Investigation of the Effect of a New Type of Copper–Sucrose Complex Compound on the Yield and Quality Parameters of Winter Wheat (Triticum aestivum L.)
Abstract
1. Introduction
1.1. The Importance of Copper in Agricultural Production
- -
- Develop a copper-complex compound that is less toxic;
- -
- Ensure that the complex does not have excessively high stability;
- -
- Assess how the anionic component of the applied copper salt negatively affects the plant by causing foliar burn;
- -
- Use a ligand that is beneficial for plant nutrition;
- -
- Create a compound that is suitable for foliar application;
- -
- Enhance wheat yield and quality through the application of the complex;
- -
- Provide fungicidal effects to reduce the fungal contamination of the crop.
1.2. The Role of Copper in Plants
1.3. Effects of Copper on the Quantitative and Qualitative Parameters of Winter Wheat
2. Materials and Methods
2.1. Meteorological Conditions During the Study Period
2.2. Soil Analysis
2.3. Cultivation Technology
2.4. Foliar Treatment Trials
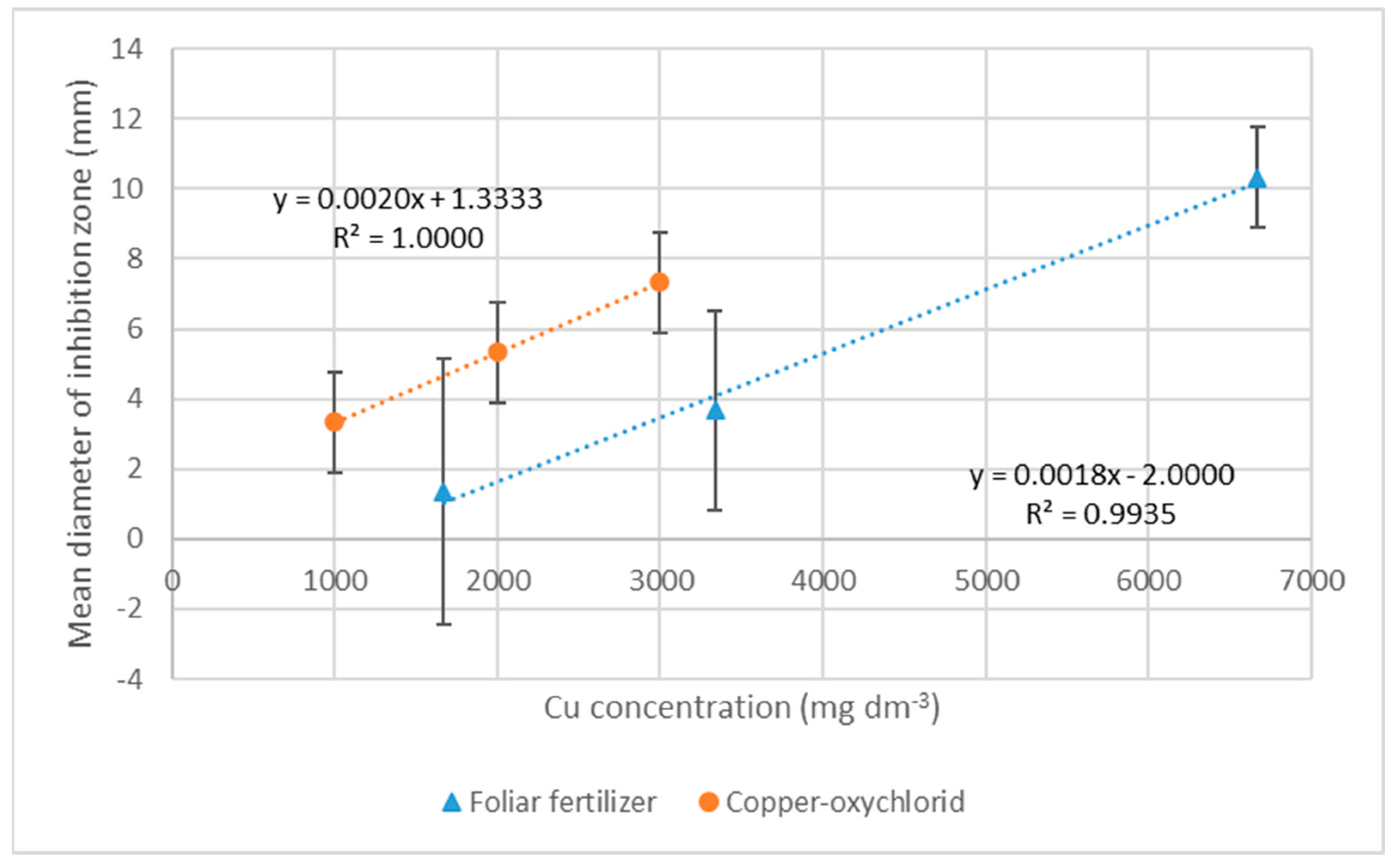
2.5. Fungicide Tests
2.6. Deoxinivalenol (DON) Test
2.7. Statistical Evaluation
3. Results
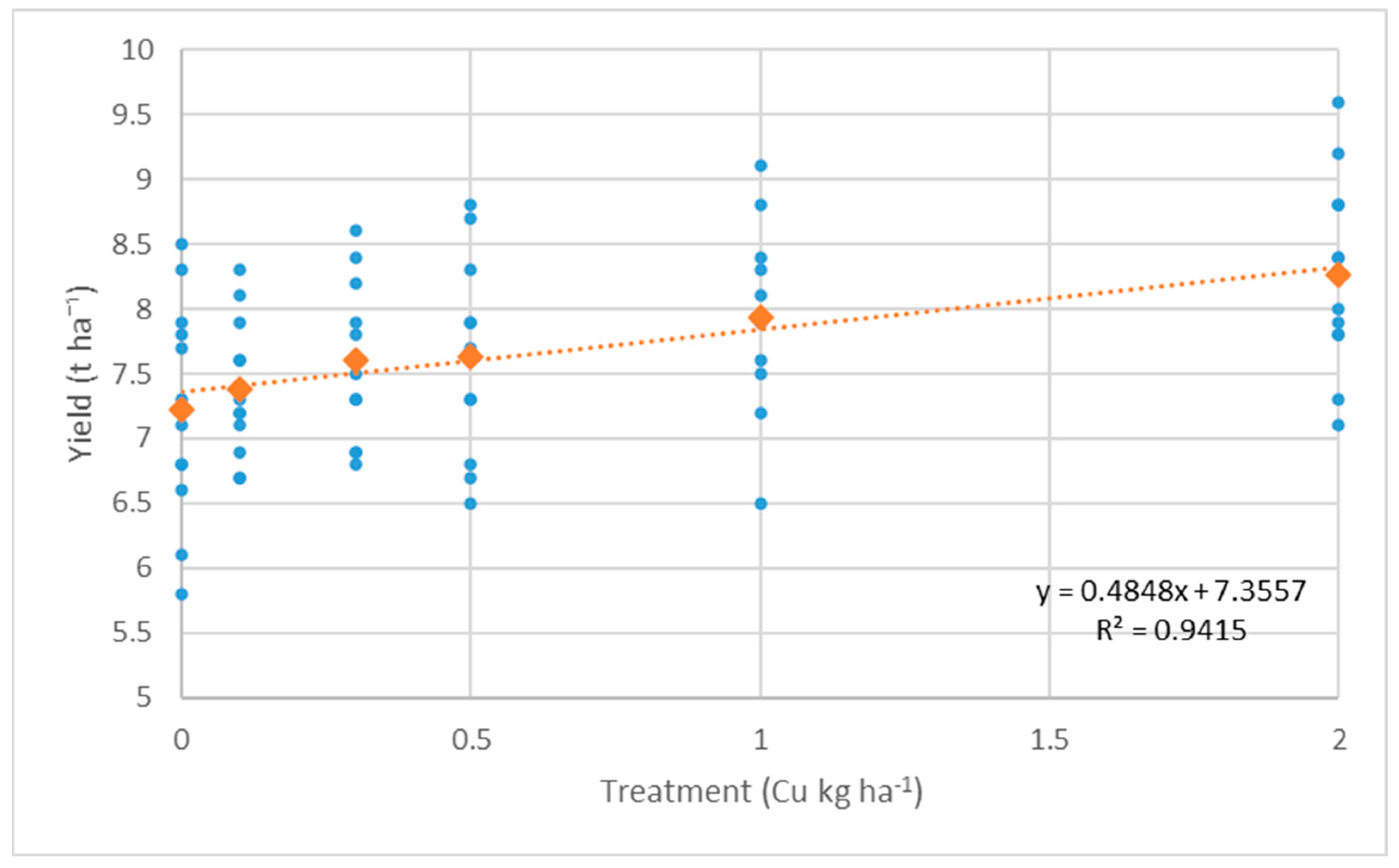
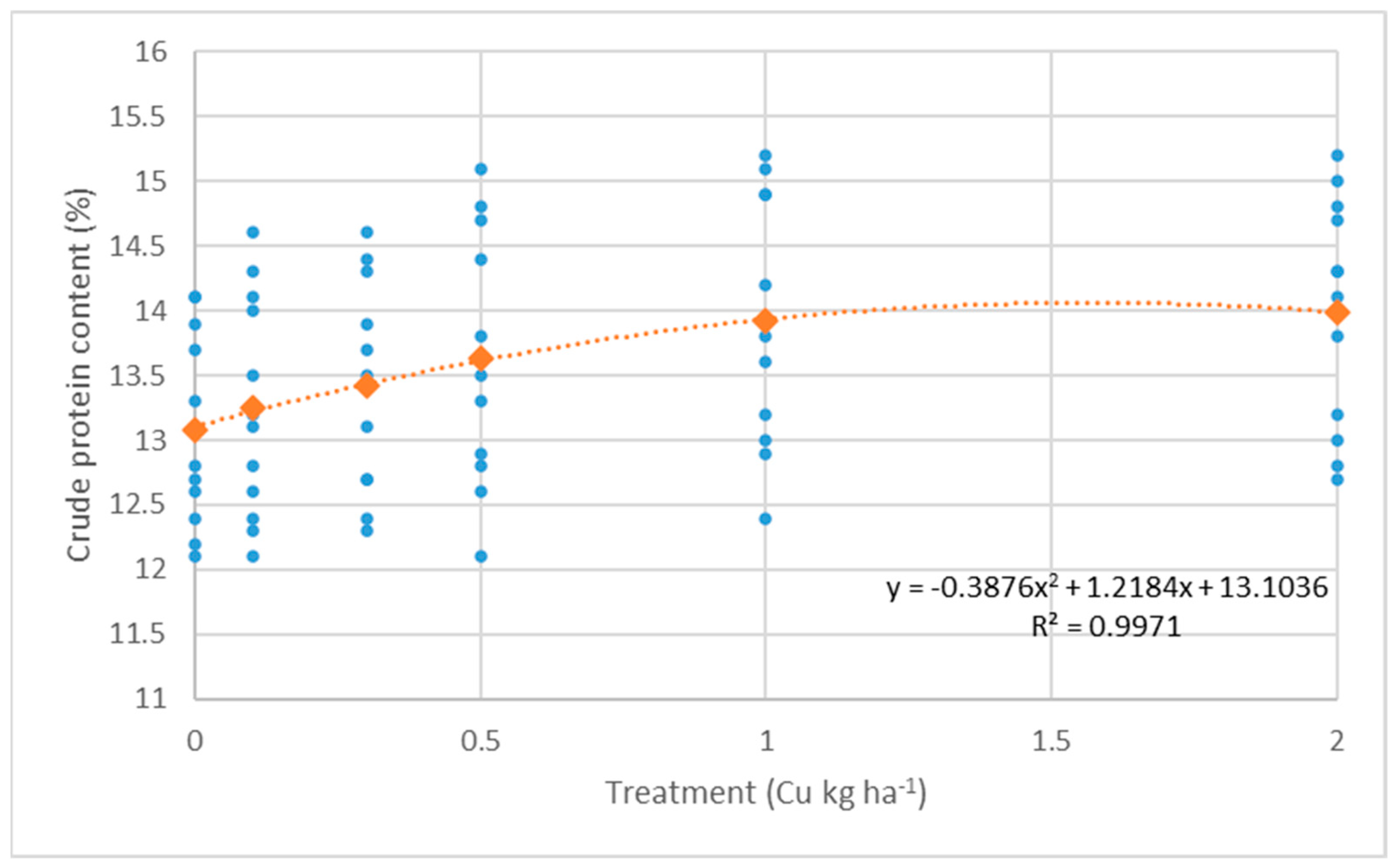
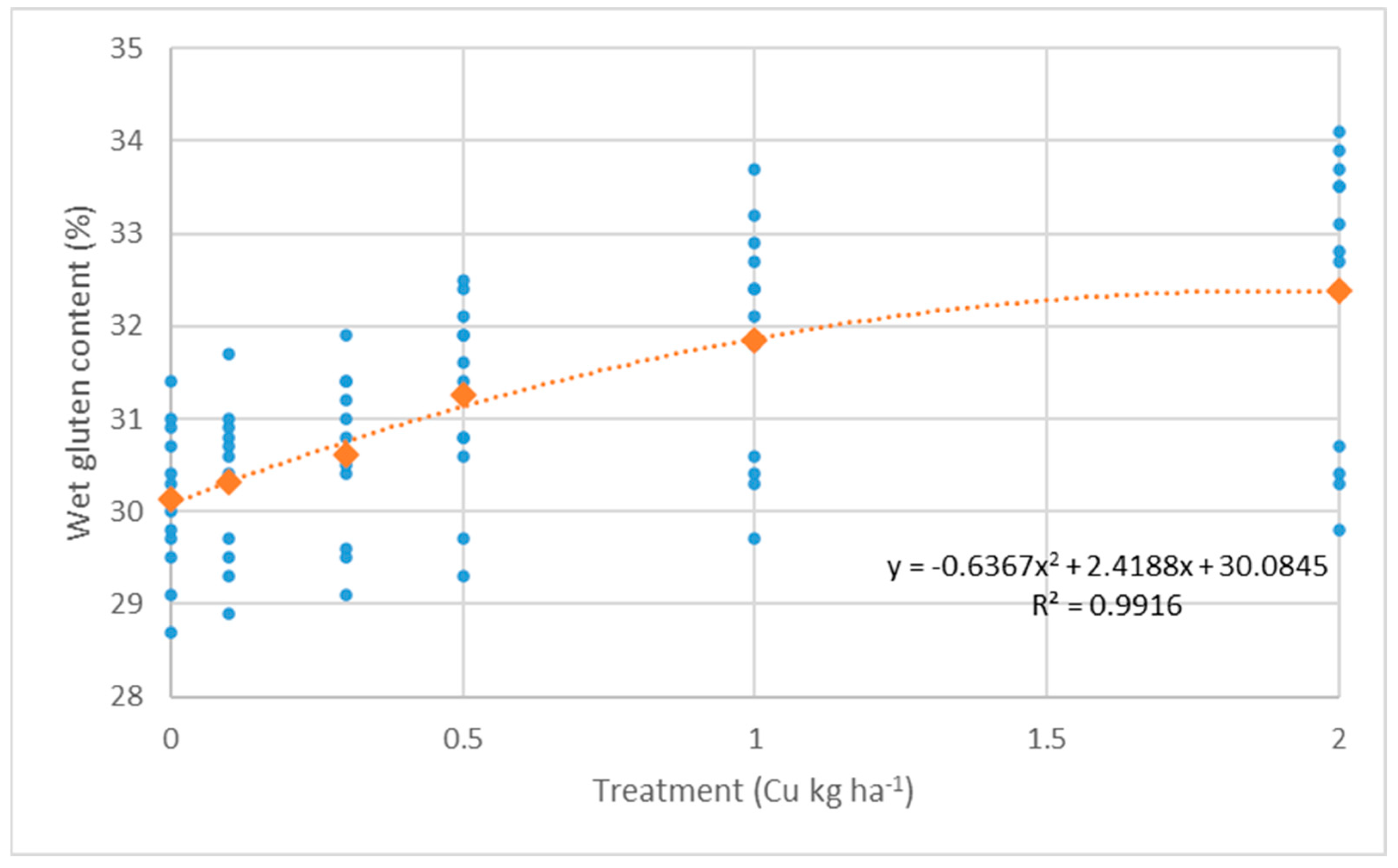
3.1. Effect of Foliar Fertilization on Yield Components
- Yield: F(5,15) = 3.91, p = 0.018 (2019); F(5,15) = 3.85, p = 0.019 (2020); F(5,15) = 3.32, p = 0.032 (2022)
- Crude protein content: F(5,15) = 11.4, p < 0.01; F(5,15) = 13.7, p < 0.01; F(5,15) = 3.65, p = 0.023
- Wet gluten content: F(5,15) = 3.74, p = 0.021; F(5,15) = 35.4, p < 0.01; F(5,15) = 28.8, p < 0.01
- Yield: F(5,10) = 14.9, p < 0.01
- Crude protein content: F(5,10) = 23.3, p < 0.01
- Wet gluten content: F(5,10) = 12.8, p < 0.01 [54]
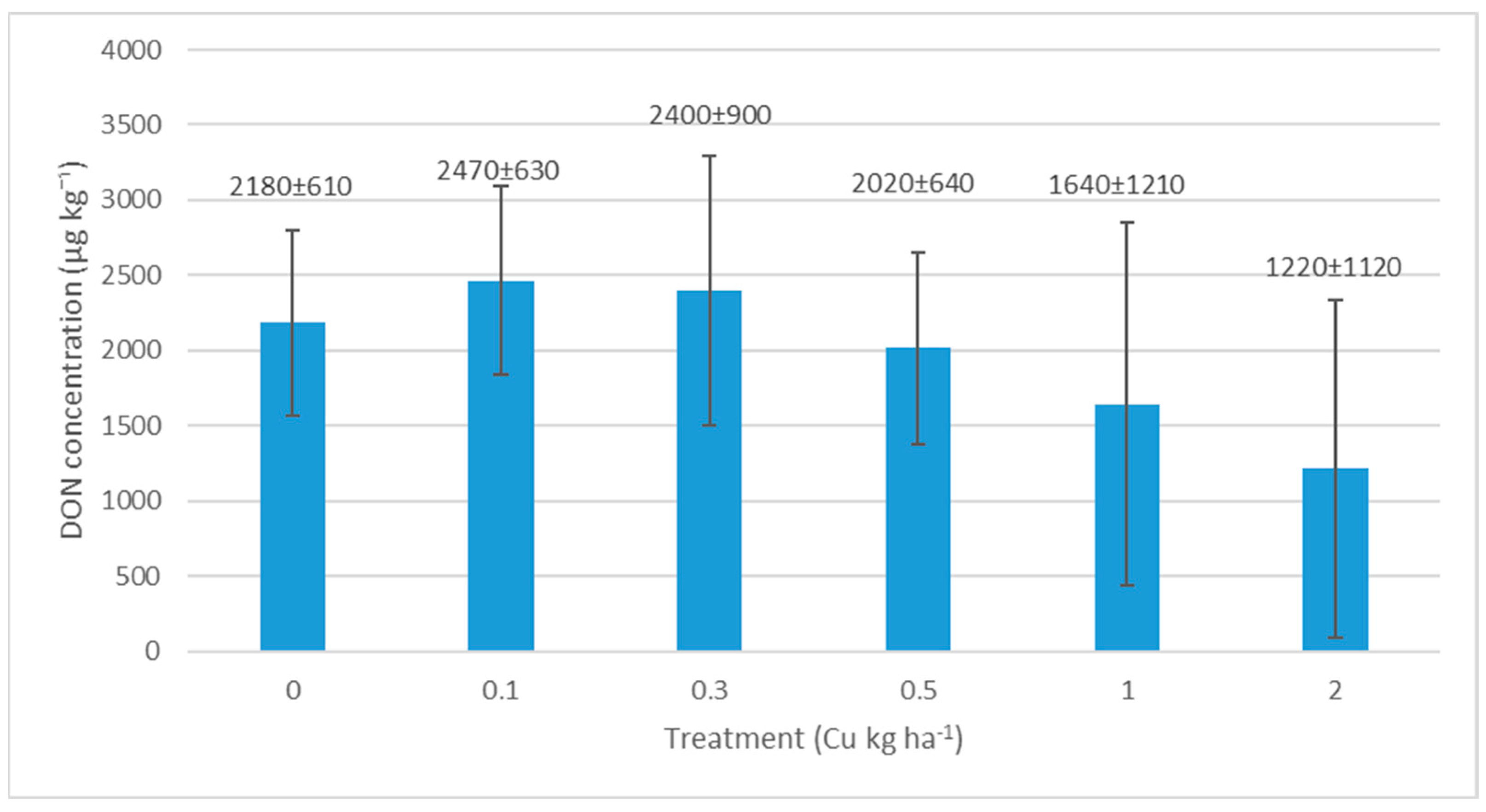
3.2. Results of Deoxynivalenol (DON) Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DON test | Deoxinivalenol test |
| EDTA | Ethylenediaminetetraacetic acid disodium salt |
| MSD | Minimum significant difference |
| FHB | Fusarium head blight |
References
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops That Feed the World 10. Past Successes and Future Challenges to the Role Played by Wheat in Global Food Security. Food Sec. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Mottaleb, K.A.; Sonder, K.; Donovan, J.; Braun, H.-J. Global Trends in Wheat Production, Consumption and Trade. In Wheat Improvement; Reynolds, M.P., Braun, H.-J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 47–66. ISBN 978-3-030-90672-6. [Google Scholar]
- Kraamwinkel, C.T.; Beaulieu, A.; Dias, T.; Howison, R.A. Planetary Limits to Soil Degradation. Commun. Earth Environ. 2021, 2, 249. [Google Scholar] [CrossRef]
- Kalocsai, R.; Szalka, É.; Svétlíková, A.; Kukurová, K.; Szakál, T.; Giczi, Z.; Vona, V. The Principles of Sustainable Agricultural Cultivation and the Supply of Nutrients to Our Cultivated Plants. Acta Agron. Óváriensis 2022, 63, 51–65. [Google Scholar]
- Brockington, N.R. Modelling of Agricultural Production: Weather, Soils and Crops. Agric. Syst. 1987, 25, 79–81. [Google Scholar] [CrossRef]
- Goli, M.B.; Pande, M.; Bellaloui, N.; Wrachien, D.D. Effects of Soil Applications of Micro-Nutrients and Chelating Agent Citric Acid on Mineral Nutrients in Soybean Seeds. Agric. Sci. 2015, 6, 1404–1411. [Google Scholar] [CrossRef][Green Version]
- Marschner, H.; Marschner, P. (Eds.) Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier/Academic Press: London, UK; Waltham, MA, USA, 2012; ISBN 978-0-12-384905-2. [Google Scholar]
- Szakál, T.; Giczi, Z.; Vasas, D.; Molnár, J.; Szüle, B.; Szakál, P.; Gubó, E. Foliar Application of Copper-Tetramine-Sulphate from Microelectronic Waste to Improve Yield and Quality Parameters of Winter Wheat. Chem. Eng. Trans. 2024, 114, 1069–1074. [Google Scholar] [CrossRef]
- Korzeniowska, J.; Stanisławska-Glubiak, E. The Effect of Foliar Application of Copper on Content of This Element in Winter Wheat Grain. Pol. J. Agron. 2011, 4, 3–6. [Google Scholar]
- Yan, Y.; Yang, Z.; Yang, S.; Xu, A.; Xu, D. The Geochemical Characteristics and Exploitation Threshold of Copper in the Cultivated Soils of Guanzhong Plain, Shaanxi Province. Agronomy 2025, 15, 256. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A.; Kosegarten, H.; Appel, T. (Eds.) Principles of Plant Nutrition; Springer Netherlands: Dordrecht, The Netherlands, 2001; ISBN 978-1-4020-0008-9. [Google Scholar]
- Flemming, C.A.; Trevors, J.T. Copper Toxicity and Chemistry in the Environment: A Review. Water Air Soil Pollut. 1989, 44, 143–158. [Google Scholar] [CrossRef]
- Szakál, P.; Schmidt, R.; Barkóczi, M.; Kalocsai, R.; Beke, D.; Csatai, O. N-Containing Copper Complexes in Wheat Production. Cereal Res. Commun. 2006, 34, 681–684. [Google Scholar] [CrossRef]
- Yruela, I. Copper in Plants: Acquisition, Transport and Interactions. Funct. Plant Biol. 2009, 36, 409. [Google Scholar] [CrossRef] [PubMed]
- Nenova, V.; Bogoeva, I. Separate and Combined Effects of Excess Copper and Fusarium Culmorum Infection on Growth and Antioxidative Enzymes in Wheat (Triticum aestivum L.) Plants. J. Plant Interact. 2014, 9, 259–266. [Google Scholar] [CrossRef]
- Nanda, R.; Agrawal, V. Elucidation of Zinc and Copper Induced Oxidative Stress, DNA Damage and Activation of Defence System during Seed Germination in Cassia Angustifolia Vahl. Environ. Exp. Bot. 2016, 125, 31–41. [Google Scholar] [CrossRef]
- Giczi, Z.; Kalocsai, R.; Vona, V.; Szakál, T.; Lakatos, E.; Ásványi, B. Study of the Antifungal Effect of a Copper-Containing Foliar Fertilizer. Cereal Res. Commun. 2021, 49, 337–341. [Google Scholar] [CrossRef]
- Tölgyessy, J.; Horak, O.; Nielsen, N.E.; Schmidt, R.; Lesny, J.; Cik, G.; Végh, D.; Szakál, P. Agricultural Reutilization of Non-Ferrous Metal (Cu, Zn) Containing Waste from Microelectronic and Surface Treating Industry (Part I.). Acta Univ. Matthiae Belii Ser. Chem. 1998, 1, 9–15. [Google Scholar]
- Fageria, N.K.; Filho, M.P.B.; Moreira, A.; Guimarães, C.M. Foliar Fertilization of Crop Plants. J. Plant Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]
- Schmidt, R.; Barkóczi, M.; Szakál, P.; Kalocsai, R. The Impact of Copper Tetramine Hydroxide Treatments on Wheat Yield. Agrokémia és Talajt. 2002, 51, 193–200. [Google Scholar] [CrossRef]
- Debreczeni, B.; Szakál, P.; Leitner, M.; Schmidt, R. The Effect of Cu Complexes Containing Different Ligands on the Yield and the Chemical Composition of Spring Barley. In Proceedings of the Analytical and Environmental Conference; Schmidt, R., Szakál, P., Eds.; University of West Hungary, Faculty of Agriculture: Mosonmagyaróvár, Hungary, 2000; pp. 104–108. [Google Scholar]
- Arnon, D.I.; Stout, P.R. The Essentiality of Certain Elements in Minute Quantity For Plants With Special Reference to Copper. Plant Physiol. 1939, 14, 371–375. [Google Scholar] [CrossRef]
- Burkhead, J.L.; Collins, J.F. Nutrition Information Brief—Copper. Adv. Nutr. 2022, 13, 681–683. [Google Scholar] [CrossRef]
- Charkiewicz, A.E. Is Copper Still Safe for Us? What Do We Know and What Are the Latest Literature Statements? Curr. Issues Mol. Biol. 2024, 46, 8441–8463. [Google Scholar] [CrossRef]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of Copper on Mitochondrial Function and Metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Hey, S.J. The Contribution of Wheat to Human Diet and Health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Epstein, E.; Bloom, A.J. Mineral Nutrition of Plants: Principles and Perspectives, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2005; ISBN 978-0-87893-172-9. [Google Scholar]
- Hänsch, R.; Mendel, R.R. Physiological Functions of Mineral Micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of Nutrients. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 191–248. ISBN 978-0-12-384905-2. [Google Scholar]
- Xu, E.; Liu, Y.; Gu, D.; Zhan, X.; Li, J.; Zhou, K.; Zhang, P.; Zou, Y. Molecular Mechanisms of Plant Responses to Copper: From Deficiency to Excess. Int. J. Mol. Sci. 2024, 25, 6993. [Google Scholar] [CrossRef] [PubMed]
- Yruela, I. Copper in Plants. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef]
- Mydy, L.S.; Chigumba, D.N.; Kersten, R.D. Plant Copper Metalloenzymes As Prospects for New Metabolism Involving Aromatic Compounds. Front. Plant Sci. 2021, 12, 692108. [Google Scholar] [CrossRef]
- Gerwien, F.; Skrahina, V.; Kasper, L.; Hube, B.; Brunke, S. Metals in Fungal Virulence. FEMS Microbiol. Rev. 2018, 42, fux050. [Google Scholar] [CrossRef]
- Burkhead, J.L.; Gogolin Reynolds, K.A.; Abdel-Ghany, S.E.; Cohu, C.M.; Pilon, M. Copper Homeostasis. New Phytol. 2009, 182, 799–816. [Google Scholar] [CrossRef]
- Chaudhry, F.; Loneragan, J. Effects of Nitrogen, Copper, and Zinc Fertilizers on the Copper and Zinc Nutrition of Wheat Plants. Aust. J. Agric. Res. 1970, 21, 865. [Google Scholar] [CrossRef]
- Szakál, T.; Szalka, É.; Giczi, Z.; Vasas, D.; Světlíková, A.; Kukurová, K. Effect of the Copper-Sucrose Complex from Copper-Containing Waste on Yield and Quality of Winter Wheat. Acta Agron. Óváriensis 2022, 63, 22–35. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer: Sunderland, MA, USA, 2002; ISBN 978-0-87893-823-0. [Google Scholar]
- Chen, G.; Li, J.; Han, H.; Du, R.; Wang, X. Physiological and Molecular Mechanisms of Plant Responses to Copper Stress. Int. J. Mol. Sci. 2022, 23, 12950. [Google Scholar] [CrossRef] [PubMed]
- Fariduddin, Q.; Yusuf, M.; Hayat, S.; Ahmad, A. Effect of 28-Homobrassinolide on Antioxidant Capacity and Photosynthesis in Brassica Juncea Plants Exposed to Different Levels of Copper. Environ. Exp. Bot. 2009, 66, 418–424. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Adnan, M.; Ali, M.; Rana, M.S.; Kamran, M.; Ali, Q.; Hashem, I.A.; Bhantana, P.; Ali, M.; et al. Foliar Application of Gibberellic Acid Endorsed Phytoextraction of Copper and Alleviates Oxidative Stress in Jute (Corchorus capsularis L.) Plant Grown in Highly Copper-Contaminated Soil of China. Environ. Sci. Pollut. Res. 2020, 27, 37121–37133. [Google Scholar] [CrossRef]
- Letelier, M.E.; Lepe, A.M.; Faúndez, M.; Salazar, J.; Marín, R.; Aracena, P.; Speisky, H. Possible Mechanisms Underlying Copper-Induced Damage in Biological Membranes Leading to Cellular Toxicity. Chem. Biol. Interact. 2005, 151, 71–82. [Google Scholar] [CrossRef]
- Weitbrecht, K.; Schwab, S.; Rupp, C.; Bieler, E.; Dürrenberger, M.; Bleyer, G.; Schumacher, S.; Kassemeyer, H.-H.; Fuchs, R.; Schlücker, E. Microencapsulation—An Innovative Technique to Improve the Fungicide Efficacy of Copper against Grapevine Downy Mildew. Crop Prot. 2021, 139, 105382. [Google Scholar] [CrossRef]
- Patnaik, A.; Meher, R. Effect of Copper Fungicide on Earthworm, Lampito Mauritii. Int. J. Environ. Clim. Change 2023, 13, 184–194. [Google Scholar] [CrossRef]
- Burandt, Q.C.; Deising, H.B.; Von Tiedemann, A. Further Limitations of Synthetic Fungicide Use and Expansion of Organic Agriculture in Europe Will Increase the Environmental and Health Risks of Chemical Crop Protection Caused by Copper-Containing Fungicides. Environ. Toxicol. Chem. 2024, 43, 19–30. [Google Scholar] [CrossRef]
- Loch, J.; Nosticzius, Á. Agrochemistry and Plant Protection Chemistry (Agrokémia És Növényvédelmi Kémia); Mezőgazda Kiadó: Budapest, Hungary, 2004; ISBN 963-286-053-5. [Google Scholar]
- Liu, X.; Jiang, Y.; He, D.; Fang, X.; Xu, J.; Lee, Y.-W.; Keller, N.P.; Shi, J. Copper Tolerance Mediated by FgAceA and FgCrpA in Fusarium graminearum. Front. Microbiol. 2020, 11, 1392. [Google Scholar] [CrossRef]
- Ibarra-Laclette, E.; Blaz, J.; Pérez-Torres, C.-A.; Villafán, E.; Lamelas, A.; Rosas-Saito, G.; Ibarra-Juárez, L.A.; García-Ávila, C.D.J.; Martínez-Enriquez, A.I.; Pariona, N. Antifungal Effect of Copper Nanoparticles against Fusarium kuroshium, an Obligate Symbiont of Euwallacea Kuroshio Ambrosia Beetle. J. Fungi 2022, 8, 347. [Google Scholar] [CrossRef]
- Garutti, M.; Nevola, G.; Mazzeo, R.; Cucciniello, L.; Totaro, F.; Bertuzzi, C.A.; Caccialanza, R.; Pedrazzoli, P.; Puglisi, F. The Impact of Cereal Grain Composition on the Health and Disease Outcomes. Front. Nutr. 2022, 9, 888974. [Google Scholar] [CrossRef]
- Poutanen, K.S.; Kårlund, A.O.; Gómez-Gallego, C.; Johansson, D.P.; Scheers, N.M.; Marklinder, I.M.; Eriksen, A.K.; Silventoinen, P.C.; Nordlund, E.; Sozer, N.; et al. Grains—A Major Source of Sustainable Protein for Health. Nutr. Rev. 2022, 80, 1648–1663. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, W.; Nowicki, B.; Bełka, D.; Kazimierowicz, A.; Kulwicki, M.; Grzebisz, W. Effect of Foliar Application of Micronutrients and Fungicides on the Nitrogen Use Efficiency in Winter Wheat. Agronomy 2022, 12, 257. [Google Scholar] [CrossRef]
- Chisté, L.; Silva, C.A.; Rabêlo, F.H.S.; Jindo, K.; Melo, L.C.A. Evaluating Copper-Doped Biochar Composites for Improving Wheat Nutrition and Growth in Oxisols. Agronomy 2025, 15, 144. [Google Scholar] [CrossRef]
- Abd EL-Aziz, M.A.; Omar, E.H.; Ragab, M.M.S.; Said, M.M. Response of Wheat (Triticum aestivum L.) Plant Grown on Light Textured Soil to Copper Foliar Application Under Different Rates of Nitrogen Fertilization. Alex. Sci. Exch. J. Int. Q. J. Sci. Agric. Environ. 2008, 29, 91–98. [Google Scholar] [CrossRef][Green Version]
- Stepien, A.; Wojtkowiak, K. Effect of Foliar Application of Cu, Zn, and Mn on Yield and Quality Indicators of Winter Wheat Grain. Chil. J. Agric. Res. 2016, 76, 219–226. [Google Scholar] [CrossRef]
- Berzsenyi, Z. Design and Evaluation of Crop Production Experiments (Növénytermesztési Kísérletek Tervezése és értékelése); Agroinform: Budapest, Hungary, 2016; ISBN 978-963-502-954-9. [Google Scholar]
- Braun, D.M.; Wang, L.; Ruan, Y.-L. Understanding and Manipulating Sucrose Phloem Loading, Unloading, Metabolism, and Signalling to Enhance Crop Yield and Food Security. J. Exp. Bot. 2014, 65, 1713–1735. [Google Scholar] [CrossRef]
- Stein, O.; Granot, D. An Overview of Sucrose Synthases in Plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, F.; Song, P.; Liu, X.; Liu, Q.; Kou, T. Nighttime Warming Reduced Copper Concentration and Accumulation in Wheat Grown in Copper-Contaminated Soil by Affecting Physiological Traits. Agronomy 2024, 14, 1302. [Google Scholar] [CrossRef]
- Flynn, A.G.; Panozzo, J.F.; Gardner, W.K. The Effect of Copper Deficiency on the Baking Quality and Dough Properties of Wheat Flour. J. Cereal Sci. 1987, 6, 91–98. [Google Scholar] [CrossRef]
- Svecnjak, Z.; Jenel, M.; Bujan, M.; Vitali, D.; Vedrina Dragojević, I. Trace Element Concentrations in the Grain of Wheat Cultivars as Affected by Nitrogen Fertilization. Agric. Food Sci. 2013, 22, 445–451. [Google Scholar] [CrossRef][Green Version]
- Arshad, M.; Murtaza, G.; Ali, M.A.; Shafiq, M.; Dumat, C. Wheat Growth and Phytoavailability of Copper and Zinc as Affected by Soil Texture in Saline-Sodic Conditions. Pak. J. Bot. 2011, 43, 2433–2439. [Google Scholar]
- Karamanos, R.E.; Pomarenski, Q.; Goh, T.B.; A Flore, N. The Effect of Foliar Copper Application on Grain Yield and Quality of Wheat. Can. J. Plant Sci. 2004, 84, 47–56. [Google Scholar] [CrossRef]
- Moreira, A.; Moraes, L.A.C.; Schroth, G. Copper Fertilization in Soybean–Wheat Intercropping under No–till Management. Soil Tillage Res. 2019, 193, 133–141. [Google Scholar] [CrossRef]
- Jankowski, K.; Jankowski, K.; Hulanicki, P.S.; Sokólski, M.; Dubis, B.; Hulanicki, P. Yield and Quality of Winter Wheat (Triticum aestivum L.) in Response Differ. Syst. Foliar Fertilization. J. Elem. 2016, 21, 3. [Google Scholar] [CrossRef]
- Kumar, R.; Mehrotra, N.K.; Nautiyal, B.D.; Kumar, P.; Singh, P.K. Effect of Copper on Growth, Yield and Concentration of Fe, Mn, Zn and Cu in Wheat Plants (Triticum aestivum L.). J. Environ. Biol. 2009, 30, 485–488. [Google Scholar]
- Nazir, F.; Hussain, A.; Fariduddin, Q. Hydrogen Peroxide Modulate Photosynthesis and Antioxidant Systems in Tomato (Solanum lycopersicum L.) Plants Under Copp. Stress. Chemosphere 2019, 230, 544–558. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen Peroxide Metabolism and Functions in Plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef] [PubMed]
- Alhammad, B.A.; Seleiman, M.F.; Harrison, M.T. Hydrogen Peroxide Mitigates Cu Stress in Wheat. Agriculture 2023, 13, 862. [Google Scholar] [CrossRef]
- Lacey, J.; Bateman, G.L.; Mirocha, C.J. Effects of Infection Time and Moisture on Development of Ear Blight and Deoxynivalenol Production by Fusarium Spp. in Wheat. Ann. Appl. Biol. 1999, 134, 277–283. [Google Scholar] [CrossRef]
- Windels, C.E. Economic and Social Impacts of Fusarium Head Blight: Changing Farms and Rural Communities in the Northern Great Plains. Phytopathology 2000, 90, 17–21. [Google Scholar] [CrossRef]
- Worrall, E.; Hamid, A.; Mody, K.; Mitter, N.; Pappu, H. Nanotechnology for Plant Disease Management. Agronomy 2018, 8, 285. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A Review of the Antibacterial Effects of Silver Nanomaterials and Potential Implications for Human Health and the Environment. J. Nanopartic. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
| Meteorological Element | Year | March | April | May | June | March–June |
|---|---|---|---|---|---|---|
| Average temperature (°C) | 2019 | 8.6 | 12.1 | 12.7 | 23.0 | 14.0 |
| 2020 | 6.9 | 11.9 | 14.0 | 19.0 | 12.9 | |
| 2022 | 5.5 | 9.7 | 17.6 | 21.9 | 13.6 | |
| 1991–2020 | 6.0 | 11.4 | 15.9 | 19.6 | 13.2 | |
| Average relative humidity (%) | 2019 | 58 | 57 | 75 | 68 | 64 |
| 2020 | 60 | 49 | 65 | 74 | 62 | |
| 2022 | 54 | 63 | 66 | 67 | 63 | |
| 1991–2020 | 71 | 64 | 68 | 68 | 68 | |
| Precipitation sum (mm) | 2019 | 15 | 17 | 134 | 60 | 226 |
| 2020 | 40 | 3 | 40 | 92 | 175 | |
| 2022 | 12 | 19 | 60 | 118 | 208 | |
| 1991–2020 | 38 | 36 | 61 | 68 | 202 |
| Year | Depth (cm) | pH (KCl) | KA * | CaCO3 (w/w%) | Humus (w/w%) | P2O5 (mg kg−1) | K2O (mg kg−1) | Na (mg kg−1) | Zn (mg kg−1) | Cu (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 0–30 | 7.42 | 36 | 11.16 | 2.1 | 128 | 146 | 38 | 1.82 | 0.68 |
| 30–60 | 7.41 | 36 | 9.4 | 1.4 | 87 | 124 | 34 | 1.64 | 0.41 | |
| 60–90 | 7.24 | 38 | 6.8 | 1.2 | 63 | 89 | 37 | 0.76 | 0.29 | |
| 2020 | 0–30 | 7.47 | 37 | 10.6 | 2.4 | 144 | 218 | 38 | 2.82 | 0.73 |
| 30–60 | 7.38 | 36 | 9.8 | 2.1 | 122 | 196 | 41 | 1.94 | 0.64 | |
| 60–90 | 7.32 | 37 | 7.4 | 1.7 | 87 | 135 | 48 | 1.27 | 0.31 | |
| 2022 | 0–30 | 7.42 | 36 | 11.16 | 2.1 | 128 | 146 | 38 | 1.82 | 0.68 |
| 30–60 | 7.41 | 36 | 9.4 | 1.4 | 87 | 124 | 34 | 1.64 | 0.41 | |
| 60–90 | 7.24 | 39 | 6.8 | 1.2 | 63 | 89 | 37 | 0.76 | 0.29 |
| Parameters | Year 2019 | Year 2020 | Year 2022 |
|---|---|---|---|
| Preceding crop | Sunflower | Sunflower | Sunflower |
| Soil cultivation | Chisel plowing (up to 25 cm depth) and disk harrowing | Chisel plowing (up to 25 cm depth) and disk harrowing | Chisel plowing (up to 25 cm depth) and disk harrowing |
| Fertilizer applied to the soil (kg ha−1) | N = 120 | N = 120 | N = 120 |
| P = 80 | P = 80 | P = 80 | |
| K = 100 | K = 100 | K = 100 | |
| Winter wheat variety | early-maturing Montecarlo | early-maturing Montecarlo | early-maturing Obiwan |
| Sowing rate (seeds/m2) | 500 | 500 | 500 |
| Inter-row spacing (cm) | 12 | 12 | 12 |
| Herbicide used | Moderator | Moderator | Moderator |
| Treatment (kg ha−1) | Yield (t ha−1) | Crude Protein (%) | Wet Gluten (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2022 | Avg | 2019 | 2020 | 2022 | Avg | 2019 | 2020 | 2022 | Avg | ||||
| Control | 7.28 | 8.08 | 6.33 | 7.23 ± 1.34 | c | 13.0 | 14.0 | 12.3 | 13.1 ± 1.2 | e | 29.3 | 31.0 | 30.1 | 30.1 ± 1.3 | d |
| 0.1 (kg ha−1) Cu | 7.20 | 7.98 | 6.98 | 7.38 ± 0.84 | c | 13.2 | 14.3 | 12.4 | 13.3 ± 1.3 | de | 29.4 | 31.0 | 30.6 | 30.3 ± 1.3 | d |
| 0.3 (kg ha−1) Cu | 7.38 | 8.28 | 7.15 | 7.60 ± 0.95 | bc | 13.4 | 14.3 | 12.5 | 13.4 ± 1.3 | cd | 29.7 | 31.3 | 30.9 | 30.6 ± 1.4 | d |
| 0.5 (kg ha−1) Cu | 7.40 | 8.35 | 7.13 | 7.63 ± 1.19 | bc | 13.6 | 14.8 | 12.6 | 13.6 ± 1.5 | bc | 30.1 | 32.1 | 31.6 | 31.3 ± 1.6 | c |
| 1.0 (kg ha−1) Cu | 7.95 | 8.55 | 7.30 | 7.93 ± 1.11 | ab | 13.9 | 15.0 | 12.9 | 13.9 ± 1.5 | ab | 30.3 | 33.1 | 32.3 | 31.9 ± 2.1 | b |
| 2.0 (kg ha−1) Cu | 8.23 | 8.90 | 7.65 | 8.26 ± 1.19 | a | 14.1 | 14.9 | 12.9 | 14.0 ± 1.4 | a | 30.3 | 33.8 | 33.0 | 32.4 ± 2.5 | a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalocsai, R.; Giczi, Z.; Vasas, D.; Molnár, J.; Szakál, P.; Varga, Z.; Gubó, E.; Szakál, P.; Vona, V.M.; Krániczné Mayer, E.; et al. Investigation of the Effect of a New Type of Copper–Sucrose Complex Compound on the Yield and Quality Parameters of Winter Wheat (Triticum aestivum L.). Agronomy 2025, 15, 1506. https://doi.org/10.3390/agronomy15071506
Kalocsai R, Giczi Z, Vasas D, Molnár J, Szakál P, Varga Z, Gubó E, Szakál P, Vona VM, Krániczné Mayer E, et al. Investigation of the Effect of a New Type of Copper–Sucrose Complex Compound on the Yield and Quality Parameters of Winter Wheat (Triticum aestivum L.). Agronomy. 2025; 15(7):1506. https://doi.org/10.3390/agronomy15071506
Chicago/Turabian StyleKalocsai, Renátó, Zsolt Giczi, Dávid Vasas, Judit Molnár, Pál Szakál, Zoltán Varga, Eduárd Gubó, Pál Szakál, Viktória Margit Vona, Erika Krániczné Mayer, and et al. 2025. "Investigation of the Effect of a New Type of Copper–Sucrose Complex Compound on the Yield and Quality Parameters of Winter Wheat (Triticum aestivum L.)" Agronomy 15, no. 7: 1506. https://doi.org/10.3390/agronomy15071506
APA StyleKalocsai, R., Giczi, Z., Vasas, D., Molnár, J., Szakál, P., Varga, Z., Gubó, E., Szakál, P., Vona, V. M., Krániczné Mayer, E., Ásványi, B., & Szakál, T. (2025). Investigation of the Effect of a New Type of Copper–Sucrose Complex Compound on the Yield and Quality Parameters of Winter Wheat (Triticum aestivum L.). Agronomy, 15(7), 1506. https://doi.org/10.3390/agronomy15071506







