Temporal Stability of Plant Species α-Diversity in Alpine Grasslands of the Tibetan Plateau and Their Implications for Biodiversity Conservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data
2.3. Statistical Analyses
3. Results
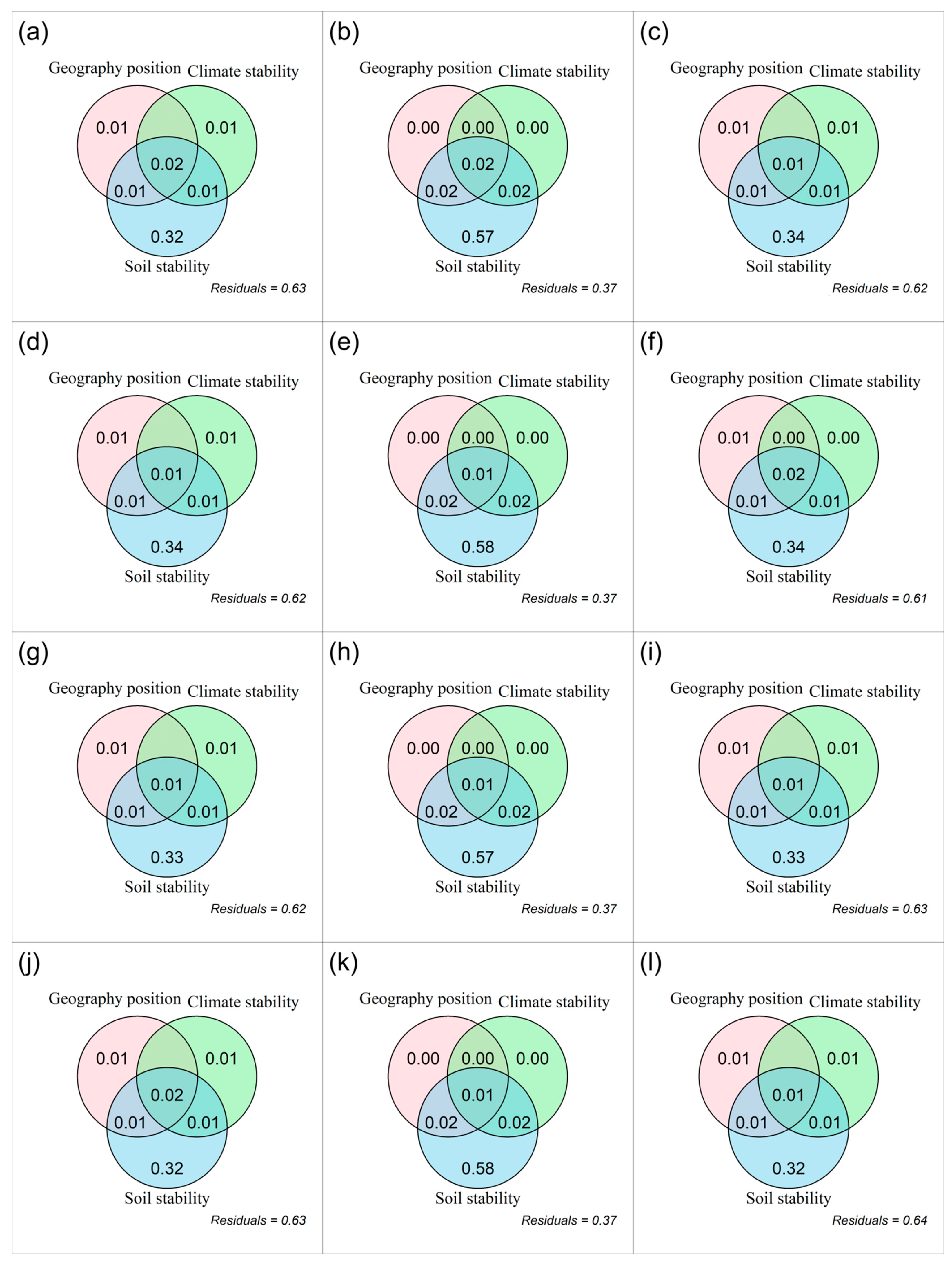
3.1. Spatial Distributions of Temporal Stability of Plant α-Diversity and Its Drivers
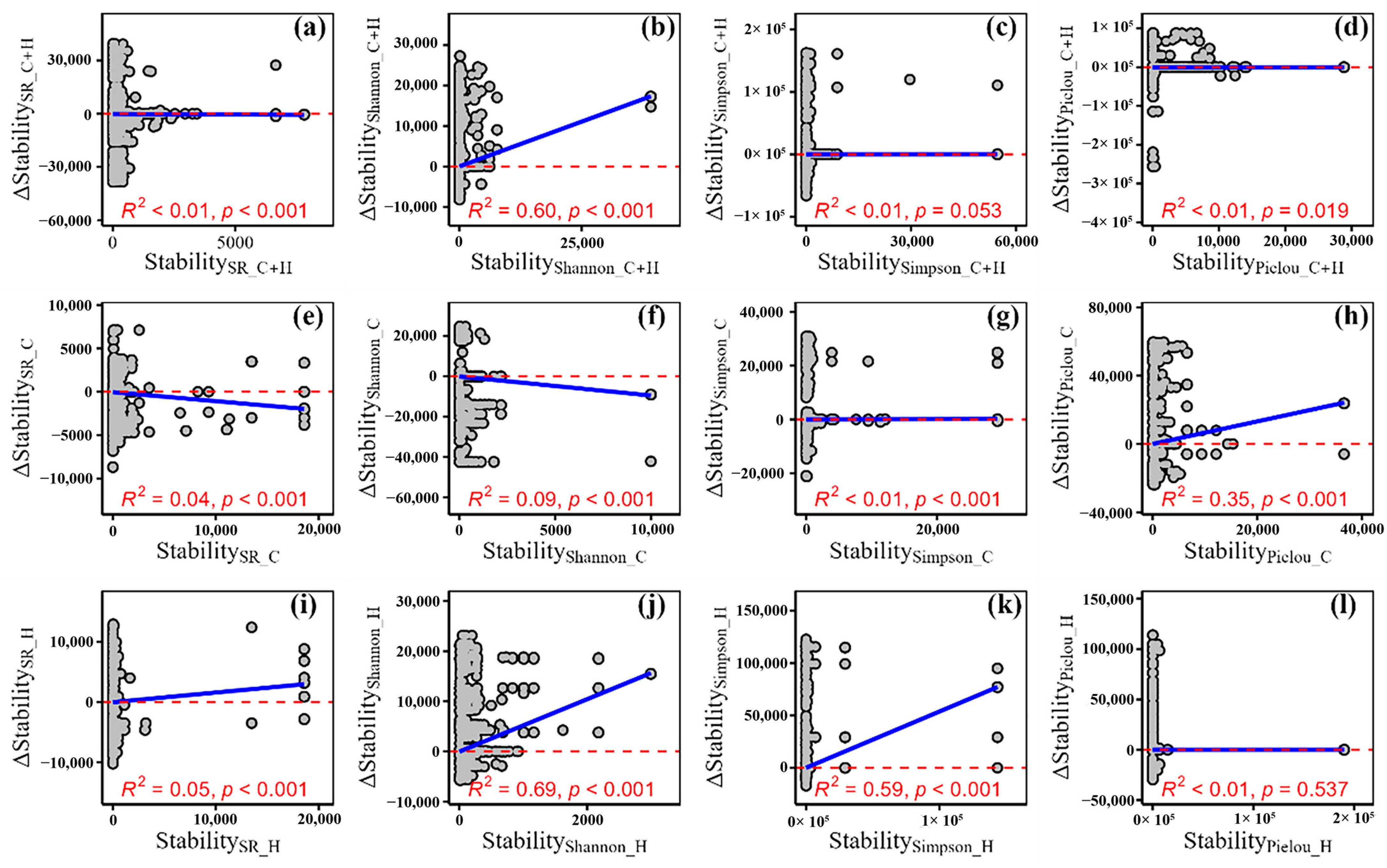
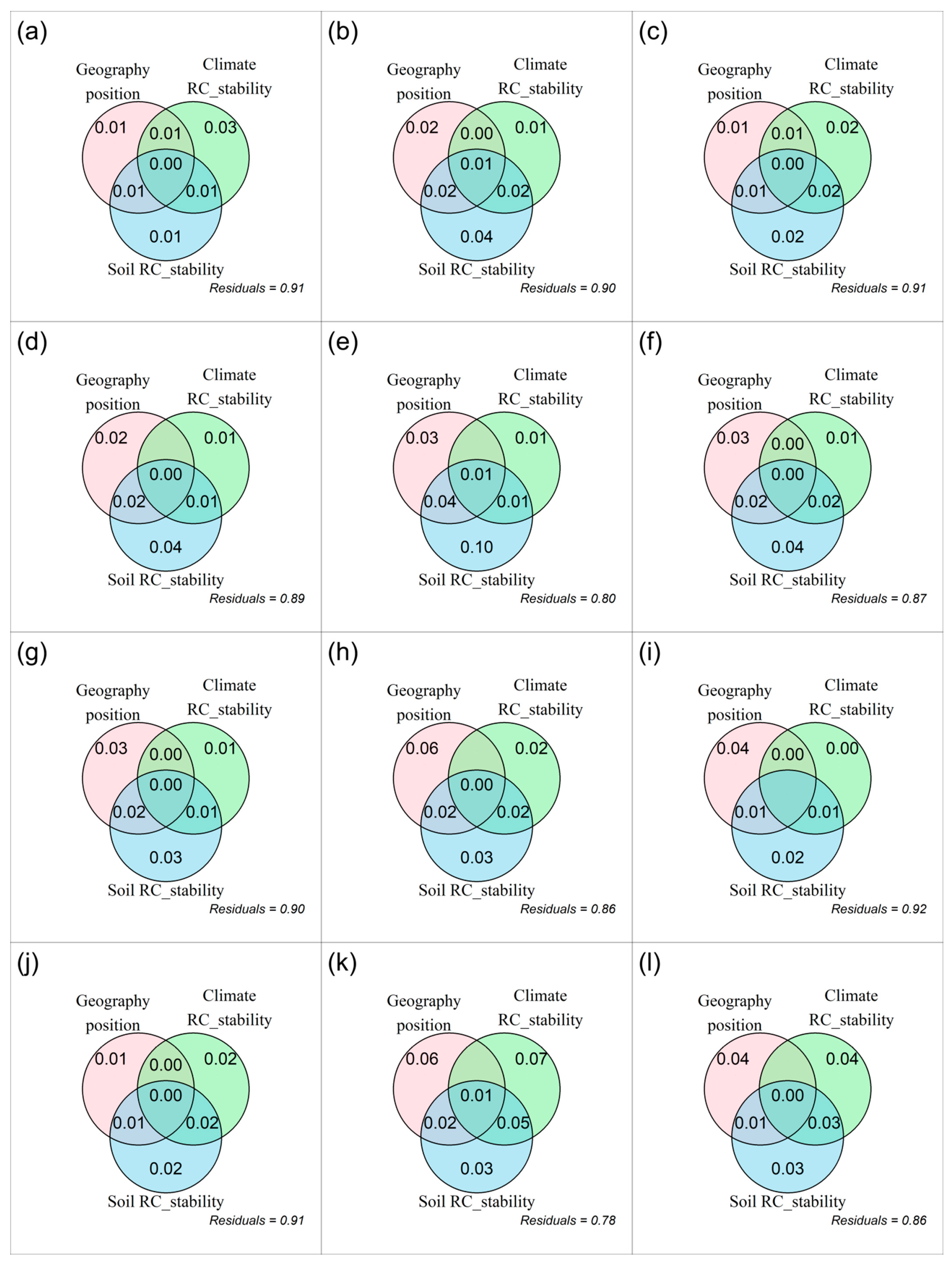
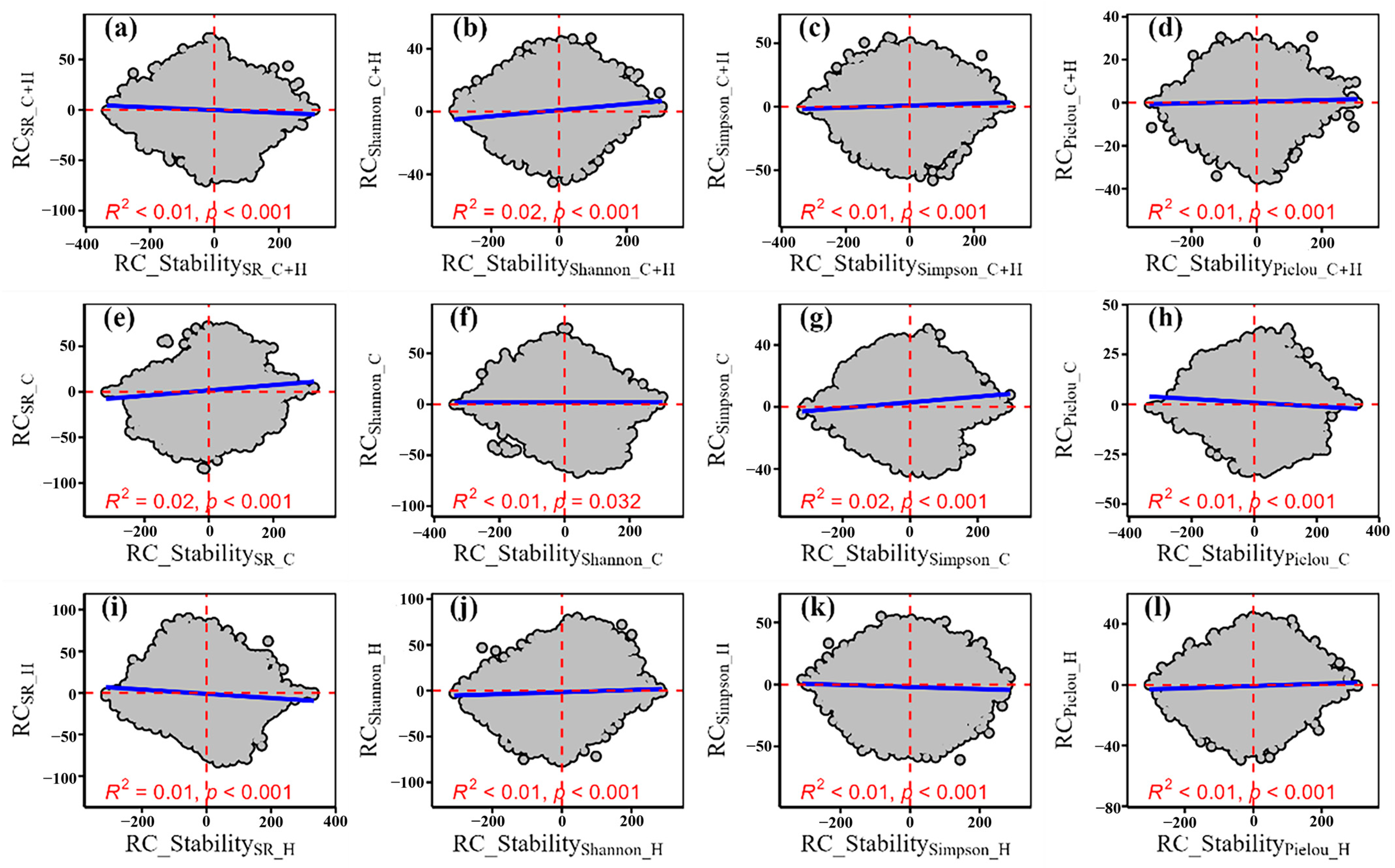
3.2. The Relative Change of Temporal Stability of Plant α-Diversity and Its Drivers
4. Discussion
4.1. Spatiotemporal Patterns of the Temporal Stability of Plant α-Diversity and Its Drivers
4.2. The Effects of Precipitation, Temperature, and Radiation on the Temporal Stability of Plant α-Diversity
4.3. The Relationship Between the Relative Change of Plant α-Diversity and That of Its Temporal Stability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lei, T.J.; Feng, J.; Lv, J.; Wang, J.B.; Song, H.Q.; Song, W.L.; Gao, X.F. Net Primary Productivity Loss under different drought levels in different grassland ecosystems. J. Environ. Manag. 2020, 274, 111144. [Google Scholar] [CrossRef] [PubMed]
- Kamp, J.; Koshkin, M.A.; Bragina, T.M.; Katzner, T.E.; Milner-Gulland, E.J.; Schreiber, D.; Sheldon, R.; Shmalenko, A.; Smelansky, I.; Terraube, J.; et al. Persistent and novel threats to the biodiversity of Kazakhstan’s steppes and semi-deserts. Biodivers. Conserv. 2016, 25, 2521–2541. [Google Scholar] [CrossRef]
- Zhao, Y.; Peth, S.; Krümmelbein, J.; Horn, R.; Wang, Z.Y.; Steffens, M.; Hoffmann, C.; Peng, X.H. Spatial variability of soil properties affected by grazing intensity in Inner Mongolia grassland. Ecol. Model. 2007, 205, 241–254. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Guo, H.B.; Quan, Q.; Niu, S.L.; Li, T.T.; He, Y.C.; Fu, Y.W.; Li, J.P.; Wang, J.S.; Zhang, R.Y.; Li, Z.L.; et al. Shifting biomass allocation and light limitation co-regulate the temporal stability of an alpine meadow under eutrophication. Sci. Total Environ. 2023, 860, 160411. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Ma, W.J.; Li, F.Y.; Zhang, Q.; Niu, J.M.; Ding, Y.; Han, F.; Sun, X.L. Functional Redundancy Instead of Species Redundancy Determines Community Stability in a Typical Steppe of Inner Mongolia. PLoS ONE 2015, 10, e0145605. [Google Scholar] [CrossRef]
- Magurran, A.E.; Deacon, A.E.; Moyes, F.; Shimadzu, H.; Dornelas, M.; Phillip, D.A.T.; Ramnarine, I.W. Divergent biodiversity change within ecosystems. Proc. Natl. Acad. Sci. USA 2018, 115, 1843–1847. [Google Scholar] [CrossRef]
- Qiao, X.T.; Geng, Y.; Zhang, C.Y.; Han, Z.X.; Zhang, Z.H.; Zhao, X.H.; Gadow, K. Spatial asynchrony matters more than alpha stability in stabilizing ecosystem productivity in a large temperate forest region. Glob. Ecol. Biogeogr. 2022, 31, 1133–1146. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Ma, Y.; Ma, W.; Liang, C.; Flynn, D.F.B.; Schmid, B.; Fang, J.; He, J.S. Field-based observations of regional-scale, temporal variation in net primary production in Tibetan alpine grasslands. Biogeosciences 2014, 11, 2003–2016. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.K.; Gao, Q.Z.; Zhang, Y.; Liu, S.L.; Ganjurjav, H.; Hu, G.Z.; Wang, X.X.; Yan, Y.L.; Wu, H.B.; et al. Rotational grazing promotes grassland aboveground plant biomass and its temporal stability under changing weather conditions on the Qinghai-Tibetan plateau. Land Degrad. Dev. 2020, 31, 2662–2671. [Google Scholar] [CrossRef]
- Huang, S.L.; Fu, G. Impacts of Climate Change and Human Activities on Plant Species a-Diversity across the Tibetan Grasslands. Remote Sens. 2023, 15, 2947. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Fu, G. Changes in soil organic carbon, total nitrogen and total phosphorus in 2000-2020 and their driving mechanisms in Tibetan alpine grasslands. Glob. Planet. Chang. 2024, 239, 104484. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.Z.; Niu, B.; He, Y.T.; Wang, X.T.; Wu, J.S. Changes in plant species richness distribution in Tibetan alpine grasslands under different precipitation scenarios. Glob. Ecol. Conserv. 2020, 21, e00848. [Google Scholar] [CrossRef]
- Campana, S.; Tognetti, P.M.; Alberti, J.; Graff, P.; Molina, C.D.; Silvoso, M.C.; Yahdjian, L. The spatiotemporal stability of plant diversity is disconnected from biomass stability in response to human activities in a South American temperate grassland. Sci. Total Environ. 2024, 955, 177031. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Calderon, A.P.; Meijer, M.J.J.; Wang, S.P.; van Kuijk, M.; Verweij, P.; Hautier, Y. Andean grassland stability across spatial scales increases with camelid grazing intensity despite biotic homogenization. J. Ecol. 2025, 113, 931–942. [Google Scholar] [CrossRef]
- Lama, G.; Villarroel, M. Behavioural biology of South American domestic camelids: An overview from a welfare perspective. Small Rumin. Res. 2023, 220, 106918. [Google Scholar] [CrossRef]
- Liu, F.; Liu, F.; Zhou, Q.; Chen, Q.; Wang, S.; Guo, R.; Ma, W. Ecological risk and regional differentiation in the Qinghai-Tibet Plateau. J. Nat. Resour. 2021, 36, 3232–3246. [Google Scholar] [CrossRef]
- Zhu, Q.A.; Chen, H.; Peng, C.H.; Liu, J.X.; Piao, S.L.; He, J.S.; Wang, S.P.; Zhao, X.Q.; Zhang, J.; Fang, X.Q.; et al. An early warning signal for grassland degradation on the Qinghai-Tibetan Plateau. Nat. Commun. 2023, 14, 6406. [Google Scholar] [CrossRef]
- Zhang, W.J.; Xue, X.; Peng, F.; You, Q.G.; Hao, A.H. Meta-analysis of the effects of grassland degradation on plant and soil properties in the alpine meadows of the Qinghai-Tibetan Plateau. Glob. Ecol. Conserv. 2019, 20, e00774. [Google Scholar] [CrossRef]
- Duan, X.Y.; Chen, Y.; Wang, L.Q.; Zheng, G.D.; Liang, T. The impact of land use and land cover changes on the landscape pattern and ecosystem service value in Sanjiangyuan region of the Qinghai-Tibet Plateau. J. Environ. Manag. 2023, 325, 116539. [Google Scholar] [CrossRef]
- Ran, Q.W.; Hao, Y.B.; Xia, A.Q.; Liu, W.J.; Hu, R.H.; Cui, X.Y.; Xue, K.; Song, X.N.; Xu, C.; Ding, B.Y.; et al. Quantitative Assessment of the Impact of Physical and Anthropogenic Factors on Vegetation Spatial-Temporal Variation in Northern Tibet. Remote Sens. 2019, 11, 1183. [Google Scholar] [CrossRef]
- Ganjurjav, H.; Gornish, E.S.; Hu, G.Z.; Wan, Y.F.; Li, Y.; Danjiu, L.B.; Gao, Q.Z. Temperature leads to annual changes of plant community composition in alpine grasslands on the Qinghai-Tibetan Plateau. Environ. Monit. Assess. 2018, 190, 585. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Wulf, H.; Schmid, B.; He, J.S.; Schaepman, M.E. Estimating Plant Traits of Alpine Grasslands on the Qinghai-Tibetan Plateau Using Remote Sensing. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2018, 11, 2263–2275. [Google Scholar] [CrossRef]
- Xu, H.J.; Wang, X.P.; Zhang, X.X. Alpine grasslands response to climatic factors and anthropogenic activities on the Tibetan Plateau from 2000 to 2012. Ecol. Eng. 2016, 92, 251–259. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, W.; Shi, P.; Zhao, C.; Liu, R.; Tang, H.; Wang, J.a.; He, B. The differences of vegetation characteristics and environmental conditions among main vegetation types on the Qinghai-Tibet Plateau. Acta Ecol. Sin. 2024, 44, 2955–2970. [Google Scholar]
- Duan, H.; Xue, X.; Wang, T.; Kang, W.; Liao, J.; Liu, S. Spatial and Temporal Differences in Alpine Meadow, Alpine Steppe and All Vegetation of the Qinghai-Tibetan Plateau and Their Responses to Climate Change. Remote Sens. 2021, 13, 669. [Google Scholar] [CrossRef]
- Yan, Y.J.; Ma, F.F.; Wang, J.S.; Zhang, R.Y.; Peng, J.L.; Liao, J.Q.; Zhou, Q.P.; Niu, S.L. Warming stabilizes alpine ecosystem facing extreme rainfall events by changing plant species composition. J. Ecol. 2023, 111, 2064–2076. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Liu, H.Y.; Mi, Z.R.; Zhang, Z.H.; Wang, Y.H.; Xu, W.; Jiang, L.; He, J.S. Climate warming reduces the temporal stability of plant community biomass production. Nat. Commun. 2017, 8, 15378. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, S.B.; Liu, H.H. Estimation of alpine grassland above-ground biomass and its response to climate on the Qinghai-Tibet Plateau during 2001 to 2019. Glob. Ecol. Conserv. 2022, 35, e02065. [Google Scholar] [CrossRef]
- Yuan, Z.R.; Liang, Y.L.; Zhao, H.; Wei, D.; Wang, X.D. Trade-offs and synergies between ecosystem services on the Tibetan Plateau. Ecol. Indic. 2024, 158, 111384. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, J.B.; Zhang, F.W.; Yang, Y.S.; Luo, F.L.; Li, Y.N.; Li, J.X. Stability response of alpine meadow communities to temperature and precipitation changes on the Northern Tibetan Plateau. Ecol. Evol. 2022, 12, e8592. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wu, Y.T.; Cui, J.H.; Li, H.Y.; Li, H.; Zhang, J.H.; Miao, B.L.; Wang, L.X.; Li, Z.Y.; Liang, C.Z. Temporal Stability of Grazed Grassland Ecosystems Alters Response to Climate Variability, While Resistance Stability Remains Unchanged. Agronomy 2023, 13, 2030. [Google Scholar] [CrossRef]
- Li, L.; Huang, C.; Zhang, Y.; Liu, L.; Wang, Z.; Zhang, H.; Ding, M.; Zhang, H. Mapping the multi-temporal grazing intensity on the Qinghai-Tibet Plateau using geographically weighted random forest. Sci. Geogr. Sin. 2023, 43, 398–410. [Google Scholar]
- Qiu, J. The third pole. Nature 2008, 454, 393–396. [Google Scholar] [CrossRef]
- Liu, J.S.; Wang, S.Y.; Yu, S.M.; Yang, D.Q.; Zhang, L. Climate warming and growth of high-elevation inland lakes on the Tibetan Plateau. Glob. Planet. Chang. 2009, 67, 209–217. [Google Scholar] [CrossRef]
- Yang, K.; Wu, H.; Qin, J.; Lin, C.G.; Tang, W.J.; Chen, Y.Y. Recent climate changes over the Tibetan Plateau and their impacts on energy and water cycle: A review. Glob. Planet. Chang. 2014, 112, 79–91. [Google Scholar] [CrossRef]
- Gong, X.W.; Wang, X.Y.; Li, Y.Q.; Ma, L.; Li, M.Y.; Si, H.T. Observed Changes in Extreme Temperature and Precipitation Indices on the Qinghai-Tibet Plateau, 1960–2016. Front. Environ. Sci. 2022, 10, 888937. [Google Scholar] [CrossRef]
- Tang, L.; Dong, S.K.; Sherman, R.; Liu, S.L.; Liu, Q.R.; Wang, X.X.; Su, X.K.; Zhang, Y.; Li, Y.Y.; Wu, Y.; et al. Changes in vegetation composition and plant diversity with rangeland degradation in the alpine region of Qinghai-Tibet Plateau. Rangel. J. 2015, 37, 107–115. [Google Scholar] [CrossRef]
- Solow, A.R.; Polasky, S. Measuring biological diversity. Environ. Ecol. Stat. 1994, 1, 95–103. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Knight, R. Species divergence and the measurement of microbial diversity. Fems Microbiol. Rev. 2008, 32, 557–578. [Google Scholar] [CrossRef]
- Gorelick, R. Combining richness and abundance into a single diversity index using matrix analogues of Shannon’s and Simpson’s indices. Ecography 2006, 29, 525–530. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Wilsey, B.J.; Potvin, C. Biodiversity and ecosystem functioning: Importance of species evenness in an old field. Ecology 2000, 81, 887–892. [Google Scholar] [CrossRef]
- Isbell, F.; Craven, D.; Connolly, J.; Loreau, M.; Schmid, B.; Beierkuhnlein, C.; Bezemer, T.M.; Bonin, C.; Bruelheide, H.; de Luca, E.; et al. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 2015, 526, 574–577. [Google Scholar] [CrossRef]
- Gu, Y.T.; Meng, L.; Wang, Y.T.; Wu, Z.R.; Pan, Y.H.; Zhao, Y.Y.; Detto, M.; Wu, J. Uncovering the role of solar radiation and water stress factors in constraining decadal intra-site spring phenology variability in diverse ecosystems across the Northern Hemisphere. New Phytol. 2025, 246, 1986–2003. [Google Scholar] [CrossRef]
- van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Kardol, P.; Klironomos, J.N.; Kulmatiski, A.; Schweitzer, J.A.; et al. Plant-soil feedbacks: The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Fu, Z.; Xiao, Y.; Xu, H.; Shi, X.; Luo, H.; Ben, C.; Yang, Z.; Li, M. Interannual dynamics and homeostasis of soil carbon, nitrogen, and phosphorus stoichiometry in a subtropical evergreen broad-leaved forest: Based on ten years observation. Chin. J. Ecol. 2025, 44, 1–10. [Google Scholar]
- Liu, K.; Liu, Z.C.; Zhou, N.; Shi, X.R.; Lock, T.R.; Kallenbach, R.L.; Yuan, Z.Y. Diversity-stability relationships in temperate grasslands as a function of soil pH. Land Degrad. Dev. 2022, 33, 1704–1717. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, N.; Zhang, S.Y.; Zhu, X.Y.; Wang, H.; Xiu, W.M.; Zhao, J.N.; Liu, H.M.; Zhang, H.F.; Yang, D.L. Soil bacterial community composition is altered more by soil nutrient availability than pH following long-term nutrient addition in a temperate steppe. Front. Microbiol. 2024, 15, 1455891. [Google Scholar] [CrossRef]
- Dai, E.R.; Zhang, G.Y.; Fu, G.; Zha, X.J. Can meteorological data and normalized difference vegetation index be used to quantify soil pH in grasslands? Front. Ecol. Evol. 2023, 11, 1206581. [Google Scholar] [CrossRef]
- Wang, S.H.; Qi, H.X.; Li, T.Y.; Qin, Y.; Fu, G.; Pan, X.; Zha, X.J. Can normalized difference vegetation index and climate data be used to estimate soil carbon, nitrogen, and phosphorus and their ratios in the Xizang grasslands? Front. Earth Sci. 2024, 11, 1340020. [Google Scholar] [CrossRef]
- Sun, W.; Qi, H.X.; Li, T.Y.; Qin, Y.; Fu, G.; Han, F.S.; Wang, S.H.; Pan, X. Modelling Soil Ammonium Nitrogen, Nitrate Nitrogen and Available Phosphorus Using Normalized Difference Vegetation Index and Climate Data in Xizang’s Grasslands. Sustainability 2024, 16, 4695. [Google Scholar] [CrossRef]
- Tian, Y.; Fu, G. Quantifying Plant Species α-Diversity Using Normalized Difference Vegetation Index and Climate Data in Alpine Grasslands. Remote Sens. 2022, 14, 5007. [Google Scholar] [CrossRef]
- Tilman, D. The ecological consequences of changes in biodiversity: A search for general principles. Ecology 1999, 80, 1455–1474. [Google Scholar] [CrossRef]
- Sandoval-Calderon, A.P.; Soons, M.B.; van Kuijk, M.; Verweij, P.A.; Barry, K.E.; Hautier, Y. Camelid herding may homogenize Andean grassland plant communities. Ecol. Indic. 2024, 167, 112590. [Google Scholar] [CrossRef]
- Song, X.X.; Wang, L.; Zhao, X.; Liu, C.; Chang, Q.; Wang, Y.; Xu, T.T.; Wang, D.L. Sheep grazing and local community diversity interact to control litter decomposition of dominant species in grassland ecosystem. Soil Biol. Biochem. 2017, 115, 364–370. [Google Scholar] [CrossRef]
- Xian, Y.Y.; Wang, T.X.; Leng, W.C.; Letu, H.; Shi, J.C.; Wang, G.F.; Yan, X.W.; Yuan, H.Y. Can Topographic Effects on Solar Radiation Be Ignored: Evidence From the Tibetan Plateau. Geophys. Res. Lett. 2024, 51, e2024GL108653. [Google Scholar] [CrossRef]
- Jia, A.L.; Liang, S.L.; Wang, D.D.; Jiang, B.; Zhang, X.T. Air pollution slows down surface warming over the Tibetan Plateau. Atmos. Chem. Phys. 2020, 20, 881–899. [Google Scholar] [CrossRef]
- Buzhdygan, O.Y.; Tietjen, B.; Rudenko, S.S.; Nikorych, V.A.; Petermann, J.S. Direct and indirect effects of land-use intensity on plant communities across elevation in semi-natural grasslands. PLoS ONE 2020, 15, e0231122. [Google Scholar] [CrossRef]
- Ding, R.; Qin, Y.; Li, T.Y.; Fu, G. Exploring spatiotemporal dynamics in temporal stability of soil carbon, nitrogen, phosphorus, and pH in Tibetan grasslands. Geoderma 2024, 451, 117062. [Google Scholar] [CrossRef]
- Zhan, T.Y.; Zhang, S.R.; Zhao, W.W. Adaptive management for alpine grassland of the Tibetan Plateau based on a multi-criteria assessment. Front. Plant Sci. 2025, 16, 1518721. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.G.; Zhang, X.K.; Du, X.D.; Du, Z.Y.; Sun, M.Z. Alpine grassland greening on the Northern Tibetan Plateau driven by climate change and human activities considering extreme temperature and soil moisture. Sci. Total Environ. 2024, 916, 169995. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; de Jong, R.; Schmid, B.; Wulf, H.; Schaepman, M.E. Changes in grassland cover and in its spatial heterogeneity indicate degradation on the Qinghai-Tibetan Plateau. Ecol. Indic. 2020, 119, 106641. [Google Scholar] [CrossRef]
- Wang, S.S.; Jia, L.Z.; Cai, L.P.; Wang, Y.J.; Zhan, T.Y.; Huang, A.Q.; Fan, D.L. Assessment of Grassland Degradation on the Tibetan Plateau Based on Multi-Source Data. Remote Sens. 2022, 14, 6011. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Reiter, K.; Klettner, C.; Grabherr, G. Signals of range expansions and contractions of vascular plants in the high Alps: Observations (1994-2004) at the GLORIA*master site Schrankogel, Tyrol, Austria. Glob. Change Biol. 2007, 13, 147–156. [Google Scholar] [CrossRef]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barancok, P.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, M.R.F.; et al. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Chang. 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Dullinger, S.; Abdaladze, O.; Akhalkatsi, M.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, R.F.; et al. Recent Plant Diversity Changes on Europe’s Mountain Summits. Science 2012, 336, 353–355. [Google Scholar] [CrossRef]
- Yan, Y.; Lu, X.Y. Is grazing exclusion effective in restoring vegetation in degraded alpine grasslands in Tibet, China? PeerJ 2015, 3, e1020. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Guo, Y.; Liang, L. Ecological protection and restoration model of multi-ecological elements in Qinghai-Tibet Plateau: A case study of Lhasa River Basin. Acta Ecol. Sin. 2019, 39, 8966–8974. [Google Scholar]
- Wang, L.M.; Han, S.J.; Tian, F.Q.; Li, K.B.; Li, Y.K.; Tudaji, M.; Cao, X.J.; Nan, Y.; Cui, T.; Zheng, X.Y.; et al. The Evaporation on the Tibetan Plateau Stops Increasing in the Recent Two Decades. J. Geophys. Res.-Atmos. 2022, 127, e2022JD037377. [Google Scholar] [CrossRef]
- Sun, J.; Yang, K.; Yu, Y.; Lu, H.; Lin, Y.L. Land-Atmosphere Interactions Partially Offset the Accelerated Tibetan Plateau Water Cycle through Dynamical Processes. J. Clim. 2023, 36, 3867–3880. [Google Scholar] [CrossRef]
- Zarzycki, J.; Kopec, M. Effect of Habitat and Historical Factors on the Distribution of Meadow Plant Species in the Radziejowa Range (Beskid Sadecki Mountains). Pol. J. Environ. Stud. 2009, 18, 949–955. [Google Scholar]
- Khamidov, M.; Ishchanov, J.; Hamidov, A.; Shermatov, E.; Gafurov, Z. Impact of Soil Surface Temperature on Changes in the Groundwater Level. Water 2023, 15, 3865. [Google Scholar] [CrossRef]
- Luo, H.; Quaas, J.; Han, Y. Diurnally asymmetric cloud cover trends amplify greenhouse warming. Sci. Adv. 2024, 10, eado5179. [Google Scholar] [CrossRef]
- Kejna, M.; Uscka-Kowalkowska, J.; Kejna, P. The influence of cloudiness and atmospheric circulation on radiation balance and its components. Theor. Appl. Climatol. 2021, 144, 823–838. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Wu, L.; Wang, B.; Xu, M. Response of organic carbon mineralization to temperature changing pattern under long-term fertilization in red soil. Soil Fertil. Sci. China 2020, 10–16. [Google Scholar]
- Chen, Q.; Tao, B.; Shang, Y.; Zhan, M.; Li, H.; Li, G.; Li, Y. Combined effects of nitrogen forms and temperature on soil organic carbon mineralization of forest in the sandy area of western Shandong Province, China. J. Agro-Environ. Sci. 2023, 42, 414–423. [Google Scholar]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Griffiths, B.; Ritz, K.; Wheatley, R. Root-induced nitrogen mineralisation: A theoretical analysis. Plant Soil 1989, 117, 185–193. [Google Scholar] [CrossRef]
- Connell, J.H.; Slatyer, R.O. Mechanisms of succession in natural communities and their role in community stability and organization. Am. Nat. 1977, 111, 1119–1144. [Google Scholar] [CrossRef]
- Xiang, X.M.; De, K.J.; Lin, W.S.; Feng, T.X.; Li, F.; Wei, X.J. Effects of warming and nitrogen deposition on species and functional diversity of plant communities in the alpine meadow of Qinghai-Tibet Plateau. PLoS ONE 2025, 20, e0319581. [Google Scholar] [CrossRef] [PubMed]
- Spohn, M.; Bagchi, S.; Bakker, J.D.; Borer, E.T.; Carbutt, C.; Catford, J.A.; Dickman, C.R.; Eisenhauer, N.; Eskelinen, A.; Hagenah, N.; et al. Interactive and unimodal relationships between plant biomass, abiotic factors, and plant diversity in global grasslands. Commun. Biol. 2025, 8, 97. [Google Scholar] [CrossRef]
- Wu, X.; Gong, K.; Jin, G.; Ma, J.; Yomaerjiang, I.; Abdukutus, K. Effects of Different Animals on Seed Dispersal Characteristics of Achnatherum inebrians. Acta Agrestia Sin. 2021, 29, 2240–2246. [Google Scholar]
- Qin, X.P.; Liang, W.; Liu, Z.M.; Liu, M.H.; Baskin, C.C.; Baskin, J.M.; Xin, Z.M.; Wang, Z.G.; Zhou, Q.L. Plant canopy may promote seed dispersal by wind. Sci. Rep. 2022, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.J.; Hernández, D.L.; Pasari, J.R.; Zavaleta, E.S. Grazing maintains native plant diversity and promotes community stability in an annual grassland. Ecol. Appl. 2015, 25, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Steidl, R.J.; Litt, A.R.; Matter, W.J. Effects of Plant Invasions on Wildlife in Desert Grasslands. Wildl. Soc. Bull. 2013, 37, 527–536. [Google Scholar] [CrossRef]
- Liang, M.W.; Cappelli, S.L.; Borer, E.T.; Tilman, D.; Seabloom, E.W. Consumers Modulate Effects of Plant Diversity on Community Stability. Ecol. Lett. 2025, 28, e70103. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, S.P.; Zhao, L.Q.; Liang, C.Z.; Miao, B.L.; Zhang, Q.; Niu, X.X.; Ma, W.H.; Schmid, B. Stability and asynchrony of local communities but less so diversity increase regional stability of Inner Mongolian grassland. Elife 2022, 11, e74881. [Google Scholar] [CrossRef]
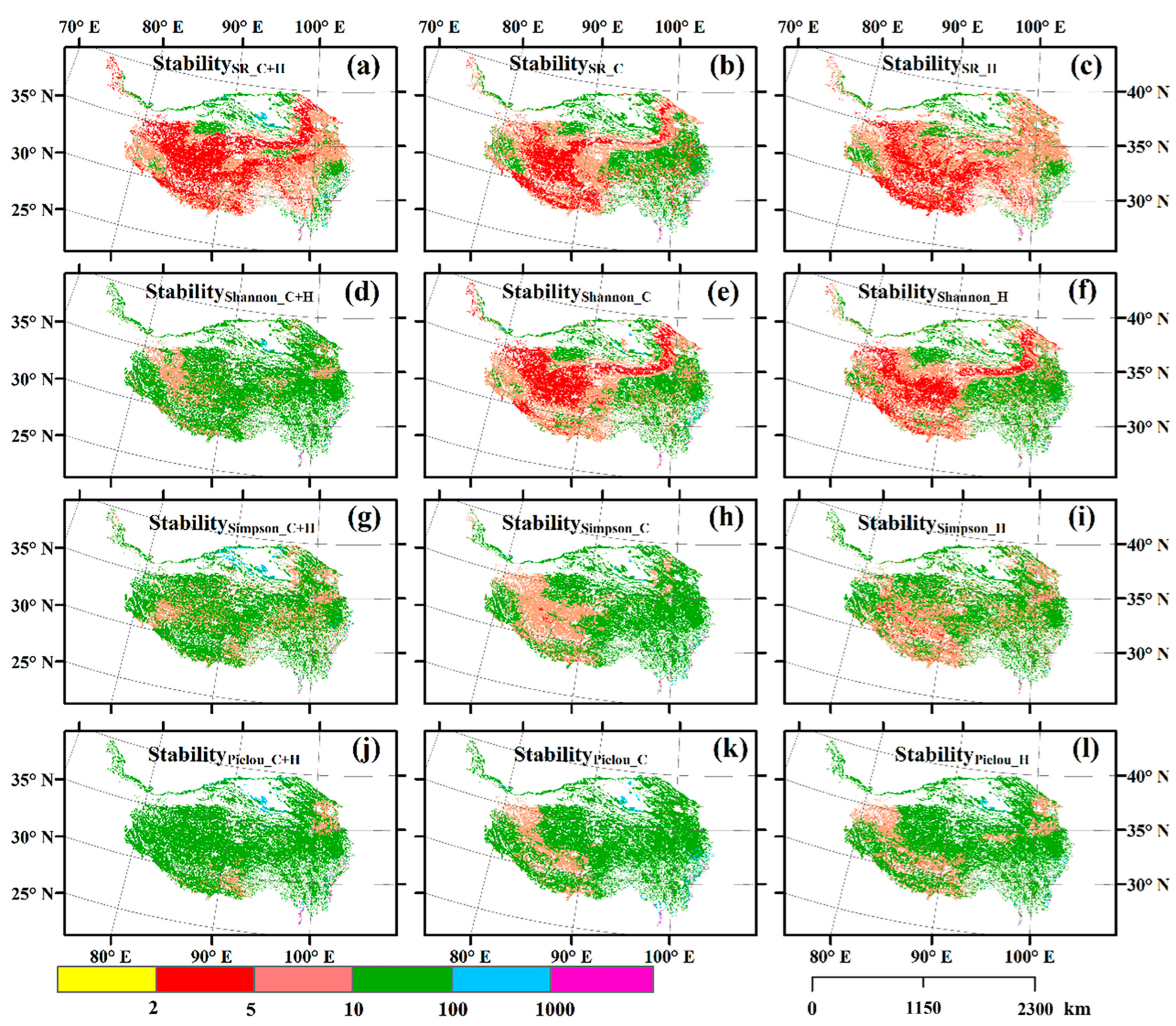


| <2 | 2–5 | 5–10 | 10–100 | 100–1000 | >1000 | Min | Max | Mean | |
|---|---|---|---|---|---|---|---|---|---|
| StabilitySR_C+H | 0.00 | 41.02 | 41.49 | 16.21 | 1.10 | 0.18 | 2.18 | 7822.00 | 23.55 |
| StabilityShannon_C+H | 0.00 | 0.00 | 18.61 | 80.77 | 0.44 | 0.17 | 4.30 | 39,138.98 | 75.47 |
| StabilitySimpson_C+H | 0.00 | 0.04 | 24.35 | 73.69 | 1.74 | 0.18 | 4.24 | 54,677.60 | 103.48 |
| StabilityPielou_C+H | 0.00 | 0.00 | 6.37 | 91.76 | 1.51 | 0.36 | 2.96 | 28,863.64 | 93.17 |
| StabilitySR_C | 0.00 | 23.05 | 38.64 | 37.46 | 0.55 | 0.30 | 1.94 | 18,588.08 | 63.79 |
| StabilityShannon_C | 0.00 | 25.39 | 38.75 | 34.97 | 0.68 | 0.21 | 2.00 | 9995.65 | 31.06 |
| StabilitySimpson_C | 0.00 | 0.31 | 28.57 | 70.20 | 0.73 | 0.20 | 3.26 | 29,259.66 | 74.37 |
| StabilityPielou_C | 0.00 | 0.02 | 13.83 | 84.15 | 1.77 | 0.23 | 4.13 | 36,580.27 | 92.42 |
| StabilitySR_H | 0.10 | 27.45 | 55.02 | 17.15 | 0.13 | 0.15 | 1.39 | 18,582.66 | 35.77 |
| StabilityShannon_H | 0.00 | 21.46 | 39.85 | 38.24 | 0.30 | 0.15 | 2.03 | 2993.54 | 15.74 |
| StabilitySimpson_H | 0.00 | 1.99 | 39.55 | 58.08 | 0.25 | 0.14 | 2.38 | 143,375.86 | 204.73 |
| StabilityPielou_H | 0.00 | 0.39 | 21.29 | 77.24 | 0.92 | 0.16 | 2.87 | 190,054.64 | 278.30 |
| <−100 | −100 to −50 | −50 to 0 | 0–50 | 50–100 | >100 | Min | Max | Mean | |
|---|---|---|---|---|---|---|---|---|---|
| RC_StabilitySR_C+H | 5.91 | 9.46 | 42.81 | 35.42 | 4.59 | 1.81 | −335.44 | 312.21 | −11.75 |
| RC_StabilityShannon_C+H | 4.42 | 13.31 | 38.5 | 33.72 | 8.21 | 1.84 | −309.89 | 304.89 | −8.83 |
| RC_StabilitySimpson_C+H | 7.35 | 14.28 | 39.01 | 31.5 | 6.04 | 1.8 | −336.88 | 313.16 | −16.40 |
| RC_StabilityPielou_C+H | 4.77 | 11.45 | 42.53 | 33.35 | 6.3 | 1.61 | −328.54 | 310.01 | −10.69 |
| RC_StabilitySR_C | 5.27 | 14 | 36.93 | 34.38 | 7.18 | 2.24 | −319.37 | 327.21 | −9.99 |
| RC_StabilityShannon_C | 6.58 | 12.4 | 37.77 | 33.79 | 6.62 | 2.83 | −337.92 | 301.02 | −10.87 |
| RC_StabilitySimpson_C | 5.76 | 14.35 | 36.67 | 32.85 | 8.31 | 2.06 | −319.43 | 295.41 | −11.14 |
| RC_StabilityPielou_C | 6.18 | 15.02 | 36.26 | 31.93 | 8.21 | 2.4 | −335.04 | 330.42 | −11.43 |
| RC_StabilitySR_H | 5.33 | 14.17 | 38.33 | 32.15 | 8.13 | 1.88 | −311.58 | 333.04 | −10.69 |
| RC_StabilityShannon_H | 6.94 | 14.49 | 34.98 | 32.38 | 8.46 | 2.76 | −308.82 | 288.22 | −11.22 |
| RC_StabilitySimpson_H | 5.65 | 15.35 | 37.36 | 29.92 | 9.06 | 2.67 | −310.06 | 286.72 | −10.68 |
| RC_StabilityPielou_H | 5.69 | 14.96 | 36.59 | 30.78 | 9.12 | 2.85 | −303.74 | 304.18 | −9.83 |
| RC_StabilitySR_C+H | RC_StabilityShannon_C+H | RC_StabilitySimpson_C+H | RC_StabilityPielou_C+H | |
|---|---|---|---|---|
| ICC | 19.00 | 19.26 | 18.99 | 19.41 |
| IHA | 21.60 | 24.02 | 19.40 | 20.72 |
| ICCHA | 0.01 | 0.01 | 0.01 | 0.01 |
| DCC | 28.20 | 24.69 | 28.90 | 28.55 |
| DHA | 29.98 | 31.52 | 31.72 | 30.18 |
| DCCHA | 0.01 | 0.01 | 0.04 | 0.00 |
| NCC | 0.66 | 0.17 | 0.37 | 0.75 |
| NHA | 0.53 | 0.30 | 0.55 | 0.34 |
| NCCHA | 0.01 | 0.02 | 0.03 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Sun, W.; Li, S.; Dai, E.; Fu, G. Temporal Stability of Plant Species α-Diversity in Alpine Grasslands of the Tibetan Plateau and Their Implications for Biodiversity Conservation. Agronomy 2025, 15, 1502. https://doi.org/10.3390/agronomy15071502
Li T, Sun W, Li S, Dai E, Fu G. Temporal Stability of Plant Species α-Diversity in Alpine Grasslands of the Tibetan Plateau and Their Implications for Biodiversity Conservation. Agronomy. 2025; 15(7):1502. https://doi.org/10.3390/agronomy15071502
Chicago/Turabian StyleLi, Tianyu, Wei Sun, Shaowei Li, Erfu Dai, and Gang Fu. 2025. "Temporal Stability of Plant Species α-Diversity in Alpine Grasslands of the Tibetan Plateau and Their Implications for Biodiversity Conservation" Agronomy 15, no. 7: 1502. https://doi.org/10.3390/agronomy15071502
APA StyleLi, T., Sun, W., Li, S., Dai, E., & Fu, G. (2025). Temporal Stability of Plant Species α-Diversity in Alpine Grasslands of the Tibetan Plateau and Their Implications for Biodiversity Conservation. Agronomy, 15(7), 1502. https://doi.org/10.3390/agronomy15071502







