Screening Terminal Drought Tolerance in Dry Bean Genotypes and Commercial Bean Cultivars in Chile
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site Conditions and Genetic Materials
2.2. Crop Management and Experimental Design
2.3. Agronomic Evaluations
2.4. The Drought Intensity and Susceptibility Index Calculations
2.5. Phenological Evaluations
2.6. Physiological Evaluations
2.7. Statistical Analysis
3. Results and Discussion
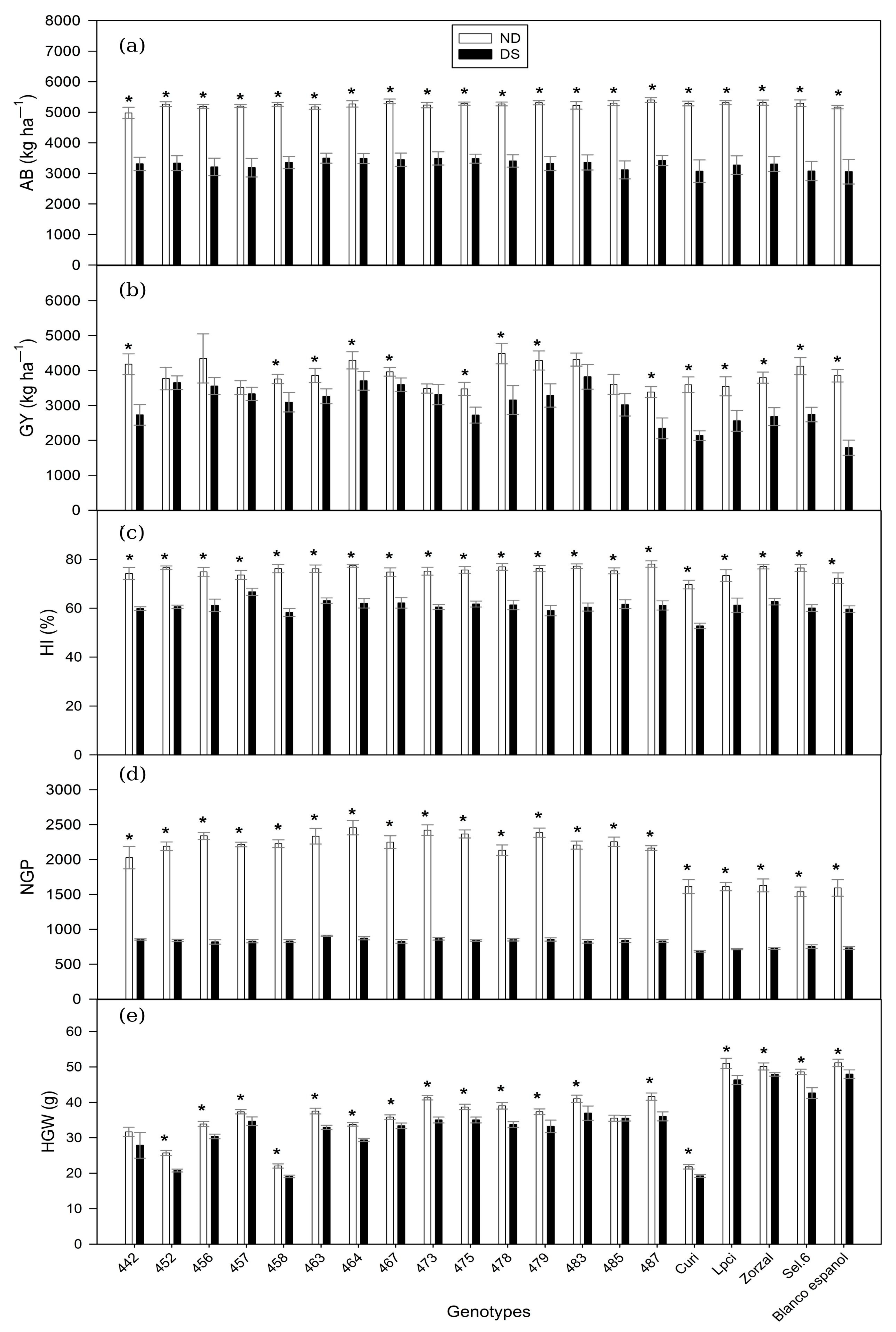
3.1. Agronomic and Productive Traits
3.2. Phenological Traits
3.3. Physiological Traits
3.4. Relationships Between Traits and Yield Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uebersax, M.A.; Cichy, K.A.; Gomez, F.E.; Porch, T.G.; Heitholt, J.J.; Osorno, J.M.; Kamfwa, K.; Snapp, S.S.; Bales, S.R. Dry Beans (Phaseolus vulgaris L.) as a Vital Component of Sustainable Agriculture and Food Security—A Review. Legume Sci. 2022, 5, 1. [Google Scholar] [CrossRef]
- Didinger, C.; Foster, M.T.; Bunning, M.; Thompson, H.J. Nutrition and Human Health Benefits of Dry Beans and Other Pulses. In Dry Beans and Pulses: Production, Processing, and Nutrition; Siddiq, M., Uebersax, M.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 481–504. [Google Scholar]
- Lisciani, S.; Marconi, S.; Donne, C.L.; Camilli, E.; Aguzzi, A.; Gabrielli, P.; Gambelli, L.; Kunert, K.; Marais, D.; Vorster, J.; et al. Legumes and Common Beans in Sustainable Diets: Nutritional Quality, Environmental Benefits, Spread and Use in Food Preparations. Front. Nutr. 2024, 11, 1385232. [Google Scholar] [CrossRef] [PubMed]
- Cominelli, E.; Sparvoli, F.; Lisciani, F.; Forti, C.; Camilli, G.; Ferrari, S.; Le Donne, M.; Marconi, S.; Vorster, B.J.; Botha, A.-M.; et al. Antinutritional factors, nutritional improvement, and future food use of common beans: A perspective. Front. Plant Sci. 2022, 13, 992169. [Google Scholar] [CrossRef]
- Papanikolaou, Y.; Slavin, J.; Fulgoni, V.L. Adult Dietary Patterns with Increased Bean Consumption Are Associated with Greater Overall Shortfall Nutrient Intakes, Lower Added Sugar, Improved Weight-Related Outcomes and Better Diet Quality. Nutr. J. 2024, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Jain, P.; Ujinwal, M.; Langyan, S. Escalate protein plates from legumes for sustainable human nutrition. Front. Nutr. 2022, 9, 977986. [Google Scholar] [CrossRef]
- Parker, T.A.; Acosta-Gallegos, J.; Beaver, J.; Brick, M.; Brown, J.K.; Cichy, K.A.; Debouck, D.G.; Delgado-Salinas, A.; Dohle, S.; Ernest, E.; et al. Genetic Resources and Breeding Priorities in Phaseolus Beans: Vulnerability, Resilience, and Future Challenges. Plant Breed. Rev. 2023, 46, 289–420. [Google Scholar]
- Farrow, A.; Muthoni-Andriatsitohaina, R. Atlas of Common Bean Production in Africa, 2nd ed.; Pan-Africa Bean Research Alliance (PABRA) International Center for Tropical Agriculture (CIAT): Nairobi, Kenya, 2020; p. 242. [Google Scholar]
- Jha, P.K.; Beebe, S.; Alvarez-Toro, P.; Mukankusi, C.; Ramirez-Villegas, J. Characterizing Patterns of Seasonal Drought Stress for Use in Common Bean Breeding in East Africa under Present and Future Climates. Agric. For. Meteorol. 2023, 342, 109735. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Young, R.; Reig, P. Aqueduct projected water stress country rankings. World Resources Institute. Tech. Note 2015, 16, 1–16. [Google Scholar]
- Araya-Osses, D.; Casanueva, A.; Román-Figueroa, C.; Jaramillo, J.M.; Paneque, M. Climate change projections of temperature and precipitation in Chile based on statistical downscaling. Clim. Dyn. 2020, 54, 4309–4330. [Google Scholar] [CrossRef]
- Rai, A.; Sharma, V.; Heitholt, J. Dry Bean (Phaseolus vulgaris L.) Growth and Yield Response to Variable Irrigation in the Arid to Semi-Arid Climate. Sustainability 2020, 12, 3851. [Google Scholar] [CrossRef]
- Labastida, D.; Ingvarsson, P.K.; Rendón-Anaya, M. Dissecting the genetic basis of drought responses in common bean using natural variation. Front. Plant Sci. 2023, 14, 1143873. [Google Scholar] [CrossRef] [PubMed]
- González, J.; Tay, K. Producción de Poroto y Garbanzo en el Secano Costero del Valle del Mataquito: Línea Base Productiva, Económica y Comercial; Instituto de Investigaciones Agropecuarias (INIA): Santiago, Chile, 2017; Volume 349, p. 146. [Google Scholar]
- Martínez-Barradas, V.; Inostroza-Blancheteau, C.; Tighe-Neira, R.; Romero-Romero, J.L.; Schwember, A.R.; Arce-Johnson, P. Drought Tolerance Evaluation of ‘Zorzal,’ the Most Cultivated Common Bean in Chile, a Country Facing Desertification. Agric. Res. 2023, 13, 41–54. [Google Scholar] [CrossRef]
- Garreaud, R.; Boisier, J.P.; Rondanelli, R.; Montecinos, A.; Sepúlveda, H.H.; Veloso-Aguila, D. The Central Chile Mega Drought (2010–2018): A climate dynamics perspective. Int. J. Climatol. 2019, 40, 421–439. [Google Scholar] [CrossRef]
- Jódar, J.; Urrutia, J.; Herrera, C.; Custódio, E.; Martos-Rosillo, S.; Lambán, L.J. The catastrophic effects of groundwater intensive exploitation and Mega-drought on aquifers in Central Chile: Global change impact projections in water re-sources based on groundwater balance modeling. Sci. Total Environ. 2023, 914, 169651. [Google Scholar] [CrossRef]
- Mutari, B.; Sibiya, J.; Shayanowako, A.; Chidzanga, C.; Matova, P.M.; Gasura, E. Genome-wide association mapping for component traits of drought tolerance in dry beans (Phaseolus vulgaris L.). PLoS ONE 2023, 18, e0278500. [Google Scholar] [CrossRef] [PubMed]
- Mladenov, P.; Aziz, S.; Tonev, T.; Georgieva, T.; Uzundzhalieva, K.; Nikolova, K. Physiological Responses of Common Bean Genotypes to Drought Stress. Agronomy 2023, 13, 1022. [Google Scholar] [CrossRef]
- Memari, M.; Dadashi, M.R.; Sheikh, F.; Mohammadi, M. Evaluation of drought stress tolerance in some faba bean genotypes using drought tolerance indices. Rom. Agric. Res. 2022, 39, 59–66. [Google Scholar] [CrossRef]
- Mutari, B.; Sibiya, J.; Gasura, E.; Mabasa, S.; Chirwa, R. Genetic Analysis of Grain Yield and Yield-Attributing Traits in Navy Bean (Phaseolus vulgaris L.) under Drought Stress. Euphytica 2022, 218, 51. [Google Scholar] [CrossRef]
- Khadka, K.; Earl, H.J.; Raizada, M.N.; Navabi, A. A Physio-Morphological Trait-Based Approach for Breeding Drought Tolerant Wheat. Front. Plant Sci. 2020, 11, 715. [Google Scholar] [CrossRef]
- Gracia-Romero, A.; Segarra, J.; Rezzouk, F.Z.; Aparicio, N.; Kefauver, S.C.; Araus, J.L. In-Field Phenotyping Using the Low-Cost and Open Access Fluorescence PhotosynQ Multispeq Sensor Together with NDVI: A Case Study with Durum Wheat. Agriculture 2025, 15, 385. [Google Scholar] [CrossRef]
- Tefera, A.T.; O’Leary, G.J.; Thayalakumaran, T.; Rao, S.; Silva-Perez, V.; Shunmugam, A.S.K.; Armstrong, R.; Rosewarne, G.M. Identification of agro-physiological traits of lentil that reduce risks of drought. Front. Plant Sci. 2022, 13, 1019491. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, F.; Ninou, E.; Mylonas, I.; Baxevanos, D.; Papadopoulou, F.; Avdikos, I.; Sistanis, I.; Koskosidis, A.; Vlachostergios, D.N.; Stefanou, S.; et al. The Evaluation of Common Bean (Phaseolus vulgaris L.) Genotypes under Water Stress Based on Physiological and Agronomic Parameters. Plants 2022, 11, 2432. [Google Scholar] [CrossRef] [PubMed]
- Traub, J.; Porch, T.G.; Naeem, M.; Urrea, C.A.; Austic, G.; Kelly, J.D.; Loescher, W.H. Screening for Heat Tolerance in Phaseolus spp. Using Multiple Methods. Crop Sci. 2018, 58, 2459–2469. [Google Scholar] [CrossRef]
- Gonçalves, J.G.R.; de Andrade, E.R.; da Silva, D.A.; de Fátima Esteves, J.A.; Chiorato, A.F.; Carbonell, S.A.M. Drought tolerance evaluated in common bean genotypes. Ciênc. Agrotec. 2019, 43, e01719. [Google Scholar] [CrossRef]
- Mickelbart, M.V.; Hasegawa, P.M.; Bailey-Serres, J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet. 2015, 16, 237–251. [Google Scholar] [CrossRef]
- USDA. Keys to Soil Taxonomy, 12th ed.; United States Department of Agriculture (USDA): Washington, DC, USA, 2014.
- Suárez, J.C.; Polanía, J.A.; Contreras, A.T.; Beebe, S.E.; Rao, I.M. Adaptation of Common Bean Lines to High Temperature Conditions: Genotypic Differences in Phenological and Agronomic Performance. Euphytica 2020, 216, 28. [Google Scholar] [CrossRef]
- Beebe, S.; Rao, I.M.; Blair, M.W.; Acosta-Gallegos, J.A. Phenotyping common beans for adaptation to drought. Front. Physiol. 2013, 4, 35. [Google Scholar] [CrossRef]
- Dramadri, I.O.; Nkalubo, S.T.; Kramer, D.; Kelly, J.D. Genome-wide association analysis of drought adaptive traits in common bean. Crop Sci. 2021, 61, 3232–3248. [Google Scholar] [CrossRef]
- Rodriguez, D.F.C.; Urban, M.O.; Santaella, M.; Gereda, J.M.; Contreras, A.D.; Wenzl, P. Using Phenomics to Identify and Integrate Traits of Interest for Better-Performing Common Beans: A Validation Study on an Interspecific Hybrid and Its Acutifolii Parents. Front. Plant Sci. 2022, 13, 1008666. [Google Scholar] [CrossRef]
- Oliveira Neto, S.S.; Pereira, F.F.S.; Zoz, T.; Oliveira, C.E.S.; Cirino, V.M. Effect of water deficit on morphoagronomic traits of black common bean genotypes (Phaseolus vulgaris L.) with contrasting drought tolerance. J. Agron. Crop Sci. 2022, 208, 460–468. [Google Scholar] [CrossRef]
- Polanía, J.; Poschenrieder, C.; Beebe, S.; Rao, I.M. Effective Use of Water and Increased Dry Matter Partitioned to Grain Contribute to Yield of Common Bean Improved for Drought Resistance. Front. Plant Sci. 2016, 7, 660. [Google Scholar] [CrossRef] [PubMed]
- Androcioli, L.G.; Zeffa, D.M.; Alves, D.S.; Tomaz, J.P.; Moda-Cirino, V. Effect of Water Deficit on Morpho agronomic and Physiological Traits of Common Bean Genotypes with Contrasting Drought Tolerance. Water 2020, 12, 217. [Google Scholar] [CrossRef]
- Rezene, Y.; Gebeyehu, S.; Zelleke, H. Morpho-physiological response to post-flowering drought stress in small red seeded common bean (Phaseolus vulgaris L.) genotypes. J. Plant Stud. 2012, 2, 42–52. [Google Scholar] [CrossRef]
- Darkwa, K.; Ambachew, D.; Mohammed, H.; Asfaw, A.; Blair, M.W. Evaluation of common bean (Phaseolus vulgaris L.) genotypes for drought stress adaptation in Ethiopia. Crop Sci. 2016, 56, 568–583. [Google Scholar] [CrossRef]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef]
- Sita, K.; Sehgal, A.; Hanumantha Rao, B.; Nair, R.M.; Prasad, P.V.V.; Kumar, S.; Gaur, P.M.; Farooq, M.; Siddique, K.H.M.; Varshney, R.K.; et al. Food Legumes and Rising Temperatures: Effects, Adaptive Functional Mechanisms Specific to Repro-ductive Growth Stage and Strategies to Improve Heat Tolerance. Front. Plant Sci. 2017, 8, 1658. [Google Scholar] [CrossRef] [PubMed]
- Gebeyehu, S. Physiological Response to Drought Stress of Common Bean (Phaseolus vulgaris L.) Genotypes Differing in Drought Resistance. J. Agron. Crop Sci. 2006, 192, 257–263. [Google Scholar]
- Hageman, A.; Van Volkenburgh, E. Sink Strength Maintenance Underlies Drought Tolerance in Common Bean. Plants 2021, 10, 489. [Google Scholar] [CrossRef]
- Sánchez-Reinoso, A.D.; Moreno, G.A.L.; Restrepo-Díaz, H. Evaluation of drought indices to identify tolerant genotypes in common bean bush (Phaseolus vulgaris L.). J. Integr. Agric. 2020, 19, 99–107. [Google Scholar] [CrossRef]
- Assefa, T.; Rao, I.M.; Cannon, S.B.; Wu, J.; Gutema, Z.; Blair, M.; Otyama, P.; Alemayehu, F.; Dagne, B. Improving adaptation to drought stress in white pea bean (Phaseolus vulgaris L.): Genotypic effects on grain yield, yield components and pod harvest index. Plant Breed. 2017, 136, 548–561. [Google Scholar] [CrossRef]
- Polanía, J.; Salazar-Chavarría, V.; González-Lemes, I.; Acosta-Maspons, A.; Chater, C.C.C.; Covarrubias, A.A. Contrasting Phaseolus Crop Water Use Patterns and Stomatal Dynamics in Response to Terminal Drought. Front. Plant Sci. 2022, 13, 894657. [Google Scholar] [CrossRef] [PubMed]
- Tapia, G.; Méndez, J.; Inostroza, L.; Lozano, C. Water Shortage Affects Vegetative and Reproductive Stages of Common Bean (Phaseolus vulgaris) Chilean Landraces, Differentially Impacting Grain Yield Components. Plants 2022, 11, 749. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2008, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Bota, J.; Galmés, J.; Medrano, H.; Ribas-Carbó, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Bashir, N.; Athar, H.; Kalaji, H.M.; Wróbel, J.; Mahmood, S.; Zafar, Z.U.; Ashraf, M. Is Photoprotection of PSII One of the Key Mechanisms for Drought Tolerance in Maize? Int. J. Mol. Sci. 2021, 22, 13490. [Google Scholar] [CrossRef]
- Ruban, A.V. Non-photochemical Chlorophyll Fluorescence Quenching: Mechanism and Effectiveness in Protecting Plants from Photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef]
- Živčák, M.; Kalaji, H.M.; Shao, H.; Olšovská, K.; Brestič, M. Photosynthetic proton and electron transport in wheat leaves under prolonged moderate drought stress. J. Photochem. Photobiol. 2014, 137, 107–115. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.-P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Tyystjärvi, E.; Atherton, J.; van der Tol, C.; Flexas, J.; Pfündel, E.E.; Moreno, J.; Frankenberg, C.; Berry, J.A. Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: Mechanisms and challenges. J. Exp. Bot. 2014, 65, 4065–4095. [Google Scholar] [CrossRef]
- Rosales, M.A.; Ocampo, E.; Rodríguez-Valentín, R.; Olvera-Carrillo, Y.; Acosta-Gallegos, J.; Covarrubias, A.A. Physiological Analysis of Common Bean (Phaseolus vulgaris L.) Cultivars Uncovers Characteristics Related to Terminal Drought Resistance. Plant Physiol. Biochem. 2012, 56, 24–34. [Google Scholar] [CrossRef]
- Lanna, A.C.; Silva, R.A.; Ferraresi, T.M.; Mendonça, J.A.; Coelho, G.R.C.; Moreira, A.S.; Valdisser, P.A.M.R.; Brondani, C.; Vianello, R.P. Physiological Characterization of Common Bean (Phaseolus vulgaris L.) under Abiotic Stresses for Breeding Purposes. Environ. Sci. Pollut. Res. Int. 2018, 25, 31149–33116. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, M.; Murchie, E.H.; Gray, J.E.; Villegas, D.; Pastenes, C.; Pinto, M.; Horton, P. Differential Adaptation of Two Varieties of Common Bean to Abiotic Stress: II. Acclimation of Photosynthesis. J. Exp. Bot. 2006, 57, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Rao, I.M.; Beebe, S.E.; Polania, J.; Grajales, M.; Cajiao, C.; Ricaurte, J.; Garcia, R.; Rivera, M. Evidence for Genotypic Differences among Elite Lines of Common Bean in Their Ability to Remobilize Photosynthate to Increase Yield under Drought. J. Agric. Sci. 2017, 155, 857–875. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Blair, M.W.; Butare, L. Breeding for Abiotic Stress Tolerance in Common Bean: Present and Future Challenges. SABRAO J. Breed. Genet. 2009, 41, 1–11. [Google Scholar]
- Keller, B.; Soto, J.; Steier, A.; Portilla-Benavides, A.E.; Raatz, B.; Studer, B.; Walter, A.; Muller, O.; Urban, M.O. Linking photosynthesis and yield reveals a strategy to improve light use efficiency in a climbing bean breeding population. J. Exp. Bot. 2024, 75, 901–916. [Google Scholar] [CrossRef]
- Macedo, D.C.; Lima, G.R.; Barros, R.L.N.; Pimentel, C. The Intensity of and Recovery from Photoinhibition under Drought in a Thermotolerant Common Bean Compared to Drought Tolerant Genotypes. Biol. Plant. 2019, 63, 465–473. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Regulation in Photosynthetic Organisms: Signaling, Acclimation, and Practical Implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Gregersen, P.L.; Culetic, A.; Boschian, L.; Krupinska, K. Plant Senescence and Crop Productivity. Plant Mol. Biol. 2013, 82, 603–622. [Google Scholar] [CrossRef]
- Esan, V.I.; Oke, G.O.; Ogunbode, T.O.; Obisesan, I.A. AMMI and GGE Biplot Analyses of Bambara Groundnut [Vigna subterranea (L.) Verdc.] for Agronomic Performances under Three Environmental Conditions. Front. Plant Sci. 2022, 13, 997429. [Google Scholar] [CrossRef]
- Omrani, A.; Omrani, S.; Khodarahmi, M.; Shojaei, S.H.; Illés, Á.; Bojtor, C.; Mousavi, S.M.N.; Nagy, J. Evaluation of Grain Yield Stability in Some Selected Wheat Genotypes Using AMMI and GGE Biplot Methods. Agronomy 2022, 12, 1130. [Google Scholar] [CrossRef]
- Wang, R.; Wang, H.; Huang, S.; Zhao, Y.; Chen, E.; Li, F.; Qin, L.; Yang, Y.; Guan, Y.; Liu, B.; et al. Assessment of Yield Performances for Grain Sorghum Varieties by AMMI and GGE Biplot Analyses. Front. Plant Sci. 2023, 14, 1261323. [Google Scholar] [CrossRef] [PubMed]
- Gauch, H.G.; Piepho, H.P.; Annicchiarico, P. Statistical Analysis of Yield Trials by AMMI and GGE: Further Considerations. Crop Sci. 2008, 48, 866–889. [Google Scholar] [CrossRef]
- Acosta-Díaz, E.; Acosta-Gallegos, J.A.; Trejo-López, C.; Padilla-Ramírez, J.S.; Amador-Ramírez, M.D. Adaptation Traits in Dry Bean Cultivars Grown under Drought Stress. Agric. Téc. Méx. 2009, 35, 419–428. [Google Scholar]



| Season | Month | T° Max (°C) | T° Min (°C) | T° Ave (°C) | ET0 (mm) | Rf (mm) | Irr (mm) | ND (mm) | DS (mm) |
|---|---|---|---|---|---|---|---|---|---|
| 2021/2022 | Nov. | 25.2 | 6.7 | 16.0 | 119.5 | 6.0 | 60 | 66.0 | 66.0 |
| Dec. | 29.3 | 9.3 | 19.3 | 147.0 | 13.0 | 100 | 113.0 | 113.0 | |
| Jan. | 29.2 | 9.0 | 19.1 | 143.2 | 1.6 | 120 | 121.6 | 1.6 | |
| Feb. | 29.7 | 8.3 | 19.0 | 130.4 | 9.5 | 90 | 99.5 | 9.5 | |
| 2022/2023 | Nov. | 26.5 | 9.3 | 17.9 | 123.1 | 25.7 | 60 | 85.7 | 85.7 |
| Dec. | 29.0 | 9.7 | 19.4 | 142.2 | 0.0 | 100 | 100 | 100 | |
| Jan. | 30.3 | 9.9 | 20.1 | 159.5 | 7.4 | 140 | 147.4 | 7.4 | |
| Feb. | 31.1 | 8.6 | 19.8 | 128.8 | 0.0 | 90 | 90 | 00 |
| Code | Genotype | Market Class | Growth Habit | Origen |
|---|---|---|---|---|
| 442 | GN16-7 | Great Northern | II | NE |
| 452 | SB2-171 | Cream | II | NE |
| 456 | NE1-09-19 | Great Northern | II | NE |
| 457 | NE2-17-6 | Pinto | II | NE |
| 458 | NE14-17-2 | Black | II | NE |
| 463 | Matterhorn | Great Northern | II | NE |
| 464 | Marquis | Great Northern | II | NE |
| 467 | NE1-18-9 | Great Northern | II | NE |
| 473 | NE1-18-42 | Great Northern | II | NE |
| 475 | NE3-18-3 | Great Northern | II | NE |
| 478 | NE3-18-9 | Great Northern | II | NE |
| 479 | NE3-18-22 | Great Northern | II | NE |
| 483 | NE3-18-40 | Great Northern | II | NE |
| 485 | NE3-18-58 | Great Northern | II | NE |
| 487 | NE3-18-99 | Great Northern | II | NE |
| Curi | Curi | Black | I | INIA |
| Lpci | Lpci | Coscorrón | III | INIA |
| Zorzal | Zorzal | Tórtola | III | INIA |
| Sel 6 | Sel 6 | Tórtola | III | INIA |
| Blanco Español | Blanco Español | Great Northern | III | INIA |
| AGB | GY | HI | NGP | HGW | |
|---|---|---|---|---|---|
| G | NS | *** | *** | *** | *** |
| WR | *** | *** | *** | *** | *** |
| G × WR | NS | ** | NS | *** | NS |
| ND | 5264.2 ± 17.2 b | 3890.8 ± 62.6 b | 75.49 ± 0.4 b | 2105.1 ± 29.1 b | 37.7 ± 0.7 b |
| DS | 3310.7 ± 54.9 a | 3022.8 ± 70.8 a | 60.81 ± 0.4 a | 815.0 ± 6.3 a | 33.9 ± 0.7 a |
| DF | DM | Chl | ΦII | ΦNPQ | ΦNO | |
|---|---|---|---|---|---|---|
| G | *** | *** | *** | *** | NS | NS |
| WR | NS | *** | ** | *** | *** | *** |
| G × WR | NS | *** | NS | NS | NS | NS |
| ND | 47.9 ± 0.3 a | 91.2 ± 0.5 b | 56.4 ± 0.3 a | 0.29 ± 0.00 b | 0.52 ± 0.01 a | 0.19 ± 0.01 b |
| DS | 48.2 ± 0.3 a | 83.6 ± 0.2 a | 58.0 ± 0.4 b | 0.26 ± 0.00 a | 0.59 ± 0.01 b | 0.15 ± 0.00 a |
| Code | Yield (kg ha−1) | 100-Seed Weight | Days to Maturity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NS | DS | PR (%) | GMP | DSI | ND | DS | ND | DS | |
| 442 | 4390 | 2727 | 38 | 3460 | 1.7 | 31.5 | 27.9 | 88 | 83 |
| 452 | 3768 | 3650 | 3 | 3708 | 0.1 | 25.7 | 20.7 | 89 | 83 |
| 456 | 4345 | 3555 | 18 | 3930 | 0.8 | 33.9 | 30.4 | 87 | 83 |
| 457 | 3509 | 3330 | 5 | 3419 | 0.2 | 37.3 | 34.7 | 88 | 83 |
| 458 | 3757 | 3091 | 18 | 3408 | 0.8 | 22 | 19.1 | 88 | 83 |
| 463 | 3857 | 3263 | 15 | 3512 | 0.6 | 37.5 | 32.9 | 89 | 83 |
| 464 | 4292 | 3703 | 14 | 3987 | 0.6 | 33.8 | 29.4 | 89 | 83 |
| 467 | 3963 | 3594 | 9 | 3774 | 0.4 | 35.8 | 33.4 | 89 | 83 |
| 473 | 3484 | 3311 | 5 | 3427 | 0.1 | 41.3 | 35 | 88 | 83 |
| 475 | 3473 | 2722 | 22 | 3074 | 1 | 38.7 | 35 | 89 | 83 |
| 478 | 4486 | 3154 | 30 | 3761 | 1.3 | 39 | 33.7 | 89 | 83 |
| 479 | 4287 | 3284 | 23 | 3752 | 1.1 | 37.3 | 33.2 | 89 | 83 |
| 483 | 4312 | 3819 | 11 | 4058 | 0.5 | 41 | 37 | 90 | 83 |
| 485 | 3604 | 3017 | 16 | 3297 | 0.7 | 35.5 | 35.5 | 88 | 83 |
| 487 | 3382 | 2343 | 31 | 2815 | 1.4 | 41.6 | 36 | 88 | 83 |
| Curi | 3591 | 2134 | 41 | 2624 | 2.4 | 21.8 | 19.2 | 90 | 83 |
| Lpci | 3548 | 2558 | 28 | 2768 | 1.8 | 51 | 46.3 | 104 | 87 |
| Zorzal | 3796 | 2678 | 29 | 3012 | 1.3 | 50.1 | 47.9 | 105 | 87 |
| Sel 6 | 4125 | 2738 | 34 | 3361 | 1.5 | 48.6 | 42.6 | 102 | 86 |
| Blanco Español | 3850 | 1788 | 54 | 3189 | 1.3 | 51.5 | 47.9 | 95 | 85 |
| Overall mean | 3891 | 3023 | 0.98 | 37.7 | 33.8 | 91 | 84 | ||
| LSD (0.05) | 684.601 | 643.149 | 1.93 | 3.51 | 2.04 | 1.87 | |||
| CV% | 20.33 | 29.64 | 23.0 | 26.1 | 7.47 | 9.1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tay, K.; Zapata, N.; Urrea, C.A.; Elazab, A.; Garriga, M.; León, L. Screening Terminal Drought Tolerance in Dry Bean Genotypes and Commercial Bean Cultivars in Chile. Agronomy 2025, 15, 1499. https://doi.org/10.3390/agronomy15071499
Tay K, Zapata N, Urrea CA, Elazab A, Garriga M, León L. Screening Terminal Drought Tolerance in Dry Bean Genotypes and Commercial Bean Cultivars in Chile. Agronomy. 2025; 15(7):1499. https://doi.org/10.3390/agronomy15071499
Chicago/Turabian StyleTay, Kianyon, Nelson Zapata, Carlos A. Urrea, Abdelhalim Elazab, Miguel Garriga, and Lorenzo León. 2025. "Screening Terminal Drought Tolerance in Dry Bean Genotypes and Commercial Bean Cultivars in Chile" Agronomy 15, no. 7: 1499. https://doi.org/10.3390/agronomy15071499
APA StyleTay, K., Zapata, N., Urrea, C. A., Elazab, A., Garriga, M., & León, L. (2025). Screening Terminal Drought Tolerance in Dry Bean Genotypes and Commercial Bean Cultivars in Chile. Agronomy, 15(7), 1499. https://doi.org/10.3390/agronomy15071499





