Abstract
The polygalacturonase (PG) gene family plays a crucial role in plant cell wall metabolism and participates in various biological processes, such as fruit ripening, pod dehiscence, and pollen tube growth. However, the members of the PG gene family in Vicia sativa remain largely unexplored. We identified and analyzed the PG gene family members in V. sativa to investigate their gene expansion, functional evolution, and potential associations with agronomic traits. A total of 83 V. sativa PG genes (VsPGs) were identified, 51 of which retained all four characteristic PG domains (I–IV). We classified the VsPGs into seven subgroups (A–G) based on the results of phylogenetic analysis, and collinearity analysis suggested that segmental duplication was the primary driver of family expansion. The VsPG promoters were enriched with elements responsive to abscisic acid, low temperatures, and aluminum stress. Transcriptomic and qPCR analyses revealed tissue-specific and stress-responsive expression patterns of the VsPGs. Notably, VsPG48 and VsPG60 were highly expressed in the ventral sutures of pod-dehiscent varieties, whereas VsPG2 and VsPG41, among others, were co-upregulated under cold and aluminum stress. This study provides a foundation for further exploration of the biological functions of VsPGs.
1. Introduction
Functioning as a rigid peripheral barrier, the plant cell wall primarily comprises hemicellulose, cellulose, structural proteins, and pectin [1,2]. The plant cell wall not only provides mechanical support but also plays a crucial role in physiological processes, such as cell development, regulation, signal transduction, and defense responses, as the interface between plants and their environment [3]. Within the cell wall matrix, pectin, a dominant polymer of α-1,4-glycosidically bonded galacturonic acid chains, mediates wall dynamics [2,3]. Pectin degradation is critical for dynamic cell wall remodeling, cell separation, organ abscission, and fruit softening, which are primarily mediated through the cooperative activity of pectic enzymes [4]. The pectic enzymes, a family of enzymes responsible for pectin degradation, include polygalacturonases (PGs), pectin methylesterases, β-galactosidases, and rhamnogalacturonases [5,6]. These enzymes cleave specific chemical bonds in pectic polysaccharides and directly contribute to the dynamic remodeling of the cell wall [7]. As pivotal pectinases, PGs critically regulate key processes, including pod shattering, pollen tube growth, and fruit maturation [4,7,8,9,10,11,12,13]. Four evolutionarily conserved domains orchestrate PG catalysis: domains I(SPNTDG)–II(GDDC) form the active site, III(CGPGHG) modulates spatial conformation, and IV(RIK) enables substrate recognition, collectively ensuring pectin hydrolysis [5,14,15]. This precise molecular mechanism indicates the molecular basis of the dynamic regulation of plant cell walls.
The functional roles of PG in model plants have been extensively studied. AtPGs regulate pectin metabolism in the cell wall and participate in various developmental processes in Arabidopsis thaliana. For instance, ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE 1 (ADPG1) and 2 (ADPG2) mediate silique dehiscence by degrading the pectin layer in the abscission zone [16,17], whereas QUARTET2 (QTR2) and QUARTET3 (QTR3) play key roles in anther and floral organ abscission [11,18]. POLYGALAC TURONASE INVOLVED IN EXPANSION1 (PGX1) and 2 (PGX2) influence hypocotyl elongation by regulating cell wall loosening and expansion [19,20]. Notably, silencing ADPG1, ADPG2, and QTR2 inhibits pectin degradation, preventing the separation of the gelatinous layer in the cell wall and resulting in pollen grain adhesion and abnormal anther dehiscence in A. thaliana [17]. PG genes have diverse biological functions in crops. GmPG031 regulates seed coat permeability and weight in soybean (Glycine max) by modulating the structure of the seed coat cell wall and intercellular spaces [21]. SlPG (solyc10g080210) directly regulates the rate of pectin degradation in tomatoes (Solanum lycopersicum), thereby controlling fruit softening [10]. OsPG1 dynamically regulates pectin metabolism around the stomata in rice (Oryza sativa) to influence water homeostasis, whereas OsPG3 determines rice plant height by modulating cell wall synthesis in the stems [22,23]. Similarly, the expression levels of CitPG2, CitPG29, and CitPG34 positively correlate with fruit abscission rates in citrus (Citrus sinensis), which are induced by ethylene and inhibited by auxin (indole acetic acid (IAA)), indicating their regulatory roles in abscission zone development [24]. Additionally, antisense RNA technology has been used to suppress PG expression, substantially enhancing fruit firmness by reducing pectin depolymerization and intercellular separation. This approach has been applied to strawberry (Fragaria × ananassa Duch) FaPG1 [25,26,27], apple (Malus domestica) MdPG1 [28,29], and peach (Prunus persica) PpPG21 and PpPG22 [30], providing critical targets for increasing post-harvest quality in fruits and vegetables [10].
Common vetch (Vicia sativa) is a significant annual leguminous crop characterized by excellent palatability, rich nutrient contents, and strong adaptability [31]. It is primarily utilized as a forage and green manure. Owing to its high protein content, robust stress tolerance, and nitrogen-fixing capacity, it is extensively cultivated in arid and impoverished regions globally, with a planting area exceeding 573,769 hectares worldwide [32,33,34]. However, its yield is severely constrained by pod shattering and abiotic stresses (e.g., cold stress and aluminum toxicity) [31,32,33]. PGs play critical roles in plant development and abiotic stress tolerance [4]. In Medicago ruthenica and Brassica napus L., PG plays a critical role in pod dehiscence [34,35,36]. MsPG1 and MsPG4 enhance aluminum stress tolerance in alfalfa (Medicago sativa) via reducing the number of aluminum binding sites through targeted pectin hydrolysis and improving the physical properties of the cell wall (e.g., porosity and extensibility) [9,37]. Although the PG gene family has been systematically characterized in G. max [6], the functional evolution of the PG family in V. sativa and its association with agronomic traits remain poorly understood. Thus, we systematically analyzed the PG gene family in V. sativa using whole-genome data [38,39], identifying and characterizing its members as well as its gene duplication patterns, structural diversification, and evolution of cis-regulatory elements. Transcriptomic and qPCR analyses were conducted to identify the key PG genes involved in pod dehiscence and abiotic stress responses, and their expression profiles were examined in various tissues under different stress conditions. Our findings provide valuable insights into the contributions of VsPGs to plant development and abiotic stress tolerance as well as offer new targets for breeding crack-resistant varieties and strengthening stress-resilient traits.
2. Materials and Methods
2.1. Comprehensive Annotation of V. sativa PG Genes
An integrated HMM and BLASTP approach was employed for the genome-wide identification of PG genes in V. sativa. The proteome and corresponding genome annotation files (Gigabyte database, http://dx.doi.org/10.5524/100954, accessed 25 November 2024) were acquired from the Gigabyte Database [38,39]. Concurrently, the specific HMM profile for the GH28 glycosyl hydrolase domain (Pfam entry PF00295) was downloaded as the source data from the Pfam database (http://pfam.xfam.org, accessed 25 November 2024) [6]. This profile was utilized to scan the V. sativa proteome using HMMER v3.3.2 with a stringent E-value ≤ 1 × 10−5. Separately, 67 experimentally validated PG protein sequences from A. thaliana were retrieved from the TAIR resource (http://www.arabidopsis.org, accessed 25 November 2024) [5]. Using TBtools-II v2.149, a local BLASTP database was constructed, and homology searches against the V. sativa proteome were performed with thresholds set at E-value ≤ 1 × 10−5, sequence identity ≥ 50%, and query coverage ≥ 70% [5,40]. Candidate genes identified through both methodologies were subsequently merged, with redundant entries eliminated. Candidate genes were filtered using a cross-validation strategy, with dual verification performed using the NCBI.
The Conserved Domain Database (CDD, https://www.ncbi.nlm.nih.gov/cdd, accessed on 25 November 2024) and SMART database (http://smart.embl.de, accessed on 25 November 2024) [5] were used to obtain genes containing at least two core PG domains [41]. Homologs of A. thaliana QRT3 (At4g20050), which lack typical domains but participate in pollen mother cell wall degradation during the tetrad stage [42], were retained for subsequent evolutionary analyses. We utilized the Expasy Decrease Redundancy tool (https://web.expasy.org/decrease_redundancy/, accessed on 25 November 2024) under the default configuration to remove redundant sequences [43]. The remaining sequences were identified as VsPG proteins and were subjected to further analysis. The identified VsPG family members were systematically characterized. Computational analysis of protein properties was performed using the ExPASy ProtParam tool (http://web.expasy.org/protparam/, accessed on 25 November 2024) to determine molecular weight and isoelectric point. Signal peptide prediction was conducted with SignalP 5.0 (https://services.healthtech.dtu.dk/services/SignalP-5.0, accessed on 25 November 2024) [5], while subcellular localization was assessed via WoLF PSORT (https://wolfpsort.hgc.jp, accessed on 25 November 2024) [44].
2.2. Integrative Analysis of VsPG Evolution and Regulatory Features
Proteins from A. thaliana and V. sativa were aligned using MUSCLE v3.8.31. TrimAL v1.4 was subsequently applied in automatic mode to refine alignment quality. An ML phylogenetic tree was generated via IQ-TREE v1.6.12, employing the VT + F + R10 amino acid substitution model identified as optimal by the integrated ModelFinder module. Branch support values were derived from 1000 ultrafast bootstrap replications [45]. VsPGs were classified into distinct evolutionary subgroups, following A. thaliana PG-based phylogeny [46]. Gene exon–intron architectures were analyzed via TBtools-II v2.149 to visualize structural features. For conserved motif discovery, MEME Suite v5.5.5 (http://meme-suite.org/, accessed on 25 November 2024) was employed, with a maximum motif limit of eight; the results were subsequently visualized using the same TBtools-II platform. Promoter regions spanning 2 kb upstream of VsPG transcription start sites were retrieved and screened for cis-regulatory elements via the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 25 November 2024). Statistically enriched CREs were then plotted using R v4.3.1 [47].
2.3. Chromosome-Wide Analysis of Gene Duplication and Synteny
The physical chromosomal locations of VsPGs were mapped using the “Gene Location Visualization” module in TBtools-II v2.149. The identified 83 PG genes were sequentially named VsPG1–VsPG83 based on their chromosomal positions. The following steps were performed using TBtools-II v2.149 to investigate the mechanisms underlying the expansion of the VsPG family. The MCScanX algorithm was used to identify collinear blocks within the genome. Output files were reformatted using the “File Trans-format for MicroSynteny Viewer” module, and the genome-wide VsPG collinearity network was visualized using the “Advanced Circos” function. The genomic data (FASTA and GFF3 files) for six species (A. thaliana, Medicago truncatula, M. sativa, G. max, Zea mays, and O. sativa) were retrieved from TAIR (http://www.arabidopsis.org, accessed on 25 November 2024), MODMS (https://modms.lzu.edu.cn/, accessed on 25 November 2024) [48], and Ensembl Plants [49]. The MCScan algorithm (TBtools-II) identified synteny blocks. Ka/Ks ratios derived from the integrated calculator evaluated duplication-induced divergence, defining Ka/Ks > 1 = positive selection, Ka/Ks < 1 = purifying selection, and Ka/Ks = 1 = neutral evolution [5,41,47,50].
2.4. Analysis of VsPG Expression
The transcriptomic datasets from NCBI SRA (https://www.ncbi.nlm.nih.gov/sra/, accessed on 28 November 2024) were integrated to analyze the tissue-specific functions and abiotic stress responses of the VsPGs. The datasets included project IDs SRX2400610, SRR17967534-SRR17967539, PRJNA778324, SRR20083627-SRR20083644, and PRJNA820149, covering the pod ventral suture [33], stem apex [51], seeds at different developmental stages [52], leaves under cold stress [53], and roots under aluminum toxicity stress [32]. Gene expression quantification was performed using fragments per kilobase of transcript per million mapped reads (FPKM) normalization. Expression patterns were visualized via the HeatMap Illustrator module in TBtools v2.149.
2.5. Plant Materials
The V. sativa cv. Lan Jian No. 3 served as the experimental material for spatiotemporal expression profiling of VsPGs. Seeds were surface sterilized in 1% NaClO (w/v; 20 min), thoroughly rinsed with sterile ddH2O (5×), and germinated in a growth chamber under controlled conditions (16 h light/25 °C, 8 h dark/23 °C, 60% ± 5% RH) for 72 h. Thirty uniform seedlings were transferred to aerated Hoagland solution and maintained under identical conditions. Six biological replicates (independent plants) were sampled at 7 d (seedling stage), 21 d (vegetative stage), and 45 d (reproductive stage). Apical root tissues (≤1 cm), third-internode stems, and fully expanded second pair leaves were collected per biological replicate, immediately cryopreserved in liquid N2, and maintained at −80 °C prior to RNA extraction.
2.6. Quantitative Real-Time PCR Analysis
Gene-specific primers (Table S7) were designed with Primer Premier 6.0 (accessed on 28 November 2024). Total RNA isolation employed TRIzol® reagent (Invitrogen, Waltham, MA, USA), followed by cDNA synthesis from 1 μg RNA using FastKing RT Kit (TIANGEN Biotech, Beijing, China). qRT-PCR was conducted on a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) with biological–technical triplicates (3 × 3). The reaction mixture (10 μL) consisted of Taq SYBR® Green qPCR Premix (Yugong Biotech, Nanjing, China), gene-specific primers, and cDNA template. Cycling conditions followed the SYBR Green Premix guidelines. V. sativa β-actin (Unigene68614) served as an endogenous control. VsPG expression was quantified via 2−ΔΔCt [54], with statistical significance (p < 0.05) determined by one-way ANOVA and Tukey’s test.
3. Results
3.1. Identification and Physicochemical Properties of VsPGs
Eighty-three PG genes were identified in the V. sativa genome through a homologous search with A. thaliana PG queries. These genes were distributed across six chromosomes and were sequentially named VsPG1 to VsPG83 based on their chromosomal positions (Tables S1 and S2). The results of conserved domain analysis showed that 51 members (61.4%) retained all four characteristic PG family domains (I–IV), with conservation being lowest for domain III. Furthermore, 32 family members (38.6%) displayed domain deletions: a total of 16 (19.3%) lacked domain III, 5 (6.0%) lacked domain IV, and 2 (2.4%) lacked domain I.
Additionally, five members (6.0%) lacked both domains III and IV, two members (2.4%) lacked domains I and II, and one member (1.2%) lacked domains I and III. Notably, VsPG12 was the only member lacking all four domains (Figure S1, Table S3). Domain losses (e.g., absence of catalytic domain III in 19.3% of VsPGs) may indicate neofunctionalization, as seen in A. thaliana QRT3 [18], or pseudogenization. VsPG12 (lacking all domains) warrants functional validation due to its homolog QRT3’s role in pollen development. The results of physicochemical analysis indicated a wide variation in the amino acid sequence length of the VsPG proteins, ranging from 100 (VsPG59) to 591 (VsPG41) amino acids, and molecular weights ranging from 10.7 (VsPG59) to 65.7 (VsPG41) kDa, with an average of 42.5 kDa.
The isoelectric points ranged from 4.77 (VsPG73) to 9.36 (VsPG23), with 56% and 44% of the proteins being basic (pI > 7) and acidic (pI < 7), respectively. Approximately 20% of the VsPG proteins (17 members, e.g., VsPG36 and VsPG72) were identified as unstable, with instability indices exceeding 40. The results of hydrophobicity analysis revealed that the grand average hydropathicity (GRAVY) was negative for 93% of the VsPG proteins (77 members), indicating hydrophilicity. Among these, the hydrophilicity of VsPG31 (GRAVY = −0.41) and VsPG73 (GRAVY = −0.294) was the highest. Only six members (VsPG52 and GRAVY = 0.096) displayed weak hydrophobicity. The results of signal peptide analysis indicated that 62% of the VsPG proteins (52 members) contained signal peptides and were predominantly localized in the extracellular spaces, vacuoles, and membrane systems, such as the plasma membrane and endoplasmic reticulum, consistent with the characteristics of secreted proteins. The remaining 31 members without signal peptides were primarily localized in the nucleus, cytoplasm, and chloroplasts (Table S3).
3.2. Evolutionary Phylogeny and Classification of VsPGs
A maximum likelihood phylogenetic tree was constructed using the 83 VsPG and 67 Arabidopsis AtPG protein sequences (Figure 1). The VsPGs were classified into the same seven subgroups, with 9 members in A, 7 in B, 5 in C, 23 in D, 18 in E, 20 in F, and 1 in G, based on the established classification of Arabidopsis PG proteins into seven subgroups (A–G) [5].
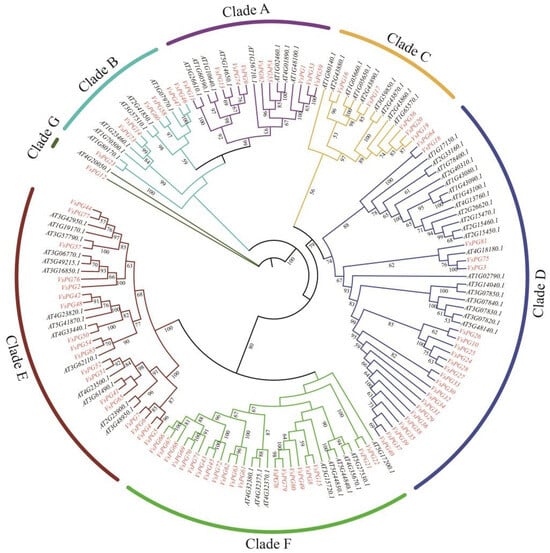
Figure 1.
Phylogenetic analysis of VsPGs and AtPGs constructed by IQ-TREE v1.6.12 using the maximum likelihood method. The colored blocks indicate seven subclasses (Clade A–G) of the PG gene family. The VsPG family was divided into 7 clades containing 9 (A), 7 (B), 5 (C), 23 (D), 18 (E), 20 (F), and 1 (G) members, respectively. Bootstrap values below 50 were hidden for clarity. The scale bar represents the number of amino acid substitutions per site. Gene names are labeled adjacent to corresponding branches, with red font denoting VsPG proteins from Vicia sativa and black font representing AtPG proteins from Arabidopsis thaliana.
3.3. Genomic Architecture and Motif Conservation
The exon–intron structures and eight conserved motifs were analyzed to determine the structural diversity of the VsPGs (Figure 2). The number of exons varied from one (VsPG28) to eleven (VsPG73), with most genes having three to eight exons. The exon–intron structure varied among evolutionary clades and was relatively conserved within the same clade. Among the eight conserved motifs (motifs one to eight), motifs one, two, and four were widely present in most PG genes and corresponded to domains I, II, and III/IV, respectively (Figure S2).
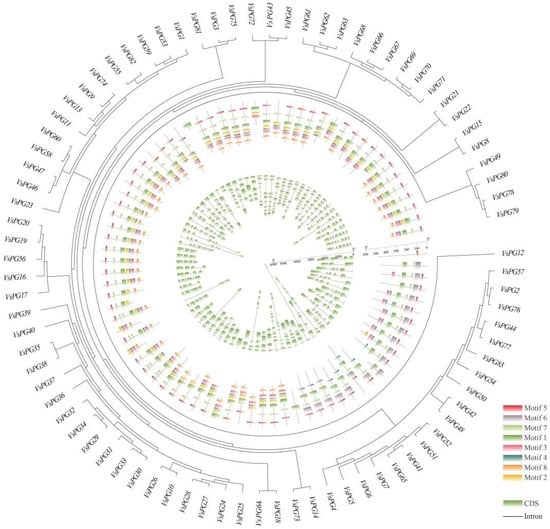
Figure 2.
Phylogenetic relationships, motif patterns, and gene structures of Vicia sativa VsPGs. Outer circle: an unrooted phylogenetic tree constructed based on the full-length sequences of VsPG proteins. Middle circle: motif compositions of VsPG proteins identified using MEME. Gray lines represent non-conserved regions. Each motif is represented by a colored box (Motif 1–6) numbered at the bottom. Detailed sequences and SeqLogo information are provided in Table S3. Inner circle: gene structures of Vicia sativa VsPGs visualized using the GSDS2.0 (https://gsds.gao-lab.org/, accessed on 25 November 2024) online tool. Exons (colored blocks) and introns (black connecting lines) are displayed proportionally to their lengths. Gene names (e.g., VsPG38, VsPG72, VsPG60) are labeled next to the corresponding branches. Different colors indicate motif types, while exons and introns are represented by colored blocks and black lines, respectively.
3.4. Genome Organization: Synteny, Duplication Events, and Evolutionary Selection
We explored the mechanisms through which the VsPG family expanded and evolved using chromosomal localization, collinearity, gene duplication, and Ka/Ks analyses. Chromosomal mapping placed all 83 VsPGs on six chromosomes (Chr1–Chr6; Figure 3). Specifically, VsPGs 15, 30, 5, 13, 14, and 6 were distributed on Chr1, Chr2, Chr3, Chr4, Chr5, and Chr6, respectively. The results of gene duplication analysis revealed that the VsPG family expanded via two duplication mechanisms: tandem and segmental duplication. A total of 25 VsPGs were involved in five tandem duplication pairs and nine segmental duplication pairs (Figure 4, Table S4), indicating that the VsPG family likely expanded through both mechanisms, with segmental duplication being the primary driver. Additionally, collinearity maps were constructed between V. sativa and six model species (A. thaliana, M. truncatula, M. sativa, G. max, Z. mays, and O. sativa). The results showed 109 homologous gene pairs between V. sativa and G. max, followed by 60 pairs with M. truncatula, 39 pairs with M. sativa, 32 pairs with A. thaliana, 11 pairs with Z. mays, and 4 pairs with O. sativa (Figure 5). V. sativa shared a larger number of homologous sequences with leguminous species than with grasses. Additionally, phylogenetic analysis revealed that VsPG16 and VsPG21 are orthologous to MsPG4 and MsPG1 (M. sativa alfalfa aluminum resistance genes, whose function is described in the Introduction), suggesting their potential involvement in the aluminum stress response in V. sativa. VsPG60 shares homology with the A. thaliana gene ADPG1 (AT3G57510), known for its role in anther/silique development, indicating its potential function in regulating pod shattering in common vetch. Conversely, VsPG13 is homologous to the A. thaliana pollen tube gene AtPGLR (AT3G57510), implying a potential role in pollen tube function [55]. Future work needs to elucidate the functional conservation of these pathways (e.g., pod shattering) within a leguminous plant context. The evolutionary selection analysis results revealed that all homologous VsPG pairs had Ka/Ks ratios of <1, indicating strong purifying selection and conserved functions during evolution (Table S4). For example, the Ka/Ks ratios for VsPG6-VsPG51 and VsPG2-VsPG76 were 0.065354 and 0.16462, respectively. Purification selection suggests that these gene pairs retain essential metabolic functions and participate in critical physiological processes, such as cell wall degradation.
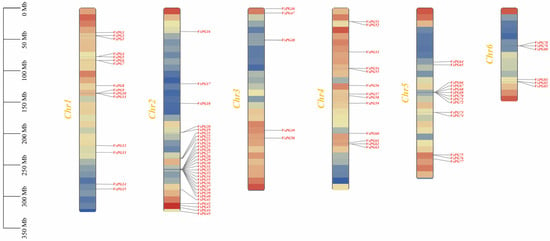
Figure 3.
Distribution of 83 VsPGs on six chromosomes. Vertical bars represent chromosomes, with chromosome numbers labeled on the left side of each chromosome. The scale on the left indicates chromosome length (Mb).
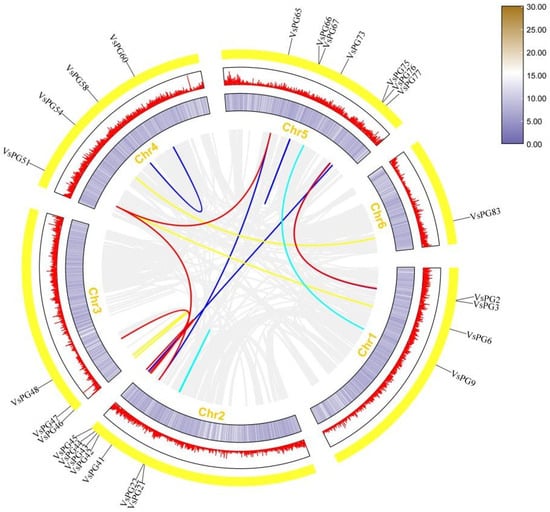
Figure 4.
Collinearity analysis of VsPGs. Lines of different colors represent collinear VsPG pairs with duplication events, while gray lines in the background indicate collinear blocks across the Vicia sativa genome. Chromosome numbers are shown at the bottom of each chromosome. The outer circle displays lines and a heatmap indicating gene density along the chromosomes.
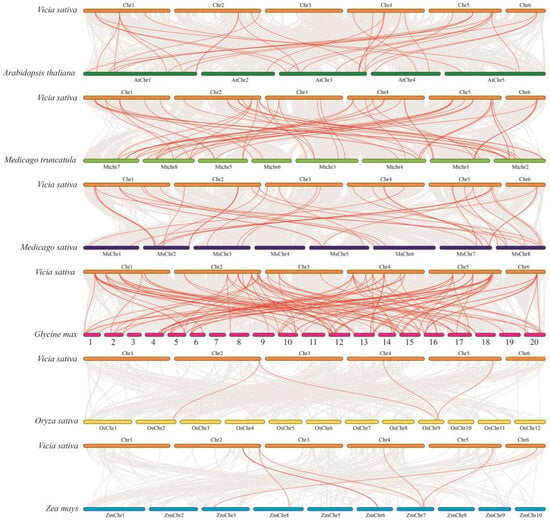
Figure 5.
Gene collinearity analysis between Vicia sativa and six other species. Collinearity analysis of gene sequences was performed between Vicia sativa and Arabidopsis thaliana, Medicago truncatula, Medicago sativa, Glycine max, Oryza sativa, and Zea mays using TBtools. Gray lines in the background represent homologous genes between V. sativa and the six species, while red lines highlight collinear PG gene pairs.
3.5. Cis-Regulatory Element Analysis in VsPGs
Promoter CRE prediction for the 83 VsPGs (2 kb upstream regions) via PlantCARE revealed 40 functional categories associated with stress responses, hormone signaling, and developmental regulation (Figure S3, Table S5). These regulatory elements inform potential VsPG functions and pathways [56]. Among the VsPGs, VsPG65 exhibited the highest CRE density (57 elements), consistent with multifaceted roles in stress adaptation, hormone signaling, and developmental control. Three genes (VsPG28, VsPG33, VsPG80) each contained 21 stress-responsive CREs, which was the maximum observed—indicating specialized environmental stress engagement. Similarly, VsPG41 and VsPG63 possessed peak hormone-responsive CRE counts (21 each), suggesting extensive hormonal pathway modulation. Notably, VsPG41 harbored 11 abscisic acid-responsive elements (ABRE) and three methyl jasmonate-responsive elements (CGTCA motif), suggesting its potential roles in ABA- and JA-mediated stress adaptation. VsPG4 and VsPG33 were enriched in most development-related CREs (20 each), suggesting roles in the regulation of specific developmental processes. Hormone-responsive elements, such as abscisic acid (ABA), gibberellin (GA), salicylic acid (SA), and jasmonic acid (JA), are widely present. Among these, 58 VsPGs contained ABA-responsive elements, with VsPG39 exhibiting the highest number (15). These findings indicate that ABA plays a central role in regulating PG genes.
3.6. Abiotic Stress-Responsive Profiling of VsPGs
We analyzed the expression patterns of VsPGs using transcriptome data under cold and aluminum stress treatments to elucidate the mechanisms underlying the response of VsPGs to abiotic stress. The heatmap for cold stress (Figure 6A,B) revealed that VsPG2 was upregulated across all six varieties after 48 h of treatment, whereas VsPG50 exhibited a rapid response pattern (strong upregulation at 6 h, returning to baseline at 48 h). The results of a comparative analysis between cold-tolerant and cold-sensitive varieties indicated that VsPG2, VsPG22, VsPG41, VsPG46, VsPG51, and VsPG82 showed coordinated upregulation at 48 h in cold-tolerant varieties, whereas only VsPG58 and VsPG2 expression levels increased in cold-sensitive varieties. This gene module likely enables the fine-tuned modulation of cell wall modifications (particularly within the pectin metabolism pathway) through transcriptional activation. This strategy facilitates beneficial alterations while preventing detrimental degradation, indicating that the module plays a synergistic role in regulating cold tolerance [57]. VsPG9, VsPG14, VsPG42, VsPG50, VsPG51, VsPG73, and VsPG74 were continuously upregulated during the later stages of aluminum stress (24–48 h) in aluminum-tolerant varieties (Figure 6C), whereas VsPG15, VsPG24, VsPG55, and VsPG67 were upregulated during the later stages of stress in aluminum-sensitive varieties. This finding indicates that these genes participate in responses to aluminum stress by coregulating cell wall modification pathways. This finding indicates that these genes participate in responses to aluminum stress by coregulating cell wall modification pathways [37]. Notably, cis-regulatory element (CRE) analysis revealed that VsPG51, which was consistently upregulated during late stages of both cold and aluminum stress, contains 15 abiotic stress-responsive elements (including five MYB and seven MYC binding sites). Meanwhile, VsPG41, which showed co-upregulation under cold and aluminum stress, harbored 11 ABRE (ABA-responsive elements) and three CGTCA motifs (JA-responsive elements) in its promoter. This enrichment pattern aligns with their stress-induced expression profiles, suggesting potential regulation by ABA and JA signaling pathways.
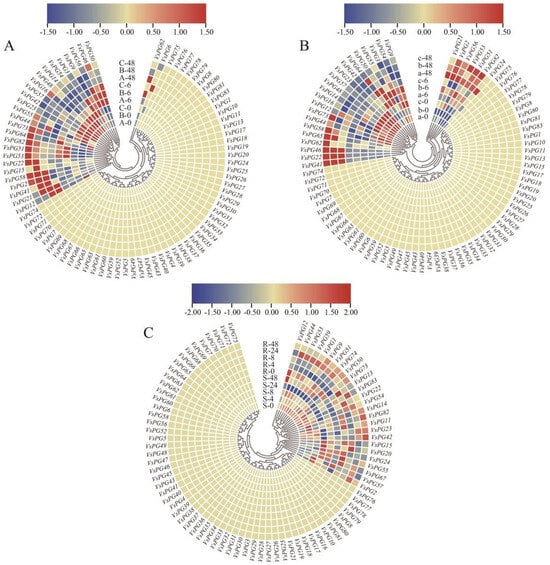
Figure 6.
Expression patterns of VsPGs under cold and aluminum stress treatments. (A) Expression patterns of VsPGs in the leaves of three cold-tolerant varieties at 0, 6, and 48 h of cold stress treatment. (B) Expression patterns of VsPGs in the leaves of three cold-sensitive varieties at 0, 6, and 48 h of cold stress treatment. Lowercase letters (a, b, c) represent cold-tolerant varieties, while uppercase letters (A, B, C) denote cold-sensitive varieties. (C) Expression patterns of VsPGs in roots under aluminum stress. “R” represents aluminum-tolerant varieties, while “S” represents aluminum-sensitive varieties. Seedlings were treated for 0, 4, 8, 24, and 48 h, and root tips (1–1.5 cm) were collected. Red blocks indicate higher relative expression levels, while blue blocks indicate lower relative expression levels.
3.7. Tissue-Specific Expression of VsPGs
We integrated our transcriptomic data from the roots, leaves, flowers, seeds, and ventral suture tissues with those from previously published studies to investigate the expression characteristics of the 83 VsPGs (Figure 6 and Figure 7, Table S6). The results showed that the VsPGs were expressed in all the examined tissues, although distinct tissue-specific expression patterns were observed. A total of 31 of the 83 VsPGs were not detected in any of the transcriptomic datasets, suggesting that they exhibit stage-specific expression or underwent pseudogenization. Three genes (VsPG11, VsPG59, and VsPG67) were highly expressed in the roots, whereas two genes (VsPG6 and VsPG21) were specifically expressed in the leaves. Five genes each were specifically expressed in the flowers (VsPG19, VsPG33, VsPG47, VsPG75, and VsPG81) and seeds (VsPG4, VsPG8, VsPG27, VsPG63, and VsPG71), indicating that this gene family participates in tissue-specific developmental regulation via functional differentiation and indicating functional differentiation within this gene family. Further analysis revealed that VsPG2 and VsPG9 were highly expressed during early seed development, suggesting potential roles in endosperm differentiation. VsPG48 and VsPG60 showed higher expression in ventral sutures of pod-dehiscent varieties than in resistant varieties, implying possible contributions to pod dehiscence through cell wall modification in the abscission layer.
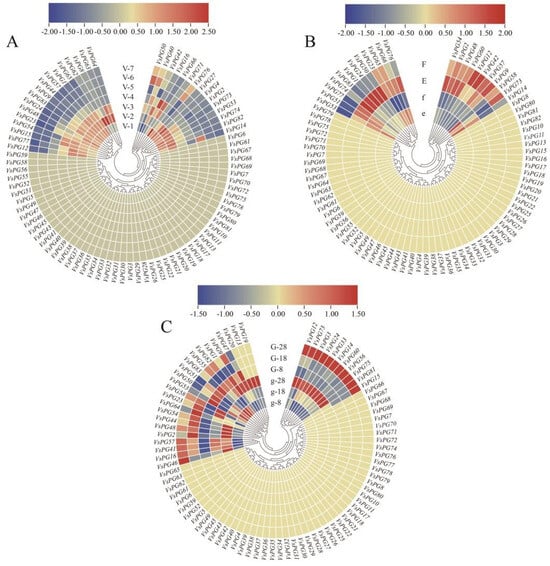
Figure 7.
Expression patterns of VsPGs in specific tissues of V. sativa. (A) Expression levels of VsPGs in seeds at seven different developmental stages. Sampling began at the time of full petal expansion and occurred every five days until 35 days post-anthesis. (B) Expression levels of VsPGs in the ventral sutures of pod-shattering-resistant and pod-shattering-susceptible varieties. Lowercase letters (e, f) represent shattering-resistant varieties, while uppercase letters (E, F) represent shattering-susceptible varieties. (C) Expression levels of VsPGs in stem apices of early-flowering and late-flowering varieties. Lowercase letters (g) represent early-flowering varieties, while uppercase letters (G) represent late-flowering varieties.
Additionally, VsPG33 and VsPG41 were upregulated during late flower development (77 days), consistent with potential functions in floral organ abscission. Therefore, these highly expressed genes represent potential targets for genome editing to study their biological functions.
3.8. qRT-PCR Validation
To characterize the developmental stage-associated expression patterns of VsPGs under standard growth conditions, reverse transcription quantitative PCR (RT-qPCR) analysis was performed for nine selected genes (VsPG1, VsPG9, VsPG12, VsPG24, VsPG42, VsPG48, VsPG51, VsPG60, and VsPG77) in roots, stems, and leaves of V. sativa cv. Lan Jian No. 3 at three key growth stages: seedling (7 d), vegetative (21 d), and reproductive (45 d) phases (Figure 8, Table S7). This analysis complements the stress- and tissue-specific expression profiles in Figure 6 and Figure 7 by revealing constitutive expression dynamics during development. The results showed substantial differences in VsPG expression among the tissues and time points. Specifically, VsPG1, VsPG9, VsPG42, VsPG48, and VsPG51 expression levels were the highest in leaves at 45 d, whereas VsPG12 was highly expressed in the roots at 21 d. These findings indicate that different VsPGs respond to distinct tissues and developmental stages, reflecting their functional differentiation during different growth phases.
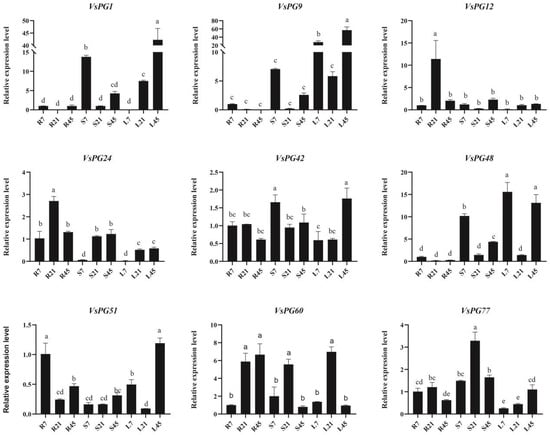
Figure 8.
qPCR analysis of developmental stage-dependent expression patterns of selected VsPGs in roots, stems, and leaves of Vicia sativa under standard growth conditions. Data represent mean ± SD of three biological replicates. Different lowercase letters above bars indicate significant differences (p < 0.05, one-way ANOVA with Tukey’s test). The β-actin gene served as an internal reference.
4. Discussion
4.1. Evolutionary and Functional Insights into the PG Gene Family
The PG gene family plays a critical role in adaptive plant evolution through the dynamic evolution of domains and functional differentiation, as an important member of the glycoside hydrolase family 28 (GH28) [58]. These genes are primarily involved in the plant cell wall, with key functions in pectin degradation and cell wall remodeling [59]. The PG gene families have been systematically identified in several species, including in model plants such as A. thaliana (68 members) [58] and O. sativa (46 members) [60]; major crops such as G. max (112 members) [6], Z. mays (55 members) [15], and Triticum aestivum (113 members) [61]; and horticultural crops such as S. lycopersicum (tomato, 54 members) [42], Cucumis sativus (cucumber, 53 members) [62], and Citrus sinensis (citrus, 38 members) [24]. PG genes exhibit species-specific functional innovations. For example, A. thaliana QRT3 (At4G20050) has evolved a noncanonical function in pollen separation through domain loss [11]. The tandem expansion of the four SlPG genes in tomatoes contributes to species-specific traits or the development of certain organs or tissues [42]. We systematically identified 83 VsPG gene family members in V. sativa, which were randomly distributed across six chromosomes, with the highest and lowest density on chromosomes 2 (30 genes) and 3 (5 genes), respectively. We classified the VsPG family into seven clades (A–G) based on the results of phylogenetic analysis, with members within the same clade exhibiting similar gene structures, conserved motifs, and physicochemical properties. These findings are consistent with those of previous studies on G. max and S. lycopersicum [6,42]. Clades D (23 members), F (20 members), and E (18 members) constituted the major branches, whereas clade G contained only one member. The systematic absence of clade G in species such as Ipomoea batatas (sweet potato) and Z. mays suggests species-specific functional divergence [5,15]. Domains I and II form the catalytic core, domain III participates in the catalytic process, and domain IV mediates carboxylic acid group recognition, among the 51 VsPG proteins retaining all four characteristic domains (I–IV) [42]. Members of clade E commonly lack domain III, which is also observed in I. batatas, tomatoes, and Brassicaoleracea (cabbage), suggesting that domain loss may be associated with specific functional evolution [5,42,63]. A unique case is VsPG12, which lacks all conserved domains but retains PG activity, similar to its homolog AtQRT3 in A. thaliana [11]. These findings systematically reveal the evolutionary dynamics of the PG gene family. The PG gene family achieves functional innovation through molecular mechanisms, such as domain rearrangement (e.g., tandem duplication in SlPG genes) and domain loss (e.g., QRT3 and clade E members), while maintaining conserved core functions. This dynamic balance likely underlies the ability of plants to adapt to diverse ecological environments.
4.2. Gene Duplication as a Driver of PG Gene Family Expansion
Gene duplication events are critical mechanisms that drive the expansion and evolution of gene families. These events primarily occur in three modes: segmental duplication, tandem duplication, and transposition [6,60]. The dominant duplication mechanism widely varies among species. For example, the expansion of the G. max PG gene family has largely been driven by segmental duplication, with members nonrandomly distributed across 20 chromosomes [6]. BoPG genes exhibit nonrandom chromosomal distribution in B. oleracea, and the results of phylogenetic analysis revealed that tandem duplication has driven their evolutionary trajectory [63]. Intraspecies collinearity analysis in Akebia trifoliata showed that the expansion of the AktPG gene family has mainly occurred through whole-genome duplication (48.94%) and dispersed duplication (23.40%), whereas tandem duplication has accounted for only 21.28% of the total expansion [64]. We identified five pairs of tandemly duplicated and nine pairs of segmentally duplicated VsPGs, with segmental duplication being the dominant mode of family expansion (64.3%). The results of clade distribution analysis revealed tandem duplications concentrated in clades F (three pairs) and B (two pairs), whereas segmental duplications were enriched in clade E (seven pairs). This pattern aligns with that of I. batatas (sweet potato), in which segmental duplication has been the primary driver of PG family expansion, particularly in clade E [5]. Ka/Ks ratios of all the VsPG duplication events ranged from 0.065 to 0.90, all of which were less than 1, indicating strong purification selection and high functional conservation within the VsPG gene family. This finding is consistent with those of a study on B. oleracea, where all 20 pairs of duplicated BoPG genes were under purifying selection (Ka/Ks < 1) [63]. Similarly, only one pair (AktPG15/AktPG17) of 467 homologous AktPG genes had a Ka/Ks ratio greater than 1 (1.122) in A. trifoliata, and the rest experienced purifying selection [64]. These results further confirm the essential roles of PG genes in plant growth and development as well as indicate the functional constraints during adaptive evolution.
4.3. CREs and Functional Implications in VsPGs
Promoters regulate the precise spatiotemporal expression of genes through CREs, which play vital roles in plant development and evolution [65,66,67]. CREs participate in transcriptional regulation, and their types and quantities within promoter regions can be used to predict the potential functions of genes [65]. Combined patterns of CREs and their biological functions are strongly correlated [5,15,68]. A total of 35 ZmPG promoters in Z. mays contain pathogen-responsive MYB binding site (MBS) elements, which, along with 36 endosperm-specific expression elements and 32 zein metabolic regulatory elements, establish a molecular basis for seed development [15]. Similarly, the I. batatas PG gene family exhibits unique stress-responsive characteristics, with 97 light-responsive, 34 defense-stress-responsive, and 29 low-temperature-responsive elements systematically distributed across the promoters. All IbPG promoters integrate five hormone-responsive elements, including IAA and ABA, highlighting the pivotal role of PG genes in environmental adaptation [5]. We identified 40 types of CREs in the promoters of VsPGs, which are primarily associated with environmental stress responses, hormone signaling, and plant growth and development. Each VsPG contains at least two of these CRE types, indicating that most PG genes respond to diverse abiotic stresses. Many stress-related CREs, such as ABA response (ABRE), anaerobic induction (ARE), low-temperature response, and MBSs (involved in the drought response), have been identified in the promoter sequences of VsPGs. These elements have also been reported in PG gene families of I. batatas and B oleracea, among other species [5,63]. For example, ABRE interacts with upstream transcription factors to activate ABA-responsive genes, enhancing plant stress tolerance [52,69]. We found that 58 VsPG promoters contained ABA-responsive elements, with VsPG39 harboring the highest number (15). This suggests that VsPGs play a central role in regulating the growth and development of V. sativa in response to biotic and abiotic stresses.
4.4. Gene Expression Patterns Provide Insights into VsPG Functions
Gene expression profiles provide critical clues for understanding gene function. PG genes play essential roles in the regulation of plant growth, development, and stress responses. In I. batatas, IbPGs are indispensable during vegetative growth, with clade D members exhibiting heightened sensitivity to abiotic stressors [5]. Similarly, in Z. mays, clade E ZmPGs show broad tissue expression, while clade D members are another specific [15]. The G. max GmPGs also display distinct spatiotemporal patterns, such as floral and seed-specific expression [6]. Consistent with these patterns, VsPGs were ubiquitously expressed across V. sativa tissues (Figure 7), implying broad functional roles in growth and development, analogous to PGs in A. thaliana [16,17] and G. max [6]. Notably, qRT-PCR analysis under standard growth conditions revealed developmentally programmed expressions of selected VsPGs (e.g., VsPG1, VsPG9, VsPG48) in roots, stems, and leaves across seedling (7 d), vegetative (21 d), and reproductive (45 d) stages (Figure 8). This constitutive expression dynamic provides a baseline reference for distinguishing developmental versus stress-induced regulation. Clade-specific functional divergence was evident: VsPGs in clades D and E showed heightened sensitivity to abiotic stresses (Figure 6), mirroring findings in I. batatas and suggesting specialized roles in stress adaptation. While expression patterns (Figure 6, Figure 7 and Figure 8) and promoter CRE enrichment (e.g., development-related elements in VsPG4/VsPG33; Section 3.5) support putative roles in pod dehiscence, floral abscission, and stress responses, causal relationships require validation via molecular genetics. Future studies should employ CRISPR/Cas9-mediated mutagenesis (e.g., targeting VsPG48/VsPG60 for pod shattering) or heterologous expression assay approaches proven effective in validating PG functions in tomato and strawberry.
5. Conclusions
We systematically identified and functionally analyzed the PG gene family of V. sativa. We identified 83 VsPGs in the V. sativa genome that were randomly distributed across six chromosomes. We used phylogenetic analysis to cluster VsPG proteins into seven subgroups, with relatively conserved gene structures within each clade. Approximately 61.4% of the VsPGs retained all four characteristic PG family domains (I–IV), whereas 38.6% exhibited domain loss. The expansion of the VsPG gene family was primarily driven by segmental (64.3%) and tandem duplications (35.7%). The results of promoter analysis revealed that the upstream regions of the VsPGs contained CREs associated with plant growth and development, environmental stress responses, and hormone signaling. Some VsPGs were implicated in critical biological processes, including pod dehiscence, organ abscission, and abiotic stress response. These findings provide insights into the functional characteristics of the VsPG gene family and lay the foundation for further research on their roles in V. sativa. Our findings enable concrete molecular breeding strategies: CRISPR/Cas9-mediated knockout of VsPG48 and VsPG60 to suppress pod shattering combined with overexpression of VsPG41 to enhance aluminum tolerance through targeted pectin modification in the cell wall. Validating these approaches could simultaneously improve stability and stress resilience in V. sativa cultivation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15061457/s1, Figure S1: Multiple sequence alignment of PG peptides in Vicia sativa showing four conserved domains. The four typical conserved domains of PG, labeled as I, II, III, and IV, are underlined. The consensus sequence is represented at the bottom with corresponding letters. The consensus sequence uses uppercase letters to indicate highly conserved residues and lowercase letters to indicate moderately conserved residues. Gaps are represented by dashes (-). Figure S2: Sequence logos of conserved motifs in the VsPG gene family. The sequence logos were generated using the MEME online tool. The X-axis represents the conserved sequences of the motifs, with the specific amino acid residues shown at each position. The Y-axis indicates the conservation level of amino acids, represented by the height of the letters, where taller letters denote higher conservation. Figure S3: Cis-regulatory element (CRE) analysis of VsPG promoters. (A) An ML phylogenetic tree of VsPGs, with bootstrap values indicated on branches ranging from 0 to 100. (B) Analysis of the number, distribution, and classification of cis-regulatory elements in VsPGs. Colors represent three functional categories of elements: green (abiotic and biotic stress response), red (hormone response), and blue (plant growth and development regulation). Numeric labels indicate the specific distribution of elements for each gene. Table S1: Protein sequences of identified VsPGs. Table S2: Protein sequences of PGs of Arabidopsis thaliana. Table S3: Information on 83 VsPGs from the pear genome. Table S4: Ka/Ks ratios of paralogous genes in VsPG gene family. Table S5: Cis-acting regulatory elements identified in the promoters of VsPGs. Table S6: Expression data of VsPGs in different tissues and different treatments. Table S7: Primers used in qRT-PCR analysis of VsPGs.
Author Contributions
All authors contributed to the study conception and design. Material preparation was performed by X.Y., T.L., Z.Y., and R.D.; data collection and analysis were performed by X.Y., Z.L., X.Z., J.C., X.G., J.H., and C.C.; the first draft of the manuscript was written by X.Y. and R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (32060392), Qian Ke He Cheng Guo ([2022] Zhong Dian 005), and the GZMARS-Forage Industry Technology System of Guizhou Province. Teaching Content and Curriculum System Reform Project for Higher Education Institutions in Guizhou Province (2023024).
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank Qiang Yu (Guizhou University, China) for assistance with the experiments.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
References
- Popper, Z.A. Evolution and diversity of green plant cell walls. Curr. Opin. Plant Biol. 2008, 11, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Gro eMalinovsky, F.; Fangel, J.U.; Willats, W.G.T. The role of the cell wall in plant immunity. Front. Plant Sci. 2014, 5, 178. [Google Scholar] [CrossRef]
- Wan, J.; He, M.; Hou, Q.; Zou, L.; Yang, Y.; Wei, Y.; Chen, X. Cell wall associated immunity in plants. Stress Biol. 2021, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, K.A.; Bennett, A.B. Polygalacturonases: Many genes in search of a function. Plant Physiol. 1998, 117, 337–343. [Google Scholar] [CrossRef]
- He, P.; Zhang, J.; Lv, Z.; Cui, P.; Xu, X.; George, M.S.; Lu, G. Genome-wide identification and expression analysis of the polygalacturonase gene family in sweetpotato. BMC Plant Biol. 2023, 23, 300. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, X.; Shi, X.; Zhai, H.; Tian, C.; Kong, F.; Liu, B.; Yuan, X. A global analysis of the polygalacturonase gene family in soybean (Glycine max). PLoS ONE 2016, 11, e0163012. [Google Scholar] [CrossRef]
- Bosch, M.; Hepler, P.K. Pectin methylesterases and pectin dynamics in pollen tubes. Plant Cell 2005, 17, 3219–3226. [Google Scholar] [CrossRef]
- Jiang, C.-Z.; Lu, F.; Imsabai, W.; Meir, S.; Reid, M.S. Silencing polygalacturonase expression inhibits tomato petiole abscission. J. Exp. Bot. 2008, 59, 973–979. [Google Scholar] [CrossRef]
- Li, J.; Su, L.; Lv, A.; Li, Y.; Zhou, P.; An, Y. MsPG1 alleviated aluminum-induced inhibition of root growth by decreasing aluminum accumulation and increasing porosity and extensibility of cell walls in alfalfa (Medicago sativa). Environ. Exp. Bot. 2020, 175, 104045. [Google Scholar] [CrossRef]
- Nie, H.; Shi, Y.; Geng, X.; Xing, G. CRISRP/Cas9-mediated targeted mutagenesis of tomato polygalacturonase gene (SlPG) delays fruit softening. Front. Plant Sci. 2022, 13, 729128. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Osborne, E.; Poindexter, P.D.; Somerville, C.R. Microspore separation in the quartet 3 mutants of Arabidopsis is impaired by a defect in a developmentally regulated polygalacturonase required for pollen mother cell wall degradation. Plant Physiol. 2003, 133, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.Y.; Somerville, C.R. Tetrad pollen formation in quartet mutants of Arabidopsis thaliana is associated with persistence of pectic polysaccharides of the pollen mother cell wall. Plant J. 1998, 15, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzis, P.; Solomos, T.; Tucker, M.L. Three different polygalacturonases are expressed in tomato leaf and flower abscission, each with a different temporal expression pattern. Plant Physiol. 1997, 113, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Markovič, O.; Janeček, Š. Pectin degrading glycoside hydrolases of family 28: Sequence-structural features, specificities and evolution. Protein Eng. 2001, 14, 615–631. [Google Scholar] [CrossRef]
- Lu, L.; Hou, Q.; Wang, L.; Zhang, T.; Zhao, W.; Yan, T.; Zhao, L.; Li, J.; Wan, X. Genome-wide identification and characterization of polygalacturonase gene family in maize (Zea mays L.). Int. J. Mol. Sci. 2021, 22, 10722. [Google Scholar] [CrossRef]
- Sander, L.; Child, R.; Ulvskov, P.; Albrechtsen, M.; Borkhardt, B. Analysis of a dehiscence zone endo-polygalacturonase in oilseed rape (Brassica napus) and Arabidopsis thaliana: Evidence for roles in cell separation in dehiscence and abscission zones, and in stylar tissues during pollen tube growth. Plant Mol. Biol. 2001, 46, 469–479. [Google Scholar] [CrossRef]
- Ogawa, M.; Kay, P.; Wilson, S.; Swain, S.M. Arabidopsis Dehiscence Zone Polygalacturonase1 (ADPG1), ADPG2, and QUARTET2 are polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell 2009, 21, 216–233. [Google Scholar] [CrossRef]
- Preuss, D.; Rhee, S.Y.; Davis, R.W. Tetrad analysis possible in Arabidopsis with mutation of the QUARTET (QRT) genes. Science 1994, 264, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Somerville, C.; Anderson, C.T. Polygalacturonase Involved in Expansion1 functions in cell elongation and flower development in Arabidopsis. Plant Cell 2014, 26, 1018–1035. [Google Scholar] [CrossRef]
- Xiao, C.; Barnes, W.J.; Zamil, M.S.; Yi, H.; Puri, V.M.; Anderson, C.T. Activation tagging of Arabidopsis Polygalacturonase Involved in Expansion 2 promotes hypocotyl elongation, leaf expansion, stem lignification, mechanical stiffening, and lodging. Plant J. 2017, 89, 1159–1173. [Google Scholar] [CrossRef]
- Wang, F.; Sun, X.; Liu, B.; Kong, F.; Pan, X.; Zhang, H. A polygalacturonase gene PG031 regulates seed coat permeability with a pleiotropic effect on seed weight in soybean. Appl. Genet. 2022, 135, 1603–1618. [Google Scholar] [CrossRef]
- Zou, Q.; Tu, R.; Wu, J.; Huang, T.; Sun, Z.; Ruan, Z.; Cao, H.; Yang, S.; Shen, X.; He, G. A polygalacturonase gene OsPG1 modulates water homeostasis in rice. CROP J. 2024, 12, 79–91. [Google Scholar] [CrossRef]
- Peng, S.; Liu, Y.; Xu, Y.; Zhao, J.; Gao, P.; Liu, Q.; Yan, S.; Xiao, Y.; Zuo, S.-M.; Kang, H. Genome-Wide Association Study Identifies a Plant-Height—Associated Gene OsPG3 in a Population of Commercial Rice Varieties. Int. J. Mol. Sci. 2023, 24, 11454. [Google Scholar] [CrossRef]
- Ge, T.; Huang, X.; Pan, X.; Zhang, J.; Xie, R. Genome-wide identification and expression analysis of citrus fruitlet abscission-related polygalacturonase genes. 3 Biotech 2019, 9, 250. [Google Scholar] [CrossRef]
- Quesada, M.A.; Blanco-Portales, R.; Posé, S.; García-Gago, J.A.; Jiménez-Bermúdez, S.; Muñoz-Serrano, A.; Caballero, J.L.; Pliego-Alfaro, F.; Mercado, J.A.; Munoz-Blanco, J. Antisense down-regulation of the FaPG1 gene reveals an unexpected central role for polygalacturonase in strawberry fruit softening. Plant Physiol. 2009, 150, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Posé, S.; Paniagua, C.; Cifuentes, M.; Blanco-Portales, R.; Quesada, M.A.; Mercado, J.A. Insights into the effects of polygalacturonase FaPG1 gene silencing on pectin matrix disassembly, enhanced tissue integrity, and firmness in ripe strawberry fruits. J. Exp. Bot. 2013, 64, 3803–3815. [Google Scholar] [CrossRef] [PubMed]
- López-Casado, G.; Sánchez-Raya, C.; Ric-Varas, P.D.; Paniagua, C.; Blanco-Portales, R.; Muñoz-Blanco, J.; Pose, S.; Matas, A.J.; Mercado, J.A. CRISPR/Cas9 editing of the polygalacturonase FaPG1 gene improves strawberry fruit firmness. Hortic. Res. 2023, 10, uhad011. [Google Scholar] [CrossRef]
- Wakasa, Y.; Kudo, H.; Ishikawa, R.; Akada, S.; Senda, M.; Niizeki, M.; Harada, T. Low expression of an endopolygalacturonase gene in apple fruit with long-term storage potential. Postharvest Biol. Technol. 2006, 39, 193–198. [Google Scholar] [CrossRef]
- Poles, L.; Gentile, A.; Giuffrida, A.; Valentini, L.; Endrizzi, I.; Aprea, E.; Gasperi, F.; Distefano, G.; Artioli, G.; La Malfa, S. Role of fruit flesh cell morphology and MdPG1 allelotype in influencing juiciness and texture properties in apple. Postharvest Biol. Technol. 2020, 164, 111161. [Google Scholar] [CrossRef]
- Qian, M.; Xu, Z.; Zhang, Z.; Li, Q.; Yan, X.; Liu, H.; Han, M.; Li, F.; Zheng, J.; Zhang, D. The downregulation of PpPG21 and PpPG22 influences peach fruit texture and softening. Planta 2021, 254, 22. [Google Scholar] [CrossRef]
- Dong, R.; Luo, B.; Tang, L.; Wang, Q.-x.; Lu, Z.-J.; Chen, C.; Yang, F.; Wang, S.; He, J. A comparative transcriptomic analysis reveals a coordinated mechanism activated in response to cold acclimation in common vetch (Vicia sativa L.). BMC Genom. 2022, 23, 814. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Tian, Z.; Yang, Z.; Yin, X.; Dong, R. Comparative transcriptomic analysis reveals coordinated mechanisms of different genotypes of common vetch root in response to Al stress. Environ. Exp. Bot. 2023, 213, 105450. [Google Scholar] [CrossRef]
- Dong, R.; Dong, D.; Luo, D.; Zhou, Q.; Chai, X.; Zhang, J.; Xie, W.; Liu, W.; Dong, Y.; Wang, Y. Transcriptome analyses reveal candidate pod shattering-associated genes involved in the pod ventral sutures of common vetch (Vicia sativa L.). Front. Plant Sci. 2017, 8, 649. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, N.; Chen, T.; Xu, H.; Li, R.; Wang, L.; Zhou, S.; Cai, Q.a.; Hou, X.; Wang, L. Genome-wide identification of GH28 family and insight into its contributions to pod shattering resistance in Brassica napus L. BMC Genom. 2024, 25, 492. [Google Scholar] [CrossRef]
- Guo, M.W.; Zhu, L.; Li, H.Y.; Liu, W.P.; Wu, Z.N.; Wang, C.H.; Liu, L.; Li, Z.Y.; Li, J. Mechanism of pod shattering in the forage legume Medicago ruthenica. Plant Physiol. Biochem. 2022, 185, 260–267. [Google Scholar] [CrossRef]
- Wang, D.; Zuo, S.; Zhang, Y.; Zhao, P.; Tuoheti, G.; Zhao, B.; Wan, P.; Chu, L.; Yang, K. Transcriptome analyses reveal key genes related to pod dehiscence of adzuki bean (Vigna angularis L.). 3 Biotech 2025, 15, 80. [Google Scholar] [CrossRef]
- Fan, N.; Wen, W.; Gao, L.; Lv, A.; Su, L.; Zhou, P.; An, Y. MsPG4-mediated hydrolysis of pectins increases the cell wall extensibility and aluminum resistance of alfalfa. Plant Soil 2022, 477, 357–371. [Google Scholar] [CrossRef]
- Xi, H.; Nguyen, V.; Ward, C.; Liu, Z.; Searle, I.R. Chromosome-level assembly of the common vetch (Vicia sativa) reference genome. Gigabyte 2022, 2022, gigabyte38. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pu, J.; Jia, C.; Luo, D.; Zhou, Q.; Fang, X.; Nie, B.; Liu, W.; Nan, Z.; Searle, I.R. The genome of Vicia sativa ssp. amphicarpa provides insights into the role of terpenoids in antimicrobial resistance within subterranean fruits. Plant J. 2024, 119, 2654–2671. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, M.; Zhang, H.; Zhang, S.; Qian, M.; Zhang, Z.; Luo, W.; Fan, J.; Liu, Z.; Wang, L. Genome-wide analysis of polygalacturonase gene family from pear genome and identification of the member involved in pear softening. BMC Plant Biol. 2019, 19, 587. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Wang, H.; Li, Y.; Zhu, B.; Zang, Y.; He, Y.; Cao, J.; Zhu, Z.; Yu, Y. Genome-wide identification and analysis of polygalacturonase genes in Solanum lycopersicum. Int. J. Mol. Sci. 2018, 19, 2290. [Google Scholar] [CrossRef]
- Yan, W.; Dong, X.; Li, R.; Zhao, X.; Zhou, Q.; Luo, D.; Liu, Z. Genome-wide identification of JAZ gene family members in autotetraploid cultivated alfalfa (Medicago sativa subsp. sativa) and expression analysis under salt stress. BMC Genom. 2024, 25, 636. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Yang, Z.; Nie, G.; Feng, G.; Xu, X.; Li, D.; Wang, X.; Huang, L.; Zhang, X. Genome-wide identification of MADS-box gene family in orchardgrass and the positive role of DgMADS114 and DgMADS115 under different abiotic stress. Int. J. Biol. Macromol. 2022, 223, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-C.; Kwon, S.-J.; Kim, N.-S. Intron loss mediated structural dynamics and functional differentiation of the polygalacturonase gene family in land plants. Genes Genom. 2010, 32, 570–577. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z.; Peng, J.; Mou, H.; Wang, Z.; Dao, Y.; Liu, T.; Kong, D.; Liu, S.; Xiong, Y. Exploring evolutionary pathways and abiotic stress responses through genome-wide identification and analysis of the alternative oxidase (AOX) gene family in common oat (Avena sativa). Int. J. Mol. Sci. 2024, 25, 9383. [Google Scholar] [CrossRef]
- Fang, L.; Liu, T.; Li, M.; Dong, X.; Han, Y.; Xu, C.; Li, S.; Zhang, J.; He, X.; Zhou, Q. MODMS: A multi-omics database for facilitating biological studies on alfalfa (Medicago sativa L.). Hortic. Res. 2024, 11, uhad245. [Google Scholar] [CrossRef] [PubMed]
- Bolser, D.; Staines, D.M.; Pritchard, E.; Kersey, P. Ensembl plants: Integrating tools for visualizing, mining, and analyzing plant genomics data. Plant Bioinform. MPs 2016, 1374, 115–140. [Google Scholar] [CrossRef]
- Zhao, M.; Hu, R.; Lin, Y.; Yang, Y.; Chen, Q.; Li, M.; Zhang, Y.; Zhang, Y.; Wang, Y.; He, W.; et al. Genome-Wide Analysis of Polygalacturonase Gene Family Reveals Its Role in Strawberry Softening. Plants 2024, 13, 1838. [Google Scholar] [CrossRef]
- Zhou, Q.; Cui, Y.; Dong, R.; Luo, D.; Fang, L.; Nan, Z.; Liu, Z. Integrative analyses of transcriptomes and metabolomes reveal associated genes and metabolites with flowering regulation in common Vetch (Vicia sativa L.). Int. J. Mol. Sci. 2022, 23, 6818. [Google Scholar] [CrossRef]
- Li, M.; Zhao, L.; Zhou, Q.; Fang, L.; Luo, D.; Liu, W.; Searle, I.R.; Liu, Z. Transcriptome and Coexpression Network Analyses Provide In-Sights into the Molecular Mechanisms of Hydrogen Cyanide Synthesis during Seed Development in Common Vetch (Vicia sativa L.). Int. J. Mol. Sci. 2022, 23, 2275. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Cui, Y.; Dong, S.; Luo, D.; Fang, L.; Shi, Z.; Liu, W.; Wang, Z.; Nan, Z.; Liu, Z. Integrative physiological, transcriptome, and metabolome analyses reveal the associated genes and metabolites involved in cold stress response in common vetch (Vicia sativa L.). Food Energy Secur. 2023, 12, e484. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hocq, L.; Guinand, S.; Habrylo, O.; Voxeur, A.; Tabi, W.; Safran, J.; Fournet, F.; Domon, J.M.; Mollet, J.C.; Pilard, S. The exogenous application of AtPGLR, an endo-polygalacturonase, triggers pollen tube burst and repair. Plant J. 2020, 103, 617–633. [Google Scholar] [CrossRef]
- Mengarelli, D.A.; Zanor, M.I. Genome-wide characterization and analysis of the CCT motif family genes in soybean (Glycine max). Planta 2021, 253, 15. [Google Scholar] [CrossRef]
- Baldwin, L.; Domon, J.-M.; Klimek, J.F.; Fournet, F.; Sellier, H.; Gillet, F.; Pelloux, J.; Lejeune-Hénaut, I.; Carpita, N.C.; Rayon, C. Structural alteration of cell wall pectins accompanies pea development in response to cold. Phytochemistry 2014, 104, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.L.; Liu, H.J.; Wang, X.R.; Zeng, Q.Y. Molecular evolution and expression divergence of the Populus polygalacturonase supergene family shed light on the evolution of increasingly complex organs in plants. New Phytol. 2013, 197, 1353–1365. [Google Scholar] [CrossRef]
- Safran, J.; Tabi, W.; Ung, V.; Lemaire, A.; Habrylo, O.; Bouckaert, J.; Rouffle, M.; Voxeur, A.; Pongrac, P.; Bassard, S.; et al. Plant polygalacturonase structures specify enzyme dynamics and processivities to fine-tune cell wall pectins. Plant Cell 2023, 35, 3073–3091. [Google Scholar] [CrossRef]
- Kim, J.; Shiu, S.-H.; Thoma, S.; Li, W.-H.; Patterson, S.E. Patterns of expansion and expression divergence in the plant polygalacturonase gene family. Genome Biol. 2006, 7, R87. [Google Scholar] [CrossRef]
- Ye, J.; Yang, X.; Yang, Z.; Niu, F.; Chen, Y.; Zhang, L.; Song, X. Comprehensive analysis of polygalacturonase gene family highlights candidate genes related to pollen development and male fertility in wheat (Triticum aestivum L.). Planta 2020, 252, 31. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liang, Y.; Lv, M.; Wu, J.; Lu, G.; Cao, J. Genome-wide identification and characterization of polygalacturonase genes in Cucumis sativus and Citrullus lanatus. Plant Physiol. Biochem. 2014, 74, 263–275. [Google Scholar] [CrossRef]
- Lyu, M.; Iftikhar, J.; Guo, R.; Wu, B.; Cao, J. Patterns of expansion and expression divergence of the polygalacturonase gene family in Brassica oleracea. Int. J. Mol. Sci. 2020, 21, 5706. [Google Scholar] [CrossRef]
- Yi, X.; Chen, W.; Guan, J.; Zhu, J.; Zhang, Q.; Yang, H.; Yang, H.; Zhong, S.; Chen, C.; Tan, F. Genome-Wide Analysis of the Polygalacturonase Gene Family Sheds Light on the Characteristics, Evolutionary History, and Putative Function of Akebia trifoliata. Int. J. Mol. Sci. 2023, 24, 16973. [Google Scholar] [CrossRef]
- Marand, A.P.; Eveland, A.L.; Kaufmann, K.; Springer, N.M. cis-Regulatory elements in plant development, adaptation, and evolution. Annu. Rev. Plant Biol. 2023, 74, 111–137. [Google Scholar] [CrossRef]
- Chen, D.; Yan, W.; Fu, L.-Y.; Kaufmann, K. Architecture of gene regulatory networks controlling flower development in Arabidopsis thaliana. Nat. Commun. 2018, 9, 4534. [Google Scholar] [CrossRef] [PubMed]
- Adrian, J.; Farrona, S.; Reimer, J.J.; Albani, M.C.; Coupland, G.; Turck, F. cis-Regulatory elements and chromatin state coordinately control temporal and spatial expression of Flowering Locus T in Arabidopsis. Plant Cell 2010, 22, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Duan, Z.; Wang, Z.; Li, Y.; Wang, Y.; Li, C.; Mao, W.; Que, Q.; Chen, X.; Li, P. Genome-wide identification, expression pattern and subcellular localization analysis of the JAZ gene family in Toona ciliata. Ind. Crop. Prod. 2022, 178, 114582. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).