Molecular Mechanisms Underlying Root Nodule Formation and Activity
Abstract
1. Introduction
2. Organogenesis and Function of Root Nodules
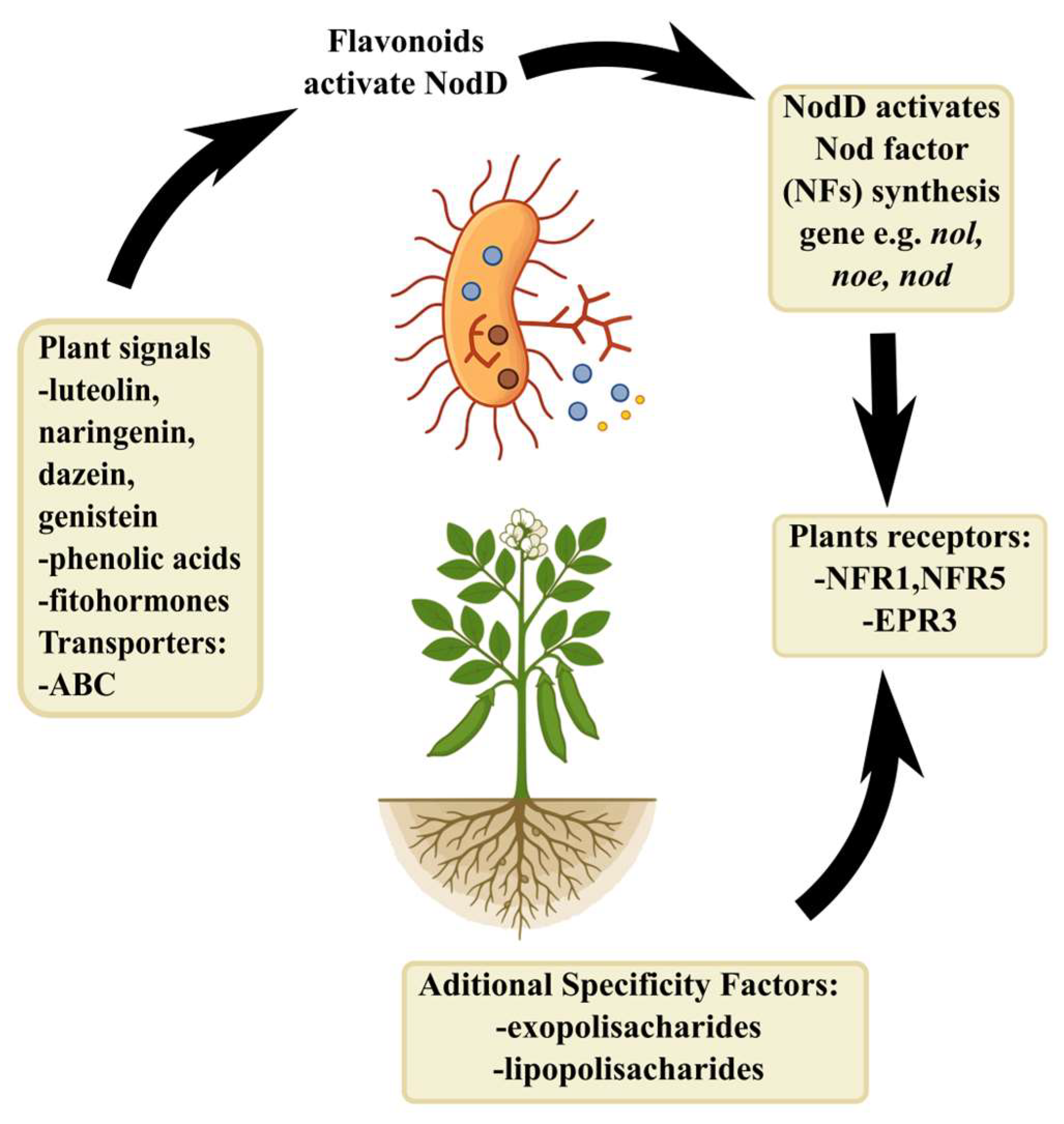
3. The Selection of a Symbiotic Partner and the Exchange of Chemical Signals Are Specific Processes
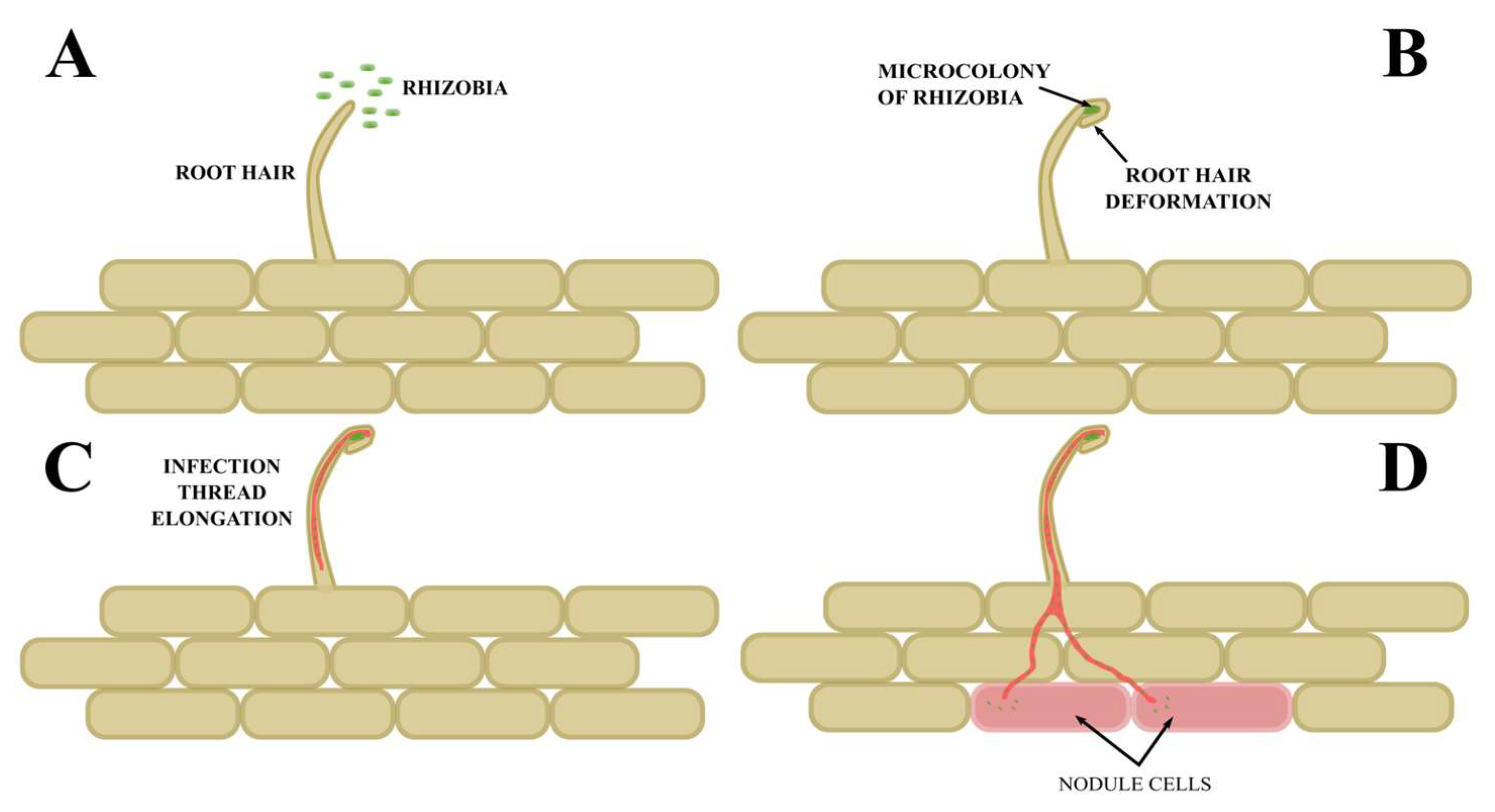
4. The Early Stages of the Infection
5. Infection of the Root by Rhizobia
6. Types of Root Nodules
7. Biological Nitrogen Fixation
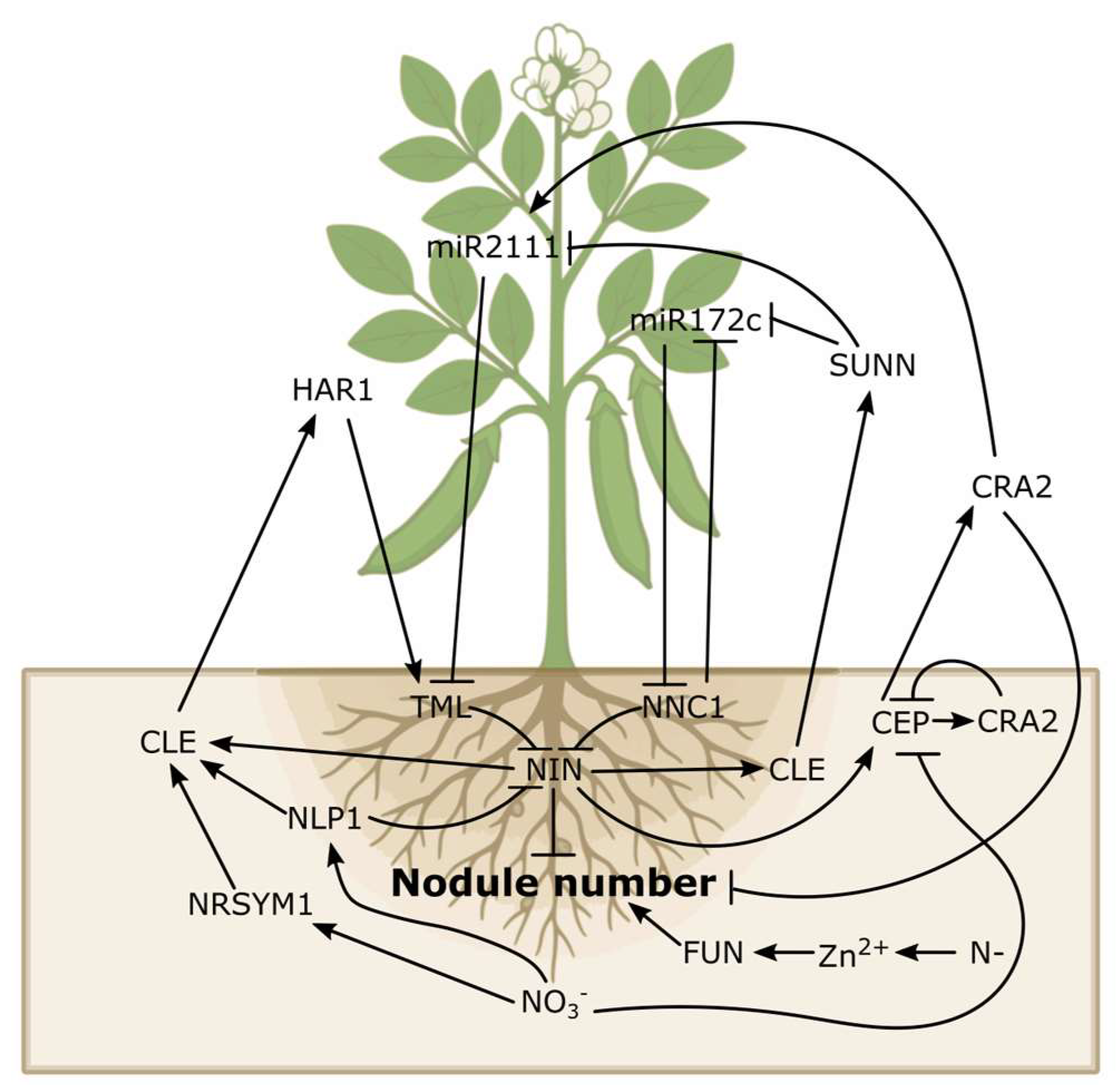
8. Autoregulation of Nodulation (AON)
9. Cyclophilins
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aboagye, I.A.; Oba, M.; Koenig, K.M.; Zhao, G.Y.; Beauchemin, K.A. Use of Gallic Acid and Hydrolyzable Tannins to Reduce Methane Emission and Nitrogen Excretion in Beef Cattle Fed a Diet Containing Alfalfa Silage. J. Anim. Sci. 2019, 97, 2230–2244. [Google Scholar] [CrossRef] [PubMed]
- Adrain, C.; Slee, E.A.; Harte, M.T.; Martin, S.J. Regulation of Apoptotic Protease Activating Factor-1 Oligomerization and Apoptosis by the WD-40 Repeat Region. J. Biol. Chem. 1999, 274, 20855–20860. [Google Scholar] [CrossRef]
- Ahn, J.C.; Kim, D.-W.; You, Y.N.; Seok, M.S.; Park, J.M.; Hwang, H.; Kim, B.-G.; Luan, S.; Park, H.-S.; Cho, H.S. Classification of Rice (Oryza sativa L. japonica Nipponbare) Immunophilins (FKBPs, CYPs) and Expression Patterns under Water Stress. BMC Plant Biol. 2010, 10, 253. [Google Scholar] [CrossRef]
- Stewart, D.E.; Sarkar, A.; Wampler, J.E. Occurrence and Role of Cis Peptide Bonds in Protein Structures. J. Mol. Biol. 1990, 214, 253–260. [Google Scholar] [CrossRef]
- Cheng, H.N.; Bovey, F.A. Cis-Trans Equilibrium and Kinetic Studies of Acetyl-L-Proline and Glycyl-L-Proline. Biopolymers 1977, 16, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Hanes, S.D. The Ess1 Prolyl Isomerase: Traffic Cop of the RNA Polymerase II Transcription Cycle. Biochim. Biophys. Acta 2014, 1839, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.P.; Hanes, S.D.; Hunter, T. A Human Peptidyl-Prolyl Isomerase Essential for Regulation of Mitosis. Nature 1996, 380, 544–547. [Google Scholar] [CrossRef]
- Joseph, J.D.; Yeh, E.S.; Swenson, K.I.; Means, A.R. Winkler, null The Peptidyl-Prolyl Isomerase Pin1. Prog. Cell Cycle Res. 2003, 5, 477–487. [Google Scholar]
- Hirsch, A.M.; Larue, T.A.; Doyle, J. Is the Legume Nodule a Modified Root or Stem or an Organ Sui Generis? Crit. Rev. Plant Sci. 1997, 16, 361–392. [Google Scholar] [CrossRef]
- Arévalo-Rodríguez, M.; Heitman, J. Cyclophilin A Is Localized to the Nucleus and Controls Meiosis in Saccharomyces Cerevisiae. Eukaryot. Cell 2005, 4, 17–29. [Google Scholar] [CrossRef]
- Andreeva, L.; Heads, R.; Green, C.J. Cyclophilins and Their Possible Role in the Stress Response. Int. J. Exp. Pathol. 1999, 80, 305–315. [Google Scholar] [CrossRef]
- Lin, D.-T.; Lechleiter, J.D. Mitochondrial Targeted Cyclophilin D Protects Cells from Cell Death by Peptidyl Prolyl Isomerization. J. Biol. Chem. 2002, 277, 31134–31141. [Google Scholar] [CrossRef]
- Pushkarsky, T.; Yurchenko, V.; Vanpouille, C.; Brichacek, B.; Vaisman, I.; Hatakeyama, S.; Nakayama, K.I.; Sherry, B.; Bukrinsky, M.I. Cell Surface Expression of CD147/EMMPRIN Is Regulated by Cyclophilin 60. J. Biol. Chem. 2005, 280, 27866–27871. [Google Scholar] [CrossRef] [PubMed]
- Nuc, K.; Lesniewicz, K.; Nuc, P.; Slomski, R. Yellow Lupine Cyclophilin Interacts with Nucleic Acids. Protein Pept. Lett. 2008, 15, 719–723. [Google Scholar] [CrossRef]
- Trivedi, D.K.; Ansari, M.W.; Tuteja, N. Multiple Abiotic Stress Responsive Rice Cyclophilin: (OsCYP-25) Mediates a Wide Range of Cellular Responses. Commun. Integr. Biol. 2013, 6, e25260. [Google Scholar] [CrossRef] [PubMed]
- Bannikova, O.; Zywicki, M.; Marquez, Y.; Skrahina, T.; Kalyna, M.; Barta, A. Identification of RNA Targets for the Nuclear Multidomain Cyclophilin atCyp59 and Their Effect on PPIase Activity. Nucleic Acids Res. 2013, 41, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Nuc, K.; Nuc, P.; Słomski, R. Yellow Lupine Cyclophilin Transcripts Are Highly Accumulated in the Nodule Meristem Zone. Mol. Plant Microbe Interact. 2001, 14, 1384–1394. [Google Scholar] [CrossRef]
- Trupkin, S.A.; Mora-García, S.; Casal, J.J. The Cyclophilin ROC1 Links Phytochrome and Cryptochrome to Brassinosteroid Sensitivity. Plant J. 2012, 71, 712–723. [Google Scholar] [CrossRef]
- Nuc, K.; Olejnik, P.; Samardakiewicz, M.; Nuc, P. Functional Analysis of the Lupinus Luteus Cyclophilin Gene Promoter Region in Lotus japonicus. Agriculture 2021, 11, 435. [Google Scholar] [CrossRef]
- Aloui, A.; Recorbet, G.; Gollotte, A.; Robert, F.; Valot, B.; Gianinazzi-Pearson, V.; Aschi-Smiti, S.; Dumas-Gaudot, E. On the Mechanisms of Cadmium Stress Alleviation in Medicago truncatula by Arbuscular Mycorrhizal Symbiosis: A Root Proteomic Study. Proteomics 2009, 9, 420–433. [Google Scholar] [CrossRef]
- Gullerova, M.; Barta, A.; Lorkovic, Z.J. AtCyp59 Is a Multidomain Cyclophilin from Arabidopsis thaliana That Interacts with SR Proteins and the C-Terminal Domain of the RNA Polymerase II. RNA 2006, 12, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Poole, P.; Ramachandran, V.; Terpolilli, J. Rhizobia: From Saprophytes to Endosymbionts. Nat. Rev. Microbiol. 2018, 16, 291–303. [Google Scholar] [CrossRef]
- Mitsch, M.J.; diCenzo, G.C.; Cowie, A.; Finan, T.M. Succinate Transport Is Not Essential for Symbiotic Nitrogen Fixation by Sinorhizobium meliloti or Rhizobium leguminosarum. Appl. Environ. Microbiol. 2017, 84, e01561-17. [Google Scholar] [CrossRef]
- Aroney, S.T.N.; Poole, P.S.; Sánchez-Cañizares, C. Rhizobial Chemotaxis and Motility Systems at Work in the Soil. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Compton, K.K.; Scharf, B.E. Rhizobial Chemoattractants, the Taste and Preferences of Legume Symbionts. Front. Plant Sci. 2021, 12, 686465. [Google Scholar] [CrossRef]
- Ji, Y.-Y.; Zhang, B.; Zhang, P.; Chen, L.-C.; Si, Y.-W.; Wan, X.-Y.; Li, C.; Wang, R.-H.; Tian, Y.; Zhang, Z.; et al. Rhizobial Migration toward Roots Mediated by FadL-ExoFQP Modulation of Extracellular Long-Chain AHLs. ISME J. 2023, 17, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Liu, W.; Nandety, R.S.; Crook, A.; Mysore, K.S.; Pislariu, C.I.; Frugoli, J.; Dickstein, R.; Udvardi, M.K. Celebrating 20 Years of Genetic Discoveries in Legume Nodulation and Symbiotic Nitrogen Fixation. Plant Cell 2020, 32, 15–41. [Google Scholar] [CrossRef]
- Biała-Leonhard, W.; Zanin, L.; Gottardi, S.; de Brito Francisco, R.; Venuti, S.; Valentinuzzi, F.; Mimmo, T.; Cesco, S.; Bassin, B.; Martinoia, E.; et al. Identification of an Isoflavonoid Transporter Required for the Nodule Establishment of the Rhizobium-Fabaceae Symbiotic Interaction. Front. Plant Sci. 2021, 12, 758213. [Google Scholar] [CrossRef]
- Sugiyama, A.; Shitan, N.; Yazaki, K. Involvement of a Soybean ATP-Binding Cassette-Type Transporter in the Secretion of Genistein, a Signal Flavonoid in Legume-Rhizobium Symbiosis. Plant Physiol. 2007, 144, 2000–2008. [Google Scholar] [CrossRef]
- Berillo, D.; Kozhahmetova, M.; Lebedeva, L. Overview of the Biological Activity of Anthraquinons and Flavanoids of the Plant Rumex Species. Molecules 2022, 27, 1204. [Google Scholar] [CrossRef]
- Liu, C.-W.; Murray, J.D. The Role of Flavonoids in Nodulation Host-Range Specificity: An Update. Plants 2016, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.A.; Mathesius, U. Flavonoids: Their Structure, Biosynthesis and Role in the Rhizosphere, Including Allelopathy. J. Chem. Ecol. 2013, 39, 283–297. [Google Scholar] [CrossRef]
- Cooper, J.E. Early Interactions between Legumes and Rhizobia: Disclosing Complexity in a Molecular Dialogue. J. Appl. Microbiol. 2007, 103, 1355–1365. [Google Scholar] [CrossRef]
- Skorupska, A.; Wielbo, J.; Kidaj, D.; Marek-Kozaczuk, M. Enhancing Rhizobium–Legume Symbiosis Using Signaling Factors. In Microbes for Legume Improvement; Khan, M.S., Musarrat, J., Zaidi, A., Eds.; Springer: Vienna, Austria, 2010; pp. 27–54. ISBN 978-3-211-99753-6. [Google Scholar]
- Shimamura, M.; Kumaki, T.; Hashimoto, S.; Saeki, K.; Ayabe, S.-I.; Higashitani, A.; Akashi, T.; Sato, S.; Aoki, T. Phenolic Acids Induce Nod Factor Production in Lotus japonicus-Mesorhizobium Symbiosis. Microbes Environ. 2022, 37, ME21094. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lan, L.; Jin, Y.; Yu, N.; Wang, D.; Wang, E. Mechanisms Underlying Legume–Rhizobium Symbioses. J. Integr. Plant Biol. 2022, 64, 244–267. [Google Scholar] [CrossRef]
- Mbengue, M.D.; Hervé, C.; Debellé, F. Nod Factor Signaling in Symbiotic Nodulation. In Advances in Botanical Research; Frendo, P., Frugier, F., Masson-Boivin, C., Eds.; Regulation of Nitrogen-Fixing Symbioses in Legumes; Academic Press: Cambridge, MA, USA, 2020; Volume 94, pp. 1–39. [Google Scholar]
- Bag, S.; Mondal, A.; Majumder, A.; Mondal, S.K.; Banik, A. Flavonoid Mediated Selective Cross-Talk between Plants and Beneficial Soil Microbiome. Phytochem. Rev. 2022, 21, 1739–1760. [Google Scholar] [CrossRef] [PubMed]
- Limpens, E.; van Zeijl, A.; Geurts, R. Lipochitooligosaccharides Modulate Plant Host Immunity to Enable Endosymbioses. Annu. Rev. Phytopathol. 2015, 53, 311–334. [Google Scholar] [CrossRef]
- Ghantasala, S.; Roy Choudhury, S. Nod Factor Perception: An Integrative View of Molecular Communication during Legume Symbiosis. Plant Mol. Biol. 2022, 110, 485–509. [Google Scholar] [CrossRef]
- Nelson, M.S.; Sadowsky, M.J. Secretion Systems and Signal Exchange between Nitrogen-Fixing Rhizobia and Legumes. Front. Plant Sci. 2015, 6, 491. [Google Scholar] [CrossRef]
- Downie, J.A. The Roles of Extracellular Proteins, Polysaccharides and Signals in the Interactions of Rhizobia with Legume Roots. FEMS Microbiol. Rev. 2010, 34, 150–170. [Google Scholar] [CrossRef]
- Perret, X.; Staehelin, C.; Broughton, W.J. Molecular Basis of Symbiotic Promiscuity. Microbiol. Mol. Biol. Rev. 2000, 64, 180–201. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C.; Oldroyd, G.E.D. Plant Signalling in Symbiosis and Immunity. Nature 2017, 543, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Bek, A.S.; Sauer, J.; Thygesen, M.B.; Duus, J.Ø.; Petersen, B.O.; Thirup, S.; James, E.; Jensen, K.J.; Stougaard, J.; Radutoiu, S. Improved Characterization of Nod Factors and Genetically Based Variation in LysM Receptor Domains Identify Amino Acids Expendable for Nod Factor Recognition in Lotus Spp. Mol. Plant Microbe Interact. 2010, 23, 58–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Geurts, R.; Bisseling, T. Rhizobium Nod Factor Perception and Signalling. Plant Cell 2002, 14, S239–S249. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D.; Murray, J.D.; Poole, P.S.; Downie, J.A. The Rules of Engagement in the Legume-Rhizobial Symbiosis. Annu. Rev. Genet. 2011, 45, 119–144. [Google Scholar] [CrossRef]
- Bozsoki, Z.; Cheng, J.; Feng, F.; Gysel, K.; Vinther, M.; Andersen, K.R.; Oldroyd, G.; Blaise, M.; Radutoiu, S.; Stougaard, J. Receptor-Mediated Chitin Perception in Legume Roots Is Functionally Separable from Nod Factor Perception. Proc. Natl. Acad. Sci. USA 2017, 114, E8118–E8127. [Google Scholar] [CrossRef]
- Murakami, E.; Cheng, J.; Gysel, K.; Bozsoki, Z.; Kawaharada, Y.; Hjuler, C.T.; Sørensen, K.K.; Tao, K.; Kelly, S.; Venice, F.; et al. Epidermal LysM Receptor Ensures Robust Symbiotic Signalling in Lotus japonicus. eLife 2018, 7, e33506. [Google Scholar] [CrossRef]
- Zhou, N.; Li, X.; Zheng, Z.; Liu, J.; Downie, J.A.; Xie, F. RinRK1 Enhances NF Receptors Accumulation in Nanodomain-like Structures at Root-Hair Tip. Nat. Commun. 2024, 15, 3568. [Google Scholar] [CrossRef] [PubMed]
- Skorupska, A.; Janczarek, M.; Marczak, M.; Mazur, A.; Król, J. Rhizobial Exopolysaccharides: Genetic Control and Symbiotic Functions. Microb. Cell Fact. 2006, 5, 7. [Google Scholar] [CrossRef]
- Fraysse, N.; Couderc, F.; Poinsot, V. Surface Polysaccharide Involvement in Establishing the Rhizobium–Legume Symbiosis. Eur. J. Biochem. 2003, 270, 1365–1380. [Google Scholar] [CrossRef]
- Acosta-Jurado, S.; Fuentes-Romero, F.; Ruiz-Sainz, J.-E.; Janczarek, M.; Vinardell, J.-M. Rhizobial Exopolysaccharides: Genetic Regulation of Their Synthesis and Relevance in Symbiosis with Legumes. Int. J. Mol. Sci. 2021, 22, 6233. [Google Scholar] [CrossRef]
- Kawaharada, Y.; Kelly, S.; Nielsen, M.W.; Hjuler, C.T.; Gysel, K.; Muszyński, A.; Carlson, R.W.; Thygesen, M.B.; Sandal, N.; Asmussen, M.H.; et al. Receptor-Mediated Exopolysaccharide Perception Controls Bacterial Infection. Nature 2015, 523, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Kawaharada, Y.; Nielsen, M.W.; Kelly, S.; James, E.K.; Andersen, K.R.; Rasmussen, S.R.; Füchtbauer, W.; Madsen, L.H.; Heckmann, A.B.; Radutoiu, S.; et al. Differential Regulation of the Epr3 Receptor Coordinates Membrane-Restricted Rhizobial Colonization of Root Nodule Primordia. Nat. Commun. 2017, 8, 14534. [Google Scholar] [CrossRef]
- Madsen, L.H.; Tirichine, L.; Jurkiewicz, A.; Sullivan, J.T.; Heckmann, A.B.; Bek, A.S.; Ronson, C.W.; James, E.K.; Stougaard, J. The Molecular Network Governing Nodule Organogenesis and Infection in the Model Legume Lotus japonicus. Nat. Commun. 2010, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Sieberer, B.J.; Chabaud, M.; Timmers, A.C.; Monin, A.; Fournier, J.; Barker, D.G. A Nuclear-Targeted Cameleon Demonstrates Intranuclear Ca2+ Spiking in Medicago truncatula Root Hairs in Response to Rhizobial Nodulation Factors. Plant Physiol. 2009, 151, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Zeng, W.; Bernard, S.; Liao, J.; Venkateshwaran, M.; Ane, J.-M.; Jiang, Y. Ca2+-Regulated Ca2+ Channels with an RCK Gating Ring Control Plant Symbiotic Associations. Nat. Commun. 2019, 10, 3703. [Google Scholar] [CrossRef]
- Kanamori, N.; Madsen, L.H.; Radutoiu, S.; Frantescu, M.; Quistgaard, E.M.H.; Miwa, H.; Downie, J.A.; James, E.K.; Felle, H.H.; Haaning, L.L.; et al. A Nucleoporin Is Required for Induction of Ca2+ Spiking in Legume Nodule Development and Essential for Rhizobial and Fungal Symbiosis. Proc. Natl. Acad. Sci. USA 2006, 103, 359–364. [Google Scholar] [CrossRef]
- Saito, K.; Yoshikawa, M.; Yano, K.; Miwa, H.; Uchida, H.; Asamizu, E.; Sato, S.; Tabata, S.; Imaizumi-Anraku, H.; Umehara, Y.; et al. NUCLEOPORIN85 Is Required for Calcium Spiking, Fungal and Bacterial Symbioses, and Seed Production in Lotus japonicus. Plant Cell 2007, 19, 610–624. [Google Scholar] [CrossRef]
- Yokota, K.; Fukai, E.; Madsen, L.H.; Jurkiewicz, A.; Rueda, P.; Radutoiu, S.; Held, M.; Hossain, M.S.; Szczyglowski, K.; Morieri, G.; et al. Rearrangement of Actin Cytoskeleton Mediates Invasion of Lotus japonicus Roots by Mesorhizobium Loti. Plant Cell 2009, 21, 267–284. [Google Scholar] [CrossRef]
- Yano, K.; Shibata, S.; Chen, W.-L.; Sato, S.; Kaneko, T.; Jurkiewicz, A.; Sandal, N.; Banba, M.; Imaizumi-Anraku, H.; Kojima, T.; et al. CERBERUS, a Novel U-Box Protein Containing WD-40 Repeats, Is Required for Formation of the Infection Thread and Nodule Development in the Legume-Rhizobium Symbiosis. Plant J. 2009, 60, 168–180. [Google Scholar] [CrossRef]
- Ibáñez, F.; Wall, L.; Fabra, A. Starting Points in Plant-Bacteria Nitrogen-Fixing Symbioses: Intercellular Invasion of the Roots. J. Exp. Bot. 2017, 68, 1905–1918. [Google Scholar] [CrossRef]
- Quilbé, J.; Montiel, J.; Arrighi, J.-F.; Stougaard, J. Molecular Mechanisms of Intercellular Rhizobial Infection: Novel Findings of an Ancient Process. Front. Plant Sci. 2022, 13, 922982. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.X.; Qiu, L.P.; Xie, F. SPIKE1 Activates the GTPase ROP6 to Guide the Polarized Growth of Infection Threads in Lotus japonicus. Plant Cell 2020, 32, 3774–3791. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, H.; Xie, F. Cellular and Molecular Basis of Symbiotic Nodule Development. Curr. Opin. Plant Biol. 2023, 76, 102478. [Google Scholar] [CrossRef]
- Su, C. Pectin Modifications at the Symbiotic Interface. New Phytol. 2023, 238, 25–32. [Google Scholar] [CrossRef]
- Su, C.; Zhang, G.; Rodriguez-Franco, M.; Hinnenberg, R.; Wietschorke, J.; Liang, P.; Yang, W.; Uhler, L.; Li, X.; Ott, T. Transcellular Progression of Infection Threads in Medicago truncatula Roots Is Associated with Locally Confined Cell Wall Modifications. Curr. Biol. 2023, 33, 533–542.e5. [Google Scholar] [CrossRef]
- Xie, F.; Murray, J.D.; Kim, J.; Heckmann, A.B.; Edwards, A.; Oldroyd, G.E.D.; Downie, J.A. Legume Pectate Lyase Required for Root Infection by Rhizobia. Proc. Natl. Acad. Sci. USA 2012, 109, 633–638. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Brewin, N.J.; Tsyganov, V.E. Structure and Development of the Legume-Rhizobial Symbiotic Interface in Infection Threads. Cells 2021, 10, 1050. [Google Scholar] [CrossRef]
- Fournier, J.; Teillet, A.; Chabaud, M.; Ivanov, S.; Genre, A.; Limpens, E.; de Carvalho-Niebel, F.; Barker, D.G. Remodeling of the Infection Chamber before Infection Thread Formation Reveals a Two-Step Mechanism for Rhizobial Entry into the Host Legume Root Hair. Plant Physiol. 2015, 167, 1233–1242. [Google Scholar] [CrossRef]
- Liu, C.-W.; Breakspear, A.; Stacey, N.; Findlay, K.; Nakashima, J.; Ramakrishnan, K.; Liu, M.; Xie, F.; Endre, G.; de Carvalho-Niebel, F.; et al. A Protein Complex Required for Polar Growth of Rhizobial Infection Threads. Nat. Commun. 2019, 10, 2848. [Google Scholar] [CrossRef]
- Gao, J.-P.; Jiang, S.; Su, Y.; Xu, P.; Wang, J.; Liang, W.; Liu, C.-W.; Murray, J.D. Intracellular Infection by Symbiotic Bacteria Requires the Mitotic Kinase AURORA1. Proc. Natl. Acad. Sci. USA 2022, 119, e2202606119. [Google Scholar] [CrossRef]
- Fournier, J.; Timmers, A.C.J.; Sieberer, B.J.; Jauneau, A.; Chabaud, M.; Barker, D.G. Mechanism of Infection Thread Elongation in Root Hairs of Medicago truncatula and Dynamic Interplay with Associated Rhizobial Colonization. Plant Physiol. 2008, 148, 1985–1995. [Google Scholar] [CrossRef]
- Deng, J.-L.; Zhao, L.; Wei, H.; Ye, H.-X.; Yang, L.; Sun, L.; Zhao, Z.; Murray, J.D.; Liu, C.-W. A Deeply Conserved Amino Acid Required for VAPYRIN Localization and Function during Legume–Rhizobial Symbiosis. New Phytol. 2024, 243, 14–22. [Google Scholar] [CrossRef]
- Liu, M.; Jia, N.; Li, X.; Liu, R.; Xie, Q.; Murray, J.D.; Downie, J.A.; Xie, F. CERBERUS Is Critical for Stabilization of VAPYRIN during Rhizobial Infection in Lotus japonicus. New Phytol. 2021, 229, 1684–1700. [Google Scholar] [CrossRef]
- Genre, A.; Timmers, T. The Symbiotic Role of the Actin Filament Cytoskeleton. New Phytol. 2019, 221, 611–613. [Google Scholar] [CrossRef]
- Jhu, M.-Y.; Oldroyd, G.E.D. Dancing to a Different Tune, Can We Switch from Chemical to Biological Nitrogen Fixation for Sustainable Food Security? PLOS Biol. 2023, 21, e3001982. [Google Scholar] [CrossRef]
- Wolf, S. Cell Wall Signaling in Plant Development and Defense. Annu. Rev. Plant Biol. 2022, 73, 323–353. [Google Scholar] [CrossRef]
- Medina-Puche, L.; Lozano-Durán, R. Plasma Membrane-to-Organelle Communication in Plant Stress Signaling. Curr. Opin. Plant Biol. 2022, 69, 102269. [Google Scholar] [CrossRef]
- Zeng, Y.; Liang, Z.; Liu, Z.; Li, B.; Cui, Y.; Gao, C.; Shen, J.; Wang, X.; Zhao, Q.; Zhuang, X.; et al. Recent Advances in Plant Endomembrane Research and New Microscopical Techniques. New Phytol. 2023, 240, 41–60. [Google Scholar] [CrossRef]
- Cai, G.; Ahmed, M.A. The Role of Root Hairs in Water Uptake: Recent Advances and Future Perspectives. J. Exp. Bot. 2022, 73, 3330–3338. [Google Scholar] [CrossRef]
- Pei, W.; Du, F.; Zhang, Y.; He, T.; Ren, H. Control of the Actin Cytoskeleton in Root Hair Development. Plant Sci. 2012, 187, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zepeda, I.; Sánchez-López, R.; Kunkel, J.G.; Bañuelos, L.A.; Hernández-Barrera, A.; Sánchez, F.; Quinto, C.; Cárdenas, L. Visualization of Highly Dynamic F-Actin Plus Ends in Growing Phaseolus Vulgaris Root Hair Cells and Their Responses to Rhizobium Etli Nod Factors. Plant Cell Physiol. 2014, 55, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Schmitz, C.; Lace, B.; Ditengou, F.A.; Su, C.; Schulze, E.; Knerr, J.; Grosse, R.; Keller, J.; Libourel, C.; et al. Formin-Mediated Bridging of Cell Wall, Plasma Membrane, and Cytoskeleton in Symbiotic Infections of Medicago truncatula. Curr. Biol. 2021, 31, 2712–2719.e5. [Google Scholar] [CrossRef]
- Su, C.; Klein, M.-L.; Hernández-Reyes, C.; Batzenschlager, M.; Ditengou, F.A.; Lace, B.; Keller, J.; Delaux, P.-M.; Ott, T. The Medicago truncatula DREPP Protein Triggers Microtubule Fragmentation in Membrane Nanodomains during Symbiotic Infections. Plant Cell 2020, 32, 1689–1702. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.J.; Ivanov, S. Exocytosis for Endosymbiosis: Membrane Trafficking Pathways for Development of Symbiotic Membrane Compartments. Curr. Opin. Plant Biol. 2017, 38, 101–108. [Google Scholar] [CrossRef]
- Kondorosi, E.; Mergaert, P.; Kereszt, A. A Paradigm for Endosymbiotic Life: Cell Differentiation of Rhizobium Bacteria Provoked by Host Plant Factors. Annu. Rev. Microbiol. 2013, 67, 611–628. [Google Scholar] [CrossRef]
- Oono, R.; Schmitt, I.; Sprent, J.I.; Denison, R.F. Multiple Evolutionary Origins of Legume Traits Leading to Extreme Rhizobial Differentiation. New Phytol. 2010, 187, 508–520. [Google Scholar] [CrossRef]
- Mergaert, P.; Uchiumi, T.; Alunni, B.; Evanno, G.; Cheron, A.; Catrice, O.; Mausset, A.-E.; Barloy-Hubler, F.; Galibert, F.; Kondorosi, A.; et al. Eukaryotic Control on Bacterial Cell Cycle and Differentiation in the Rhizobium-Legume Symbiosis. Proc. Natl. Acad. Sci. USA 2006, 103, 5230–5235. [Google Scholar] [CrossRef]
- Kereszt, A.; Mergaert, P.; Kondorosi, E. Bacteroid Development in Legume Nodules: Evolution of Mutual Benefit or of Sacrificial Victims? Mol. Plant Microbe Interact. 2011, 24, 1300–1309. [Google Scholar] [CrossRef]
- Montiel, J.; Downie, J.A.; Farkas, A.; Bihari, P.; Herczeg, R.; Bálint, B.; Mergaert, P.; Kereszt, A.; Kondorosi, É. Morphotype of Bacteroids in Different Legumes Correlates with the Number and Type of Symbiotic NCR Peptides. Proc. Natl. Acad. Sci. USA 2017, 114, 5041–5046. [Google Scholar] [CrossRef]
- Van de Velde, W.; Guerra, J.C.P.; Keyser, A.D.; De Rycke, R.; Rombauts, S.; Maunoury, N.; Mergaert, P.; Kondorosi, E.; Holsters, M.; Goormachtig, S. Aging in Legume Symbiosis. A Molecular View on Nodule Senescence in Medicago truncatula. Plant Physiol. 2006, 141, 711–720. [Google Scholar] [CrossRef]
- Alunni, B.; Kevei, Z.; Redondo-Nieto, M.; Kondorosi, A.; Mergaert, P.; Kondorosi, E. Genomic Organization and Evolutionary Insights on GRP and NCR Genes, Two Large Nodule-Specific Gene Families in Medicago truncatula. Mol. Plant Microbe Interact. 2007, 20, 1138–1148. [Google Scholar] [CrossRef]
- Czernic, P.; Gully, D.; Cartieaux, F.; Moulin, L.; Guefrachi, I.; Patrel, D.; Pierre, O.; Fardoux, J.; Chaintreuil, C.; Nguyen, P.; et al. Convergent Evolution of Endosymbiont Differentiation in Dalbergioid and Inverted Repeat-Lacking Clade Legumes Mediated by Nodule-Specific Cysteine-Rich Peptides. Plant Physiol. 2015, 169, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Farkas, A.; Maróti, G.; Dürgő, H.; Györgypál, Z.; Lima, R.M.; Medzihradszky, K.F.; Kereszt, A.; Mergaert, P.; Kondorosi, É. Medicago truncatula Symbiotic Peptide NCR247 Contributes to Bacteroid Differentiation through Multiple Mechanisms. Proc. Natl. Acad. Sci. USA 2014, 111, 5183–5188. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, C.L.; Viollier, P.H. New(s) to the (Z-)Ring. Curr. Opin. Microbiol. 2011, 14, 691–697. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, F.J. Structure of Indeterminate Medicago truncatula Nodules: Introduction. In The Model Legume Medicago truncatula; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 706–707. ISBN 978-1-119-40914-4. [Google Scholar]
- Monahan-Giovanelli, H.; Pinedo, C.A.; Gage, D.J. Architecture of Infection Thread Networks in Developing Root Nodules Induced by the Symbiotic Bacterium Sinorhizobium meliloti on Medicago truncatula. Plant Physiol. 2006, 140, 661–670. [Google Scholar] [CrossRef]
- Timmers, A.C.; Soupène, E.; Auriac, M.C.; de Billy, F.; Vasse, J.; Boistard, P.; Truchet, G. Saprophytic Intracellular Rhizobia in Alfalfa Nodules. Mol. Plant Microbe Interact. 2000, 13, 1204–1213. [Google Scholar] [CrossRef]
- Vasse, J.; de Billy, F.; Camut, S.; Truchet, G. Correlation between Ultrastructural Differentiation of Bacteroids and Nitrogen Fixation in Alfalfa Nodules. J. Bacteriol. 1990, 172, 4295–4306. [Google Scholar] [CrossRef]
- Newcomb, W.; Sippell, D.; Peterson, R.L. The Early Morphogenesis of Glycine Max and Pisum Sativum Root Nodules. Can. J. Bot. 1979, 57, 2603–2616. [Google Scholar] [CrossRef]
- Calvert, H.E.; Pence, M.K.; Pierce, M.; Malik, N.S.A.; Bauer, W.D. Anatomical Analysis of the Development and Distribution of Rhizobium Infections in Soybean Roots. Can. J. Bot. = J. Can. De. Bot. 1984, 62, 2375–2384. [Google Scholar] [CrossRef]
- Mathews, A.; Carroll, B.J.; Gresshoff, P.M. Development of Bradyrhizobium Infections in Supernodulating and Non-Nodulating Mutants of Soybean (Glycine Max [L.] Merrill). Protoplasma 1989, 150, 40–47. [Google Scholar] [CrossRef]
- Turgeon, B.G.; Bauer, W.D. Early Events in the Infection of Soybean by Rhizobium Japonicum. Time Course and Cytology of the Initial Infection Process. Can. J. Bot. 1982, 60, 152–161. [Google Scholar] [CrossRef]
- Rolfe, B.G.; Gresshoff, P.M. Genetic Analysis of Legume Nodule Initiation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 297–319. [Google Scholar] [CrossRef]
- Kazmierczak, T.; Yang, L.; Boncompagni, E.; Meilhoc, E.; Frugier, F.; Frendo, P.; Bruand, C.; Gruber, V.; Brouquisse, R. Chapter Seven–Legume Nodule Senescence: A Coordinated Death Mechanism between Bacteria and Plant Cells. In Advances in Botanical Research; Frendo, P., Frugier, F., Masson-Boivin, C., Eds.; Regulation of Nitrogen-Fixing Symbioses in Legumes; Academic Press: Cambridge, MA, USA, 2020; Volume 94, pp. 181–212. [Google Scholar]
- Day, D.A.; Poole, P.S.; Tyerman, S.D.; Rosendahl, L. Ammonia and Amino Acid Transport across Symbiotic Membranes in Nitrogen-Fixing Legume Nodules. Cell Mol. Life Sci. 2001, 58, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.E.; Kobayashi, H.; Walker, G.C. Molecular Determinants of a Symbiotic Chronic Infection. Annu. Rev. Genet. 2008, 42, 413–441. [Google Scholar] [CrossRef]
- White, J.; Prell, J.; James, E.K.; Poole, P. Nutrient Sharing between Symbionts. Plant Physiol. 2007, 144, 604–614. [Google Scholar] [CrossRef]
- Egener, T.; Martin, D.E.; Sarkar, A.; Reinhold-Hurek, B. Role of a Ferredoxin Gene Cotranscribed with the nifHDK Operon in N(2) Fixation and Nitrogenase “Switch-off” of Azoarcus Sp. Strain BH72. J. Bacteriol. 2001, 183, 3752–3760. [Google Scholar] [CrossRef]
- Ledbetter, R.N.; Garcia Costas, A.M.; Lubner, C.E.; Mulder, D.W.; Tokmina-Lukaszewska, M.; Artz, J.H.; Patterson, A.; Magnuson, T.S.; Jay, Z.J.; Duan, H.D.; et al. The Electron Bifurcating FixABCX Protein Complex from Azotobacter Vinelandii: Generation of Low-Potential Reducing Equivalents for Nitrogenase Catalysis. Biochemistry 2017, 56, 4177–4190. [Google Scholar] [CrossRef]
- Dixon, R.O.D.; Wheeler, C.T. Nitrogen Fixation in Plants; Blackie Chapman and Hall: Glasgow, NY, USA, 1986; ISBN 978-0-412-01381-2. [Google Scholar]
- Igarashi, R.Y.; Seefeldt, L.C. Nitrogen Fixation: The Mechanism of the Mo-Dependent Nitrogenase. Crit. Rev. Biochem. Mol. Biol. 2003, 38, 351–384. [Google Scholar] [CrossRef]
- Minchin, F.R. Regulation of Oxygen Diffusion in Legume Nodules. Soil. Biol. Biochem. 1997, 29, 881–888. [Google Scholar] [CrossRef]
- Tjepkema, J.D.; Yocum, C.S. Measurement of Oxygen Partial Pressure within Soybean Nodules by Oxygen Microelectrodes. Planta 1974, 119, 351–360. [Google Scholar] [CrossRef]
- Minguillón, S.; Román, Á.; Pérez-Rontomé, C.; Wang, L.; Xu, P.; Murray, J.D.; Duanmu, D.; Rubio, M.C.; Becana, M. Dynamics of Hemoglobins during Nodule Development, Nitrate Response, and Dark Stress in Lotus japonicus. J. Exp. Bot. 2024, 75, 1547–1564. [Google Scholar] [CrossRef] [PubMed]
- Ott, T.; van Dongen, J.T.; Günther, C.; Krusell, L.; Desbrosses, G.; Vigeolas, H.; Bock, V.; Czechowski, T.; Geigenberger, P.; Udvardi, M.K. Symbiotic Leghemoglobins Are Crucial for Nitrogen Fixation in Legume Root Nodules but Not for General Plant Growth and Development. Curr. Biol. 2005, 15, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, R.S.; Watmough, N.J. The Bacterial Cytochrome Cbb3 Oxidases. Biochim. Biophys. Acta 2004, 1655, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Tanaka, S.; Handa, Y.; Ito, M.; Sakamoto, Y.; Matsunaga, S.; Betsuyaku, S.; Miura, K.; Soyano, T.; Kawaguchi, M.; et al. A NIN-LIKE PROTEIN Mediates Nitrate-Induced Control of Root Nodule Symbiosis in Lotus japonicus. Nat. Commun. 2018, 9, 499. [Google Scholar] [CrossRef]
- Nishida, H.; Nosaki, S.; Suzuki, T.; Ito, M.; Miyakawa, T.; Nomoto, M.; Tada, Y.; Miura, K.; Tanokura, M.; Kawaguchi, M.; et al. Different DNA-Binding Specificities of NLP and NIN Transcription Factors Underlie Nitrate-Induced Control of Root Nodulation. Plant Cell 2021, 33, 2340–2359. [Google Scholar] [CrossRef]
- Pathak, P.K.; Yadav, N.; Kaladhar, V.C.; Jaiswal, R.; Kumari, A.; Igamberdiev, A.U.; Loake, G.J.; Gupta, K.J. The Emerging Roles of Nitric Oxide and Its Associated Scavengers-Phytoglobins-in Plant Symbiotic Interactions. J. Exp. Bot. 2024, 75, 563–577. [Google Scholar] [CrossRef]
- Schwember, A.R.; Schulze, J.; Del Pozo, A.; Cabeza, R.A. Regulation of Symbiotic Nitrogen Fixation in Legume Root Nodules. Plants 2019, 8, 333. [Google Scholar] [CrossRef]
- Nishida, H.; Suzaki, T. Two Negative Regulatory Systems of Root Nodule Symbiosis: How Are Symbiotic Benefits and Costs Balanced? Plant Cell Physiol. 2018, 59, 1733–1738. [Google Scholar] [CrossRef]
- Reid, D.E.; Ferguson, B.J.; Hayashi, S.; Lin, Y.-H.; Gresshoff, P.M. Molecular Mechanisms Controlling Legume Autoregulation of Nodulation. Ann. Bot. 2011, 108, 789–795. [Google Scholar] [CrossRef]
- Suzaki, T.; Yoro, E.; Kawaguchi, M. Leguminous Plants: Inventors of Root Nodules to Accommodate Symbiotic Bacteria. Int. Rev. Cell Mol. Biol. 2015, 316, 111–158. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Lin, J.; Zhu, Y.; Fu, M.; Li, X.; Xie, F. NLP1 Reciprocally Regulates Nitrate Inhibition of Nodulation through SUNN-CRA2 Signaling in Medicago truncatula. Plant Comm. 2021, 2, 100183. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Yao, X.; Li, X.; Liu, J.; Bai, M.; Fang, Z.; Gong, J.; Guan, Y.; Xie, F. GmNLP1 and GmNLP4 Activate Nitrate-Induced CLE Peptides NIC1a/b to Mediate Nitrate-Regulated Root Nodulation. Plant J. 2024, 119, 783–795. [Google Scholar] [CrossRef]
- Luo, Z.; Moreau, C.; Wang, J.; Frugier, F.; Xie, F. NLP1 Binds the CEP1 Signalling Peptide Promoter to Repress Its Expression in Response to Nitrate. New Phytol. 2022, 234, 1547–1552. [Google Scholar] [CrossRef]
- Nishida, H.; Ito, M.; Miura, K.; Kawaguchi, M.; Suzaki, T. Autoregulation of Nodulation Pathway Is Dispensable for Nitrate-Induced Control of Rhizobial Infection. Plant Signal. Behav. 2020, 15, 1733814. [Google Scholar] [CrossRef]
- Okuma, N.; Soyano, T.; Suzaki, T.; Kawaguchi, M. MIR2111-5 Locus and Shoot-Accumulated Mature miR2111 Systemically Enhance Nodulation Depending on HAR1 in Lotus japonicus. Nat. Commun. 2020, 11, 5192. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wei, S.; Li, J.; Kong, W.; Wang, B.; Pei, J.; Wu, J. Fertilization Effects on Symbiotic and Free-Living Biological Nitrogen Fixations: Similar Effects but Different Mechanisms. Appl. Soil. Ecol. 2024, 202, 105590. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Mens, C.; Hastwell, A.H.; Zhang, M.; Su, H.; Jones, C.H.; Chu, X.; Gresshoff, P.M. Legume Nodulation: The Host Controls the Party. Plant Cell Environ. 2019, 42, 41–51. [Google Scholar] [CrossRef]
- Imin, N.; Mohd-Radzman, N.A.; Ogilvie, H.A.; Djordjevic, M.A. The Peptide-Encoding CEP1 Gene Modulates Lateral Root and Nodule Numbers in Medicago truncatula. J. Exp. Bot. 2013, 64, 5395–5409. [Google Scholar] [CrossRef]
- Laffont, C.; Huault, E.; Gautrat, P.; Endre, G.; Kalo, P.; Bourion, V.; Duc, G.; Frugier, F. Independent Regulation of Symbiotic Nodulation by the SUNN Negative and CRA2 Positive Systemic Pathways. Plant Physiol. 2019, 180, 559–570. [Google Scholar] [CrossRef]
- Mohd-Radzman, N.A.; Laffont, C.; Ivanovici, A.; Patel, N.; Reid, D.; Stougaard, J.; Frugier, F.; Imin, N.; Djordjevic, M.A. Different Pathways Act Downstream of the CEP Peptide Receptor CRA2 to Regulate Lateral Root and Nodule Development. Plant Physiol. 2016, 171, 2536–2548. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Bjørk, P.K.; Kolte, M.V.; Poulsen, E.; Dedic, E.; Drace, T.; Andersen, S.U.; Nadzieja, M.; Liu, H.; Castillo-Michel, H.; et al. Zinc Mediates Control of Nitrogen Fixation via Transcription Factor Filamentation. Nature 2024, 631, 164–169. [Google Scholar] [CrossRef]
- Du, M.; Gao, Z.; Li, X.; Liao, H. Excess Nitrate Induces Nodule Greening and Reduces Transcript and Protein Expression Levels of Soybean Leghaemoglobins. Ann. Bot. 2020, 126, 61–72. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, J.; Zhou, H.; Chen, S.; Gao, Z.; Yang, Y.; Li, X.; Liao, H. The Soybean β-Expansin Gene GmINS1 Contributes to Nodule Development in Response to Phosphate Starvation. Physiol. Plant. 2021, 172, 2034–2047. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, C.; Huang, Y.; Chen, H.; Yuan, S.; Zhou, X. Characteristics and Research Progress of Legume Nodule Senescence. Plants 2021, 10, 1103. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, T.; Liang, J.; Li, R.; Xin, X.; Qi, Y.; Zhou, Y.; Fan, Q.; Ning, G.; Becana, M.; et al. A Transcription Factor of the NAC Family Regulates Nitrate-Induced Legume Nodule Senescence. New Phytol. 2023, 238, 2113–2129. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, Z.; Zhu, W.; Wang, N.; Bai, M.; Kuang, H.; Cai, C.; Zhong, X.; Kong, F.; Lü, P.; et al. The NAC Transcription Factors SNAP1/2/3/4 Are Central Regulators Mediating High Nitrogen Responses in Mature Nodules of Soybean. Nat. Commun. 2023, 14, 4711. [Google Scholar] [CrossRef]
- Deng, J.; Zhu, F.; Liu, J.; Zhao, Y.; Wen, J.; Wang, T.; Dong, J. Transcription Factor bHLH2 Represses CYSTEINE PROTEASE77 to Negatively Regulate Nodule Senescence. Plant Physiol. 2019, 181, 1683–1703. [Google Scholar] [CrossRef] [PubMed]
- Sauviac, L.; Rémy, A.; Huault, E.; Dalmasso, M.; Kazmierczak, T.; Jardinaud, M.-F.; Legrand, L.; Moreau, C.; Ruiz, B.; Cazalé, A.-C.; et al. A Dual Legume-Rhizobium Transcriptome of Symbiotic Nodule Senescence Reveals Coordinated Plant and Bacterial Responses. Plant Cell Environ. 2022, 45, 3100–3121. [Google Scholar] [CrossRef]
- Yu, H.; Xiao, A.; Wu, J.; Li, H.; Duan, Y.; Chen, Q.; Zhu, H.; Cao, Y. GmNAC039 and GmNAC018 Activate the Expression of Cysteine Protease Genes to Promote Soybean Nodule Senescence. Plant Cell 2023, 35, 2929–2951. [Google Scholar] [CrossRef]
- Fanghänel, J.; Fischer, G. Insights into the Catalytic Mechanism of Peptidyl Prolyl Cis/Trans Isomerases. Front. Biosci. 2004, 9, 3453–3478. [Google Scholar] [CrossRef] [PubMed]
- Schiene, C.; Fischer, G. Enzymes That Catalyse the Restructuring of Proteins. Curr. Opin. Struct. Biol. 2000, 10, 40–45. [Google Scholar] [CrossRef]
- Romano, P.G.N.; Horton, P.; Gray, J.E. The Arabidopsis Cyclophilin Gene Family. Plant Physiol. 2004, 134, 1268–1282. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Bang, H.; Mech, C. Determination of enzymatic catalysis for the cis-trans-isomerization of peptide binding in proline-containing peptides. Biomed. Biochim. Acta 1984, 43, 1101–1111. [Google Scholar] [PubMed]
- Handschumacher, R.E.; Harding, M.W.; Rice, J.; Drugge, R.J.; Speicher, D.W. Cyclophilin: A Specific Cytosolic Binding Protein for Cyclosporin A. Science 1984, 226, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Bierer, B.E.; Mattila, P.S.; Standaert, R.F.; Herzenberg, L.A.; Burakoff, S.J.; Crabtree, G.; Schreiber, S.L. Two Distinct Signal Transmission Pathways in T Lymphocytes Are Inhibited by Complexes Formed between an Immunophilin and Either FK506 or Rapamycin. Proc. Natl. Acad. Sci. USA 1990, 87, 9231–9235. [Google Scholar] [CrossRef]
- He, Z.; Li, L.; Luan, S. Immunophilins and Parvulins. Superfamily of Peptidyl Prolyl Isomerases in Arabidopsis. Plant Physiol. 2004, 134, 1248–1267. [Google Scholar] [CrossRef]
- Arsenijevic-Maksimovic, I.; Broughton, W.J.; Krause, A. Rhizobia Modulate Root-Hair-Specific Expression of Extensin Genes. MPMI 1997, 10, 95–101. [Google Scholar] [CrossRef]
- Muñoz, J.A.; Palomares, A.J.; Ratet, P. Plant Genes Induced in the Rhizobium-Legume Symbiosis. World J. Microbiol. Biotechnol. 1996, 12, 189–202. [Google Scholar] [CrossRef]
- Sherrier, D.J.; VandenBosch, K.A. Localization of Repetitive Proline-Rich Proteins in the Extracellular Matrix of Pea Root Nodules. Protoplasma 1994, 183, 148–161. [Google Scholar] [CrossRef]
- Wilson, R.C.; Long, F.; Maruoka, E.M.; Cooper, J.B. A New Proline-Rich Early Nodulin from Medicago truncatula Is Highly Expressed in Nodule Meristematic Cells. Plant Cell 1994, 6, 1265–1275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferguson, B.J.; Indrasumunar, A.; Hayashi, S.; Lin, M.-H.; Lin, Y.-H.; Reid, D.E.; Gresshoff, P.M. Molecular Analysis of Legume Nodule Development and Autoregulation. J. Integr. Plant Biol. 2010, 52, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Suzaki, T.; Yano, K.; Ito, M.; Umehara, Y.; Suganuma, N.; Kawaguchi, M. Positive and Negative Regulation of Cortical Cell Division during Root Nodule Development in Lotus japonicus Is Accompanied by Auxin Response. Development 2013, 139, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Nuc, K. Cyklofiliny “Lotus japonicus”; Wydawnictwo Uniwersytetu Przyrodniczego: Poznań, Poland, 2015; ISBN 978-83-7160-819-3. [Google Scholar]
| Name of the Protein | Function | Product Location |
|---|---|---|
| ENOD 2 | presumed cell wall protein | inner cortex (nodule parenchyma), may be nodule specific |
| ENOD 5 | related to arabinogalactans in plasma membrane | nodule primordium, zones 3 and 4 of mature indeterminate nodules, in infected cells only |
| ENOD l0 | presumed cell wall protein | nodule primordium, zone 2 of mature indeterminate nodules |
| ENOD 11 | presumed cell wall protein | root hair/epidermal cells, nodule primordium, invasion zone of mature nodules |
| ENOD l2 | presumed cell wall protein | root hair/epidermal cells, nodule primordium, invasion zone of mature nodules |
| PrP4 | 62-kDa proline-rich protein | meristem of mature nodule, nodule specific |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuc, K.; Olejnik, P. Molecular Mechanisms Underlying Root Nodule Formation and Activity. Agronomy 2025, 15, 1552. https://doi.org/10.3390/agronomy15071552
Nuc K, Olejnik P. Molecular Mechanisms Underlying Root Nodule Formation and Activity. Agronomy. 2025; 15(7):1552. https://doi.org/10.3390/agronomy15071552
Chicago/Turabian StyleNuc, Katarzyna, and Przemysław Olejnik. 2025. "Molecular Mechanisms Underlying Root Nodule Formation and Activity" Agronomy 15, no. 7: 1552. https://doi.org/10.3390/agronomy15071552
APA StyleNuc, K., & Olejnik, P. (2025). Molecular Mechanisms Underlying Root Nodule Formation and Activity. Agronomy, 15(7), 1552. https://doi.org/10.3390/agronomy15071552







