Film-Forming and Metabolic Antitranspirants Reduce Potato Drought Stress and Tuber Physiological Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Environment and Planting Materials
2.2. Experimental Design
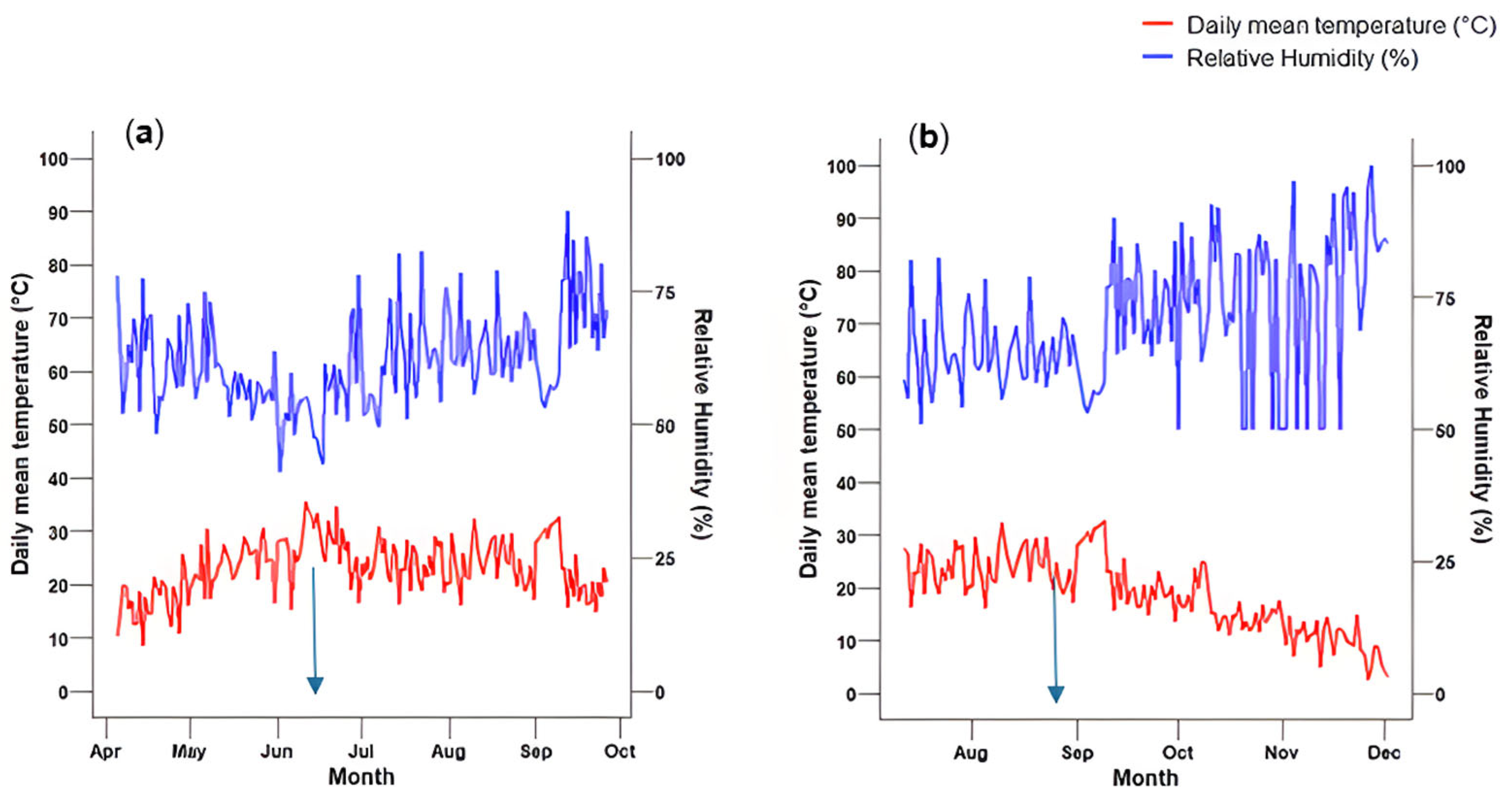
2.3. Monitoring Temperature and Humidity with Tinytag Data Logger
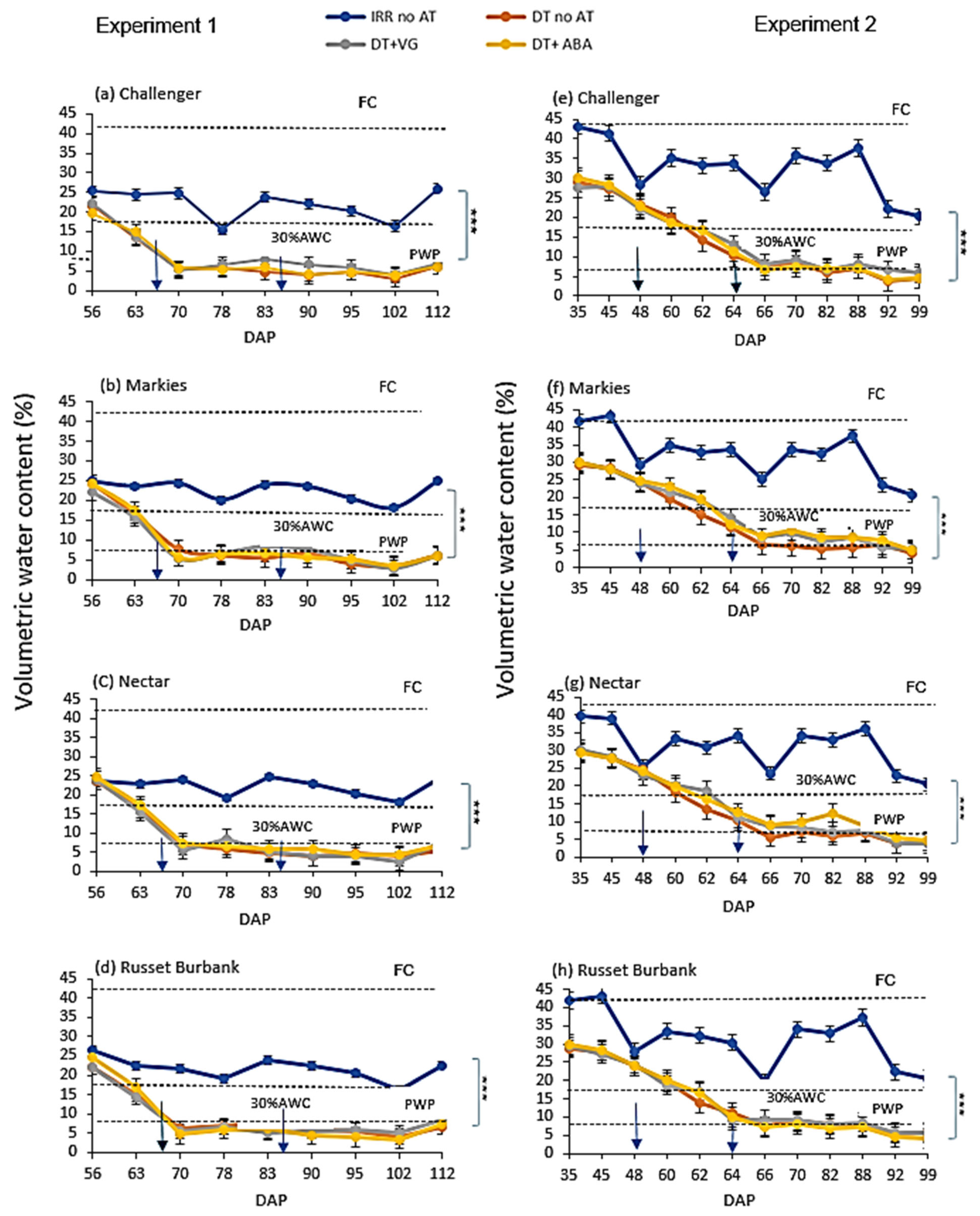
2.4. Soil Moisture Measurements, Irrigation Regimes, and Drought Imposition
| Experiment 1 | Experiment 2 | |||||
|---|---|---|---|---|---|---|
| Week | Week | Stage | DAP at Drought Initiation | % of AWC | AT | Assessments |
| 1–4 | 1–2 | Establishment | - | 100% | - | - |
| 5–8 | 3–6 | Stolon initiation | - | 100% | - | - |
| 9–13 | 6–10 | Tuber initiation | 56 (Exp 1) 35 (Exp 2) | Dry-down | - | Porometer |
| 11 (June 22) | 8 (Sept. 2) | - | 30% | Spray 1 | Porometer | |
| 12–13 | 10 | Tuber initiation | 30% | Spray 2 | Porometer | |
| 14–19 | 11–16 | Tuber filling | 30% | RWC | ||
| 20–24 | 17–20 | Maturity | 0% | - | ||
| 25 | 21 | Maturity | 0% | - | Harvest |
2.5. Antitranspirant Application
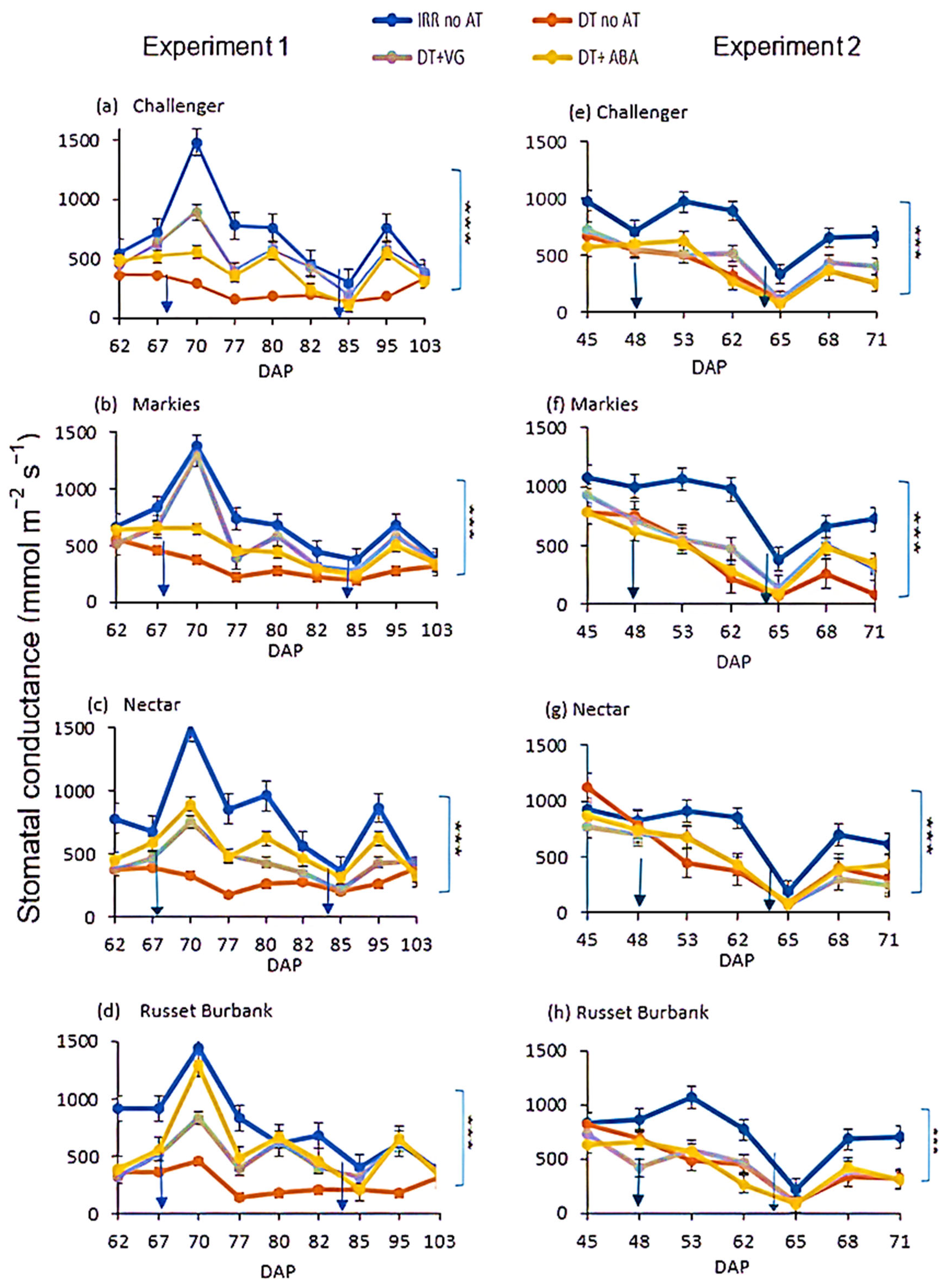
2.6. Stomatal Conductance Measurements
2.7. The Relative Water Content (RWC) of the Leaves
2.8. Yield Component Analysis and Potato Physiological Disorder Assessment
2.9. Statistical Analysis
3. Results
3.1. Temperature and Relative Humidity
3.2. Volumetric Water Content (VWC)
3.3. Stomatal Conductance
3.4. Relative Water Content (RWC)
3.5. Yield
3.6. Potato Physiological Disorders
4. Discussion
4.1. Effect of Water Stress and AT Application on RWC
4.2. Water Stress Depressed Stomatal Conductance
4.3. AT Application Effects Under Water Stress on Stomatal Conductance
4.4. Effect of Water Stress and AT Application on Potato Yield
4.5. Effect of Water Stress and AT Application on Physiological Disorders
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Global Potato Statistics: Latest FAO Data Published. Potato News Today. Published Online 21 January 2023. Available online: https://www.potatonewstoday.com/2023/01/21/global-potato-statistics-latest-fao-data-published/ (accessed on 2 June 2024).
- International Potato Center. CIP Annual Report 2022. Catalyzing Change: Food Systems for Resilience, Prosperity, and Health; International Potato Center: Lime, Peru, 2023; Available online: https://cgspace.cgiar.org/items/0c1203cf-3024-43be-a3df-3a3f448d3761 (accessed on 2 June 2024).
- Nasir, M.W.; Toth, Z. Effect of Drought Stress on Potato Production: A Review. Agronomy 2022, 12, 635. [Google Scholar] [CrossRef]
- Fleisher, D.H.; Condori, B.; Quiroz, R.; Alva, A.; Asseng, S.; Barreda, C.; Bindi, M.; Boote, K.J.; Ferrise, R.; Franke, A.C.; et al. A Potato Model Intercomparison across Varying Climates and Productivity Levels. Glob. Change Biol. 2017, 23, 1258–1281. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Li, Y.; Chen, R.; Rui, M.; Wang, Y. Stomatal Responses of Two Drought-Tolerant Barley Varieties with Different ROS Regulation Strategies under Drought Conditions. Antioxidants 2023, 12, 790. [Google Scholar] [CrossRef]
- Abid, G.; Jebara, M.; Debode, F.; Vertommen, D.; dit Ruys, S.P.; Ghouili, E.; Jebara, S.H.; Ouertani, R.N.; El Ayed, M.; de Oliveira, A.C.; et al. Comparative Physiological, Biochemical and Proteomic Analyses Reveal Key Proteins and Crucial Regulatory Pathways Related to Drought Stress Tolerance in Faba Bean (Vicia faba L.) Leaves. Curr. Plant Biol. 2024, 37, 100320. [Google Scholar] [CrossRef]
- Kumar, P.; Ginzberg, I. Potato Periderm Development and Tuber Skin Quality. Plants 2022, 11, 2099. [Google Scholar] [CrossRef]
- Jiang, M.; Shinners-Carnelley, T.; Gibson, D.; Jones, D.; Joshi, J.; Wang-Pruski, G. Irrigation Effect on Yield, Skin Blemishes, Phellem Formation, and Total Phenolics of Red Potatoes. Plants 2022, 11, 3523. [Google Scholar] [CrossRef]
- Hajjar, G.; Quellec, S.; Pépin, J.; Challois, S.; Joly, G.; Deleu, C.; Leport, L.; Musse, M. MRI Investigation of Internal Defects in Potato Tubers with Particular Attention to Rust Spots Induced by Water Stress. Postharvest Biol. Technol. 2021, 180, 111600. [Google Scholar] [CrossRef]
- Hoidal, N. Potatoes: Post-Harvest Disorders and Handling. Fruits and Vegetable News. University of Minnesota Extension. 2023. Available online: https://blog-fruit-vegetable-ipm.extension.umn.edu/2019/10/potatoes-post-harvest-disorders-and.html (accessed on 2 June 2024).
- Mphande, W.; Kettlewell, P.S.; Grove, I.G.; Farrell, A.D. The Potential of Antitranspirants in Drought Management of Arable Crops: A Review. Agric. Water Manag. 2020, 236, 106143. [Google Scholar] [CrossRef]
- Byari, S.H. The Effects of Three Antitranspirants on Yield and Transpiration of Potato Cultivars. J. King Abdulaziz Univ. Meteorol. Environ. Arid Land Agric. Sci. 1991, 2, 55–64. [Google Scholar]
- Abdel-Rehem, N.A.; Abido, A.I.A.; Abdel-Nasser, G.; Gaber, M.M. Impact of Irrigation Deficit, Soil Conditioner and Antitranspirant on Yield and Quality of Potato. Alex. J. Soil Water Sci. 2019, 3, 20–35. [Google Scholar] [CrossRef]
- Lakshmi, M.; Korav, S.; Banik, B.; Changade, N.; Sharma, V. Advancements in Understanding the Mechanisms and Applications of Anti-Transpirants in Crop Production and Stress Management: A Review. Int. J. Res. Agron. 2025, 8, 499–506. [Google Scholar] [CrossRef]
- Monnem, A.; Khalel, S. Effect of Drip Irrigation Intervals and Some Antitranspirants on the Water Status, Growth and Yield of Potato (Solanum tuberosum L.). J. Agric. Sci. Technol. B 2015, 5, 15–23. [Google Scholar]
- AbdAllah, A. Impacts of Kaolin and Pinoline Foliar Application on Growth, Yield and Water Use Efficiency of Tomato (Solanum lycopersicum L.) Grown under Water Deficit: A Comparative Study. J. Saudi Soc. Agric. Sci. 2019, 18, 256–268. [Google Scholar] [CrossRef]
- Mphande, W.; Farrell, A.D.; Grove, I.G.; Vickers, L.H.; Kettlewell, P.S. Metabolic and Film Antitranspirants Both Reduce Drought Damage to Wheat Yield despite Having Contrasting Effects on Leaf ABA. J. Agron. Crop Sci. 2022, 208, 143–157. [Google Scholar] [CrossRef]
- Shinohara, T.; Leskovar, D.I. Effects of ABA, Antitranspirants, Heat and Drought Stress on Plant Growth, Physiology and Water Status of Artichoke Transplants. Sci. Hortic. 2014, 165, 225–234. [Google Scholar] [CrossRef]
- Travaglia, C.; Reinoso, H.; Cohen, A.; Luna, C.; Tommasino, E.; Castillo, C.; Bottini, R. Exogenous ABA Increases Yield in Field-Grown Wheat with Moderate Water Restriction. J. Plant Growth Regul. 2010, 29, 366–374. [Google Scholar] [CrossRef]
- Faralli, M.; Grove, I.G.; Hare, M.C.; Boyle, R.D.; Williams, K.S.; Corke, F.M.K.; Kettlewell, P.S. Canopy Application of Film Antitranspirants over the Reproductive Phase Enhances Yield and Yield-Related Physiological Traits of Water-Stressed Oilseed Rape (Brassica napus). Crop Pasture Sci. 2016, 67, 751–765. [Google Scholar] [CrossRef]
- Win, K.; Berkowitz, G.A.; Henninger, M. Antitranspirant-Induced Increases in Leaf Water Potential Increase Tuber Calcium and Decrease Tuber Necrosis in Water-Stressed Potato Plants. Plant Physiol. 1991, 96, 116–120. [Google Scholar] [CrossRef]
- Fahey, D.J.; Rogiers, S.Y. Di-1-p-Menthene Reduces Grape Leaf and Bunch Transpiration. Aust. J. Grape Wine Res. 2019, 25, 134–141. [Google Scholar] [CrossRef]
- Palliotti, A.; Tombesi, S.; Silvestroni, O.; Lanari, V.; Gatti, M.; Poni, S. Changes in Vineyard Establishment and Canopy Management Urged by Earlier Climate-Related Grape Ripening: A Review. Sci. Hortic. 2014, 178, 43–54. [Google Scholar] [CrossRef]
- Miller Vapor Gard. Product Overview. A Huber Company. Available online: https://www.millerchemical.com/products/crop-production-aids/vapor-gard/ (accessed on 2 June 2025).
- Valent BioSciences. ProTone® Plant Growth Regulator: S-Abscisic Acid. Available online: https://www.valentbiosciences.com/agriculture/products/protone/ (accessed on 2 June 2025).
- Ellis, G.D.; Knowles, L.O.; Knowles, N.R. Developmental and Postharvest Physi ological Phenotypes of Engineered Potatoes (Solanum tuberosum L.) Grown in the Columbia Basin. Field Crops Res. 2020, 250, 107775. [Google Scholar] [CrossRef]
- Ifeduba, A.M.; Gautam, S.; Pandey, J.; Toinga-Villafuerte, S.E.; Scheuring, D.C.; Koym, J.W.; Vales, M.I. Early Tuberization: A Heat Stress Escape Strategy in the Fresh Market Potato Variety Vanguard Russet. Am. J. Potato Res. 2024, 101, 414–432. [Google Scholar] [CrossRef]
- HZPC Potato Variety Database: Challenger. Available online: https://www.hzpc.com/our-potato-varieties/challenger (accessed on 2 June 2025).
- IPM Potato Group Sustainable Robust: Nectar. Available online: https://ipmpotato.com/varieties/nectar/ (accessed on 2 June 2025).
- AGRICO Potato Variety Database: Markies. Available online: https://www.agrico.co.uk/uk-varieties/markies (accessed on 2 June 2025).
- Saeed, H.; Grove, I.G.; Kettlewell, P.S.; Hall, N.W. Potential of Partial Rootzone Drying as an Alternative Irrigation Technique for Potatoes (Solanum tuberosum). Ann. Appl. Biol. 2008, 152, 71–80. [Google Scholar] [CrossRef]
- Wheeler, R.M.; Fitzpatrick, A.H.; Tibbitts, T.W. Potatoes as a Crop for Space Life Support: Effect of CO2, Irradiance, and Photoperiod on Leaf Photosynthesis and Stomatal Conductance. Front. Plant Sci. 2019, 10, 1632. [Google Scholar] [CrossRef]
- Chandra, S.P.; Adiga, V.; Praveen, C.; Venkatesh Kikkeri, V.; Senapati, S. Modulation of Stomatal Conductance in Response to Changes in External Factors for Plants Grown in the Tropical Climate. J. High Sch. Sci. 2023, 7, 1–17. [Google Scholar]
- Barrs, H.D.; Weatherleyt, P.E. A Re-Examination of the Relative Turgidity Technique for Estimating Water Deficits in Leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- AHDB. Potato Defect Identification. Available online: https://potatoes.ahdb.org.uk/knowledge-library/potato-disease-identification (accessed on 26 September 2024).
- NDSU. Potato Tuber Second Growth. Available online: https://www.ndsu.edu/agriculture/extension/publications/potato-tuber-second-growth (accessed on 23 September 2024).
- Kettlewell, P.S.; Heath, W.L.; Haigh, I.M. Yield Enhancement of Droughted Wheat by Film Antitranspirant Application: Rationale and Evidence. Agric. Sci. 2010, 1, 143–147. [Google Scholar] [CrossRef][Green Version]
- Kocięcka, J.; Liberacki, D.; Stróżecki, M. The Role of Antitranspirants in Mitigating Drought Stress in Plants of the Grass Family (Poaceae)—A Review. Sustainability 2023, 15, 9165. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Bryan, G.J.; Jones, H.G.; Prashar, A. Coping with Drought: Stress and Adaptive Responses in Potato and Perspectives for Improvement. Front. Plant Sci. 2015, 6, 542. [Google Scholar] [CrossRef]
- Mikiciuk, G.; Mikiciuk, M.; Ptak, P. The Effects of Anitranspirant Di-1-p-Menthene on Some Physiological Traits of Strawberry. J. Ecol. Eng. 2015, 16, 161–167. [Google Scholar] [CrossRef]
- Samy, M.M. Differential Response of Some Potato Varieties Grown under Drought Conditions. J. Appl. Hortic. 2021, 23, 310–317. [Google Scholar] [CrossRef]
- Xiang, J.; Hare, M.C.; Vickers, L.H.; Kettlewell, P.S. A Comparative Study on Rapeseed Sprayed with Film Antitranspirant Under Two Contrasting Rates of Soil Water Depletion. Agronomy 2024, 14, 2944. [Google Scholar] [CrossRef]
- Mphande, W.; Farrell, A.D.; Vickers, L.H.; Grove, I.G.; Kettlewell, P.S. Yield Improvement with Antitranspirant Application in Droughted Wheat Associated with Both Reduced Transpiration and Reduced Abscisic Acid. J. Agric. Sci. 2024, 162, 33–45. [Google Scholar] [CrossRef]
- Moroni, F.J.; Gascon-Aldana, P.J.; Rogiers, S.Y. Characterizing the Efficacy of a Film-Forming Antitranspirant on Raspberry Foliar and Fruit Transpiration. Biology 2020, 9, 255. [Google Scholar] [CrossRef] [PubMed]
- de Godoi, R.G.P.; Kettlewell, P.S. Applying Sunflower Oil to Rapeseed Plants Reduces Water Loss. J. Sci. Food Agric. 2023, 103, 7941–7943. [Google Scholar] [CrossRef]
- Martínez, J.P.; Silva, H.; Ledent, J.F.; Pinto, M. Effect of Drought Stress on the Osmotic Adjustment, Cell Wall Elasticity and Cell Volume of Six Cultivars of Common Beans (Phaseolus vulgaris L.). Eur. J. Agron. 2007, 26, 30–38. [Google Scholar] [CrossRef]
- Shweta, V.G. Antitranspirants: An Effective Approach to Mitigate the Stress in Field Crops. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1671–1678. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Shen, Q.; Yang, J.; Han, X.; Tian, F.; Wu, J. Effects of Water Stress on Photosynthesis, Yield, and Water Use Efficiency in Winter Wheat. Water 2020, 12, 2127. [Google Scholar] [CrossRef]
- Gervais, T.; Creelman, A.; Li, X.Q.; Bizimungu, B.; De Koeyer, D.; Dahal, K. Potato Response to Drought Stress: Physiological and Growth Basis. Front. Plant Sci. 2021, 12, 698060. [Google Scholar] [CrossRef]
- Romero, A.P.; Alarcón, A.; Valbuena, R.I.; Galeano, C.H. Physiological Assessment of Water Stress in Potato Using Spectral Information. Front. Plant Sci. 2017, 8, 1608. [Google Scholar] [CrossRef]
- Wankmüller, F.J.P.; Carminati, A. Stomatal Regulation Prevents Plants from Critical Water Potentials during Drought: Result of a Model Linking Soil–Plant Hydraulics to Abscisic Acid Dynamics. Ecohydrology 2022, 15, e2386. [Google Scholar] [CrossRef]
- Zenes, N.; Kerr, K.L.; Trugman, A.T.; Anderegg, W.R.L. Competition and Drought Alter Optimal Stomatal Strategy in Tree Seedlings. Front. Plant Sci. 2020, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- Rudack, K.; Seddig, S.; Sprenger, H.; Köhl, K.; Uptmoor, R.; Ordon, F. Drought Stress-Induced Changes in Starch Yield and Physiological Traits in Potato. J. Agron. Crop Sci. 2017, 203, 494–505. [Google Scholar] [CrossRef]
- Topbjerg, H.B.; Kaminski, K.P.; Kørup, K.; Nielsen, K.L.; Kirk, H.G.; Andersen, M.N.; Liu, F. Screening for Intrinsic Water Use Efficiency in a Potato Dihaploid Mapping Population under Progressive Drought Conditions. Acta Agric. Scand. B Soil. Plant Sci. 2015, 65, 400–411. [Google Scholar] [CrossRef]
- Liu, T.; Dong, L.; Wang, E.; Liu, S.; Cheng, Y.; Zhao, J.; Xu, S.; Liang, Z.; Ma, H.; Nie, B.; et al. StHAB1, a Negative Regulatory Factor in Abscisic Acid Signaling, Plays Crucial Roles in Potato Drought Tolerance and Shoot Branching. J. Exp. Bot. 2023, 74, 6708–6721. [Google Scholar] [CrossRef]
- Faralli, M.; Williams, K.S.; Han, J.; Corke, F.M.K.; Doonan, J.H.; Kettlewell, P.S. Water-Saving Traits Can Protect Wheat Grain Number Under Progressive Soil Drying at the Meiotic Stage: A Phenotyping Approach. J. Plant Growth Regul. 2019, 38, 1562–1573. [Google Scholar] [CrossRef]
- Fang, G.; Yang, S.; Ruan, B.; Ye, G.; He, M.; Su, W.; Zhou, Y.; Wang, J.; Yang, S. Research Progress on Physiological, Biochemical, and Molecular Mechanisms of Potato in Response to Drought and High Temperature. Horticulturae 2024, 10, 827. [Google Scholar] [CrossRef]
- Zhang, S.H.; Xu, X.F.; Sun, Y.M.; Zhang, J.L.; Li, C.Z. Influence of Drought Hardening on the Resistance Physiology of Potato Seedlings under Drought Stress. J. Integr. Agric. 2018, 17, 336–347. [Google Scholar] [CrossRef]
- Cantore, V.; Wassar, F.; Yamaç, S.S.; Sellami, M.H.; Albrizio, R.; Stellacci, A.M.; Todorovic, M. Yield and Water Use Efficiency of Early Potato Grown under Different Irrigation Regimes. Int. J. Plant Prod. 2014, 8, 409–428. [Google Scholar]
- Chang, D.C.; Jin, Y.I.; Nam, J.H.; Cheon, C.G.; Cho, J.H.; Kim, S.J.; Yu, H.S. Early Drought Effect on Canopy Development and Tuber Growth of Potato Cultivars with Different Maturities. Field Crops Res. 2018, 215, 156–162. [Google Scholar] [CrossRef]
- Sowokinos, J.R. Internal Physiological Disorders and Nutritional and Compositional Factors That Affect Market Quality. In Potato Biology and Biotechnology: Advances and Perspectives; Elsevier: Amsterdam, The Netherlands, 2007; pp. 501–523. ISBN 9780444510181. [Google Scholar]
- Lulai, E.C.; Suttle, J.C.; Pederson, S.M. Regulatory Involvement of Abscisic Acid in Potato Tuber Wound-Healing. J. Exp. Bot. 2008, 59, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Dahal, K.; Li, X.Q.; Tai, H.; Creelman, A.; Bizimungu, B. Improving Potato Stress Tolerance and Tuber Yield under a Climate Change Scenario—A Current Overview. Front. Plant Sci. 2019, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Mike, T. Paul Bethke Stress Factors and Management Practices for Sugar End Disorder and Stem-End Chip Defect. SPUDSmart. 2019. Available online: https://spudsmart.com/stress-factors-and-management-practices-for-sugar-end-disorder-and-stem-end-chip-defect/ (accessed on 2 June 2024).
- Thompson, A.L.; Love, S.L.; Sowokinos, J.R.; Thornton, M.K.; Shock, C.C. Review of the Sugar End Disorder in Potato (Solanum tuberosum, L.). Am. J. Potato Res. 2008, 85, 375–386. [Google Scholar] [CrossRef]
| Experiment | Factors | d.f. | p Values | ||
|---|---|---|---|---|---|
| VWC | gs | VWC | gs | ||
| Exp 1 | Treatment | 3 | 3 | <0.001 | <0.001 |
| Variety | 3 | 3 | 0.414 | 0.435 | |
| Treatment × Variety | 9 | 9 | 0.887 | 0.651 | |
| Time | 17 | 8 | <0.001 | <0.001 | |
| Time × Treatment | 51 | 24 | <0.001 | <0.001 | |
| Time × Variety | 51 | 24 | 0.555 | 0.895 | |
| Time × Treatment × Variety | 153 | 72 | 0.921 | 0.955 | |
| CV (%) | 9.0 | 17.4 | |||
| Contrast p values | |||||
| IRR vs. DT | 1 | 1 | <0.001 | <0.001 | |
| DT vs. AT | 1 | 1 | 0.725 | <0.001 | |
| VG vs. ABA Interaction contrast p values | 1 | 1 | 0.952 | 0.881 | |
| Variety × Treatment | 9 | 9 | 0.889 | 0.651 | |
| Variety × IRR vs. DT | 3 | 3 | 0.585 | 0.694 | |
| Variety × DT vs. AT | 3 | 3 | 0.861 | 0.878 | |
| Variety × VG vs. ABA | 3 | 3 | 0.677 | 0.205 | |
| Time × Variety | 51 | 24 | 0.508 | 0.895 | |
| Time × Treatment | 51 | 24 | <0.001 | <0.001 | |
| Time × IRR vs. DT | 17 | 8 | <0.001 | <0.001 | |
| Time × DT vs. AT | 17 | 8 | 0.26 | 0.001 | |
| Time × VG vs. ABA | 17 | 8 | 0.97 | 0.818 | |
| Time × Variety × Treatment | 153 | 72 | 0.903 | 0.955 | |
| Time × Variety × IRR vs. DT | 51 | 24 | 0.368 | 0.703 | |
| Time × Variety × DT vs. AT | 51 | 24 | 0.976 | 0.999 | |
| Time × Variety × VG vs. ABA | 51 | 24 | 0.7906 | 0.5247 | |
| Exp 2 | Treatment | 3 | 3 | <0.001 | <0.001 |
| Variety | 3 | 3 | 0.312 | 0.199 | |
| Treatment × Variety | 9 | 9 | 0.217 | 0.228 | |
| Time | 12 | 6 | <0.001 | <0.001 | |
| Time × Treatment | 36 | 18 | <0.001 | <0.001 | |
| Time × Variety | 36 | 18 | 0.394 | 0.162 | |
| Time × Treatment × Variety | 108 | 54 | 0.942 | 0.97 | |
| CV (%) | 10.1 | 9.0 | |||
| Contrast p values | |||||
| IRR vs. DT | 1 | 1 | <0.001 | <0.001 | |
| DT vs. AT | 1 | 1 | 0.205 | 0.14 | |
| VG vs. ABA Interaction contrast p values | 1 | 1 | 0.015 | 0.439 | |
| Variety × Treatment | 9 | 9 | 0.329 | 0.092 | |
| Variety × IRR vs. DT | 3 | 3 | 0.202 | 0.073 | |
| Variety × DT vs. AT | 3 | 3 | 0.943 | 0.099 | |
| Variety × VG vs. ABA | 3 | 3 | 0.156 | 0.553 | |
| Time × Variety | 33 | 18 | 0.648 | 0.071 | |
| Time × Treatment | 33 | 18 | <0.001 | <0.001 | |
| Time × IRR vs. DT | 11 | 6 | <0.001 | <0.001 | |
| Time × DT vs. AT | 11 | 6 | 0.42 | 0.099 | |
| Time × VG vs. ABA | 11 | 6 | 0.271 | 0.18 | |
| Time × Variety × Treatment | 99 | 54 | 0.952 | 0.854 | |
| Time × Variety × IRR vs. DT | 33 | 18 | 0.626 | 0.754 | |
| Time × Variety × DT vs. AT | 33 | 18 | 0.893 | 0.736 | |
| Time × Variety × VG vs. ABA | 33 | 18 | 0.9024 | 0.618 | |
| Experiment 1 | Experiment 2 | ||
|---|---|---|---|
| Variety | Treatment | RWC (%) | RWC (%) |
| Challenger | IRR no AT | 85.8 | 96.3 |
| DT no AT | 59.6 | 54.9 | |
| DT + VG | 83.7 | 84.7 | |
| DT + ABA | 58.8 | 52.6 | |
| Mean | 72.0 | 72.1 | |
| Markies | IRR no AT | 91.8 | 85.1 |
| DT no AT | 53.5 | 57.8 | |
| DT + VG | 79.7 | 75.3 | |
| DT + ABA | 64.5 | 64.9 | |
| Mean | 72.4 | 70.8 | |
| Nectar | IRR no AT | 85.9 | 85.9 |
| DT no AT | 47.0 | 51.4 | |
| DT + VG | 74.3 | 92.7 | |
| DT + ABA | 68.3 | 77.3 | |
| Mean | 68.9 | 76.8 | |
| Russet Burbank | IRR no AT | 88.9 | 89.1 |
| DT no AT | 50.1 | 58.9 | |
| DT + VG | 66.7 | 69.5 | |
| DT + ABA | 72.6 | 46.9 | |
| Mean | 69.6 | 66.1 | |
| Mean over varieties | IRR no AT | 88.1 | 89.1 |
| DT no AT | 52.6 | 55.8 | |
| DT + VG | 76.1 | 80.6 | |
| DT + ABA | 66.1 | 60.4 | |
| CV% | 19.7 | 20.8 | |
| SED | d.f | ||
| Treatment | 60 | 4.41 | 4.70 |
| Variety | 4.41 | 4.70 | |
| Variety × Treatment | 8.83 | 9.40 | |
| p values | |||
| Treatment | <0.001 | <0.001 | |
| Variety | 0.816 | 0.164 | |
| Variety × Treatment | 0.472 | 0.075 | |
| Contrast p values | |||
| IRR vs. DT | <0.001 | <0.001 | |
| DT no AT vs. DT + ATs | <0.001 | <0.001 | |
| DT + VG vs. DT + ABA | 0.003 | 0.322 | |
| Interaction contrast p values | |||
| Variety × IRR vs. DT | 0.208 | 0.905 | |
| Variety × DT no AT vs. DT + ATs | 0.369 | 0.310 | |
| Variety × DT + VG vs. DT + ABA | 0.039 | 0.205 | |
| Experiment 1 | Experiment 2 | ||
|---|---|---|---|
| Variety | Treatment | Weight (g/plant) | Weight (g/plant) |
| Challenger | IRR no AT | 961 | 1296 |
| DT no AT | 771 | 943 | |
| DT + VG | 872 | 732 | |
| DT + ABA | 999 | 942 | |
| Mean | 901 | 978 | |
| Markies | IRR no AT | 781 | 1001 |
| DT no AT | 668 | 856 | |
| DT + VG | 700 | 694 | |
| DT + ABA | 629 | 731 | |
| Mean | 695 | 820 | |
| Nectar | IRR no AT | 1135 | 1589 |
| DT no AT | 735 | 668 | |
| DT + VG | 869 | 836 | |
| DT + ABA | 679 | 882 | |
| Mean | 854 | 994 | |
| Russet Burbank | IRR no AT | 1290 | 1507 |
| DT no AT | 741 | 637 | |
| DT + VG | 802 | 693 | |
| DT + ABA | 735 | 691 | |
| Mean | 892 | 882 | |
| Mean over varieties | |||
| IRR no AT | 1041.6 | 1348.3 | |
| DT no AT | 728.6 | 776.0 | |
| DT + VG | 810.9 | 738.8 | |
| DT + ABA | 760.7 | 811.5 | |
| CV% | 21.7 | 19.6 | |
| p-value | |||
| Treatment | 0.001 | <0.001 | |
| Variety | 0.002 | 0.177 | |
| Variety × Treatment | 0.022 | 0.099 | |
| Contrast p values | |||
| IRR vs. DT (all) | <0.001 | <0.001 | |
| DT no AT vs. DT + ATs | 0.254 | 0.988 | |
| DT + VG vs. DT + ABA | 0.037 | 0.417 | |
| Interaction contrast p values | |||
| Variety × IRR vs. DT | 0.003 | 0.012 | |
| Variety × DT no AT vs. DT + ATs | 0.645 | 0.398 | |
| Variety × DT + VG vs. DT + ABA | 0.03 | 0.841 | |
| SED | d.f | ||
| Treatment | 60 | 57.3 | 88.9 |
| Variety | 57.3 | 88.9 | |
| Variety × Treatment | 114.7 | 177.9 | |
| Challenger | Treatment | Russeting | Number of Disorder-Free Tubers | Number of Tubers Assessed |
|---|---|---|---|---|
| Experiment 1—Before Storage | ||||
| IRR no AT | 3 (20%) | 12 (80%) | 15 | |
| DT no AT | 11(79%) | 3 (21%) | 14 | |
| DT + VG | 11 (73%) | 4 (27%) | 15 | |
| DT + ABA | 11 (73%) | 4 (27%) | 15 | |
| Mean | 9 (74%) | 5.8 (39%) | 14.8 | |
| Experiment 1—After Storage | ||||
| IRR no AT | 12 (36%) | 21 (64%) | 33 | |
| DT no AT | 24 (92%) | 2 (8%) | 26 | |
| DT + VG | 16 (55%) | 13 (45%) | 29 | |
| DT + ABA | 19 (76%) | 6 (24%) | 25 | |
| Mean | 17.8 (62%) | 10.5 (37%) | 28.5 | |
| Russet Burbank | Treatment | Jelly End Rot | Number of Disorder-Free Tubers | Number of Tubers Assessed |
| Experiment 1—Before Storage | ||||
| IRR no AT | 1 (7%) | 14 (93%) | 15 | |
| DT no AT | 2 (15%) | 11 (85%) | 13 | |
| DT + VG | 3 (23%) | 12 (92%) | 13 | |
| DT + ABA | 3 (20%) | 12 (80%) | 15 | |
| Mean | 1.5 (11%) | 12.3 (88%) | 14 | |
| Experiment 1—After Storage | ||||
| IRR no AT | 2 (9%) | 20 (91(%) | 22 | |
| DT no AT | 9 (45%) | 11 (55%) | 20 | |
| DT + VG | 0 | 24 (100%) | 24 | |
| DT + ABA | 2 (8%) | 22 (92%) | 24 | |
| Mean | 3.3 (15%) | 19.3 (86%) | 22.5 | |
| Challenger | Treatment | Russeting | Number of Disorder-Free Tubers | Number of Tubers Assessed |
|---|---|---|---|---|
| Experiment 2—Before Storage | ||||
| IRR no AT | 3 (20%) | 12 (80%) | 15 | |
| DT no AT | 9 (64%) | 5 (36%) | 14 | |
| DT + VG | 2 (13%) | 13 (87%) | 15 | |
| DT + ABA | 10 (67%) | 4 (27%) | 14 | |
| Mean | 6 (41%) | 8.5 (57%) | 14.5 | |
| Experiment 2—After Storage | ||||
| IRR no AT | 5 (21%) | 19 (79%) | 24 | |
| DT no AT | 20 (95%) | 1 (5%) | 21 | |
| DT + VG | 3 (14%) | 18 (86%) | 21 | |
| DT + ABA | 23 (85%) | 4 (15%) | 27 | |
| Mean | 13 (52%) | 10.5 (44%) | 23.8 | |
| Russet Burbank | Treatment | Jelly End Rot | Number of Disorder-Free Tubers | Number of Tubers Assessed |
| Experiment 2—Before Storage | ||||
| IRR no AT | 0 | 15 (100%) | 15 | |
| DT no AT | 0 | 13 (92%) | 13 | |
| DT + VG | 0 | 12 (83%) | 12 | |
| DT + ABA | 3 (20%) | 12 (80%) | 15 | |
| Mean | 0.75 (5%) | 13 (94%) | 13.8 | |
| Experiment 2—After Storage | ||||
| IRR no AT | 1 (4%) | 23 (92%) | 24 | |
| DT no AT | 5 (23%) | 17 (73%) | 22 | |
| DT + VG | 0 | 12 (100%) | 12 | |
| DT + ABA | 2 (10%) | 18 (80%) | 20 | |
| Mean | 2 (10%) | 17.5 (90%) | 19.5 | |
| Experiment | Storage | Variety | Comparison | Russeting | Jelly End Rot | Disorder-Free |
|---|---|---|---|---|---|---|
| 1 | Before | Challenger | IRR vs. DT (all) | <0.001 | - | <0.001 |
| DT no AT vs. DT + ATs | 1.000 | - | 1.000 | |||
| DT + VG vs. DT + ABA | 1.000 | - | 1.000 | |||
| 1 | After | Challenger | IRR vs. DT (all) | <0.001 | - | <0.001 |
| DT no AT vs. DT + ATs | 0.013 | - | 0.013 | |||
| DT + VG vs. DT + ABA | 0.155 | - | 0.155 | |||
| 1 | Before | Russet Burbank | IRR vs. DT (all) | - | 0.418 | 0.257 |
| DT no AT vs. DT + ATs | - | 1.000 | 0.696 | |||
| DT + VG vs. DT + ABA | - | 1.000 | 0.682 | |||
| 1 | After | Russet Burbank | IRR vs. DT (all) | - | 0.726 | 0.348 |
| DT no AT vs. DT + ATs | - | <0.001 | <0.001 | |||
| DT + VG vs. DT + ABA | - | 0.212 | 0.050 | |||
| 2 | Before | Challenger | IRR vs. DT (all) | 0.070 | - | 0.070 |
| DT no AT vs. DT + ATs | 0.203 | - | 0.203 | |||
| DT + VG vs. DT + ABA | 0.003 | - | 0.003 | |||
| 2 | After | Challenger | IRR vs. DT (all) | <0.001 | - | <0.001 |
| DT no AT vs. DT + ATs | <0.001 | - | <0.001 | |||
| DT + VG vs. DT + ABA | <0.001 | - | <0.001 | |||
| 2 | Before | Russet Burbank | IRR vs. DT | - | 0.548 | 0.173 |
| DT no AT vs. DT + ATs | - | 0.537 | 0.643 | |||
| DT + VG vs. DT + ABA | - | 0.25 | 1.000 | |||
| 2 | After | Russet Burbank | IRR vs. all DT (all) | - | <0.001 | 0.324 |
| DT no AT vs. DT + ATs | - | 0.109 | 0.285 | |||
| DT + VG vs. DT + ABA | - | 0.503 | 0.271 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olu-Olusegun, O.F.; Farrell, A.; Monaghan, J.; Kettlewell, P. Film-Forming and Metabolic Antitranspirants Reduce Potato Drought Stress and Tuber Physiological Disorders. Agronomy 2025, 15, 1564. https://doi.org/10.3390/agronomy15071564
Olu-Olusegun OF, Farrell A, Monaghan J, Kettlewell P. Film-Forming and Metabolic Antitranspirants Reduce Potato Drought Stress and Tuber Physiological Disorders. Agronomy. 2025; 15(7):1564. https://doi.org/10.3390/agronomy15071564
Chicago/Turabian StyleOlu-Olusegun, Oluwatoyin Favour, Aidan Farrell, James Monaghan, and Peter Kettlewell. 2025. "Film-Forming and Metabolic Antitranspirants Reduce Potato Drought Stress and Tuber Physiological Disorders" Agronomy 15, no. 7: 1564. https://doi.org/10.3390/agronomy15071564
APA StyleOlu-Olusegun, O. F., Farrell, A., Monaghan, J., & Kettlewell, P. (2025). Film-Forming and Metabolic Antitranspirants Reduce Potato Drought Stress and Tuber Physiological Disorders. Agronomy, 15(7), 1564. https://doi.org/10.3390/agronomy15071564






