Microplastics in Soil–Plant Systems: Current Knowledge, Research Gaps, and Future Directions for Agricultural Sustainability
Abstract
1. Introduction
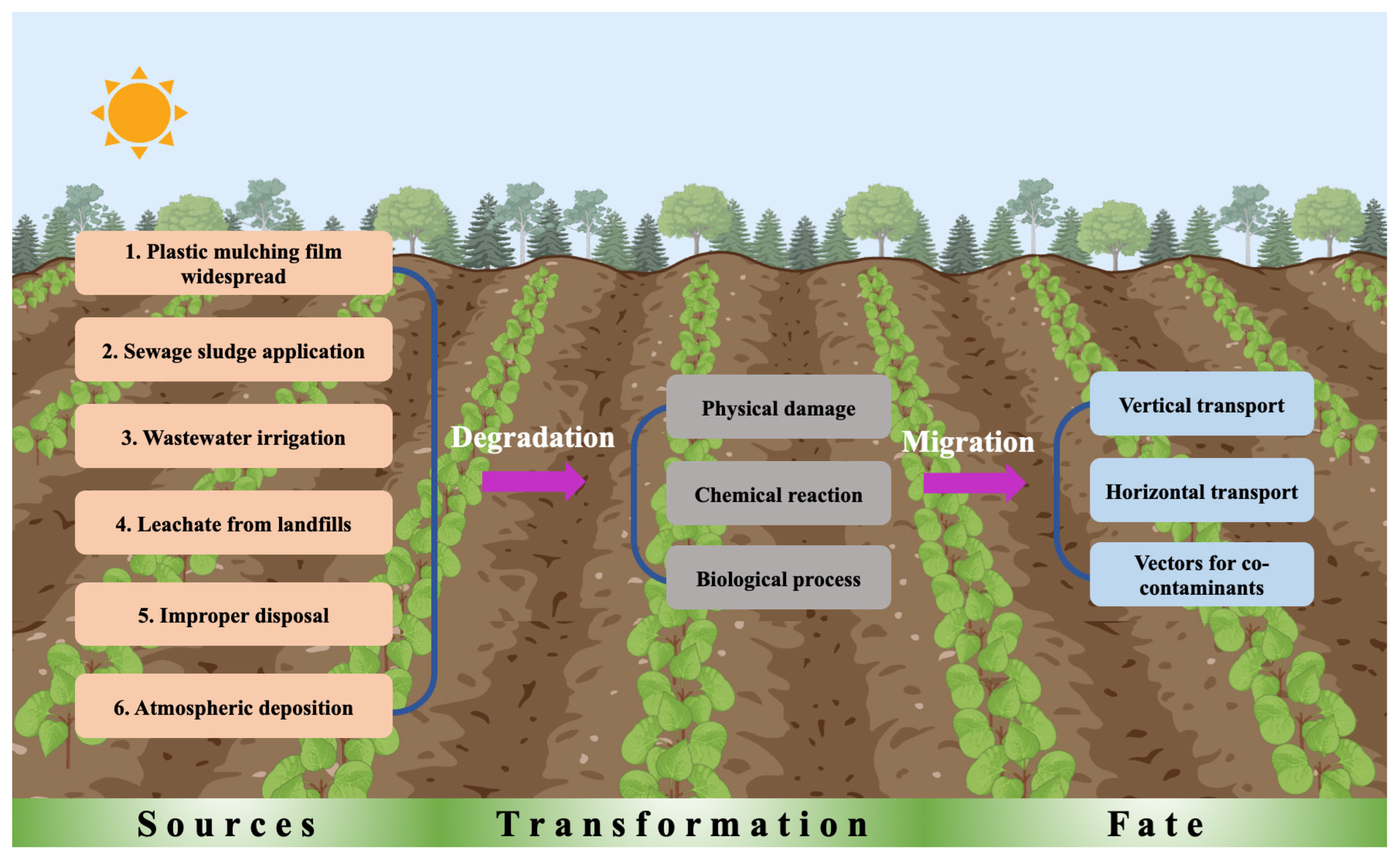
2. Sources, Migration, and Transformation of Microplastics in Agricultural Soils
2.1. Major Sources, Types, and Sizes of Microplastics in Agricultural Soils
2.2. Degradation of Microplastics in Agricultural Soils
2.3. Migration of Microplastics in Agricultural Soils
2.4. Microplastics as Vectors for Co-Contaminants in Agricultural Soils
3. Effects of Microplastics on Soil–Plant Systems: Consequences for Soil Function, Plant Development, and Food Safety
3.1. Effects of Microplastics on Soil Properties
3.1.1. Soil Physical Properties
3.1.2. Soil Chemical Properties
3.2. Effects of Microplastics on Soil Microbial Communities
3.3. Microplastic–Plant Interactions
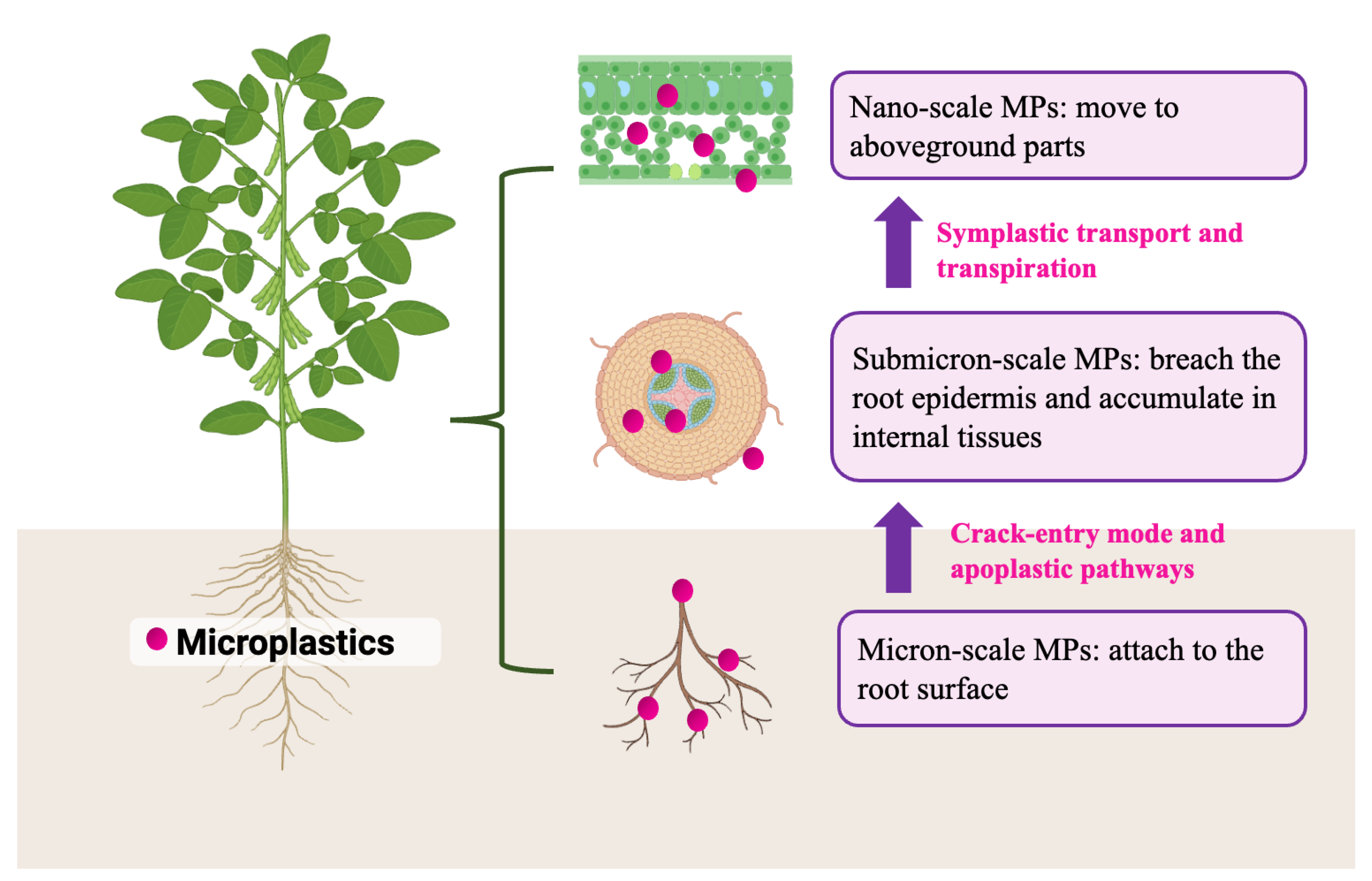
3.3.1. Entry and Uptake Pathways
3.3.2. Accumulation and Translocation Patterns
3.4. Ecotoxicological Effects of Microplastics on Plants
3.4.1. Physical Toxicity
3.4.2. Oxidative Stress and Antioxidant Responses
3.4.3. Genotoxicity and Cellular Effects
3.4.4. Carrier Effects
3.4.5. Omics-Based Molecular Evidence
3.5. Impacts on Plant Growth
3.5.1. Plant Morphology
3.5.2. Plant Physiology
3.6. Contradictory Results in Soil–Plant–Microplastic Interactions and Possible Explanations
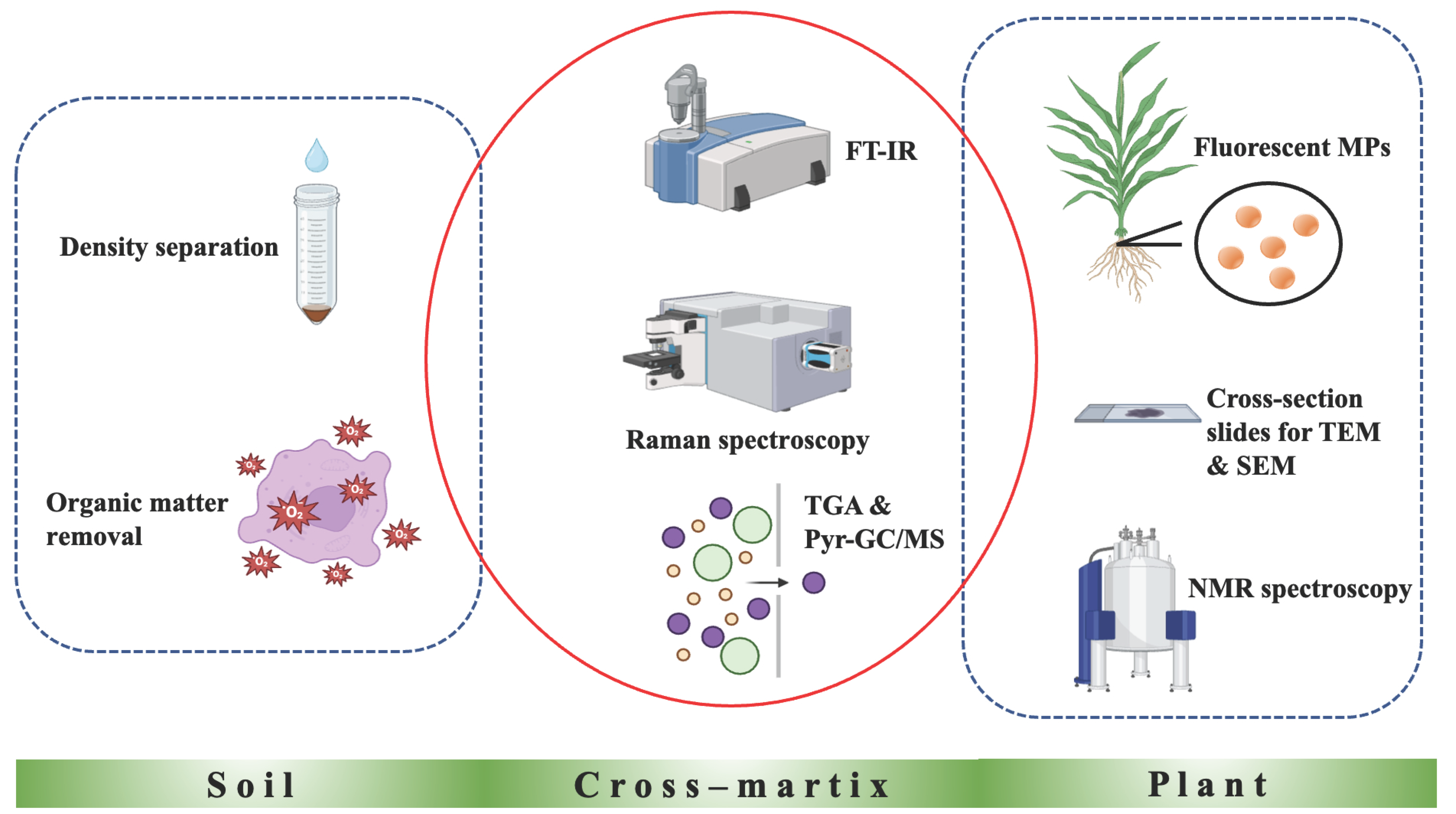
4. Microplastic Detection and Quantification in Soil–Plant Systems
4.1. Detection and Quantification of Microplastics in Soil Samples
4.2. Detection and Quantification of Microplastics in Plant Tissues
4.3. Cross-Matrix Approaches for Detection and Quantification of Microplastics
5. Knowledge Gaps in Current Research
5.1. Limited Scope of Research Subjects
5.2. Lack of Environmental Relevance in Experimental Conditions
5.3. Technical Barriers to Detection and Quantification
5.4. Underexplored Co-Contaminant Interactions and Ecological Risks
5.5. Insufficient Evidence on Long-Term Impacts of MPs on Soil Functionality
5.6. Overlooked Cross-System Transport and Environmental Spread of Microplastics
6. Future Directions for Microplastic Pollution in Agroecosystems
6.1. Bridging the Gap Between Laboratory Studies and Real-World Agroecosystems
6.2. Enhancing Microplastic Detection and Quantification in Soil–Plant Systems
6.3. Addressing Multi-Pollutant Interactions in Agroecosystems
6.4. Safeguarding Soil Health and Biogeochemical Cycling
6.5. Securing Food Safety and Crop Resilience
6.6. Advancing Systems-Level Understanding Under Global Change
6.7. Strengthening Policy Frameworks for Agricultural Microplastic Governance
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; Mcgonigle, D.F.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Bostan, N.; Ilyas, N.; Akhtar, N.; Mehmood, S.; Saman, R.U.; Sayyed, R.Z.; Shatid, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Pandiaraj, S. Toxicity assessment of microplastic (MPs); a threat to the ecosystem. Environ. Res. 2023, 234, 116523. [Google Scholar] [CrossRef]
- Sana, S.S.; Dogiparthi, L.K.; Gangadhar, L.; Chakravorty, A.; Abhishek, N. Effects of microplastics and nanoplastics on marine environment and human health. Environ. Sci. Pollut. Res. Int. 2020, 27, 44743–44756. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Guo, Z.-F.; Xu, Y.-Y.; Ka Shun Chan, F.; Xu, Y.-Y.; Johnson, M.; Zhu, Y.-G. Widespread occurrence of microplastics in marine bays with diverse drivers and environmental risk. Environ. Int. 2022, 168, 107483. [Google Scholar] [CrossRef]
- Cheng, J.; Meistertzheim, A.-L.; Leistenschneider, D.; Philip, L.; Jacquin, J.; Escande, M.-L.; Barbe, V.; ter Halle, A.; Chapron, L.; Lartaud, F.; et al. Impacts of microplastics and the associated plastisphere on physiological, biochemical, genetic expression and gut microbiota of the filter-feeder amphioxus. Environ. Int. 2023, 172, 107750. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Z.; Xiao, J.; Li, F.; Zhang, F.; Zhang, Z. Evaluation of niche, diversity, and risks of microplastics in farmland soils of different rocky desertification areas. J. Hazard. Mater. 2024, 466, 133603. [Google Scholar] [CrossRef] [PubMed]
- Sahai, H.; Aguilera del Real, A.M.; Alcayde, A.; Bueno, M.J.M.; Wang, C.; Hernando, M.D.; Fernández-Alba, A.R. Key insights into microplastic pollution in agricultural soils: A comprehensive review of worldwide trends, sources, distribution, characteristics and analytical approaches. TrAC Trends Anal. Chem. 2025, 185, 118176. [Google Scholar] [CrossRef]
- Brahney, J.; Hallerud, M.; Heim, E.; Hahnenberger, M.; Sukumaran, S. Plastic rain in protected areas of the United States. Science 2020, 368, 1257–1260. [Google Scholar] [CrossRef]
- Rochman, C.M. Microplastics research-from sink to source. Science 2018, 360, 28–29. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Xu, L.; Li, R.; Zhang, R.; Li, M.; Ran, C.; Rao, Z.; Wei, X.; Chen, M.; et al. Leaf absorption contributes to accumulation of microplastics in plants. Nature 2025, 641, 666–673. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef] [PubMed]
- Cusworth, S.J.; Davies, W.J.; McAinsh, M.R.; Stevens, C.J. A nationwide assessment of microplastic abundance in agricultural soils: The influence of plastic crop covers within the United Kingdom. Plants People Planet 2024, 6, 304–314. [Google Scholar] [CrossRef]
- Crossman, J.; Hurley, R.R.; Futter, M.; Nizzetto, L. Transfer and transport of microplastics from biosolids to agricultural soils and the wider environment. Sci. Total Environ. 2020, 724, 138334. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, M.; Li, R.; Peijnenburg, W.J.G.M.; Yang, L.; Liu, P.; Shi, Q. Transport Dynamics and Physiological Responses of Polystyrene Nanoplastics in Pakchoi: Implications for Food Safety and Environmental Health. J. Agric. Food Chem. 2025, 73, 10923–10933. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Guo, J.; Dong, Y.; Wang, Z.; Gong, L.; Li, X. Polystyrene microplastics disturb the redox homeostasis, carbohydrate metabolism and phytohormone regulatory network in barley. J. Hazard. Mater. 2021, 415, 125614. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, Y.; Li, R.; Zhou, Q.; Peijnenburg, W.J.G.M.; Yin, N.; Yang, J.; Tu, C.; Zhang, Y. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustain. 2020, 3, 929–937. [Google Scholar] [CrossRef]
- Rong, S.; Wang, S.; Liu, H.; Li, Y.; Huang, J.; Wang, W.; Han, B.; Su, S.; Liu, W. Evidence for the transportation of aggregated microplastics in the symplast pathway of oilseed rape roots and their impact on plant growth. Sci. Total Environ. 2024, 912, 169419. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, Y.; Marshall, M.R.; Zhao, J.; Gui, H.; Yang, Y.; Zeng, Z.; Jones, D.L.; Zang, H. Microplastics as an emerging threat to plant and soil health in agroecosystems. Sci. Total Environ. 2021, 787, 147444. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobučar, G. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef]
- Chen, Z.; Carter, L.J.; Banwart, S.A.; Pramanik, D.D.; Kay, P. Multifaceted effects of microplastics on soil-plant systems: Exploring the role of particle type and plant species. Sci. Total Environ. 2024, 954, 176641. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Zhang, W.; Jiang, M.; Li, S.; Liang, G.; Bu, Q.; Xu, L.; Zhu, H.; Lu, A. Species-dependent response of food crops to polystyrene nanoplastics and microplastics. Sci. Total Environ. 2021, 796, 148750. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wei, X.; Wang, C.; Zhao, R. Plastic film mulching significantly boosts crop production and water use efficiency but not evapotranspiration in China. Agric. Water Manag. 2023, 275, 108023. [Google Scholar] [CrossRef]
- Salama, K.; Geyer, M. Plastic Mulch Films in Agriculture: Their Use, Environmental Problems, Recycling and Alternatives. Environments 2023, 10, 179. [Google Scholar] [CrossRef]
- Yang, J.; Li, L.; Li, R.; Xu, L.; Shen, Y.; Li, S.; Tu, C.; Wu, L.; Christie, P.; Luo, Y. Microplastics in an agricultural soil following repeated application of three types of sewage sludge: A field study. Environ. Pollut. 2021, 289, 117943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Mathew, J.; Pulicharla, R.; Rezai, P.; Brar, S.K. Microplastics in wastewaters: Pretreatment to detection trail. J. Water Process Eng. 2024, 64, 105702. [Google Scholar] [CrossRef]
- Wojnowska-Baryła, I.; Bernat, K.; Zaborowska, M. Plastic Waste Degradation in Landfill Conditions: The Problem with Microplastics, and Their Direct and Indirect Environmental Effects. Int. J. Environ. Res. Public Health 2022, 19, 13223. [Google Scholar] [CrossRef]
- Statistics. Annual Production of Plastics Worldwide from 1950 to 2023 (in Million Metric Tons). 2024. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 29 November 2024).
- Li, J.; Zhang, J.; Ren, S.; Huang, D.; Liu, F.; Li, Z.; Zhang, H.; Zhao, M.; Cao, Y.; Mofolo, S.; et al. Atmospheric deposition of microplastics in a rural region of North China Plain. Sci. Total Environ. 2023, 877, 162947. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, S.; Allen, S.; Allen, D.; Gao, T.; Sillanpää, M. Atmospheric microplastics: A review on current status and perspectives. Earth Sci. Rev. 2020, 203, 103118. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Kim, B.-H.; Kim, K.-H. Distribution of microplastics in soil by types of land use in metropolitan area of Seoul. Appl. Biol. Chem. 2024, 67, 15. [Google Scholar] [CrossRef]
- Jia, W.; Karapetrova, A.; Zhang, M.; Xu, L.; Li, K.; Huang, M.; Wang, J.; Huang, Y. Automated identification and quantification of invisible microplastics in agricultural soils. Sci. Total Environ. 2022, 844, 156853. [Google Scholar] [CrossRef] [PubMed]
- Dimassi, S.N.; Hahladakis, J.N.; Yahia, M.N.D.; Ahmad, M.I.; Sayadi, S.; Al-Ghouti, M.A. Degradation-fragmentation of marine plastic waste and their environmental implications: A critical review. Arab. J. Chem. 2022, 15, 104262. [Google Scholar] [CrossRef]
- Liu, X.; Han, M.; Liu, T.; Liu, L. Macroscopic and Microscopic Characteristics of Strength Degradation of Silty Soil Improved by Regenerated Polyester Fibers under Dry–Wet Cycling. Polymers 2023, 15, 4367. [Google Scholar] [CrossRef]
- Bhattacharjee, L.; Gopakumar, A.N.; Beheshtimaal, A.; Jazaei, F.; Ccanccapa-Cartagena, A.; Salehi, M. Mechanisms of microplastic generation from polymer-coated controlled-release fertilizers (PC-CRFs). J. Hazard. Mater. 2025, 486, 137082. [Google Scholar] [CrossRef]
- Li, F.; Huang, D.; Wang, G.; Cheng, M.; Chen, H.; Zhou, W.; Xiao, R.; Li, R.; Du, L.; Xu, W. Microplastics/nanoplastics in porous media: Key factors controlling their transport and retention behaviors. Sci. Total Environ. 2024, 926, 171658. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, X.; Yi, K.; He, L.; Han, P.; Tong, M. Transport and deposition of microplastic particles in saturated porous media: Co-effects of clay particles and natural organic matter. Environ. Pollut. 2021, 287, 117585. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Permana, R.; Chakraborty, S.; Valsami-Jones, E. Nanoplastics in aquatic environments: The hidden impact of aging on fate and toxicity. Environ. Chem. Ecotoxicol. 2025, 7, 429–444. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, Z.; Xiang, S.; Yan, H.; Tian, H. Degradation of Polymer Materials in the Environment and Its Impact on the Health of Experimental Animals: A Review. Polymers 2024, 16, 2807. [Google Scholar] [CrossRef]
- Luo, H.; Chang, L.; Ju, T.; Li, Y. Factors Influencing the Vertical Migration of Microplastics up and down the Soil Profile. ACS Omega 2024, 9, 50064–50077. [Google Scholar] [CrossRef]
- Xu, Y.; Ou, Q.; van der Hoek, J.P.; Liu, G.; Lompe, K.M. Photo-oxidation of Micro- and Nanoplastics: Physical, Chemical, and Biological Effects in Environments. Environ. Sci. Technol. 2024, 58, 991–1009. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-G.; Wen, P.-P.; Yang, Y.-F.; Jia, P.-P.; Li, W.-G.; Pei, D.-S. Plastic biodegradation by in vitro environmental microorganisms and in vivo gut microorganisms of insects. Front. Microbiol. 2023, 13, 1001750. [Google Scholar] [CrossRef] [PubMed]
- Zurier, H.S.; Goddard, J.M. Biodegradation of microplastics in food and agriculture. Curr. Opin. Food Sci. 2021, 37, 37–44. [Google Scholar] [CrossRef]
- Gao, J.; Pan, S.; Li, P.; Wang, L.; Hou, R.; Wu, W.-M.; Luo, J.; Hou, D. Vertical migration of microplastics in porous media: Multiple controlling factors under wet-dry cycling. J. Hazard. Mater. 2021, 419, 126413. [Google Scholar] [CrossRef] [PubMed]
- Wahl, A.; Davranche, M.; Rabiller-Baudry, M.; Pédrot, M.; Khatib, I.; Labonne, F.; Canté, M.; Cuisinier, C.; Gigault, J. Condition of composted microplastics after they have been buried for 30 years: Vertical distribution in the soil and degree of degradation. J. Hazard. Mater. 2024, 462, 132686. [Google Scholar] [CrossRef]
- Weber, C.J.; Santowski, A.; Chifflard, P. Investigating the dispersal of macro- and microplastics on agricultural fields 30 years after sewage sludge application. Sci. Rep. 2022, 12, 6401. [Google Scholar] [CrossRef]
- Ren, Z.; Gui, X.; Wei, Y.; Chen, X.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. Chemical and photo-initiated aging enhances transport risk of microplastics in saturated soils: Key factors, mechanisms, and modeling. Water Res. 2021, 202, 117407. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; Jiao, M.; Zhao, Z.; Li, R.; Qin, C. Distinct microplastics abundance variation in root-associated sediments revealed the underestimation of mangrove microplastics pollution. Sci. Total Environ. 2023, 899, 165611. [Google Scholar] [CrossRef]
- Rillig, M.C.; Bonkowski, M. Microplastic and soil protists: A call for research. Environ. Pollut. 2018, 241, 1128–1131. [Google Scholar] [CrossRef]
- Mitzel, M.R.; Sand, S.; Whalen, J.K.; Tufenkji, N. Hydrophobicity of biofilm coatings influences the transport dynamics of polystyrene nanoparticles in biofilm-coated sand. Water Res. 2016, 92, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Laermanns, H.; Lehmann, M.; Klee, M.; Löder, M.G.J.; Gekle, S.; Bogner, C. Tracing the horizontal transport of microplastics on rough surfaces. Microplast Nanoplast. 2021, 1, 11. [Google Scholar] [CrossRef]
- Jolaosho, T.L.; Rasaq, M.F.; Omotoye, E.V.; Araomo, O.V.; Adekoya, O.S.; Abolaji, O.Y.; Hungbo, J.J. Microplastics in freshwater and marine ecosystems: Occurrence, characterization, sources, distribution dynamics, fate, transport processes, potential mitigation strategies, and policy interventions. Ecotoxicol. Environ. Saf. 2025, 294, 118036. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Zhao, Q.; Ao, H.; Hu, H.; Wu, C. Horizontal transport of macro- and microplastics on soil surface by rainfall induced surface runoff as affected by vegetations. Sci. Total Environ. 2022, 831, 154989. [Google Scholar] [CrossRef]
- Abbasi, S.; Rezaei, M.; Mina, M.; Sameni, A.; Oleszczuk, P.; Turner, A.; Ritsema, C. Entrainment and horizontal atmospheric transport of microplastics from soil. Chemosphere 2023, 322, 138150. [Google Scholar] [CrossRef]
- Xiao, L.; You, Z.; Zhang, H.; Xie, Z.; Zhao, R.; Greenwood, P. Effects of conservation practices on global wind erosion control: Evidence from experimental data. Land Degrad. Dev. 2023, 34, 4386–4398. [Google Scholar] [CrossRef]
- Maqbool, A.; Guzmán, G.; Fiener, P.; Wilken, F.; Soriano, M.-A.; Gómez, J.A. Tracing macroplastics redistribution and fragmentation by tillage translocation. J. Hazard. Mater. 2024, 477, 135318. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, J.; Ji, Q.; Li, C.; Chen, X.; Cao, X.; Zhu, F.; Yang, S.; Li, S.; He, H. Insights into adsorption mechanisms of nitro polycyclic aromatic hydrocarbons on common microplastic particles: Experimental studies and modeling. Chemosphere 2023, 320, 138050. [Google Scholar] [CrossRef]
- Upadhyay, R.; Singh, S.; Kaur, G. Sorption of pharmaceuticals over microplastics’ surfaces: Interaction mechanisms and governing factors. Environ. Monit. Assess. 2022, 194, 803. [Google Scholar] [CrossRef]
- Tumwesigye, E.; Nnadozie, C.F.; Akamagwuna, F.C.; Noundou, X.S.; Nyakairu, G.W.; Odume, O.N. Microplastics as vectors of chemical contaminants and biological agents in freshwater ecosystems: Current knowledge status and future perspectives. Environ. Pollut. 2023, 330, 121829. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhou, S.; Zhang, C.; Zhou, Y.; Qin, W. Soil microplastic characteristics and the effects on soil properties and biota: A systematic review and meta-analysis. Environ. Pollut. 2022, 313, 120183. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, P.; Yang, X.; Wang, Z.; Lu, B.; Chen, W.; Wu, Y.; Li, G.; Zhao, Z.; Liu, G.; et al. Soil texture is an important factor determining how microplastics affect soil hydraulic characteristics. Environ. Int. 2022, 165, 107293. [Google Scholar] [CrossRef]
- Ingraffia, R.; Amato, G.; Bagarello, V.; Carollo, F.G.; Giambalvo, D.; Iovino, M.; Lehmann, A.; Rillig, M.C.; Frenda, A.S. Polyester microplastic fibers affect soil physical properties and erosion as a function of soil type. Soil 2022, 8, 421–435. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Li, W.; Yang, W.; Jing, S. Effects of microplastics on the water characteristic curve of soils with different textures. Chemosphere 2023, 317, 137762. [Google Scholar] [CrossRef]
- Zhang, G.S.; Zhang, F.X.; Li, X.T. Effects of polyester microfibers on soil physical properties: Perception from a field and a pot experiment. Sci. Total Environ. 2019, 670, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.T.; Lozano, M.Y.; Rillig, C.M. Microplastics Increase Soil pH and Decrease Microbial Activities as a Function of Microplastic Shape, Polymer Type, and Exposure. Front. Environ. Sci. 2021, 9, 675803. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Z.; Gao, J.; Wu, H. The impacts of microplastics on the cycling of carbon and nitrogen in terrestrial soil ecosystems: Progress and prospects. Sci. Total Environ. 2024, 915, 169977. [Google Scholar] [CrossRef]
- Rillig, M.C.; Leifheit, E.; Lehmann, J. Microplastic effects on carbon cycling processes in soils. PLoS Biol. 2021, 19, e3001130. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, X.; Zhang, S.; Yang, J.; Lu, Y.; Wang, Y. Impact of microplastics on the in situ, high-resolution of key nutrient dynamics at the soil-water interface in rice fields. Front. Environ. Sci. 2023, 11, 1239282. [Google Scholar] [CrossRef]
- Wang, X.; Xing, Y.; Lv, M.; Zhang, T.; Ya, H.; Jiang, B. Recent advances on the effects of microplastics on elements cycling in the environment. Sci. Total Environ. 2022, 849, 157884. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Song, B.; Zhou, C.; Almatrafi, E.; Hu, T.; Zeng, G.; Zhang, Y. Recent advances in impacts of microplastics on nitrogen cycling in the environment: A review. Sci. Total Environ. 2022, 815, 152740. [Google Scholar] [CrossRef]
- Wang, J.; Huang, M.; Wang, Q.; Sun, Y.; Zhao, Y.; Huang, Y. LDPE microplastics significantly alter the temporal turnover of soil microbial communities. Sci. Total Environ. 2020, 726, 138682. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, S.; Wang, H.; Wang, D.; Zhu, Y.; Wang, J.; He, Y.; Zheng, Q.; Zhan, X. Microplastic particles alter wheat rhizosphere soil microbial community composition and function. J. Hazard. Mater. 2022, 436, 129176. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, H.; Banfield, C.C.; Wen, Y.; Zang, H.; Dippold, M.A.; Charlton, A.; Jones, D.L. The microplastisphere: Biodegradable microplastics addition alters soil microbial community structure and function. Soil Biol. Biochem. 2021, 156, 108211. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Liu, Y.-J.; Fu, Y.-M.; Xu, J.-Y.; Zhang, T.-L.; Cui, H.-L.; Qiao, M.; Rillig, M.C.; Zhu, Y.-G.; Zhu, D. Microplastic diversity increases the abundance of antibiotic resistance genes in soil. Nat. Commun. 2024, 15, 9788. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, Y.; Liu, L.; Wang, L.; Tang, J. Interaction between microplastic biofilm formation and antibiotics: Effect of microplastic biofilm and its driving mechanisms on antibiotic resistance gene. J. Hazard. Mater. 2023, 459, 132099. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Y.; Wang, J.; Zhang, Y.; Zhang, P.; Li, X.; Zou, J.; Zhou, A. Interactions of microplastics and antibiotic resistance genes and their effects on the aquaculture environments. J. Hazard. Mater. 2021, 403, 123961. [Google Scholar] [CrossRef]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, R.; Zhang, S.; Sun, Y.; Wang, F. Uptake and translocation of nano/microplastics by rice seedlings: Evidence from a hydroponic experiment. J. Hazard. Mater. 2022, 421, 126700. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q.; Li, R.; Zhou, J.; Wang, G. The distribution and impact of polystyrene nanoplastics on cucumber plants. Environ. Sci. Pollut. Res. Int. 2021, 28, 16042–16053. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.; An, Y.-J. Nanoplastic ingestion induces behavioral disorders in terrestrial snails: Trophic transfer effects via vascular plants. Environ. Sci. Nano 2020, 7, 975–983. [Google Scholar] [CrossRef]
- Liu, X.; Shao, J.; Peng, C.; Gong, J. Novel insights related to soil microplastic abundance and vegetable microplastic contamination. J. Hazard. Mater. 2025, 484, 136727. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, Z. Nano-enabled agriculture: How do nanoparticles cross barriers in plants? Plant Commun. 2022, 3, 100346. [Google Scholar] [CrossRef]
- Li, G.; Qiu, C.; Zhang, D.; Lv, M.; Liao, X.; Li, Q.; Wang, L. Effects of polystyrene nanoplastics (PSNPs) on the physiology of Allium sativum L.: Photosynthetic pigments, antioxidant enzymes, phytohormones, and nutritional quality. Environ. Exp. Bot. 2024, 219, 105654. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- He, X.; Wang, Q.; Qian, Y.; Li, Z.; Feng, C. Microplastic accumulation and oxidative stress in sweet pepper (Capsicum annuum Linn.): Role of the size effect. Environ. Pollut. 2024, 360, 124652. [Google Scholar] [CrossRef]
- Dong, R.; Liu, R.; Xu, Y.; Liu, W.; Sun, Y. Effect of foliar and root exposure to polymethyl methacrylate microplastics on biochemistry, ultrastructure, and arsenic accumulation in Brassica campestris L. Environ. Res. 2022, 215, 114402. [Google Scholar] [CrossRef]
- Sun, Y.; Zang, J.; Xie, S.; Wu, M.; Tao, J.; Adyel, T.M.; Wang, J. Transcriptomic and metabolomic responses of maize under conventional and biodegradable microplastic stress. IMetaOmics 2025, 2, e48. [Google Scholar] [CrossRef]
- Pignattelli, S.; Broccoli, A.; Renzi, M. Physiological responses of garden cress (L. sativum) to different types of microplastics. Sci. Total Environ. 2020, 727, 138609. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wu, D.; Zhang, D.; Geng, Z.; Tong, M.; Duan, Y.; Xia, W.; Chu, J.; Yao, X. Comparative analysis of the effects of microplastics and nitrogen on maize and wheat: Growth, redox homeostasis, photosynthesis, and AsA-GSH cycle. Sci. Total Environ. 2024, 932, 172555. [Google Scholar] [CrossRef]
- Lin, Z.; Xu, D.; Zhao, Y.; Sheng, B.; Wu, Z.; Wen, X.; Zhou, J.; Chen, G.; Lv, J.; Wang, J.; et al. Micro/Nanoplastics in plantation agricultural products: Behavior process, phytotoxicity under biotic and abiotic stresses, and controlling strategies. J. Nanobiotechnol. 2025, 23, 231. [Google Scholar] [CrossRef]
- Osmani, Z.; Wang, L.; Xiao, W.; Kulka, M. Nanomaterials as tools in plant transformation: A protoplast-centric perspective. Plant Nano Biol. 2024, 10, 100100. [Google Scholar] [CrossRef]
- Thagun, C.; Horii, Y.; Mori, M.; Fujita, S.; Ohtani, M.; Tsuchiya, K.; Kodama, Y.; Odahara, M.; Numata, K. Non-transgenic Gene Modulation via Spray Delivery of Nucleic Acid/Peptide Complexes into Plant Nuclei and Chloroplasts. ACS Nano 2022, 16, 3506–3521. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.D. Genotoxicity of Microplastics on Living Organisms: Effects on Chromosomes, DNA and Gene Expression. Environments 2025, 12, 10. [Google Scholar] [CrossRef]
- Maity, S.; Guchhait, R.; De, S.; Pramanick, K. High doses of nano-polystyrene aggravate the oxidative stress, DNA damage, and the cell death in onions. Environ. Pollut. 2023, 316, 120611. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Xu, M.; Wang, L. Cyto–Genotoxic Effect Causing Potential of Polystyrene Micro-Plastics in Terrestrial Plants. Nanomaterials 2022, 12, 2024. [Google Scholar] [CrossRef]
- Biba, R.; Cvjetko, P.; Jakopčić, M.; Komazec, B.; Tkalec, M.; Dimitrov, N.; Begović, T.; Balen, B. Phytotoxic Effects of Polystyrene and Polymethyl Methacrylate Microplastics on Allium cepa Roots. Plants 2023, 12, 747. [Google Scholar] [CrossRef]
- Xu, G.; Liu, Y.; Yu, Y. Effects of polystyrene microplastics on uptake and toxicity of phenanthrene in soybean. Sci. Total Environ. 2021, 783, 147016. [Google Scholar] [CrossRef]
- Shi, J.; Wu, D.; Su, Y.; Xie, B. (Nano)microplastics promote the propagation of antibiotic resistance genes in landfill leachate. Environ. Sci. Nano 2020, 7, 3536–3546. [Google Scholar] [CrossRef]
- Fauser, P.; Vorkamp, K.; Strand, J. Residual additives in marine microplastics and their risk assessment—A critical review. Mar. Pollut. Bull. 2022, 177, 113467. [Google Scholar] [CrossRef] [PubMed]
- Maddela, N.R.; Kakarla, D.; Venkateswarlu, K.; Megharaj, M. Additives of plastics: Entry into the environment and potential risks to human and ecological health. J. Environ. Manag. 2023, 348, 119364. [Google Scholar] [CrossRef]
- Joshi, S.; Patil, S.; Shaikh, A.; Jamla, M.; Kumar, V. Modern omics toolbox for producing combined and multifactorial abiotic stress tolerant plants. Plant Stress 2024, 11, 100301. [Google Scholar] [CrossRef]
- Huang, D.; Ding, L.; Wang, S.; Ding, R.; Qiu, X.; Li, J.; Hua, Z.; Liu, S.; Wu, R.; Liang, X.; et al. Metabolomics reveals how spinach plants reprogram metabolites to cope with intense stress responses induced by photoaged polystyrene nanoplastics (PSNPs). J. Hazard. Mater. 2024, 466, 133605. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, M.S.S.; Xue, S.; Islam, F.; Ikram, A.U.; Abdullah, M.; Liu, S.; Tappiban, P.; Chen, J. A comprehensive overview of omics-based approaches to enhance biotic and abiotic stress tolerance in sweet potato. Hortic. Res. 2024, 11, uhae014. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Huerta Lwanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- Xu, H.; Chen, C.; Pang, Z.; Zhang, G.; Zhang, W.; Kan, H. Effects of microplastics concentration on plant root traits and biomass: Experiment and meta-analysis. Ecotoxicol. Environ. Saf. 2024, 285, 117038. [Google Scholar] [CrossRef]
- Meng, F.; Yang, X.; Riksen, M.; Xu, M.; Geissen, V. Response of common bean (Phaseolus vulgaris L.) growth to soil contaminated with microplastics. Sci. Total Environ. 2021, 755, 142516. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q.; Li, R.; Zhao, Y.; Geng, J.; Wang, G. Physiological responses of lettuce (Lactuca sativa L.) to microplastic pollution. Environ. Sci. Pollut. Res. Int. 2020, 27, 30306–30314. [Google Scholar] [CrossRef]
- De Silva, Y.S.K.; Rajagopalan, U.M.; Kadono, H.; Li, D. Effects of microplastics on lentil (Lens culinaris) seed germination and seedling growth. Chemosphere 2022, 303, 135162. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, M.; Meng, F.; Xiao, Y.; Dai, W.; Luan, Y. Effect of Polystyrene Microplastics on Rice Seed Germination and Antioxidant Enzyme Activity. Toxics 2021, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Shorobi, F.M.; Vyavahare, G.D.; Seok, Y.J.; Park, J.H. Effect of polypropylene microplastics on seed germination and nutrient uptake of tomato and cherry tomato plants. Chemosphere 2023, 329, 138679. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed]
- Lozano, Y.M.; Rillig, M.C. Effects of Microplastic Fibers and Drought on Plant Communities. Environ. Sci. Technol. 2020, 54, 6166–6173. [Google Scholar] [CrossRef]
- Tunali, M.; Uzoefuna, E.N.; Tunali, M.M.; Yenigun, O. Effect of microplastics and microplastic-metal combinations on growth and chlorophyll a concentration of Chlorella vulgaris. Sci. Total Environ. 2020, 743, 140479. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, W.; Cai, K.; Liu, T.; Wang, X. Effects of Microplastics on Growth and Physiological Characteristics of Tobacco (Nicotiana tabacum L.). Agronomy 2022, 12, 2692. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J.; Zhang, H.; Shi, H.; Fei, Y.; Huang, S.; Tong, Y.; Wen, D.; Luo, Y.; Barceló, D. Microplastics in agricultural soils on the coastal plain of Hangzhou Bay, east China: Multiple sources other than plastic mulching film. J. Hazard. Mater. 2020, 388, 121814. [Google Scholar] [CrossRef]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Radford, F.; Zapata-Restrepo, L.M.; Horton, A.A.; Hudson, M.D.; Shaw, P.J.; Williams, I.D. Developing a systematic method for extraction of microplastics in soils. Anal. Methods 2021, 13, 1695–1705. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, H.; Fu, C.; Zhou, Y.; Dai, Z.; Li, Y.; Luo, Y. The distribution and morphology of microplastics in coastal soils adjacent to the Bohai Sea and the Yellow Sea. Geoderma 2018, 322, 201–208. [Google Scholar] [CrossRef]
- Perez, C.N.; Carré, F.; Hoarau-Belkhiri, A.; Joris, A.; Leonards, P.E.G.; Lamoree, M.H. Innovations in analytical methods to assess the occurrence of microplastics in soil. J. Environ. Chem. Eng. 2022, 10, 107421. [Google Scholar] [CrossRef]
- Foetisch, A.; Grunder, A.; Kuster, B.; Stalder, T.; Bigalke, M. All black: A microplastic extraction combined with colour-based analysis allows identification and characterisation of tire wear particles (TWP) in soils. Microplast. Nanoplast. 2024, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Möller, J.N.; Löder, M.G.J.; Laforsch, C. Finding Microplastics in Soils: A Review of Analytical Methods. Environ. Sci. Technol. 2020, 54, 2078–2090. [Google Scholar] [CrossRef]
- Wang, W.; Ge, J.; Yu, X.; Li, H. Environmental fate and impacts of microplastics in soil ecosystems: Progress and perspective. Sci. Total Environ. 2020, 708, 134841. [Google Scholar] [CrossRef] [PubMed]
- Lastovina, T.A.; Budnyk, A.P. A review of methods for extraction, removal, and stimulated degradation of microplastics. J. Water Process Eng. 2021, 43, 102209. [Google Scholar] [CrossRef]
- Hu, H.; Qiang, L.; Xu, J.; Li, G.; Cheng, J.; Zhong, X.; Zhang, R. Enhanced microplastic retrieval efficiency from cultivated soil samples through optimized pre-treatment in density-based extraction. Soil Tillage Res. 2024, 242, 106134. [Google Scholar] [CrossRef]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 μm) in Environmental Samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef]
- Clark, N.J.; Khan, F.R.; Mitrano, D.M.; Boyle, D.; Thompson, R.C. Demonstrating the translocation of nanoplastics across the fish intestine using palladium-doped polystyrene in a salmon gut-sac. Environ. Int. 2022, 159, 106994. [Google Scholar] [CrossRef]
- Liu, Z.; Su, Z.; Chen, J.; Zou, J.; Liu, Z.; Li, Y.; Wang, J.; Wu, L.; Wei, H.; Zhang, J. Polyethylene microplastics can attenuate soil carbon sequestration by reducing plant photosynthetic carbon assimilation and transfer: Evidence from a 13C-labeling mesocosm study. J. Clean. Prod. 2023, 385, 135558. [Google Scholar] [CrossRef]
- Maurizi, L.; Iordachescu, L.; Kirstein, I.V.; Nielsen, A.H.; Vollertsen, J. It matters how we measure—Quantification of microplastics in drinking water by μFTIR and μRaman. Heliyon 2023, 9, e20119. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lei, C.; Xu, J.; Li, R. Foliar uptake and leaf-to-root translocation of nanoplastics with different coating charge in maize plants. J. Hazard. Mater. 2021, 416, 125854. [Google Scholar] [CrossRef] [PubMed]
- Peez, N.; Janiska, M.C.; Imhof, W. The first application of quantitative 1 HNMR spectroscopy as a simple and fast method of identification and quantification of microplastic particles (PE, PET, and PS). Anal. Bioanal. Chem. 2019, 411, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Ivleva, N.P. Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Ma, S.; Ouyang, Z.; Liu, P.; Qiang, H.; Guo, X. The review of nanoplastics in plants: Detection, analysis, uptake, migration and risk. TrAC Trends Anal. Chem. 2023, 158, 116889. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, K.; Yu, H.; Tan, W.; Gao, Y.; Lv, B. Effects and mechanism of microplastics on organic carbon and nitrogen cycling in agricultural soil: A review. Soil Use Manag. 2024, 40, e12971. [Google Scholar] [CrossRef]
- Wang, Q.; Adams, C.A.; Wang, F.; Sun, Y.; Zhang, S. Interactions between microplastics and soil fauna: A critical review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3211–3243. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Shu, X.; Gong, J.; Yang, J.; Li, B.; Lin, J.; Chai, Y.; Liu, J. The effects of polyethylene microplastics on the growth, reproduction, metabolic enzymes, and metabolomics of earthworms Eisenia fetida. Ecotoxicol. Environ. Saf. 2023, 263, 115390. [Google Scholar] [CrossRef]
- Kwak, J.I.; An, Y.-J. Microplastic digestion generates fragmented nanoplastics in soils and damages earthworm spermatogenesis and coelomocyte viability. J. Hazard. Mater. 2021, 402, 124034. [Google Scholar] [CrossRef]
- Chen, H.; Huang, D.; Zhou, W.; Deng, R.; Yin, L.; Xiao, R.; Li, S.; Li, F.; Lei, Y. Hotspots lurking underwater: Insights into the contamination characteristics, environmental fates and impacts on biogeochemical cycling of microplastics in freshwater sediments. J. Hazard. Mater. 2024, 476, 135132. [Google Scholar] [CrossRef] [PubMed]
- Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Change Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef]
- Fang, S.; Hua, C.; Yang, J.; Liu, F.; Wang, L.; Wu, D.; Ren, L. Combined pollution of soil by heavy metals, microplastics, and pesticides: Mechanisms and anthropogenic drivers. J. Hazard. Mater. 2025, 485, 136812. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.; Zhou, P.; Li, H.; Wan, Q.; Lu, Y.; Li, B. Differential impacts of microplastics on carbon and nitrogen cycling in plant-soil systems: A meta-analysis. Sci. Total Environ. 2024, 948, 174655. [Google Scholar] [CrossRef]
- Al-Shammary, A.A.G.; Al-Shihmani, L.S.S.; Fernández-Gálvez, J.; Caballero-Calvo, A. Optimizing sustainable agriculture: A comprehensive review of agronomic practices and their impacts on soil attributes. J. Environ. Manag. 2024, 364, 121487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yuan, Y.; Zhang, J.; Wen, T.; Wang, H.; Qu, C.; Tan, W.; Xi, B.; Hui, K.; Tang, J. Specific response of soil properties to microplastics pollution: A review. Environ. Res. 2023, 232, 116427. [Google Scholar] [CrossRef]
- Nguyen, M.-K.; Rakib, M.R.J.; Lin, C.; Hung, N.T.Q.; Le, V.-G.; Nguyen, H.-L.; Malafaia, G.; Idris, A.M. A comprehensive review on ecological effects of microplastic pollution: An interaction with pollutants in the ecosystems and future perspectives. TrAC Trends Anal. Chem. 2023, 168, 117294. [Google Scholar] [CrossRef]
- Gao, S.; Mu, X.; Li, W.; Wen, Y.; Ma, Z.; Liu, K.; Zhang, C. Invisible threats in soil: Microplastic pollution and its effects on soil health and plant growth. Environ. Geochem. Health 2025, 47, 158. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Kim, J.-W.; Pham, T.D.; Tarafdar, A.; Hong, S.; Chun, S.-H.; Lee, S.-H.; Kang, D.-Y.; Kim, J.-Y.; Kim, S.-B.; et al. Microplastics in Food: A Review on Analytical Methods and Challenges. Int. J. Environ. Res. Public Health 2020, 17, 6710. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Mahagamage, M.G.Y.L.; Gajanayake, P.; Abeynayaka, A.; Gamaralalage, P.J.D.; Ohgaki, M.; Takenaka, M.; Fukai, T.; Itsubo, N. Microplastics and Potentially Toxic Elements: Potential Human Exposure Pathways through Agricultural Lands and Policy Based Countermeasures. Microplastics 2022, 1, 102–120. [Google Scholar] [CrossRef]
- Ren, X.; Yin, S.; Wang, L.; Tang, J. Microplastics in plant-microbes-soil system: A review on recent studies. Sci. Total Environ. 2022, 816, 151523. [Google Scholar] [CrossRef] [PubMed]
- Sheng, D.; Jing, S.; He, X.; Klein, A.-M.; Köhler, H.-R.; Wanger, T.C. Plastic pollution in agricultural landscapes: An overlooked threat to pollination, biocontrol and food security. Nat. Commun. 2024, 15, 8413–8419. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Xu, J.; Arif, M.S.; Worthy, F.R.; Jones, D.L.; Khan, S.; Alharbi, S.A.; Filimonenko, E.; Nadir, S.; Bu, D.; et al. Do Added Microplastics, Native Soil Properties, and Prevailing Climatic Conditions Have Consequences for Carbon and Nitrogen Contents in Soil? A Global Data Synthesis of Pot and Greenhouse Studies. Environ. Sci. Technol. 2024, 58, 8464–8479. [Google Scholar] [CrossRef] [PubMed]
- UNEP. Intergovernmental Negotiating Committee on Plastic Pollution. 2022. Available online: https://www.unep.org/inc-plastic-pollution (accessed on 16 June 2025).
- European Commission. Farm to Fork Strategy. 2025. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 16 June 2025).
- Wallace, H.; Alexander, J.; Barregard, L. Presence of Microplastics and Nanoplastics in Food, with Particular Focus on Seafood. Eur. Food Saf. Auth. 2016, 14, 1–30. [Google Scholar] [CrossRef]
- EPRS. Reducing Microplastic Pollution from Plastic Pellet Losses. 2023. Available online: https://www.europarl.europa.eu/RegData/etudes/BRIE/2024/760442/EPRS_BRI(2024)760442_EN.pdf (accessed on 16 June 2025).
- GOV. UK. World-Leading Microbeads Ban Takes Effect. 2018. Available online: https://www.gov.uk/government/news/world-leading-microbeads-ban-takes-effect (accessed on 16 June 2025).
- GOV. UK. Agricultural Transition Plan 2021 to 2024. 2020. Available online: https://www.gov.uk/government/publications/agricultural-transition-plan-2021-to-2024 (accessed on 16 June 2025).
- DEFRA. Option Appraisal for Intentionally Added Microplastics—CB04121. 2025. Available online: https://sciencesearch.defra.gov.uk/ProjectDetails?ProjectId=21802 (accessed on 16 June 2025).
| Soil Type | MP Type | MP Size | MP Concentration | Key Results | Reference |
|---|---|---|---|---|---|
| Loam, clay and sand soil | PP-MPs | 0, 200, and 500 μm | 6% (w/w) | The effects of MPs on soil hydraulic properties were strongly modulated by soil texture. | [64] |
| Vertisol, Entisol, and Alfisol soils | PES-MPs | n/a | 0.1%, 0.4% (w/w) | PES microfibers exhibited soil type-dependent impacts on soil physical parameters. | [65] |
| Loam and sand soil | PES-MPs | 150, 550, and 950 μm | 0.5%, 1%, and 2% (w/w) | Soil texture had a stronger influence on the soil water retention curve than MP concentration and size. | [66] |
| Clay soil | PES-MPs | <0.25 mm | 0.1%, 0.3% (w/w) | PES microfibers enhanced soil aggregation in pot experiments, but not under field conditions. | [67] |
| Loam soil | PES, PET, and PS-MPs | 5 μm, 48 μm, and 1 mm | 100 mg/kg | PET and PS-MPs reduced the formation of macroaggregates while promoting microaggregate formation. | [21] |
| Loamy sandy soil | PA, PC, PE, PES, PET, PP, and PS-MPs | 1.26–2.26 mm | 0.4% (w/w) | The impact of MPs on soil pH was dependent on particle shape, polymer composition, and exposure duration. | [68] |
| Sandy clay loam soil | HDPE-MPs | 102.6 μm | 0.1% (w/w) | HDPE-MPs significantly decreased soil pH even at a low concentration (0.1% w/w). | [69] |
| Dry soil | PE-MPs | 2 mm × 2 mm × 0.01 mm | 200 pieces | MPs accelerated the turnover rate of soil bacterial communities. | [75] |
| Pot soil | PE, PVC, and PS-MPs | 200 μm | 2% (w/w) | PE-MPs caused greater reductions in rhizosphere bacterial richness and diversity than PS and PVC-MPs. | [76] |
| Field soil | PHAs-MPs | n/a | 1–20% (w/w) | Biodegradable MPs promoted microbial turnover and improved nutrient use efficiency. | [77] |
| Plant Species | MP Type | MP Size | Cultivation Environment | MP Concentration | Exposure Time | Key Results | Reference |
|---|---|---|---|---|---|---|---|
| Wheat (Triticum aestivum L.) and Lettuce (Lactuca sativa) | PS-MPs | 0.2 μm, 2.0 μm | Soil and Aqueous | 150, 500 mg/kg (Soil) 50 mg/L (Aqueous) | 20 days (Soil) 10 days (Aqueous) | Microspheres of 2 μm mainly accumulated in roots, with limited translocation to aerial tissues; in contrast, 0.2 μm particles were transported to shoots and leaves via the transpiration stream. | [17] |
| Fava bean (Vicia faba) | PS-MPs | 5 μm, 100 nm | Aqueous | 10, 50, 100 mg/L | 48 h | Micron-sized MPs were mainly adsorbed on root surfaces, whereas nanoscale MPs were able to penetrate root tissues. | [20] |
| Garden cress (Lepidium sativum) | PS-MPs | 50, 500, 4800 nm | Aqueous | 103 to 107 particles/mL | 72 h | MP exposure significantly affected seed germination and root development, primarily due to physical blockage effects. | [81] |
| Oilseed rape (Brassica napus) | PS-MPs | 80 nm, 1 μm | Aqueous | 40 mg/L | 14 days | MPs were translocated within plant tissues through the symplastic transport system. | [18] |
| Rice (Oryza sativa L.) | PS-MPs | 80 nm, 1 μm | Aqueous | 7 × 1013, 7 × 1011 particles/L | 14 days, 40 days | MPs were absorbed by roots and translocated to aerial tissues via apoplastic pathways. | [82] |
| Cucumber (Cucumis sativus.) | PS-MPs | 100, 300, 500, and 700 nm | Aqueous | 50 mg/L | 65 days | Nanoscale MPs accumulated in root tissues and were subsequently transported to aboveground organs, including leaves, flowers, and fruits. | [83] |
| Mung beans (Vigna radiata) | PS-MPs | 28 nm | Soil | 10, 100 mg/kg | 14 days | Strong fluorescence signals in leaves at 100 mg/kg exposure indicated effective translocation of MPs to aerial tissues. | [84] |
| Barley (Hordeum vulgare) | PS-MPs | 5 μm | Aqueous | 2 g/mL | 14 days | Most MPs were localised on the root surface, with fluorescence intensity significantly higher in roots than in stems or leaves. | [16] |
| Plant Species | MP Type | MP Size | MP Concentration | Key Results | Reference |
|---|---|---|---|---|---|
| Wheat (Triticum aestivum L.) | LDPE, Bio-MPs | 50 μm–1 mm | 1% (w/w) | Bio-based MPs exhibited more pronounced negative effects on plant growth compared to conventional MPs. | [108] |
| Asian shortstem sedge (Carex breviculmis) | PP-MPs | <500 μm | 0.5%, 1%, and 2% (w/w) | High MP concentrations promoted fine root proliferation, increasing total root biomass. | [109] |
| Common bean (Phaseolus vulgaris L.) | LDPE, Bio-MPs | 250–500 μm, 500–1000 μm | 0.5%, 1.0%, 1.5%, 2.0%, and 2.5% w/w (w/w) | Bio-MPs significantly reduced shoot and root biomass and fruit yield, while increasing specific root length. | [110] |
| Lettuce (Lactuca sativa L.) | PVC-MPs | 100 nm–18 μm, 18–150 μm | 0.5%, 1%, and 2% (w/w) | MP size and concentration were important factors influencing plant physiological and biochemical responses. | [111] |
| Garden cress (Lepidium sativum) | PS-MPs | 50, 500, and 4800 nm | 103 to 107 particles/mL | Exposure time significantly affected plant responses to MP contamination. | [81] |
| Lentil (Lens culinaris) | PE-MPs | 740–4990 nm | 10, 50, and 100 mg/L | Adverse effects on seed germination intensified with increasing MP concentrations. | [112] |
| Rice (Oryza sativa L.), | PS-MPs | 200 nm | 10, 1000 mg/L | No significant impact on rice seed germination; however, PS-MPs promoted root elongation and decreased antioxidant enzyme activity. | [113] |
| Tomato (Solanum lycopersicum L.) | PP-MPs | <500 μm | 0.1 g/L | Tomato germination remained largely unaffected, though MPs inhibited later vegetative growth. | [114] |
| Perennial ryegrass (Lolium perenne) | PLA, HDPE-MPs | 65.5 μm, 102.6 μm | 1 g/kg, 10 mg/kg | The impact of MPs on plant growth varied considerably depending on polymer type. | [115] |
| Spring onion (Allium fistulosum) | PES, PE, PET-MPs | 15–20 μm | 0.2%, 2% (w/w) | MP type had distinct effects on overall plant biomass. | [116] |
| Green alga (Chlorella vulgaris) | PS-MPs | 0.5 μm | 1, 5, 50, 100, and 1000 mg/L | Chlorophyll a content decreased under 50, 100, and 1000 mg/L MP treatments, but remained unchanged at 1 mg/L. | [117] |
| Tobacco (Nicotiana tabacum L.) | LDPE-MPs | 13 μm | 10, 100, and 1000 mg/L | High MP concentrations significantly inhibited root system architecture and overall growth performance. | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Carter, L.J.; Banwart, S.A.; Kay, P. Microplastics in Soil–Plant Systems: Current Knowledge, Research Gaps, and Future Directions for Agricultural Sustainability. Agronomy 2025, 15, 1519. https://doi.org/10.3390/agronomy15071519
Chen Z, Carter LJ, Banwart SA, Kay P. Microplastics in Soil–Plant Systems: Current Knowledge, Research Gaps, and Future Directions for Agricultural Sustainability. Agronomy. 2025; 15(7):1519. https://doi.org/10.3390/agronomy15071519
Chicago/Turabian StyleChen, Zhangling, Laura J. Carter, Steven A. Banwart, and Paul Kay. 2025. "Microplastics in Soil–Plant Systems: Current Knowledge, Research Gaps, and Future Directions for Agricultural Sustainability" Agronomy 15, no. 7: 1519. https://doi.org/10.3390/agronomy15071519
APA StyleChen, Z., Carter, L. J., Banwart, S. A., & Kay, P. (2025). Microplastics in Soil–Plant Systems: Current Knowledge, Research Gaps, and Future Directions for Agricultural Sustainability. Agronomy, 15(7), 1519. https://doi.org/10.3390/agronomy15071519








