Exogenous Proline Modulates Physiological Responses and Induces Stress Memory in Wheat Under Repeated and Delayed Drought Stress
Abstract
1. Introduction
2. Materials and Methods
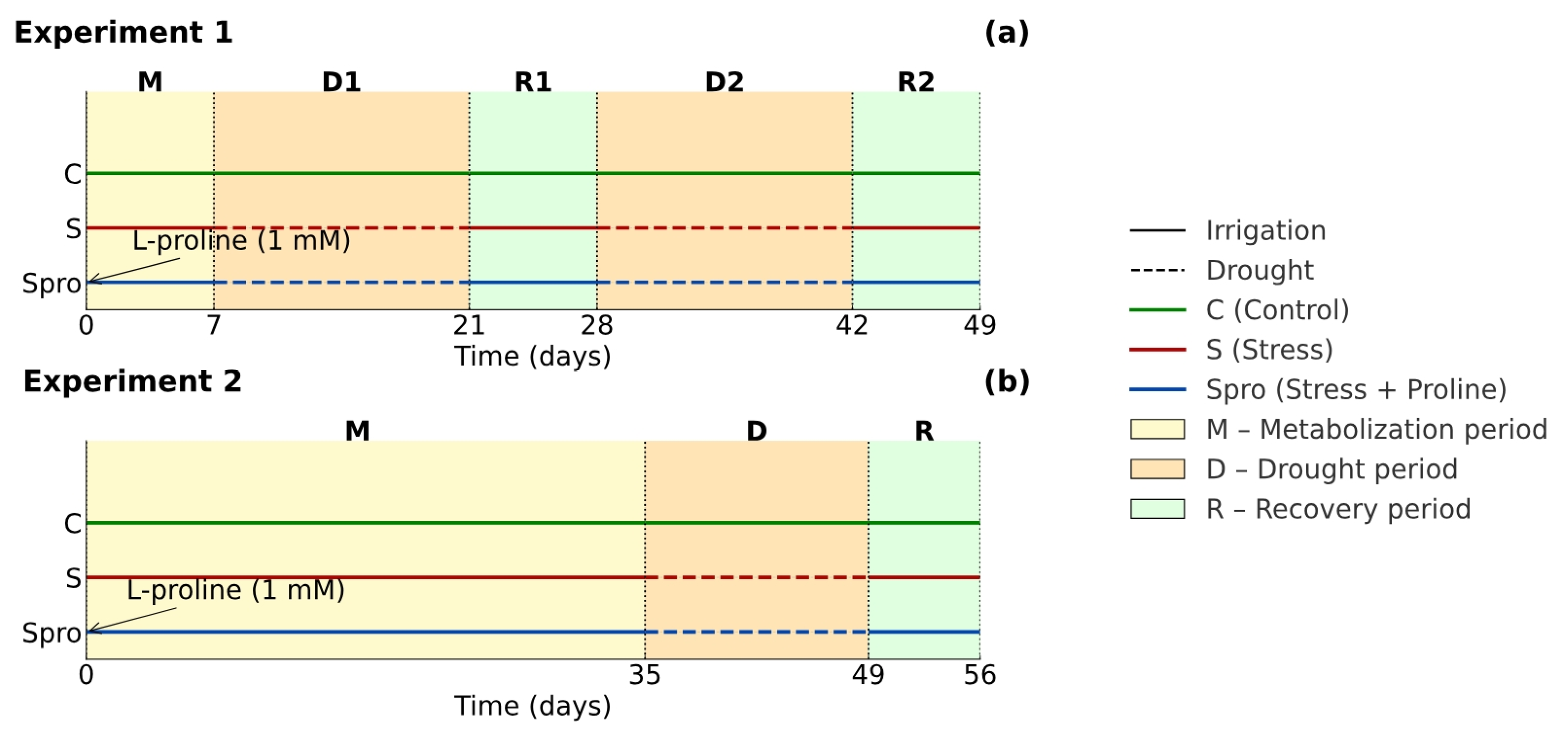
2.1. Experimental Design and Drought Stress Treatments
2.1.1. Experiment 1: Repeated Drought Stress in Two Periods
2.1.2. Experiment 2: Delayed Drought Stress
2.1.3. Preparation of the Experimental Timeline (Scheme 1)
2.2. Leaf Water Potential
2.3. Leaf Gas Exchange
2.4. Intrinsic Water Use Efficiency (WUEi)
2.5. Chlorophyll Fluorescence
2.6. Proline Content
2.7. Malondialdehyde Content
2.8. Statistical Analysis
3. Results
3.1. Repeated Drought Stress in Two Periods (Experiment 1)
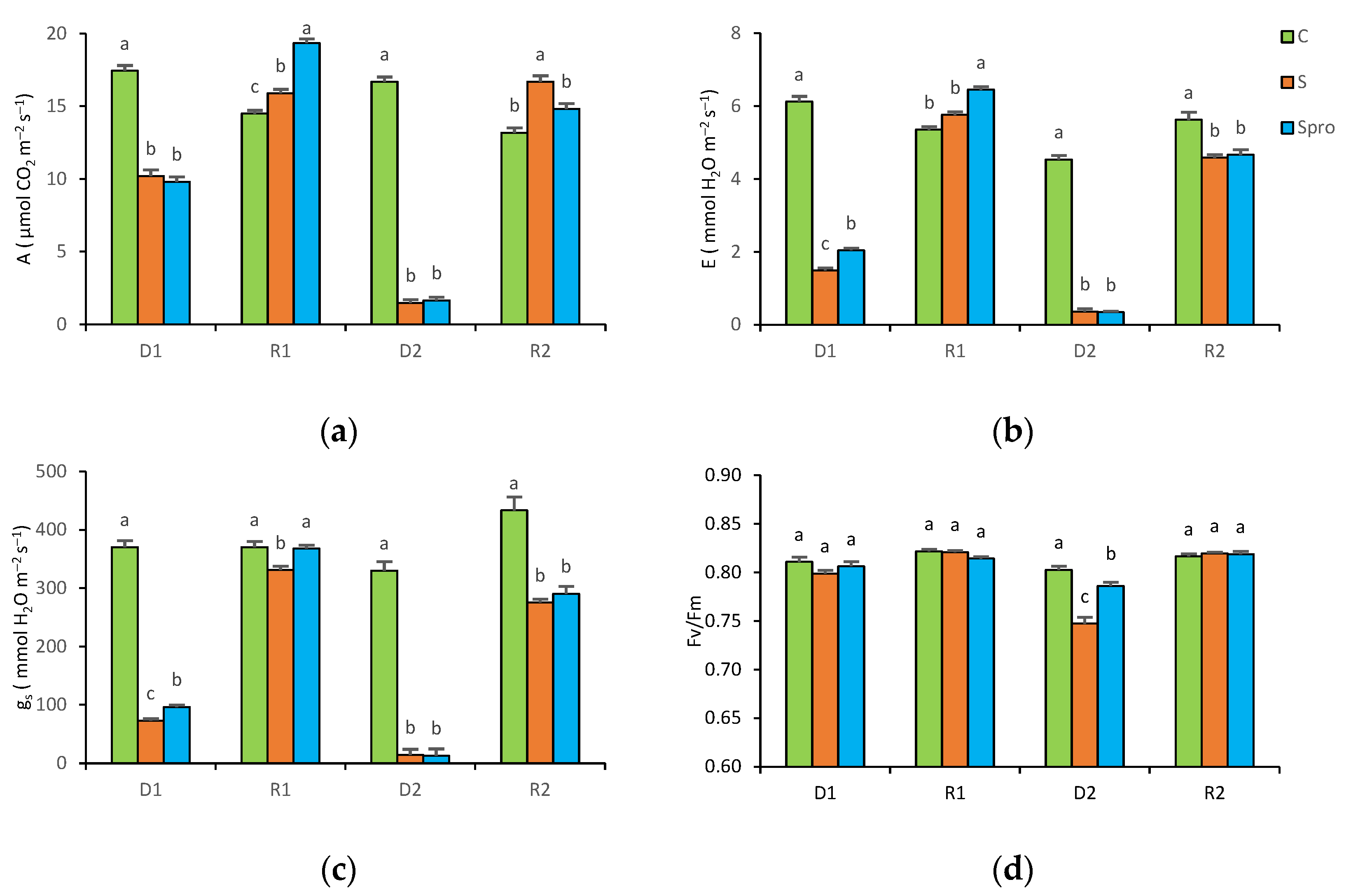
3.1.1. Gas Exchange
3.1.2. Chlorophyll Fluorescence
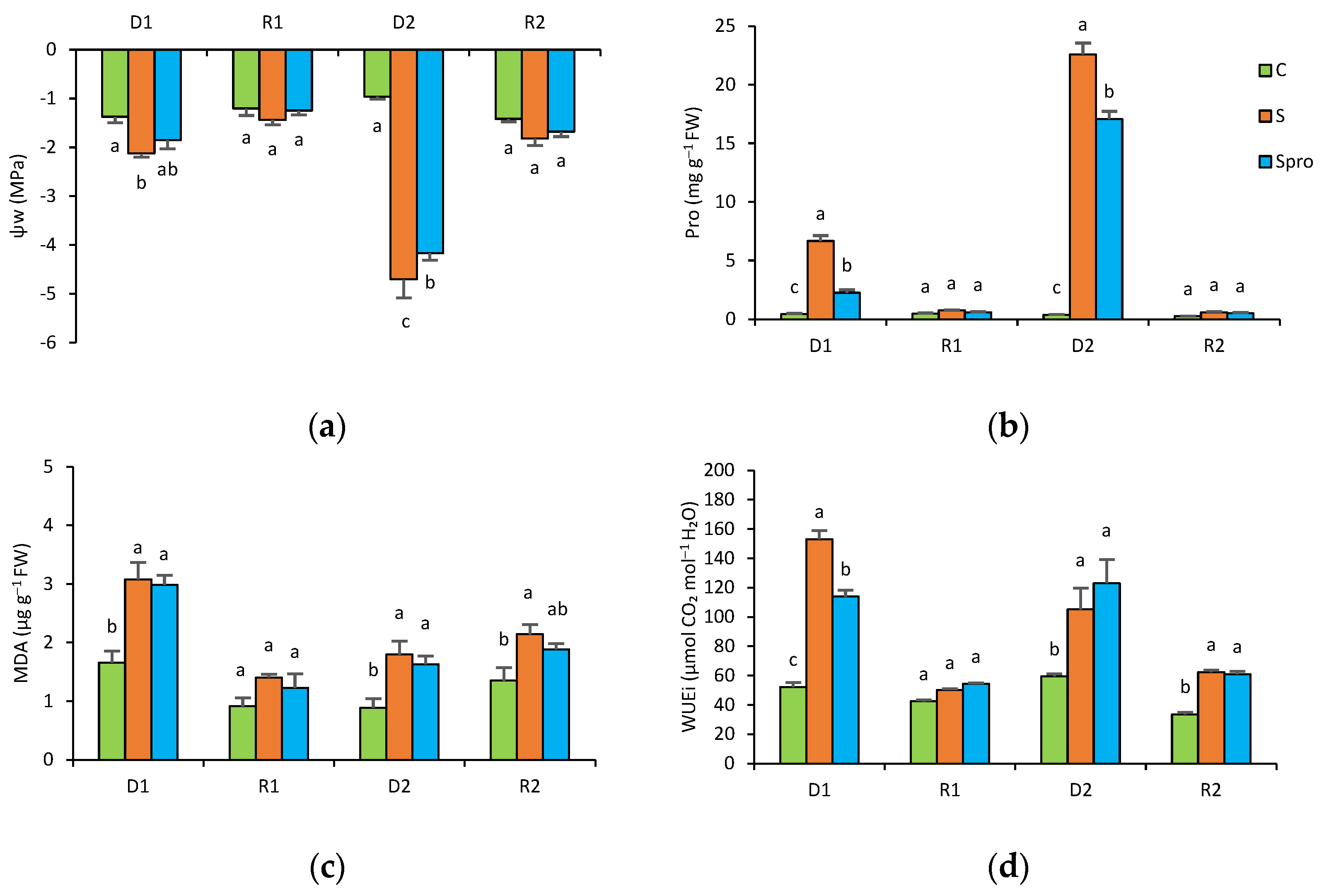
3.1.3. Leaf Water Potential
3.1.4. Proline and MDA Content
3.1.5. Intrinsic Water Use Efficiency (WUEi)
3.2. Delayed Drought Stress (Experiment 2)
3.2.1. Gas Exchange
3.2.2. Chlorophyll Fluorescence
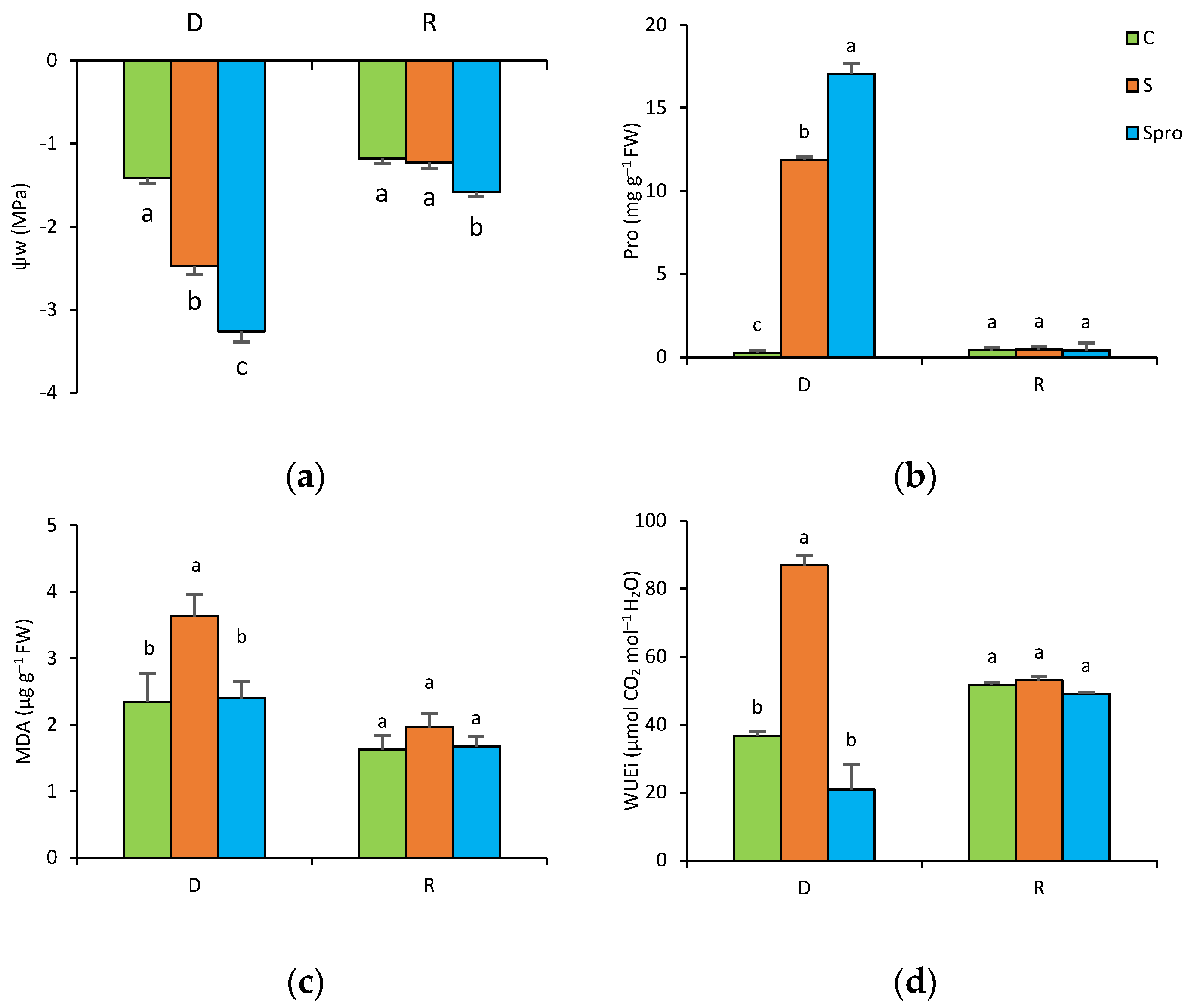
3.2.3. Leaf Water Potential
3.2.4. Proline and MDA Content
3.2.5. Intrinsic Water Use Efficiency (WUEi)
4. Discussion
4.1. Repeated Drought Stress in Two Periods (Experiment 1)
4.2. Delayed Drought Stress (Experiment 2)
5. Conclusions
- (1)
- During the first stress period, proline was demonstrably involved in stomatal regulation, leading to enhanced gas exchange. Upon rewatering, more efficient water status and rapid resumption of photosynthetic assimilation were observed.
- (2)
- Repeated stress elicited a stronger response regardless of proline treatment. The effect of exogenous proline persisted in the form of reduced endogenous proline synthesis and improved protection of PSII.
- (3)
- A long-term priming effect was demonstrated, enhancing the preparedness of plants for subsequent drought. This stress memory supported more efficient osmoregulation, lower lipid peroxidation, improved protection of photosystem II integrity, and a more effective metabolic recovery after rehydration.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Xu, J.; Li, R.; Ge, Y.; Li, Y.; Li, R. Plants’ Response to Abiotic Stress: Mechanisms and Strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef] [PubMed]
- Haghpanah, M.; Hashemipetroudi, S.; Arzani, A.; Araniti, F. Drought Tolerance in Plants: Physiological and Molecular Responses. Plants 2024, 13, 2962. [Google Scholar] [CrossRef] [PubMed]
- Fanzo, J.; Davis, C.; McLaren, R.; Choufani, J. The Effect of Climate Change across Food Systems: Implications for Nutrition Outcomes. Glob. Food Secur. 2018, 18, 12–19. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Lawson, T.; Terashima, I.; Fujita, T.; Wang, Y. Coordination Between Photosynthesis and Stomatal Behavior. In The Leaf: A Platform for Performing Photosynthesis; Adams, W., III, Terashima, I., Eds.; Advances in Photosynthesis and Respiration; Springer: Cham, Switzerland, 2018; Volume 44, pp. 141–161. ISBN 978-3-319-93594-2. [Google Scholar] [CrossRef]
- Khan, N. Decoding Phytohormone Signaling in Plant Stress Physiology: Insights, Challenges, and Future Directions. Environ. Exp. Bot. 2025, 231, 106099. [Google Scholar] [CrossRef]
- Chen, D.; Mubeen, B.; Hasnain, A.; Rizwan, M.; Adrees, M.; Naqvi, S.A.H.; Iqbal, S.; Kamran, M.; El-Sabrout, A.M.; Elansary, H.O.; et al. Role of Promising Secondary Metabolites to Confer Resistance Against Environmental Stresses in Crop Plants: Current Scenario and Future Perspectives. Front. Plant Sci. 2022, 13, 881032. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones Regulate Accumulation of Osmolytes Under Abiotic Stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef]
- Blum, A. Osmotic Adjustment Is a Prime Drought Stress Adaptive Engine in Support of Plant Production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A Key Player in Plant Abiotic Stress Tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.a.R. Proline, a Multifaceted Signalling Molecule in Plant Responses to Abiotic Stress: Understanding the Physiological Mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Charagh, S.; Abbas, S.; Hassan, M.U.; Saeed, F.; Haider, S.; Sharif, R.; Anand, A.; Corpas, F.J.; Jin, W.; et al. Assessment of Proline Function in Higher Plants under Extreme Temperatures. Plant Biol. 2023, 25, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Meena, M.; Divyanshu, K.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V.; Yadav, M.; Upadhyay, R.S. Regulation of L-Proline Biosynthesis, Signal Transduction, Transport, Accumulation and Its Vital Role in Plants during Variable Environmental Conditions. Heliyon 2019, 5, e02952. [Google Scholar] [CrossRef]
- Primo-Capella, A.; Martínez-Cuenca, M.-R.; Gil-Muñoz, F.; Forner-Giner, M.A. Physiological Characterization and Proline Route Genes Quantification under Long-Term Cold Stress in Carrizo citrange. Sci. Hortic. 2021, 276, 109744. [Google Scholar] [CrossRef]
- Renzetti, M.; Bertolini, E.; Trovato, M. Proline Metabolism Genes in Transgenic Plants: Meta-Analysis under Drought and Salt Stress. Plants 2024, 13, 1913. [Google Scholar] [CrossRef]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical Priming of Plants Against Multiple Abiotic Stresses: Mission Possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef]
- Harris, C.J.; Amtmann, A.; Ton, J. Epigenetic Processes in Plant Stress Priming: Open Questions and New Approaches. Curr. Opin. Plant Biol. 2023, 75, 102432. [Google Scholar] [CrossRef]
- Crisp, P.A.; Ganguly, D.; Eichten, S.R.; Borevitz, J.O.; Pogson, B.J. Reconsidering Plant Memory: Intersections between Stress Recovery, RNA Turnover, and Epigenetics. Sci. Adv. 2016, 2, e1501340. [Google Scholar] [CrossRef]
- Chang, Y.; Zhu, C.; Jiang, J.; Zhang, H.; Zhu, J.; Duan, C. Epigenetic Regulation in Plant Abiotic Stress Responses. J. Integr. Plant Biol. 2020, 62, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Shilpa; Thakur, R.; Prasad, P. Epigenetic Regulation of Abiotic Stress Responses in Plants. Biochim. Biophys. Acta BBA-Gen. Subj. 2024, 1868, 130661. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Uğurlar, F.; Adamakis, I.-D.S. Epigenetic Modifications of Hormonal Signaling Pathways in Plant Drought Response and Tolerance for Sustainable Food Security. Int. J. Mol. Sci. 2024, 25, 8229. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Matthes, M.C.; Napier, J.A.; Pickett, J.A. Stressful “Memories” of Plants: Evidence and Possible Mechanisms. Plant Sci. 2007, 173, 603–608. [Google Scholar] [CrossRef]
- Siddique, A.B.; Parveen, S.; Rahman, M.Z.; Rahman, J. Revisiting Plant Stress Memory: Mechanisms and Contribution to Stress Adaptation. Physiol. Mol. Biol. Plants 2024, 30, 349–367. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Liu, B.; Xu, Z.-Y. Dynamic Regulation of DNA Methylation and Histone Modifications in Response to Abiotic Stresses in Plants. J. Integr. Plant Biol. 2022, 64, 2252–2274. [Google Scholar] [CrossRef]
- Hrmova, M.; Hussain, S.S. Plant Transcription Factors Involved in Drought and Associated Stresses. Int. J. Mol. Sci. 2021, 22, 5662. [Google Scholar] [CrossRef]
- Kumar, S.; Mohapatra, T. Epigenetic Modifications in Genome Help Remembering the Stress Tolerance Strategy Adopted by the Plant. Front. Biosci.-Landmark 2024, 29, 126. [Google Scholar] [CrossRef]
- Khan, P.; Abdelbacki, A.M.M.; Albaqami, M.; Jan, R.; Kim, K.-M. Proline Promotes Drought Tolerance in Maize. Biology 2025, 14, 41. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Zhen, B.; Lv, M.; Zhou, X.; Yong, B.; Niu, Q.; Yang, S. Proline Spray Relieves the Adverse Effects of Drought on Wheat Flag Leaf Function. Plants 2024, 13, 957. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Khan, N. Climate Change and Salinity Effects on Crops and Chemical Communication Between Plants and Plant Growth-Promoting Microorganisms Under Stress. Front. Sustain. Food Syst. 2021, 5, 161. [Google Scholar] [CrossRef]
- Ahmed, M.; Fayyaz-ul-HASSAN; Qadir, G.; Shaheen, F.A.; Aslam, M.A. Response of Proline Accumulation in Bread Wheat (Triticum aestivum L.) under Rainfed Conditions. J. Agric. Meteorol. 2017, 73, 147–155. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Z.; Du, X.; Duan, H.; Zhen, W.; Zhang, Y.; Shi, Z.; He, M.; Li, R. Transcriptomic and Metabolomic Analyses Reveal the Response to Short-Term Drought Stress in Bread Wheat (Triticum aestivum L.). Agronomy 2024, 14, 704. [Google Scholar] [CrossRef]
- Du, L.; Huang, X.; Ding, L.; Wang, Z.; Tang, D.; Chen, B.; Ao, L.; Liu, Y.; Kang, Z.; Mao, H. TaERF87 and TaAKS1 Synergistically Regulate TaP5CS1/TaP5CR1-Mediated Proline Biosynthesis to Enhance Drought Tolerance in Wheat. New Phytol. 2023, 237, 232–250. [Google Scholar] [CrossRef]
- Kulkarni, M.; Soolanayakanahally, R.; Ogawa, S.; Uga, Y.; Selvaraj, M.G.; Kagale, S. Drought Response in Wheat: Key Genes and Regulatory Mechanisms Controlling Root System Architecture and Transpiration Efficiency. Front. Chem. 2017, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Sehar, Z.; Gautam, H.; Masood, A.; Khan, N.A. Ethylene- and Proline-Dependent Regulation of Antioxidant Enzymes to Mitigate Heat Stress and Boost Photosynthetic Efficacy in Wheat Plants. J. Plant Growth Regul. 2023, 42, 2683–2697. [Google Scholar] [CrossRef]
- Khan, M.A.; Waseem Akram, M.; Iqbal, M.; Ghulam Muhu-Din Ahmed, H.; Rehman, A.; Arslan Iqbal, H.S.M.; Alam, B. Multivariate and Association Analyses of Quantitative Attributes Reveal Drought Tolerance Potential of Wheat (Triticum aestivum L.) Genotypes. Commun. Soil Sci. Plant Anal. 2023, 54, 178–195. [Google Scholar] [CrossRef]
- Bekka, S.; Abrous-Belbachir, O.; Djebbar, R. Effects of Exogenous Proline on the Physiological Characteristics of Triticum Aestivum L. and Lens Culinaris Medik. under Drought Stress. Acta Agric. Slov. 2018, 111, 477–491. [Google Scholar] [CrossRef]
- Farooq, M.; Nawaz, A.; Chaudhry, M.a.M.; Indrasti, R.; Rehman, A. Improving Resistance against Terminal Drought in Bread Wheat by Exogenous Application of Proline and Gamma-Aminobutyric Acid. J. Agron. Crop Sci. 2017, 203, 464–472. [Google Scholar] [CrossRef]
- Bushra; Kiran, A.; Ahmad, M.; Shehzad, T.; Sanaullah, M. Mitigation of Drought Stress in Wheat through Exogenous Application of Proline. J. Anim. Plant Sci. 2023, 33, 1392–1401. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Priming Crops for the Future: Rewiring Stress Memory. Trends Plant Sci. 2022, 27, 699–716. [Google Scholar] [CrossRef] [PubMed]
- Ganie, S.A.; McMulkin, N.; Devoto, A. The Role of Priming and Memory in Rice Environmental Stress Adaptation: Current Knowledge and Perspectives. Plant Cell Environ. 2024, 47, 1895–1915. [Google Scholar] [CrossRef] [PubMed]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of Exogenous Proline to Abiotic Stresses Tolerance in Plants: A Review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under Drought and Salt Stress: Regulation Mechanisms from Whole Plant to Cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Xie, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Abscisic Acid and Jasmonic Acid Are Involved in Drought Priming-Induced Tolerance to Drought in Wheat. Crop J. 2021, 9, 120–132. [Google Scholar] [CrossRef]
- Kopecká, R.; Kameniarová, M.; Černý, M.; Brzobohatý, B.; Novák, J. Abiotic Stress in Crop Production. Int. J. Mol. Sci. 2023, 24, 6603. [Google Scholar] [CrossRef]
- Ghafoor, R.; Akram, N.A.; Rashid, M.; Ashraf, M.; Iqbal, M.; Lixin, Z. Exogenously Applied Proline Induced Changes in Key Anatomical Features and Physio-Biochemical Attributes in Water Stressed Oat (Avena sativa L.) Plants. Physiol. Mol. Biol. Plants 2019, 25, 1121–1135. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of Photosynthesis in C3 Plants: Stomatal and Non-stomatal Limitations Revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and Drought: Can We Make Metabolic Connections from Available Data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Kavi Kishor, P.B.; Hima Kumari, P.; Sunita, M.S.L.; Sreenivasulu, N. Role of Proline in Cell Wall Synthesis and Plant Development and Its Implications in Plant Ontogeny. Front. Plant Sci. 2015, 6, 544. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, J.; Hui, W.; Zhao, F.; Wang, P.; Su, C.; Gong, W. Physiology of Plant Responses to Water Stress and Related Genes: A Review. Forests 2022, 13, 324. [Google Scholar] [CrossRef]
- Ibrahim, A.E.-A.; Abd El Mageed, T.; Abohamid, Y.; Abdallah, H.; El-Saadony, M.; AbuQamar, S.; El-Tarabily, K.; Abdou, N. Exogenously Applied Proline Enhances Morph-Physiological Responses and Yield of Drought-Stressed Maize Plants Grown Under Different Irrigation Systems. Front. Plant Sci. 2022, 13, 897027. [Google Scholar] [CrossRef] [PubMed]
- Lukić, N.; Kukavica, B.; Davidović-Plavšić, B.; Hasanagić, D.; Walter, J. Plant Stress Memory Is Linked to High Levels of Anti-Oxidative Enzymes over Several Weeks. Environ. Exp. Bot. 2020, 178, 104166. [Google Scholar] [CrossRef]
- Jacques, C.; Salon, C.; Barnard, R.L.; Vernoud, V.; Prudent, M. Drought Stress Memory at the Plant Cycle Level: A Review. Plants 2021, 10, 1873. [Google Scholar] [CrossRef]
- Staacke, T.; Mueller-Roeber, B.; Balazadeh, S. Stress Resilience in Plants: The Complex Interplay between Heat Stress Memory and Resetting. New Phytol. 2025, 245, 2402–2421. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, N.; Virlouvet, L.; Riethoven, J.-J.; Fromm, M.; Avramova, Z. Four Distinct Types of Dehydration Stress Memory Genes in Arabidopsis thaliana. BMC Plant Biol. 2013, 13, 229. [Google Scholar] [CrossRef]

| Treatments | Time (Days) | |||||
|---|---|---|---|---|---|---|
| 7 d | 14 d | 21 d | 28 d | 35 d | Mean | |
| C | 0.28 ± 0.03 a | 0.42 ± 0.07 a | 0.46 ± 0.06 a | 0.37 ± 0.05 a | 0.25 ± 0.02 a | 0.36 ± 0.02 a |
| SPro * | 0.38 ± 0.03 a | 0.26 ± 0.01 a | 0.49 ± 0.05 a | 0.49 ± 0.05 a | 0.43 ± 0.03 a | 0.41 ± 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecka, J.; Kraus, K.; Zelený, M.; Hniličková, H. Exogenous Proline Modulates Physiological Responses and Induces Stress Memory in Wheat Under Repeated and Delayed Drought Stress. Agronomy 2025, 15, 1370. https://doi.org/10.3390/agronomy15061370
Pecka J, Kraus K, Zelený M, Hniličková H. Exogenous Proline Modulates Physiological Responses and Induces Stress Memory in Wheat Under Repeated and Delayed Drought Stress. Agronomy. 2025; 15(6):1370. https://doi.org/10.3390/agronomy15061370
Chicago/Turabian StylePecka, Jan, Kamil Kraus, Martin Zelený, and Helena Hniličková. 2025. "Exogenous Proline Modulates Physiological Responses and Induces Stress Memory in Wheat Under Repeated and Delayed Drought Stress" Agronomy 15, no. 6: 1370. https://doi.org/10.3390/agronomy15061370
APA StylePecka, J., Kraus, K., Zelený, M., & Hniličková, H. (2025). Exogenous Proline Modulates Physiological Responses and Induces Stress Memory in Wheat Under Repeated and Delayed Drought Stress. Agronomy, 15(6), 1370. https://doi.org/10.3390/agronomy15061370








