inhibition of Seed Germination and Root Growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C. ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Zhao, L.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Bandyopadhyay, S.; Peng, B.; Munoz, B.; Keller, A.A.; Gardea-Torresdey, J.L. ZnO Nanoparticle Fate in Soil and Zinc Bioaccumulation in Corn Plants (Zea mays) Influenced by Alginate. Environ. Sci. Process. Impacts 2013, 15, 260–266. [Google Scholar] [CrossRef]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Responses of Soybean (Glycine max [L.] Merr.) to Zinc Oxide Nanoparticles: Understanding Changes in Root System Architecture, Zinc Tissue Partitioning and Soil Characteristics. Sci. Total Environ. 2022, 835, 155348. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kumar, S.; Alok, A.; Upadhyay, S.K.; Rawat, M.; Tsang, D.C.W.; Bolan, N.; Kim, K.H. The Potential of Green Synthesized Zinc Oxide Nanoparticles as Nutrient Source for Plant Growth. J. Clean. Prod. 2019, 214, 1061–1070. [Google Scholar] [CrossRef]
- Zhao, L.; Peralta-Videa, J.R.; Rico, C.M.; Hernandez-Viezcas, J.A.; Sun, Y.; Niu, G.; Servin, A.; Nunez, J.E.; Duarte-Gardea, M.; Gardea-Torresdey, J.L. CeO2 and ZnO Nanoparticles Change the Nutritional Qualities of Cucumber (Cucumis sativus). J. Agric. Food Chem. 2014, 62, 2752–2759. [Google Scholar] [CrossRef] [PubMed]
- Burman, U.; Saini, M.; Kumar, P. Effect of Zinc Oxide Nanoparticles on Growth and Antioxidant System of Chickpea Seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Wu, F.; Fang, Q.; Yan, S.; Pan, L.; Tang, X.; Ye, W. Effects of Zinc Oxide Nanoparticles on Arsenic Stress in Rice (Oryza sativa L.): Germination, Early Growth, and Arsenic Uptake. Environ. Sci. Pollut. Res. 2020, 27, 26974–26981. [Google Scholar] [CrossRef]
- Pérez Velasco, E.A.; Galindo, R.B.; Valdez Aguilar, L.A.; González Fuentes, J.A.; Puente Urbina, B.A.; Lozano Morales, S.A.; Valdés, S.S. Effects of the Morphology, Surface Modification and Application Methods of ZnO-NPs on the Growth and Biomass of Tomato Plants. Molecules 2020, 25, 1282. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Gautam, K.; Singh, H.; Sinha, A.K. Nanotechnology in Plant Nanobionics: Mechanisms, Applications, and Future Perspectives. Adv. Biol. 2025, 9, e2400589. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, R.; Chen, Z.; Cui, P.; Lu, H.; Yang, Y.; Zhang, H. The Effect of Zinc Oxide Nanoparticles for Enhancing Rice (Oryza sativa L.) Yield and Quality. Agriculture 2021, 11, 1247. [Google Scholar] [CrossRef]
- Ponce-García, C.O.; Soto-Parra, J.M.; Sánchez, E.; Muñoz-Márquez, E.; Piña-Ramírez, F.J.; Flores-Córdova, M.A.; Pérez-Leal, R.; Muñoz, R.M.Y. Efficiency of Nanoparticle, Sulfate, and Zinc-Chelate Use on Biomass, Yield, and Nitrogen Assimilation in Green Beans. Agronomy 2019, 9, 128. [Google Scholar] [CrossRef]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative Effect of ZnO NPs, ZnO Bulk and ZnSO4 in the Antioxidant Defences of Two Plant Species Growing in Two Agricultural Soils under Greenhouse Conditions. Sci. Total Environ. 2017, 589, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yuan, H.; Ji, H.; Liu, H.; Zhang, Y.; Wang, G.; Chen, L.; Guo, Z. Effect of ZnO Nanoparticles on the Productivity, Zn Biofortification, and Nutritional Quality of Rice in a Life Cycle Study. Plant Physiol. Biochem. 2021, 163, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Tiwari, S.; Pandey, J.; Lata, C.; Singh, I.K. Role of Nanoparticles in Crop Improvement and Abiotic Stress Management. J. Biotechnol. 2021, 337, 57–70. [Google Scholar] [CrossRef]
- Azim, Z.; Singh, N.B.; Singh, A.; Amist, N.; Niharika; Khare, S.; Yadav, R.K.; Bano, C.; Yadav, V. A Review Summarizing Uptake, Translocation and Accumulation of Nanoparticles within the Plants: Current Status and Future Prospectus. J. Plant Biochem. Biotechnol. 2023, 32, 211–224. [Google Scholar] [CrossRef]
- Zhu, J.; Li, J.; Shen, Y.; Liu, S.; Zeng, N.; Zhan, X.; White, J.C.; Gardea-Torresdey, J.; Xing, B. Mechanism of Zinc Oxide Nanoparticle Entry into Wheat Seedling Leaves. Environ. Sci. Nano 2020, 7, 3901–3913. [Google Scholar] [CrossRef]
- Ha, N.; Seo, E.; Kim, S.; Lee, S.J. Adsorption of Nanoparticles Suspended in a Drop on a Leaf Surface of Perilla frutescens and Their Infiltration through Stomatal Pathway. Sci. Rep. 2021, 11, 11556. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Rizwan, M.; Zia ur Rehman, M.; Javed, M.R.; Imran, M.; Chatha, S.A.S.; Nazir, R. Zinc Oxide Nanoparticles Alter the Wheat Physiological Response and Reduce the Cadmium Uptake by Plants. Environ. Pollut. 2018, 242, 1518–1526. [Google Scholar] [CrossRef]
- Palacio-Márquez, A.; Ramírez-Estrada, C.A.; Gutiérrez-Ruelas, N.J.; Sánchez, E.; Barrios, D.L.O.; Chávez-Mendoza, C.; Sida-Arreola, J.P. Efficiency of Foliar Application of Zinc Oxide Nanoparticles versus Zinc Nitrate Complexed with Chitosan on Nitrogen Assimilation, Photosynthetic Activity, and Production of Green Beans (Phaseolus vulgaris L.). Sci. Hortic. 2021, 288, 110297. [Google Scholar] [CrossRef]
- Faizan, M.; Bhat, J.A.; Hessini, K.; Yu, F.; Ahmad, P. Zinc Oxide Nanoparticles Alleviates the Adverse Effects of Cadmium Stress on Oryza sativa via Modulation of the Photosynthesis and Antioxidant Defense System. Ecotoxicol. Environ. Saf. 2021, 220, 112401. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Ahmad, A.; Battaglia, M.L.; Bilal, H.M.; Alhammad, B.A.; Khan, N. Zinc Oxide Nanoparticles: A Unique Saline Stress Mitigator with the Potential to Increase Future Crop Production. S. Afr. J. Bot. 2023, 159, 208–218. [Google Scholar] [CrossRef]
- Chen, H.; Song, Y.; Wang, Y.; Wang, H.; Ding, Z.; Fan, K. Zno Nanoparticles: Improving Photosynthesis, Shoot Development, and Phyllosphere Microbiome Composition in Tea Plants. J. Nanobiotechnol. 2024, 22, 389. [Google Scholar] [CrossRef]
- Ahmad, P.; Alyemeni, M.N.; Al-Huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Kaya, C.; Bajguz, A. Zinc Oxide Nanoparticles Application Alleviates Arsenic (As) Toxicity in Soybean Plants by Restricting the Uptake of as and Modulating Key Biochemical Attributes, Antioxidant Enzymes, Ascorbate-Glutathione Cycle and Glyoxalase System. Plants 2020, 9, 825. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Khan, M.T.; Abbasi, A.; Haq, I.U.; Hina, A.; Mohiuddin, M.; Tariq, M.A.U.R.; Afzal, M.Z.; Zaman, Q.u.; Ng, A.W.M.; et al. Characterizing Stomatal Attributes and Photosynthetic Induction in Relation to Biochemical Changes in Coriandrum sativum L. by Foliar-Applied Zinc Oxide Nanoparticles under Drought Conditions. Front. Plant Sci. 2023, 13, 1079283. [Google Scholar] [CrossRef]
- Ghani, M.I.; Saleem, S.; Rather, S.A.; Rehmani, M.S.; Alamri, S.; Rajput, V.D.; Kalaji, H.M.; Saleem, N.; Sial, T.A.; Liu, M. Foliar Application of Zinc Oxide Nanoparticles: An Effective Strategy to Mitigate Drought Stress in Cucumber Seedling by Modulating Antioxidant Defense System and Osmolytes Accumulation. Chemosphere 2022, 289, 133202. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Baninasab, B.; Ghobadi, C.; Khoshgoftarmanesh, A.H. Zinc Soil Application Enhance Photosynthetic Capacity and Antioxidant Enzyme Activities in Almond Seedlings Affected by Salinity Stress. Photosynthetica 2016, 54, 267–274. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.T.; Hayat, S. Zinc Oxide Nanoparticle-Mediated Changes in Photosynthetic Efficiency and Antioxidant System of Tomato Plants. Photosynthetica 2018, 56, 678–686. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Chen, S.; Li, Q.; Wang, W.; Hou, C.; Gao, X.; Wang, L.; Wang, S. Zinc Oxide Nanoparticles Affect Biomass Accumulation and Photosynthesis in Arabidopsis. Front. Plant Sci. 2016, 6, 1243. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Ahmad, A.; Alhammad, B.A.; Tola, E. Exogenous Application of Zinc Oxide Nanoparticles Improved Antioxidants, Photosynthetic, and Yield Traits in Salt-Stressed Maize. Agronomy 2023, 13, 2645. [Google Scholar] [CrossRef]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, A.; Ram, S.; Kumar, R.; Singh, V.; Singh, V.; Salar, A.; Durgude, S.A. Synthesis and Characterization of Chitosan Encapsulated Zinc Oxide Nanoparticles and Its Application in Maize under Zinc Deficit Soil. Int. J. Plant Soil. Sci. 2024, 36, 393–401. [Google Scholar] [CrossRef]
- Faizan, M.; Sehar, S.; Rajput, V.D.; Faraz, A.; Afzal, S.; Minkina, T.; Sushkova, S.; Adil, M.F.; Yu, F.; Alatar, A.A.; et al. Modulation of Cellular Redox Status and Antioxidant Defense System after Synergistic Application of Zinc Oxide Nanoparticles and Salicylic Acid in Rice (Oryza sativa) Plant under Arsenic Stress. Plants 2021, 10, 2254. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Zia ur Rehman, M.; Adrees, M.; Arshad, M.; Qayyum, M.F.; Ali, L.; Hussain, A.; Chatha, S.A.S.; Imran, M. Alleviation of Cadmium Accumulation in Maize (Zea mays L.) by Foliar Spray of Zinc Oxide Nanoparticles and Biochar to Contaminated Soil. Environ. Pollut. 2019, 248, 358–367. [Google Scholar] [CrossRef]





| Shoot Height (cm) | Stem Diameter (mm) | Root Length (mm) | Root DW (g) | Stem DW (g) | Leaf DW (g) | Total DW (g) | ||
|---|---|---|---|---|---|---|---|---|
| Morphology ZnO-NPs | ||||||||
| Spherical | 29.73 a | 3.36 a | 65.64 a | 0.70 | 0.76 | 0.98 a | 2.44 a | |
| Hexagonal | 25.42 b | 3.15 b | 63.28 b | 0.69 | 0.75 | 0.83 b | 2.28 b | |
| ANOVA | <0.001 | <0.001 | <0.001 | 0.817 | 0.294 | <0.001 | <0.001 | |
| MD-Coated | ||||||||
| Without | 28.46 a | 3.34 a | 65.16 a | 0.68 b | 0.75 | 0.81 b | 2.25 b | |
| With | 26.68 b | 3.17 b | 63.76 b | 0.71 a | 0.76 | 1.00 a | 2.48 a | |
| ANOVA | <0.001 | <0.001 | 0.005 | <0.001 | 0.1148 | <0.001 | <0.001 | |
| Concentration (mg L−1) | ||||||||
| 0 | 17.80 e | 2.52 g | 51.40 f | 0.63 b | 0.67 c | 0.73 c | 2.02 d | |
| 250 | 30.50 ab | 3.43 bc | 68.25 ab | 0.68 ab | 0.77 ab | 0.93 ab | 2.38 bc | |
| 500 | 31.25 a | 3.80 a | 65.65 bcde | 0.73 a | 0.77 ab | 0.96 a | 2.47 ab | |
| 750 | 29.65 bc | 3.39 cd | 63.30 e | 0.69 a | 0.78 ab | 0.96 a | 2.44 ab | |
| 1000 | 30.70 ab | 3.56 b | 67.70 abc | 0.7 a | 0.77 ab | 0.93 ab | 2.42 ab | |
| 1250 | 26.20 d | 3.27 cde | 66.05 abcd | 0.68 ab | 0.74 b | 0.87 b | 2.29 c | |
| 1500 | 25.85 c | 3.12 e | 68.30 a | 0.72 a | 0.80 a | 0.95 a | 2.48 a | |
| 1750 | 26.20 d | 3.25 de | 65.15 cde | 0.73 a | 0.74 b | 0.91 ab | 2.38 bc | |
| 2000 | 27.05 d | 2.95 f | 64.40 de | 0.7 a | 0.75 ab | 0.92 ab | 2.37 bc | |
| ANOVA | <0.001 | <0.001 | 0.034 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Interactions | ||||||||
| M × MD | <0.001 | <0.001 | <0.001 | 0.0615 | 0.6775 | <0.001 | <0.001 | |
| M × C | <0.001 | <0.001 | <0.001 | 0.0272 | 0.1134 | <0.001 | <0.001 | |
| MD × C | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| M × MD × C | <0.001 | <0.001 | <0.001 | <0.001 | =0.011 | <0.001 | <0.001 | |
| Photosynthetic Rate (μmol CO2 m−2 s−1) | Stomatic Conductance (mol H2O m−2 s−1) | Internal CO2 Concentration (μmol mol−1) | Transpiration Rate (mmol H2O m−2 s−1) | SPAD Units | |
|---|---|---|---|---|---|
| Morphology ZnO-NPs | |||||
| Spherical | 11.28 | 0.37 | 304.1 | 7.7 b | 51.5 a |
| Hexagonal | 11.50 | 0.36 | 305.7 | 8.1 a | 50.4 b |
| ANOVA | 0.5579 | 0.2858 | 0.5098 | 0.0088 | 0.0011 |
| MD-coated | |||||
| Without | 11.46 | 0.32 b | 297.6 b | 7.6 b | 50.5 b |
| With | 11.33 | 0.40 a | 312.3 a | 8.2 a | 51.4 a |
| ANOVA | 0.7349 | <0.001 | <0.001 | 0.0002 | 0.0031 |
| Concentration (mg L−1) | |||||
| 0 | 6.86 d | 0.31 c | 244.6 e | 3.9 d | 40.4 e |
| 250 | 9.56 c | 0.28 c | 311.4 bc | 7.83 c | 49.8 d |
| 500 | 11.76 abc | 0.43 a | 319.9 b | 9.18 ab | 52.0 c |
| 750 | 11.54 abc | 0.32 bc | 296.1 cd | 7.55 c | 56.0 ab |
| 1000 | 13.16 bc | 0.42 ab | 363.9 a | 8.19 bc | 56.8 a |
| 1250 | 12.74 ab | 0.31 c | 301.3 cd | 9.34 a | 49.2 d |
| 1500 | 13.96 a | 0.41 ab | 308.2 bc | 9.11 ab | 54.2 b |
| 1750 | 10.60 bc | 0.35 abc | 290.2 d | 7.77 c | 51.0 cd |
| 2000 | 12.34 ab | 0.43 a | 308.6 bc | 8.31 bc | 49.0 d |
| ANOVA | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Interactions | |||||
| M × MD | <0.3411 | <0.001 | <0.001 | <0.001 | <0.001 |
| M × C | <0.5487 | <0.001 | <0.001 | <0.001 | <0.001 |
| MD × C | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| M × MD × C | <0.5966 | <0.001 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velasco, E.A.P.; Valdez-Aguilar, L.A.; Galindo, R.B.; Palomino, A.B.; Urbina, B.A.P. Morphology and Coating of ZnO Nanoparticles Affect Growth and Gas Exchange Parameters of Bell Pepper Seedlings. Agronomy 2025, 15, 1579. https://doi.org/10.3390/agronomy15071579
Velasco EAP, Valdez-Aguilar LA, Galindo RB, Palomino AB, Urbina BAP. Morphology and Coating of ZnO Nanoparticles Affect Growth and Gas Exchange Parameters of Bell Pepper Seedlings. Agronomy. 2025; 15(7):1579. https://doi.org/10.3390/agronomy15071579
Chicago/Turabian StyleVelasco, Eneida A. Pérez, Luis A. Valdez-Aguilar, Rebeca Betancourt Galindo, Adolfo Baylon Palomino, and Bertha A. Puente Urbina. 2025. "Morphology and Coating of ZnO Nanoparticles Affect Growth and Gas Exchange Parameters of Bell Pepper Seedlings" Agronomy 15, no. 7: 1579. https://doi.org/10.3390/agronomy15071579
APA StyleVelasco, E. A. P., Valdez-Aguilar, L. A., Galindo, R. B., Palomino, A. B., & Urbina, B. A. P. (2025). Morphology and Coating of ZnO Nanoparticles Affect Growth and Gas Exchange Parameters of Bell Pepper Seedlings. Agronomy, 15(7), 1579. https://doi.org/10.3390/agronomy15071579








