Severely Symptomatic Cucurbits in Croatia Dominantly Harbor a Complex of Potyviruses Including the Emerging Moroccan Watermelon Mosaic Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Virus Pre-Screening by Immunostrips
2.3. Nucleic Acid Extraction
2.4. High-Througput Sequencing
2.4.1. ONT Sequencing Preparation and Data Analyses
2.4.2. Illumina Sample Preparation and Data Analysis
2.4.3. HTS Result Confirmation and Detection by RT-PCR
2.5. Phylogenetic Analyses
2.6. Recombination Analysis
3. Results
3.1. Pre-Screened Viruses
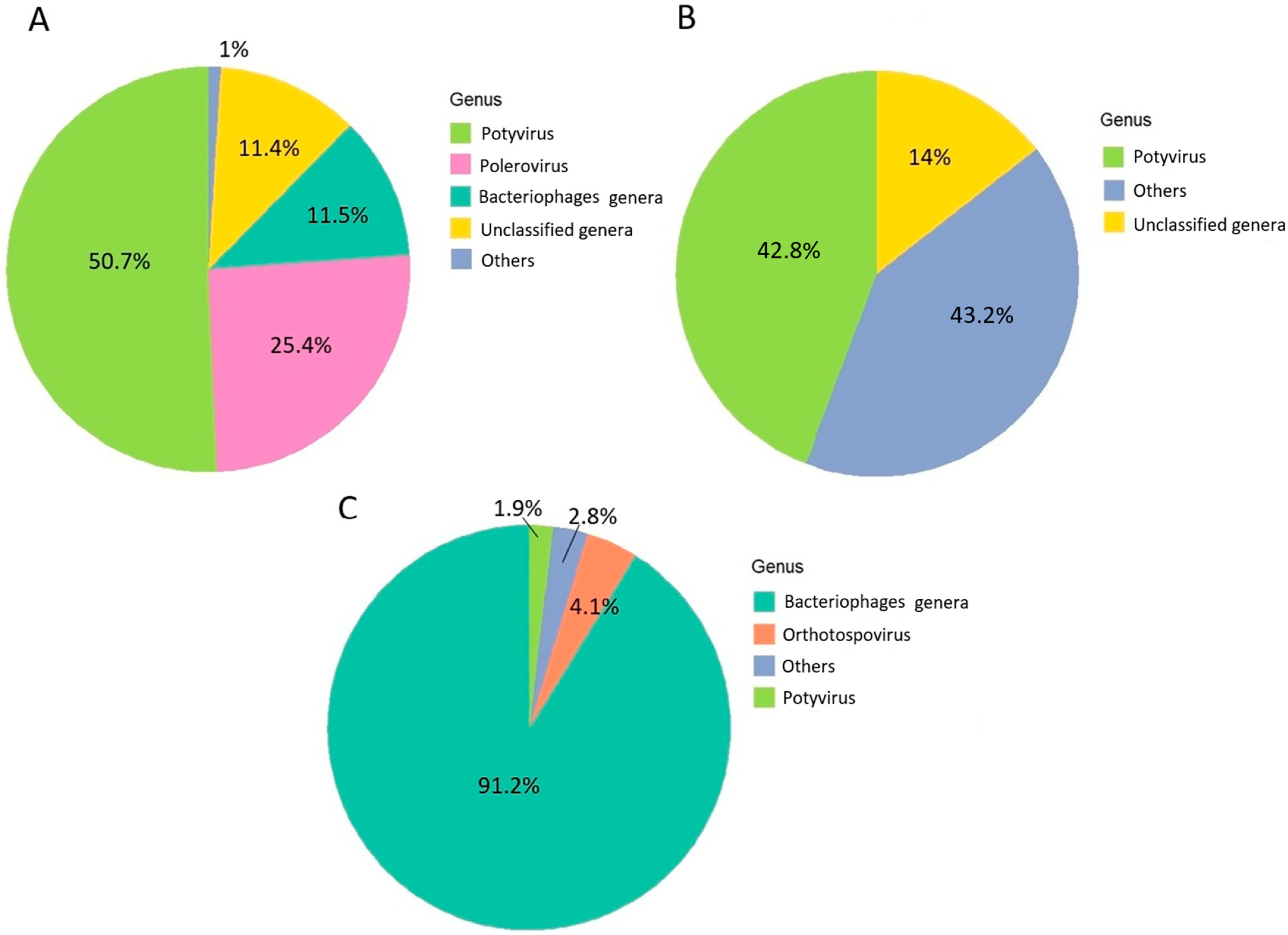
3.2. Metagenomic Results
3.3. HTS and RT-PCR Identification of Cucurbit Virome Constituents
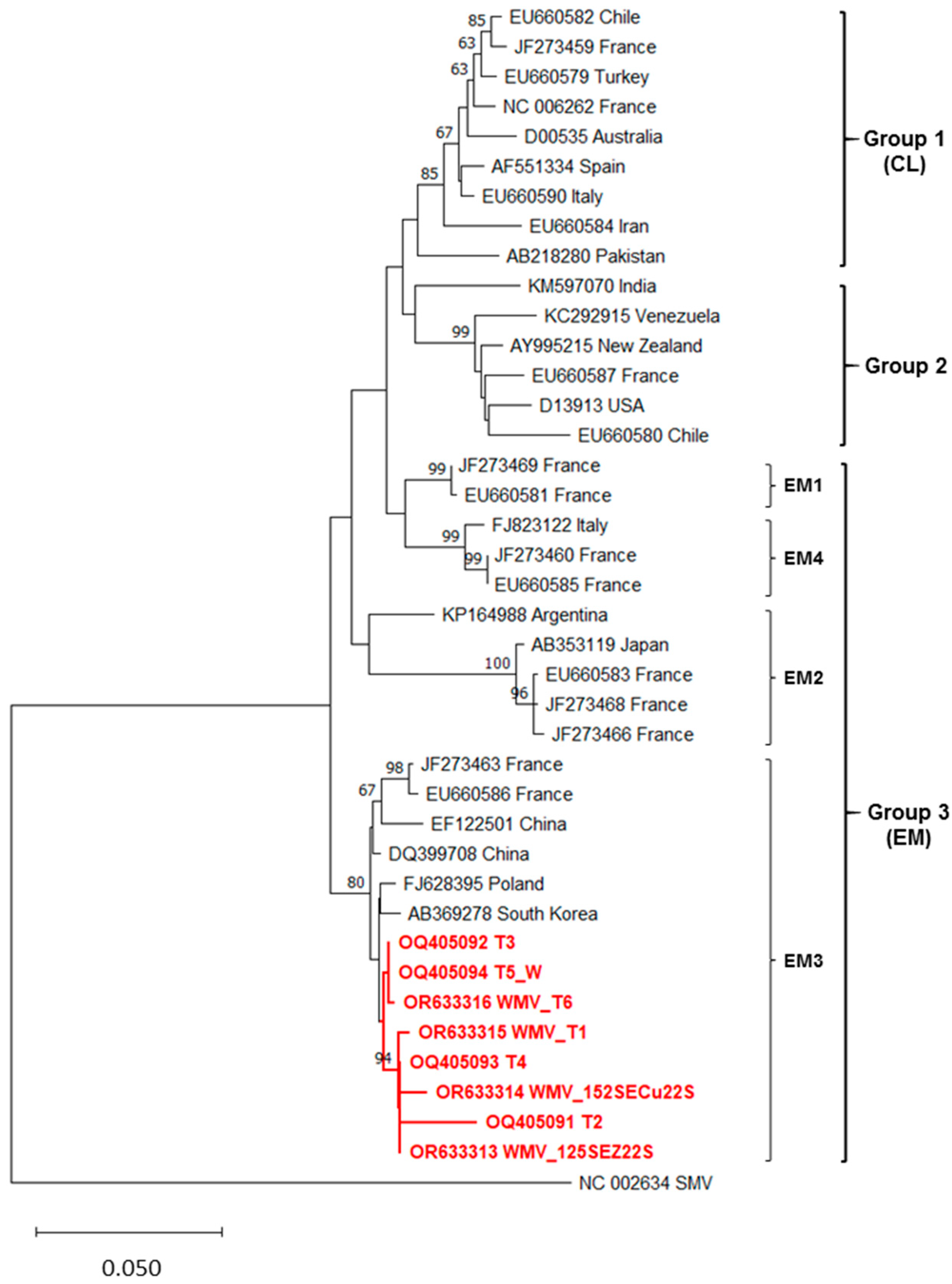
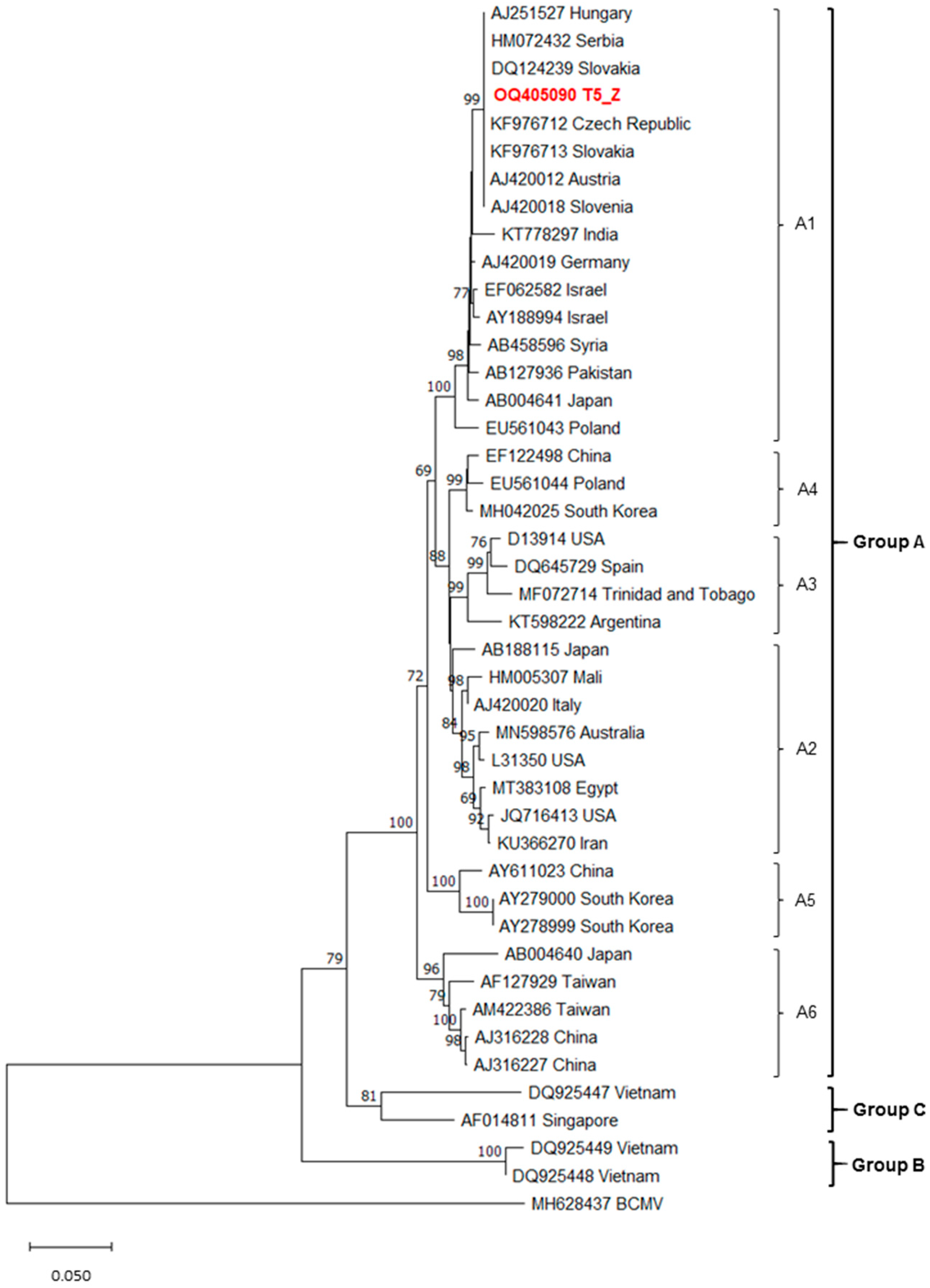
3.4. Phylogenetic Analyses Results
3.4.1. WMV
3.4.2. ZYMV
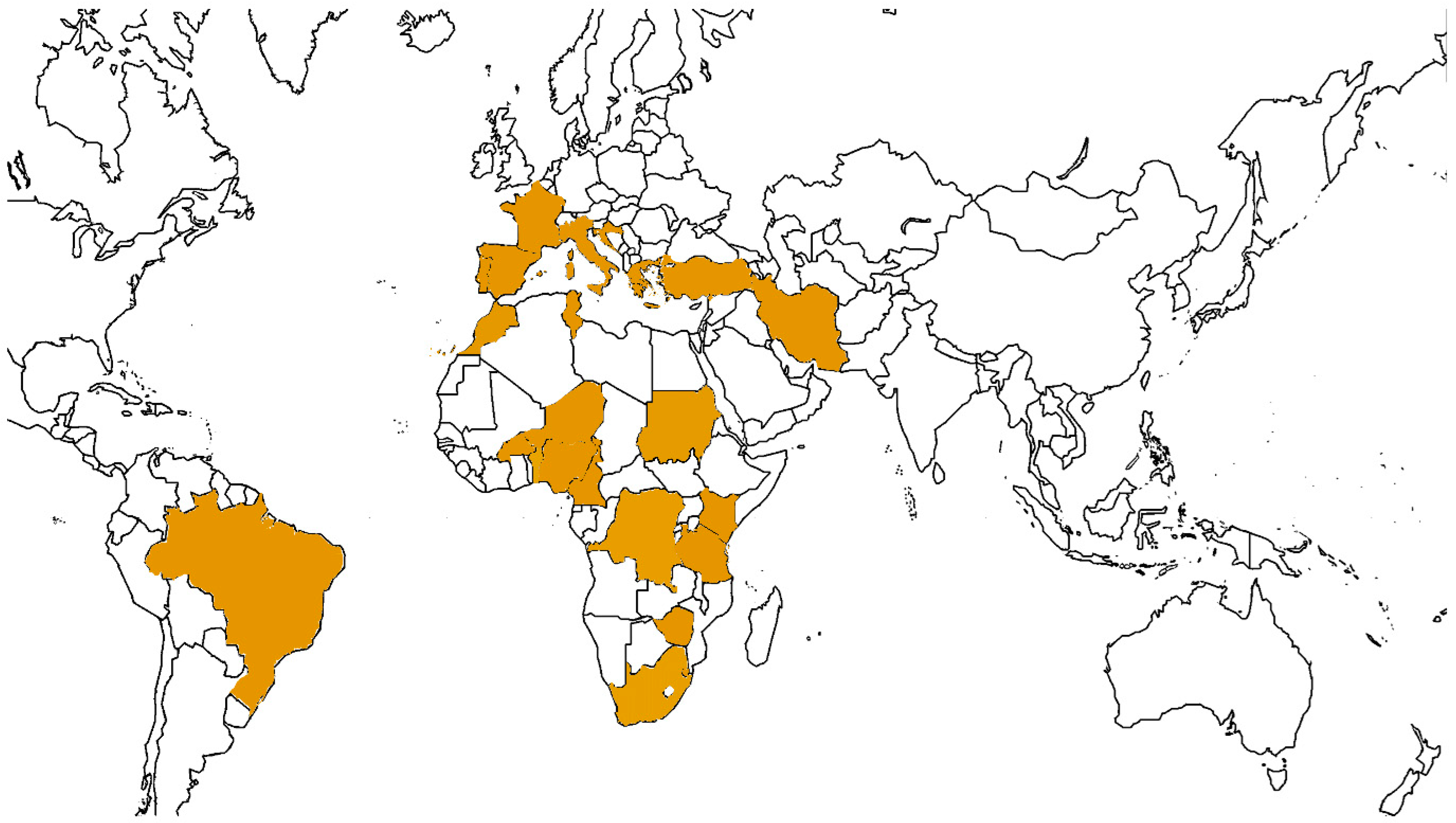
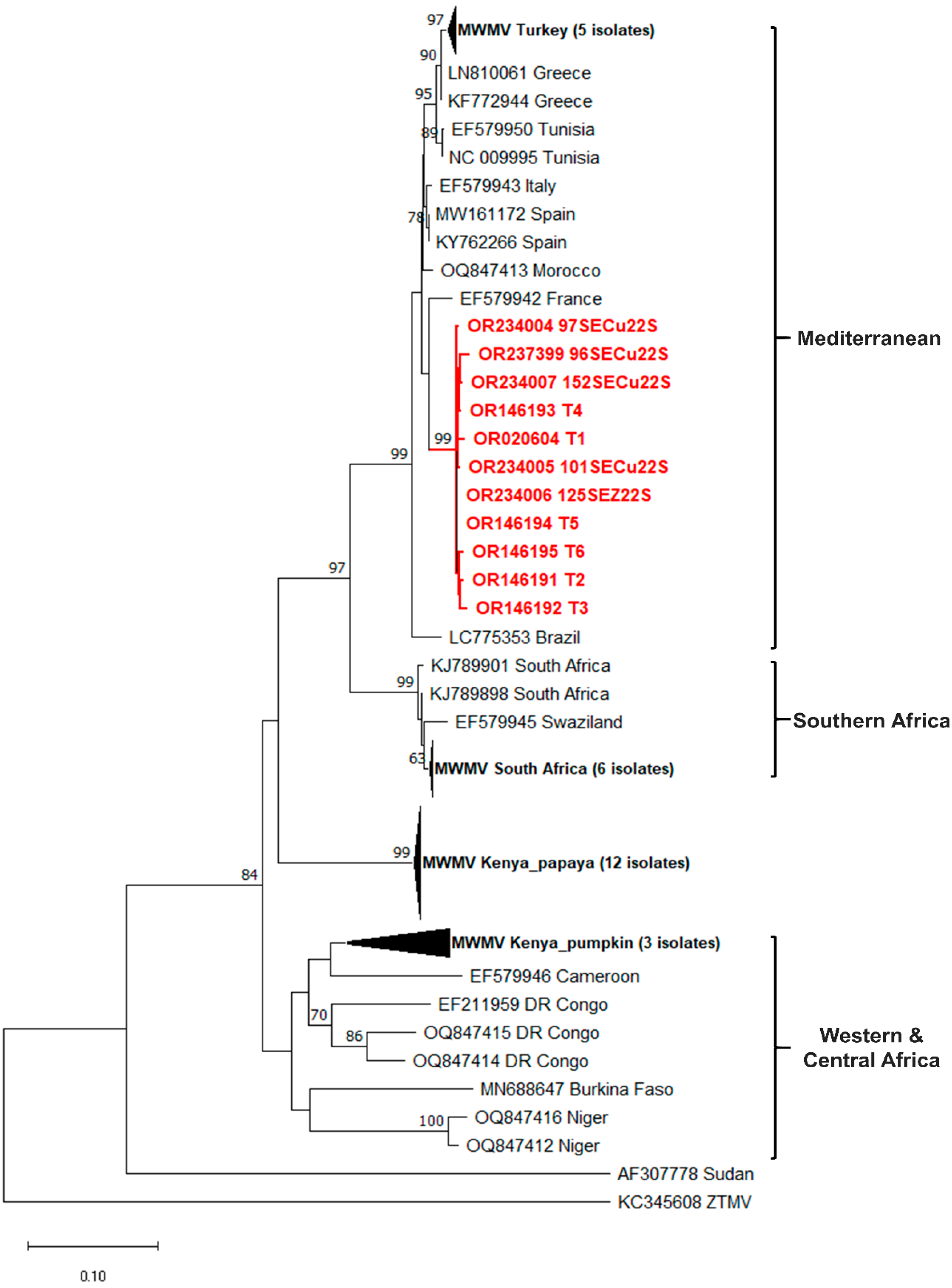
3.4.3. MWMV
3.5. Recombination Analysis Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT. Crops and Livestock Products. 2025. Available online: https://www.fao.org/faostat/en/#data/QCLDatabase (accessed on 1 May 2025).
- Croatian Bureau of Statistics. Production of Vegetables, Fruits and Grapes in 2024—Provisional Data. 2025. Available online: https://podaci.dzs.hr/2024/hr/77192 (accessed on 20 April 2025).
- Mustapić, L.; Ivić, D.; Novak, A.; Milanović, J. Neretva valley—Reservoir of economic cucurbit viruses. Glasilo Biljne Zaštite 2021, 21, 570. Available online: https://hrcak.srce.hr/267588 (accessed on 26 January 2023).
- Tuttle McGrath, M. Diseases of Cucurbits and Their Management. In Diseases of Fruits and Vegetables; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; Volume 1, pp. 455–510. [Google Scholar] [CrossRef]
- Tatineni, S.; Hein, G.L. Plant Viruses of Agricultural Importance: Current and Future Perspectives of Virus Disease Management Strategies. Phytopathology 2023, 113, 117–141. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/2072 (Annex II, Part B). Commission Implementing Regulation (EU) 2019/2072 of 28 November 2019 establishing uniform conditions for the implementation of Regulation (EU) 2016/2031 of the European Parliament and the Council, as Regards Protective Measures Against Pests of Plants, and Repealing Commission Regulation (EC) No 690/2008 and Amending Commission Implementing Regulation (EU) 2018/2019. Off. J. Eur. Union 2019, 319, 1–279. Available online: http://data.europa.eu/eli/reg_impl/2019/2072/oj (accessed on 1 May 2025).
- Acharya, N.; Kumar, M.; Bag, S.; Riley, D.G.; Diaz-Perez, J.C.; Simmons, A.M.; Coolong, T.; McAvoy, T. Prevalence of Aphid-Transmitted Potyviruses in Pumpkin and Winter Squash in Georgia, USA. Viruses 2025, 17, 233. [Google Scholar] [CrossRef]
- Lecoq, H.; Desbiez, C. Viruses of Cucurbit Crops in the Mediterranean Region. In Viruses and Virus Diseases of Vegetables in the Mediterranean Basin; Loebenstein, G., Lecoq, H., Eds.; Academic Press: Cambridge, MA, USA, 2012; Volume 84, pp. 67–126. [Google Scholar] [CrossRef]
- Mofunanya, A.A.J.; Edu, E.A. Physiological and Biochemical Changes in Cucurbita moschata Duch. Ex. Poir Inoculated with a Nigerian Strain of Moroccan Watermelon Mosaic Virus (MWMV): Lagenaria breviflora Isolate. Int. J. Plant. Pathol. 2015, 6, 36–47. [Google Scholar] [CrossRef]
- Radouane, N.; Ezrari, S.; Belabess, Z.; Tahiri, A.; Tahzima, R.; Massart, S.; Jijakli, H.; Benjelloun, M.; Lahlali, R. Viruses of Cucurbit Crops: Current Status in the Mediterranean Region. Phytopathol. Mediterr. 2021, 60, 493–519. [Google Scholar] [CrossRef]
- De Moya-Ruiz, C.; Rabadán, P.; Juárez, M.; Gómez, P. Assessment of the Current Status of Potyviruses in Watermelon and Pumpkin Crops in Spain: Epidemiological Impact of Cultivated Plants and Mixed Infections. Plants 2021, 10, 138. [Google Scholar] [CrossRef]
- Ibaba, J.D.; Laing, M.D.; Gubba, A. Pepo Aphid-Borne Yellows Virus: A New Species in the Genus Polerovirus. Virus Genes 2017, 53, 134–136. [Google Scholar] [CrossRef]
- Ibaba, J.D.; Laing, M.D.; Gubba, A. Incidence and Phylogeny of Viruses Infecting Cucurbit Crops in KwaZulu-Natal, Republic of South Africa. Crop Prot. 2015, 75, 46–54. [Google Scholar] [CrossRef]
- Zhou, C.; Zheng, M.; Du, X.; Cao, Z.; Wu, J.; Zhu, J.; Nie, C. Construction of a Full-Length Infectious CDNA Clone of Zucchini Tigre Mosaic Virus Infecting Snake Gourd and Genetic Diversity Analysis Based on Complete Genome Sequences of ZTMV Isolates. Arch. Virol. 2024, 169, 220. [Google Scholar] [CrossRef]
- Sun, K.; Liu, Y.; Zhou, X.; Yin, C.; Zhang, P.; Yang, Q.; Mao, L.; Shentu, X.; Yu, X. Nanopore Sequencing Technology and Its Application in Plant Virus Diagnostics. Front. Microbiol. 2022, 13, 939666. [Google Scholar] [CrossRef] [PubMed]
- Pecman, A.; Adams, I.; Gutiérrez-Aguirre, I.; Fox, A.; Boonham, N.; Ravnikar, M.; Kutnjak, D. Systematic Comparison of Nanopore and Illumina Sequencing for the Detection of Plant Viruses and Viroids Using Total RNA Sequencing Approach. Front. Microbiol. 2022, 13, 883921. [Google Scholar] [CrossRef] [PubMed]
- Massart, S.; Adams, I.; Al Rwahnih, M.; Baeyen, S.; Bilodeau, G.J.; Blouin, A.G.; Boonham, N.; Candresse, T.; Chandellier, A.; De Jonghe, K.; et al. Guidelines for the Reliable Use of High Throughput Sequencing Technologies to Detect Plant Pathogens and Pests. Peer Community J. 2022, 2, e62. [Google Scholar] [CrossRef]
- Vazquez-Iglesias, I.; Santala, J.; Vossenberg, B.; Gaafar, Y.; Massart, S. PM 7/151 (1) Considerations for the Use of High Throughput Sequencing in Plant Health Diagnostics 1. EPPO Bull. 2022, 52, 619–642. [Google Scholar] [CrossRef]
- Carivali, M.F.B.; Luciani, C.E.; Celli, M.G.; Olmos, S.E.; Obregon, V.G.; Silva, M.I.; Fernandez, F.D.; Mederos, D.C.; Perotto, M.C. Identification, Sequencing and Genetic Diversity of Zucchini Tigre Mosaic Virus Infecting Cucurbita Species in Argentina. Plant Pathol. 2025, 74, 997–1009. [Google Scholar] [CrossRef]
- Liu, B.; Shen, C.-C.; Xia, S.-W.; Song, S.-S.; Su, L.-H.; Li, Y.; Hao, Q.; Liu, Y.-J.; Guan, D.-L.; Wang, N.; et al. A Nanopore-Based Cucumber Genome Assembly Reveals Structural Variations at Two QTLs Controlling Hypocotyl Elongation. Plant Physiol. 2024, 195, 970–985. [Google Scholar] [CrossRef]
- Dong, Z.-X.; Lin, C.-C.; Chen, Y.-K.; Chou, C.-C.; Chen, T.-C. Identification of an Emerging Cucumber Virus in Taiwan Using Oxford Nanopore Sequencing Technology. Plant Methods 2022, 18, 143. [Google Scholar] [CrossRef]
- Mackie, J.; Kinoti, W.M.; Chahal, S.I.; Lovelock, D.A.; Campbell, P.R.; Tran-Nguyen, L.T.T.; Rodoni, B.C.; Constable, F.E. Targeted Whole Genome Sequencing (TWG-Seq) of Cucumber Green Mottle Mosaic Virus Using Tiled Amplicon Multiplex PCR and Nanopore Sequencing. Plants 2022, 11, 2716. [Google Scholar] [CrossRef]
- ICTV International Committee on Taxonomy of Viruses. Family: Potyviridae. Genus: Potyvirus. 2025. Available online: https://ictv.global/report/chapter/potyviridae/potyviridae/potyvirus (accessed on 30 April 2025).
- Sharma, P.; Sahu, A.K.; Verma, R.K.; Mishra, R.; Choudhary, D.K.; Gaur, R.K. Current Status of Potyvirus in India. Arch. Phytopathol. Pflanzenschutz 2014, 47, 906–918. [Google Scholar] [CrossRef]
- Mulholland, S.; Wildman, O.; Daly, A.; Tesoriero, L.; Chapman, T.A. Distribution and Diversity of Viruses Affecting Cucurbit Production in New South Wales, Australia. Australas. Plant Pathol. 2023, 52, 339–351. [Google Scholar] [CrossRef]
- Canto, T.; Aranda, M.A.; Fereres, A. Climate Change Effects on Physiology and Population Processes of Hosts and Vectors That Influence the Spread of Hemipteran-borne Plant Viruses. Glob. Change Biol. 2009, 15, 1884–1894. [Google Scholar] [CrossRef]
- Harrington, R.; Bale, J.S.; Tatchell, G.M. Aphids in a changing environment. In Insects in a Changing Environment; Harringon, R., Stork, N.E., Eds.; Academic Press: London, UK, 1995; pp. 125–155. [Google Scholar]
- Hou, Y.M.; Liu, S.Y.; Zhou, J.H.; Hu, Z.D.; Hu, M.R.; Zhao, Y.X. Researches on population dynamics of green peach aphid, Myzus persicae (Homoptera: Aphididae) on different host plants. Agric. Res. Arid Areas 1999, 17, 45–49. [Google Scholar]
- Norse, D.; Gommes, R. Climate change and agriculture: Physical and human dimensions. In World Agriculture Towards 2015/2030: An FAO Perspective; Bruinsma, J., Ed.; Earthscan Publications Ltd.: London, UK, 2003; pp. 357–372. [Google Scholar]
- Harrington, R.; Clark, S.J.; Welham, S.J.; Verrier, P.J.; Denholm, C.H.; Hulle, M.; Maurice, D.; Rounsevell, M.D.; Cocu, N. Environmental Change and the Phenology of European Aphids. Glob. Change Biol. 2007, 13, 1550–1564. [Google Scholar] [CrossRef]
- Hullé, M.; Cœur d’Acier, A.; Bankhead-Dronnet, S.; Harrington, R. Aphids in the Face of Global Changes. Comptes Rendus Biol. 2010, 333, 497–503. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.; Liu, H.; Qiao, G.; Huang, X. Investigating the Impact of Climate Warming on Phenology of Aphid Pests in China Using Long-Term Historical Data. Insects 2020, 11, 167. [Google Scholar] [CrossRef]
- Ali, A.; Natsuaki, T. Watermelon mosaic virus. Plant Viruses 2007, 1, 80–84. [Google Scholar]
- Leiwakabessy, M. Characterization of A Divergent Strain of Moroccan Watermelon Mosaic Virus (MWMV) from Tanzania Supports the Existence of Two Major Lineages. Master’s Thesis, University of California, Davis, CA, USA, 2021. [Google Scholar]
- Yang, X.; Du, M.; Li, S.; Zhou, X. Coinfection of Cotton Plants with Watermelon Mosaic Virus and a Novel Polerovirus in China. Viruses 2021, 13, 2210. [Google Scholar] [CrossRef]
- Cespedes, M.K.; Melgarejo, T.A.; Henry, P.M.; Al Rwahnih, M.; Gilbertson, R.L. First Report of Watermelon Mosaic Virus Naturally Infecting Coriander (Coriandrum sativum) and Causing a Leaf Mottling Disease in California. Plant Dis. 2023, 107, 1248. [Google Scholar] [CrossRef]
- Hang, C.; Huang, C.-R.; Zhu, F. First Report of Mosaic Disease of Crape Myrtle Caused by Watermelon Mosaic Virus in Jiangsu Province in China. Plant Health Prog. 2023, 24, 380–381. [Google Scholar] [CrossRef]
- Cohen, S.; Nitzany, F.E. Identity of viruses affecting Cucurbits in Israel. Phytopathology 1963, 53, 193–196. [Google Scholar]
- Mihaljevski Boltek, L. Detekcija Ekonomski Značajnih Virusa Tikvenjača u Hrvatskoj. Master’s Thesis, University of Zagreb, Zagreb, Croatia, 2019. [Google Scholar]
- Ivić, D.; Milanović, J.; Mihaljevski Boltek, L.; Novak, A.; Vončina, D. Zastupljenost i raširenost gospodarski važnih virusa tikvica (Cucurbita pepo L.) i tikvi (Cucurbita sp.) u Hrvatskoj. Agron. Glas. 2021, 83, 149–160. [Google Scholar] [CrossRef]
- Moya-Ruiz, C.D.; Gómez, P.; Juárez, M. Occurrence, Distribution, and Management of Aphid-Transmitted Viruses in Cucurbits in Spain. Pathogens 2023, 12, 422. [Google Scholar] [CrossRef] [PubMed]
- Desbiez, C.; Costa, C.; Wipf-Scheibel, C.; Girard, M.; Lecoq, H. Serological and Molecular Variability of Watermelon Mosaic Virus (Genus Potyvirus). Arch. Virol. 2007, 152, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Bertin, S.; Manglli, A.; McLeish, M.; Tomassoli, L. Genetic Variability of Watermelon Mosaic Virus Isolates Infecting Cucurbit Crops in Italy. Arch. Virol. 2020, 165, 937–946. [Google Scholar] [CrossRef]
- Desbiez, C.; Verdin, E.; Moury, B.; Lecoq, H.; Millot, P.; Wipf-Scheibel, C.; Mirzayeva, S.; Sultanova, N.; Balakishiyeva, G.; Mammadov, A.; et al. Prevalence and Molecular Diversity of the Main Viruses Infecting Cucurbit and Solanaceous Crops in Azerbaijan. Eur. J. Plant Pathol. 2019, 153, 359–369. [Google Scholar] [CrossRef]
- Perotto, M.C.; Celli, M.G.; Pozzi, E.A.; Luciani, C.E.; Conci, V.C. Occurrence and Characterization of a Severe Isolate of Watermelon Mosaic Virus from Argentina. Eur. J. Plant Pathol. 2016, 146, 213–218. [Google Scholar] [CrossRef]
- Desbiez, C.; Joannon, B.; Wipf-Scheibel, C.; Chandeysson, C.; Lecoq, H. Emergence of New Strains of Watermelon Mosaic Virus in South-Eastern France: Evidence for Limited Spread but Rapid Local Population Shift. Virus Res. 2009, 141, 201–208. [Google Scholar] [CrossRef]
- Lecoq, H.; Fabre, F.; Joannon, B.; Wipf-Scheibel, C.; Chandeysson, C.; Schoeny, A.; Desbiez, C. Search for Factors Involved in the Rapid Shift in Watermelon Mosaic Virus (WMV) Populations in South-Eastern France. Virus Res. 2011, 159, 115–123. [Google Scholar] [CrossRef]
- Borodynko, N.; Hasiów-Jaroszewska, B.; Rymelska, N.; Pospieszny, H. Watermelon Mosaic Virus Reported for the First Time in Poland. Plant Pathol. 2009, 58, 783. [Google Scholar] [CrossRef]
- Kamberoglu, M.A.; Desbiez, C.; Caliskan, A.F. Characterization of an emerging ısolate of watermelon mosaic virus in Turkey. Agric. Biol. 2015, 17, 211–215. [Google Scholar]
- Abdalla, O.A.; Ali, A. Genetic Variability and Evidence of a New Subgroup in Watermelon Mosaic Virus Isolates. Pathogens 2021, 10, 1245. [Google Scholar] [CrossRef] [PubMed]
- Salvaudon, L.; De Moraes, C.M.; Mescher, M.C. Outcomes of Co-Infection by Two Potyviruses: Implications for the Evolution of Manipulative Strategies. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122959. [Google Scholar] [CrossRef] [PubMed]
- CABI. PlantwisePlus Knowledge Bank. 2023. Available online: https://plantwiseplusknowledgebank.org/doi/10.1079/pwkb.species.57657 (accessed on 11 October 2023).
- EPPO. European Plant Protection Organisation. EPPO Global Database. 2025. Available online: https://gd.eppo.int/taxon/ (accessed on 20 April 2025).
- Lisa, V.; Boccardo, G.; D’Agostino, G.; Dellavalle, G.; D’Aquilio, M. Characterization of a potyvirus that causes zucchini yellow mosaic. Phytopathology 1981, 71, 667–672. [Google Scholar] [CrossRef]
- Kaldis, A.; Berbati, M.; Melita, O.; Reppa, C.; Holeva, M.; Otten, P.; Voloudakis, A. Exogenously Applied DsRNA Molecules Deriving from the Zucchini Yellow Mosaic Virus (ZYMV) Genome Move Systemically and Protect Cucurbits against ZYMV. Mol. Plant Pathol. 2018, 19, 883–895. [Google Scholar] [CrossRef]
- Simmons, H.E.; Holmes, E.C.; Gildow, F.E.; Bothe-Goralczyk, M.A.; Stephenson, A.G. Experimental Verification of Seed Transmission of Zucchini Yellow Mosaic Virus. Plant Dis. 2011, 95, 751–754. [Google Scholar] [CrossRef]
- Lecoq, H.; Wipf-Scheibel, C.; Chandeysson, C.; Lê Van, A.; Fabre, F.; Desbiez, C. Molecular Epidemiology of Zucchini Yellow Mosaic Virus in France: An Historical Overview. Virus Res. 2009, 141, 190–200. [Google Scholar] [CrossRef]
- Hasiów-Jaroszewska, B.; Rymelska, N.; Borodynko, N.; Pospieszny, H. Biological and molecular characterization of the Polish Zucchini yellow mosaic virus isolates. Acta Sci. Pol. Hortorum Cultus 2013, 12, 75–85. [Google Scholar]
- Lecoq, H.; Dafalla, G.; Desbiez, C.; Wipf-Scheibel, C.; Delécolle, B.; Lanina, T.; Ullah, Z.; Grumet, R. Biological and Molecular Characterization of Moroccan Watermelon Mosaic Virus and a Potyvirus Isolate from Eastern Sudan. Plant Dis. 2001, 85, 547–552. [Google Scholar] [CrossRef]
- Lecoq, H.; Justafré, I.; Wipf-Scheibel, C.; Desbiez, C. Moroccan Watermelon Mosaic Virus Newly Reported on Zucchini Squash in France. Plant Pathol. 2008, 57, 766. [Google Scholar] [CrossRef]
- Fischer, H.U.; Lockhart, B.E.L. Serious losses in cucurbits caused by watermelon mosaic virus in Morocco. Plant Dis. Rep. 1974, 58, 143–146. [Google Scholar]
- Purcifull, D.E.; Hiebert, E. Serological distinction of watermelon mosaic virus isolates. Phytopathology 1979, 69, 112–116. [Google Scholar] [CrossRef]
- McKern, N.M.; Strike, P.M.; Barnett, O.W.; Ward, C.W.; Shukla, D.D. Watermelon Mosaic Virus-Morocco Is a Distinct Potyvirus. Arch. Virol. 1993, 131, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Yakoubi, S.; Desbiez, C.; Fakhfakh, H.; Wipf-Scheibel, C.; Marrakchi, M.; Lecoq, H. Biological Characterization and Complete Nucleotide Sequence of a Tunisian Isolate of Moroccan Watermelon Mosaic Virus. Arch. Virol. 2008, 153, 117–125. [Google Scholar] [CrossRef]
- Ibaba, J.D.; Laing, M.D.; Gubba, A. First Report of a Novel Potyvirus from the Papaya Ringspot Virus Cluster Infecting Zucchini (Cucurbita pepo) in KwaZulu-Natal, Republic of South Africa. Plant Dis. 2015, 99, 1289. [Google Scholar] [CrossRef]
- Arocha, Y.; Vigheri, N.; Nkoy-Florent, B.; Bakwanamaha, K.; Bolomphety, B.; Kasongo, M.; Betts, P.; Monger, W.A.; Harju, V.; Mumford, R.A.; et al. First Report of the Identification of Moroccan Watermelon Mosaic Virus in Papaya in Democratic Republic of Congo. Plant Pathol. 2008, 57, 387. [Google Scholar] [CrossRef]
- Read, D.A.; Muoma, J.; Thompson, G.D. Metaviromic Analysis Reveals Coinfection of Papaya in Western Kenya with a Unique Strain of Moroccan Watermelon Mosaic Virus and a Novel Member of the Family Alphaflexiviridae. Arch. Virol. 2020, 165, 1231–1234. [Google Scholar] [CrossRef]
- Ibaba, J.D.; Laing, M.D.; Gubba, A. Genome Sequence Analysis of Two South African Isolates of Moroccan Watermelon Mosaic Virus Infecting Cucurbits. Virus Genes 2016, 52, 896–899. [Google Scholar] [CrossRef]
- Menzel, W.; Abang, M.M.; Winter, S. Characterization of Cucumber Vein-Clearing Virus, a Whitefly (Bemisia tabaci G.)-Transmitted Carlavirus. Arch. Virol. 2011, 156, 2309–2311. [Google Scholar] [CrossRef]
- Owolabi, A.T.; Rabenstein, F.; Ehrig, F.; Edgar, M.M.; Vetten, H.J. Strains of Moroccan Watermelon Mosaic Virus Isolated from Lagenaria breviflorus and Coccinia barteri in Calabar, Southeastern Nigeria. Int. J. Virol. 2012, 8, 258–270. [Google Scholar] [CrossRef]
- Bananej, K.; Orfanidou, C.G.; Maliogka, V.I.; Katis, N.I. First Report of Moroccan Watermelon Mosaic Virus in Zucchini in Iran. Plant Dis. 2018, 102, 2047. [Google Scholar] [CrossRef]
- Mumo, N.N.; Ateka, E.M.; Mamati, E.G.; Rimberia, F.K.; Asudi, G.O.; Machuka, E.; Njuguna, J.N.; Stomeo, F.; Pelle, R. Occurrence of a Novel Strain of Moroccan Watermelon Mosaic Virus Infecting Pumpkins in Kenya. Plant Dis. 2022, 106, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Yeşil, S. First Report of Moroccan Watermelon Mosaic Virus on Edible Seed Squash in Turkey. J. Plant Pathol. 2021, 103, 737. [Google Scholar] [CrossRef]
- Kone, D.; (West African Science Service Center on Climate Change and Adapted Land Use (WASCAL), Acrra, Ghana); Sorho, F.; (Université Félix Houphouët-Boigny, Cocody, Abidjan, Ivory Coast); Affouda, L.; (the World Bank, Niamey, Niger); Desbiez, C.; (French National Institute for Agriculture, Food and Environment (INRAE), Montfavet, France); Lecoq, H.; (French National Institute for Agriculture, Food and Environment (INRAE), Montfavet, France). Moroccan watermelon mosaic virus newly reported on zucchini squash in Benin. Unpublished Work. 2010. [Google Scholar]
- Silva, B.A.; Kauffmann, C.M.; Mota, H.B.S.; Queiroz, P.S.; Batista, A.M.V.; Cardenas, S.B.S.; Freitas, D.M.S.; Nagata, T. First Report of Moroccan Watermelon Mosaic Virus in Pumpkin Plants in Brazil. Plant Dis. 2024, 108, 539. [Google Scholar] [CrossRef]
- Šeruga, M.; Škorić, D.; Botti, S.; Paltrinieri, S.; Juretić, N.; Bertaccini, A.F. Molecular Characterization of a Phytoplasma from the Aster Yellows (16SrI) Group Naturally Infecting Populus nigra L. ‘Italica’ Trees in Croatia. For. Pathol. 2003, 33, 113–125. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 9 February 2023).
- De Coster, W.; D’Hert, S.; Schultz, D.T.; Cruts, M.; Van Broeckhoven, C. NanoPack: Visualizing and processing long-read sequencing data. Bioinformatics 2018, 34, 2666–2669. [Google Scholar] [CrossRef]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; 1000 Genome Project Data Processing Subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 2011, 27, 2987–2993. [Google Scholar] [CrossRef]
- Elbeshehy, E.K.F.; Metwali, E.M.R.; Almaghrabi, O.A. Antiviral Activity of Thuja Orientalis Extracts against Watermelon Mosaic Virus (WMV) on Citrullus Lanatus. Saudi J. Biol. Sci. 2015, 22, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Thomson, K.G.; Dietzgen, R.G.; Gibbs, A.J.; Tang, Y.C.; Liesack, W.; Teakle, D.S.; Stackebrandt, E. Identification of Zucchini Yellow Mosaic Potyvirus by RT-PCR and Analysis of Sequence Variability. J. Virol. Methods 1995, 55, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Muhire, B.M.; Varsani, A.; Martin, D.P. SDT: A Virus Classification Tool Based on Pairwise Sequence Alignment and Identity Calculation. PLoS ONE 2014, 9, e108277. [Google Scholar] [CrossRef]
- Martin, D.P.; Varsani, A.; Roumagnac, P.; Botha, G.; Maslamoney, S.; Schwab, T.; Kelz, Z.; Kumar, V.; Murrell, B. RDP5: A Computer Program for Analyzing Recombination in, and Removing Signals of Recombination from, Nucleotide Sequence Datasets. Virus Evol. 2021, 7, veaa087. [Google Scholar] [CrossRef]
- Škorić, D.; Zindović, J.; Grbin, D.; Pul, P.; Božović, V.; Margaria, P.; Mehle, N.; Pecman, A.; Kogej Zwitter, Z.; Kutnjak, D.; et al. Tomato spotted wilt virus in tomato from Croatia, Montenegro and Slovenia: Genetic diversity and evolution. Front. Microbiol. 2025, submitted.
- Grbin, D.; Pecman, A.; Musić, M.Š.; Kutnjak, D.; Škorić, D. First Report of Potato Virus S and Potato Virus Y in Tomatoes from Croatia. Plant Dis. 2023, 107, 975. [Google Scholar] [CrossRef]
- Desbiez, C.; Joannon, B.; Wipf-Scheibel, C.; Chandeysson, C.; Lecoq, H. Recombination in Natural Populations of Watermelon Mosaic Virus: New Agronomic Threat or Damp Squib? J. Gen. Virol. 2011, 92, 1939–1948. [Google Scholar] [CrossRef]
- Novakova, S.; Svoboda, J.; Glasa, M. Analysis of the Complete Sequences of Two Biologically Distinct Zucchini Yellow Mosaic Virus Isolates Further Evidences the Involvement of a Single Amino Acid in the Virus Pathogenicity. Acta Virol. 2014, 58, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Novak, A.; Ivić, D. Viral diseases of cucurbits. Glas. Biljn. Zaštite 2019, 19, 369–377. [Google Scholar]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The Impact of Climate Change on Agricultural Insect Pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Malandraki, I.; Vassilakos, N.; Xanthis, C.; Kontosfiris, G.; Katis, N.I.; Varveri, C. First Report of Moroccan Watermelon Mosaic Virus in Zucchini Crops in Greece. Plant Dis. 2014, 98, 702. [Google Scholar] [CrossRef]
- Khan, L.U.; Cao, X.; Zhao, R.; Tan, H.; Xing, Z.; Huang, X. Effect of Temperature on Yellow Leaf Disease Symptoms and Its Associated Areca Palm Velarivirus 1 Titer in Areca Palm (Areca catechu L.). Front. Plant Sci. 2022, 13, 1023386. [Google Scholar] [CrossRef]
- Moury, B.; Desbiez, C. Host Range Evolution of Potyviruses: A Global Phylogenetic Analysis. Viruses 2020, 12, 111. [Google Scholar] [CrossRef]
- Kabelka, E.; Grumet, R. Inheritance of Resistance to the Moroccan Watermelon Mosaic Virus in the Cucumber Line TMG-1 and Cosegregation with Zucchini Yellow Mosaic Virus Resistance. Euphytica 1997, 95, 237–242. [Google Scholar] [CrossRef]
- Vučurović, A.; Bulajić, A.; Stanković, I.; Ristić, D.; Berenji, J.; Jović, J.; Krstić, B. Non-Persistently Aphid-Borne Viruses Infecting Pumpkin and Squash in Serbia and Partial Characterization of Zucchini Yellow Mosaic Virus Isolates. Eur. J. Plant Pathol. 2012, 133, 935–947. [Google Scholar] [CrossRef]
- Glasa, M.; Svoboda, J.; Nováková, S. Analysis of the Molecular and Biological Variability of Zucchini Yellow Mosaic Virus Isolates from Slovakia and Czech Republic. Virus Genes 2007, 35, 415–421. [Google Scholar] [CrossRef]
- Roggero, P.; Gotta, P.; Stravato, V.M.; Dellavalle, G.; Ciuffo, M. Further spread of Moroccan watermelon mosaic potyvirus in Italy in 1998. Plant Pathol. 1999, 81, 149. [Google Scholar]
- Ciuffo, M.; (CNR-IPSP, Turin, Italy). Personal communication, 2024.
- Ng, J.C.K.; Falk, B.W. Virus-Vector Interactions Mediating Nonpersistent and Semipersistent Transmission of Plant Viruses. Annu. Rev. Phytopathol. 2006, 44, 183–212. [Google Scholar] [CrossRef]
- Chatzivassiliou, E.K.; Papapanagiotou, A.P.; Mpenardis, P.D.; Perdikis, D.C.; Menexes, G. Transmission of Moroccan Watermelon Mosaic Virus (MWMV) by Aphids in Greece. Plant Dis. 2016, 100, 601–606. [Google Scholar] [CrossRef]


| Year of Collection | Sampling Location, Croatia | Sample Name | Cucurbit Species | Plant Material |
|---|---|---|---|---|
| 2021 | Pitomača | T1 | Cucurbita pepo ‘Naxos F1’ | fruit skin |
| Pitomača | T2 | Cucurbita pepo ‘Naxos F1’ | fruit skin | |
| Pitomača | T3 | Cucurbita pepo ‘Naxos F1’ | fruit skin | |
| Stari Gradac | T4 | Cucurbita pepo ‘Naxos F1’ | fruit skin | |
| Pitomača | T5 | Cucurbita pepo ‘Naxos F1’ | fruit skin | |
| Pitomača | T6 | Cucurbita pepo ‘Naxos F1’ | fruit skin | |
| 2022 | Sedlarica | 74SECu22S | Cucumis sativus ‘Salatar’ | leaves |
| Sedlarica | 96SECu22S | Cucumis sativus ‘Salatar’ | leaves | |
| Sedlarica | 97SECu22S | Cucumis sativus ‘Salatar’ | leaves | |
| Sedlarica | 101SECu22S | Cucumis sativus ‘Salatar’ | leaves | |
| Sedlarica | 102SECu22S | Cucumis sativus ‘Salatar’ | leaves | |
| Sedlarica | 125SEZ22S | Cucurbita moschata | leaves | |
| Sedlarica | 150SECu22S | Cucumis sativus ‘Salatar’ | leaves | |
| Sedlarica | 152SECu22S | Cucumis sativus ‘Salatar’ | leaves |
| Virus | Primer Name | Orientation | Sequence 5′-3′ | Region | Product Size (bp) | Reference |
|---|---|---|---|---|---|---|
| WMV | WMV-For | forward | GAATCAGTGTCTCTGCAATCAGG | CP | 825 | [84] |
| WMV-Rev | reverse | ATTCACGTCCCTTGCAGTGTG | ||||
| ZYMV | ZY-2 | forward | GCTCCATACATAGCTGAGACAGC | NIb and CP | 1200 | [85] |
| ZY-3 | reverse | TAGGCTTGCAAACGGAGTCTAATC | ||||
| MWMV | MWMV-5 | forward | AGCAAGCGCCATACTCTGA | NIb and CP | 627 | [60] |
| MWMV-3 | reverse | CAAACTCCATTAACATTCGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagunić, M.; Grbin, D.; Marohnić, M.; Novak, A.; Čajkulić, A.M.; Škorić, D. Severely Symptomatic Cucurbits in Croatia Dominantly Harbor a Complex of Potyviruses Including the Emerging Moroccan Watermelon Mosaic Virus. Agronomy 2025, 15, 1613. https://doi.org/10.3390/agronomy15071613
Jagunić M, Grbin D, Marohnić M, Novak A, Čajkulić AM, Škorić D. Severely Symptomatic Cucurbits in Croatia Dominantly Harbor a Complex of Potyviruses Including the Emerging Moroccan Watermelon Mosaic Virus. Agronomy. 2025; 15(7):1613. https://doi.org/10.3390/agronomy15071613
Chicago/Turabian StyleJagunić, Martin, Dorotea Grbin, Marko Marohnić, Adrijana Novak, Ana Marija Čajkulić, and Dijana Škorić. 2025. "Severely Symptomatic Cucurbits in Croatia Dominantly Harbor a Complex of Potyviruses Including the Emerging Moroccan Watermelon Mosaic Virus" Agronomy 15, no. 7: 1613. https://doi.org/10.3390/agronomy15071613
APA StyleJagunić, M., Grbin, D., Marohnić, M., Novak, A., Čajkulić, A. M., & Škorić, D. (2025). Severely Symptomatic Cucurbits in Croatia Dominantly Harbor a Complex of Potyviruses Including the Emerging Moroccan Watermelon Mosaic Virus. Agronomy, 15(7), 1613. https://doi.org/10.3390/agronomy15071613







