Characteristics of Soil Nematode Communities in Pure Populus hopeiensis Forests in the Loess Hilly Region and Their Responses to Precipitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Description and Site Selection
2.2. Soil Sample Collection and Analysis
2.3. Soil Nematode Analysis
2.4. Data Analysis
3. Results
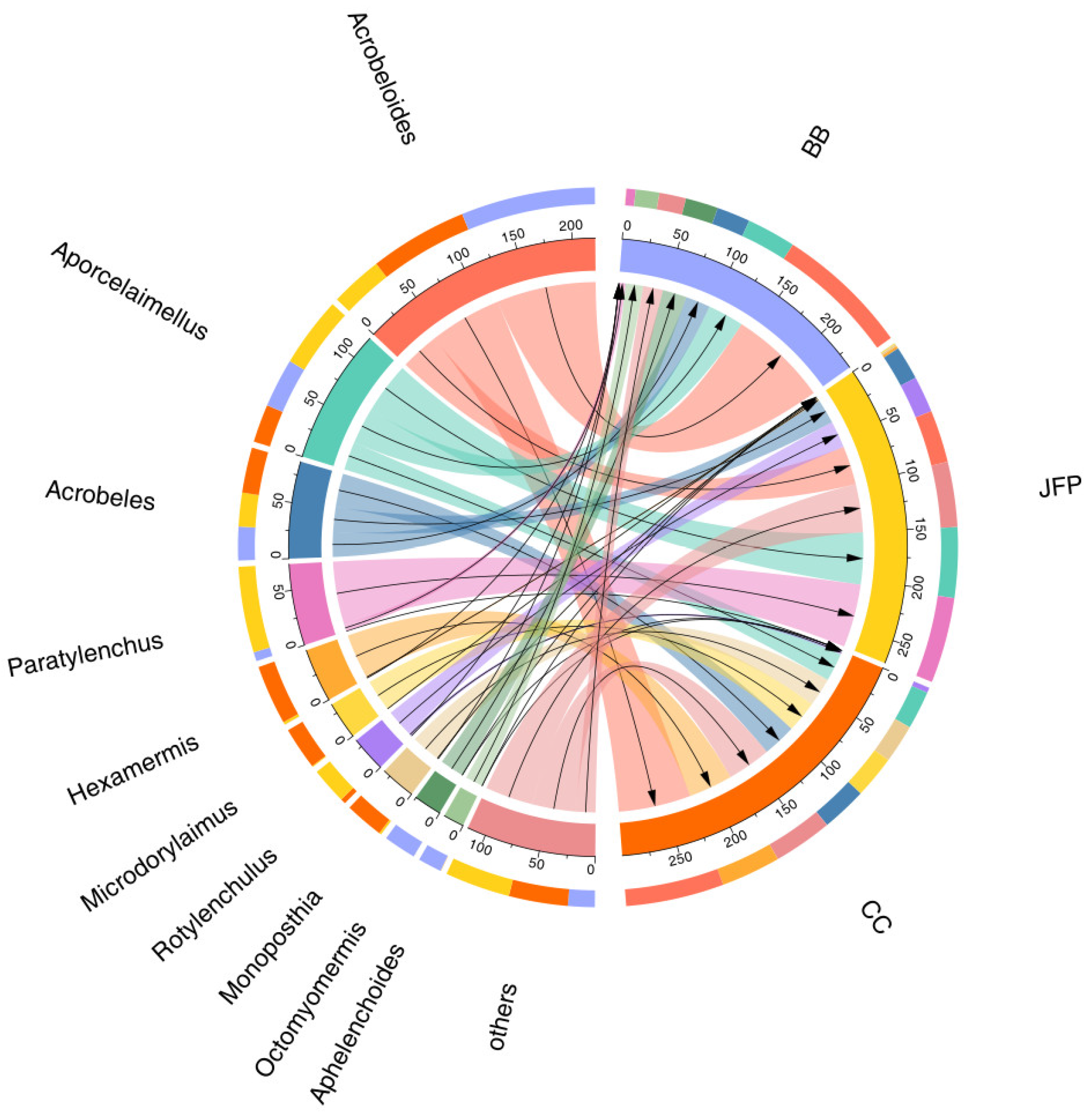
3.1. Soil Nematode Community Composition
3.2. Soil Nematode Alpha Diversity Indices
3.3. Soil Nematode Beta Diversity Indices
3.4. Soil Nematode Ecological Indices
4. Discussion
4.1. Soil Nematode Community Characteristics
4.2. Soil Nematode Diversity Indices
4.3. Soil Nematode Ecological Function Indices
4.4. Environmental Drivers of Soil Nematode Communities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Yan, F.C.; Jiao, J.Y.; Tang, B.Z.; Zhang, Y.F. Soil water availability and holding capacity of different vegetation types in hilly-gullied region of the loess plateau. Acta Ecol. Sin. 2018, 38. [Google Scholar]
- He, J.; Jiang, X.; Lei, Y.; Cai, W.; Zhang, J. Temporal and spatial variation and driving forces of soil erosion on the Loess Plateau before and after the implementation of the Grain-for-Green project: A case study in the Yanhe river basin, China. Int. J. Environ. Res. Public Health 2022, 19, 8446. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Qiang, F.; Liu, G.; Liu, C.; Ai, N. Response of soil quality to ecosystems after revegetation in a coal mine reclama-tion area. CATENA 2025, 257, 109038. [Google Scholar] [CrossRef]
- Han, S.; Wang, Q.; Zhao, Y.; Zhai, J.; Wang, X.; Hao, Y.; Li, L.; Li, X.; Li, H.; Cao, J. Response of Typical Tree Species Sap Flow to Environmental Factors in the Hilly Areas of Haihe River Basin, China. Forests 2024, 15, 294. [Google Scholar] [CrossRef]
- Zhou, H.G.; Liu, G.Q.; Jiao, X.; Wang, H. Water consumption by transpiration of several trees species in the Loess Plateau with mixed water and wind erosion. Acta Ecol. Sin. 2008, 28, 4568–4574. [Google Scholar]
- Wan, B.; Liu, T.; Gong, X.; Zhang, Y.; Li, C.; Chen, X.; Hu, F.; Griffiths, B.S.; Liu, M. Energy flux across multitrophic levels drives ecosystem multifunctionality: Evidence from nematode food webs. Soil Biol. Biochem. 2022, 169, 108656. [Google Scholar] [CrossRef]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Ferris, H. Form and function: Metabolic footprints of nematodes in the soil food web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, Y.; Lu, H.; Classen, A.T.; Wang, Z.; Li, Y.; Liu, Y.; Yang, Z.; Li, G.; Fu, S. Drought shifts soil nematode trophic groups and mediates the heterotrophic respiration. J. Plant Ecol. 2024, 17, rtae012. [Google Scholar] [CrossRef]
- Huang, J.; Chen, J.; Huang, T.; Li, G.; Wang, Z.; Zhao, S. Soil nematode biodiversity mediates the impact of altered precipitation on dryland agroecosystem multifunctionality in the loess tableland area of China. Agric. Ecosyst. Environ. 2024, 376, 109221. [Google Scholar] [CrossRef]
- Vries, D.T.F.; Griffiths, I.R.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Bardgett, R.D.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef] [PubMed]
- Siebert, J.; Sünnemann, M.; Auge, H.; Berger, S.; Cesarz, S.; Ciobanu, M.; Guerrero-Ramírez, N.R.; Eisenhauer, N. The effects of drought and nutrient addition on soil organisms vary across taxonomic groups, but are constant across seasons. Sci. Rep. 2019, 9, 639. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, C.; Luo, Y. Response of soil microbial communities to altered precipitation: A global synthesis. Glob. Ecol. Biogeogr. 2018, 27, 1121–1136. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Ma, H.; Zhang, Y.; Zhang, J.; Zhang, H.; Luo, X.; Li, J. Responses of Soil Microbial Communities and Networks to Precipitation Change in a Typical Steppe Ecosystem of the Loess Plateau. Microorganisms 2022, 10, 817. [Google Scholar] [CrossRef]
- Lu, S.; Hu, Z.; Fu, C.; Fan, W.; Wu, D. Characteristics and possible causes for extreme precipitation in summer over the Loess Plateau. Plateau Meteorol 2022, 41, 241–254. [Google Scholar]
- Puyang, X.; Wang, C.; Gou, Q.; Zhao, Z.; Huang, J. Relationship between vegetation community and soil moisture in the loess region of northern Shaanxi Province. Acta Prataculturae Sin. 2019, 28, 184. [Google Scholar]
- Soil Physics Laboratory; Nanjing Institute of Soil Science; Chinese Academy of Sciences. Methods for Determination of Soil Physical Properties; Science Press: Nanjing, China, 1978. [Google Scholar]
- Bao, S. Soil Agrochemical Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Guan, S. Soil Enzymes and Their Research Methods; China Agriculture Press: Beijing, China, 1986. [Google Scholar]
- Wang, D.; Chen, J.; Tang, Z.; Zhang, Y. Effects of Soil Physical Properties on Soil Infiltration in Forest Ecosystems of Southeast China. Forests 2024, 15, 1470. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Chen, M.; Wang, X.; Ye, C.; Li, X.; Chen, W.; Yang, Y.; Wang, B.; Wang, J.; et al. Influence of Coupling Effects between Gravel Soil Porosity and Cement Grout Weight on Diffusion Laws and Morphologies of Penetration Grouting. Appl. Sci. 2022, 12, 7601. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Xiang, X.; Sun, R.; Yang, T.; He, D.; Zhang, K.; Ni, Y.; Adams, J.M.; Chu, H.; et al. Spatial scale affects the relative role of stochasticity versus determinism in soil bacterial communities in wheat fields across the North China Plain. Microbiome 2018, 6, 27. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.; Sun, W.; Zhang, Q.; Diao, M.; Sun, J. Extreme soil salinity reduces N and P metabolism and related microbial network complexity and community immigration rate. Environ. Res. 2025, 264, 120361. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Pan, Y.; Li, L.; Wu, X.; Wang, Y. Composition and diversity of rhizosphere fungal community in Coptis chinensis Franch. continuous cropping fields. PLoS ONE 2018, 13, e0193811. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kreller, C.R.; Greenberg, M.M. Preparation and analysis of Oligonucleotides containing the C4‘-oxidized abasic site and related mechanistic probes. J. Org. Chem. 2005, 70, 8122–8129. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xie, R.; Ma, D.; Zhang, M.; Liu, L. Variations in soil microbial community structure and extracellular enzymatic activities along a forest–wetland ecotone in high-latitude permafrost regions. Ecol. Evol. 2023, 13, e10205. [Google Scholar] [CrossRef]
- De Forest, J.L. The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and L-DOPA. Soil Biol. Biochem. 2009, 41, 1180–1186. [Google Scholar] [CrossRef]
- Bell, C.W.; Fricks, B.E.; Rocca, J.D.; Steinweg, J.M.; McMahon, S.K.; Wallenstein, M.D. High-throughput fluorometric measurement of potential soil extracellular enzyme activities. J. Vis. Exp. JoVE 2013, 81, 50961. [Google Scholar]
- Liu, M.; Chen, X.; Qin, J.; Wang, D.; Griffiths, B.; Hu, F. A sequential extraction procedure reveals that water management affects soil nematode communities in paddy fields. Appl. Soil Ecol. 2008, 40, 250–259. [Google Scholar] [CrossRef]
- Sanchez, G.; Trinchera, L.; Russolillo, G. plspm: Tools for partial least squares path modeling (PLS-PM), R Package Version 0.4; R Core Team: Vienna Austria, 2015; Volume 7, p. 2015.
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Caporaso, J.G.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Čaplová, Z.; Švábová, P. Ibm Spss Statistics//Statistics and Probability in Forensic Anthropology; Academic Press: Cambridge, MA, USA, 2020; pp. 343–352. [Google Scholar]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- R Core Team, R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Van Den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Crowther, T.W.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef]
- van den Hoogen, J.; Geisen, S.; Wall, D.H.; Wardle, D.A.; Traunspurger, W.; de Goede, R.G.; Adams, B.J.; Ahmad, W.; Ferris, H.; Crowther, T.W.; et al. A global database of soil nematode abundance and functional group composition. Sci. Data 2020, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Sang, Y.; Chen, Q.; Zhu, X.; Lin, S.; Gao, Y.B. The response of soil nematode community to nitrogen, water, and grazing history in the inner Mongolian steppe, China. Ecosystems 2012, 15, 1121–1133. [Google Scholar] [CrossRef]
- Neilson, R.; Caul, S.; Fraser, F.C.; King, D.; Mitchell, S.M.; Roberts, D.M.; Giles, M.E. Microbial community size is a potential predictor of nematode functional group in limed grasslands. Appl. Soil Ecol. 2020, 156, 103702. [Google Scholar] [CrossRef]
- Guan, P.; Li, J.; Hao, C.; Yang, J.; Song, L.; Niu, X.; Wang, P.; Mahamood, M.; Wu, D. Precipitation regulated soil nematode community and footprint in cropland ecosystems. Soil Ecol. Lett. 2023, 5, 230177. [Google Scholar] [CrossRef]
- Franco, A.L.C.; Guan, P.; Cui, S.; de Tomasel, C.M. Precipitation effects on nematode diversity and carbon footprint across grasslands. Glob. Change Biol. 2022, 28, 2124–2132. [Google Scholar] [CrossRef]
- Bristol, D.; Hassan, K.; Blankinship, J.C.; Nielsen, U.N. Responses of nematode abundances to increased and reduced rainfall under field conditions: A meta-analysis. Ecosphere 2023, 14, e4364. [Google Scholar] [CrossRef]
- Wu, K.; Xu, W.; Yang, W. Effects of precipitation changes on soil bacterial community composition and diversity in the Junggar desert of Xinjiang, China. PeerJ 2020, 8, e8433. [Google Scholar] [CrossRef]
- Li, X.; Hou, Z.; Xu, C.; Shi, X.; Yang, L.; Lewis, L.A.; Zhong, B. Large phylogenomic data sets reveal deep relationships and trait evolution in chlorophyte green algae. Genome Biol. Evol. 2021, 13, evab101. [Google Scholar] [CrossRef]
- Nisa, R.U.; Tantray, A.Y.; Kouser, N.; Allie, K.A.; Wani, S.M. Influence of ecological and edaphic factors on biodiversity of soil nematodes. Saudi J. Biol. Sci. 2021, 28, 3049–3059. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Yan, W.; Sun, N.; Zhao, H. The effect of irrigation quota on straw decomposition, nitrogen release, and maize nitrogen uptake. Sci. Rep. 2025, 15, 6150. [Google Scholar] [CrossRef]
- Zhao, C.; Miao, Y.; Yu, C.; Zhu, L.; Wang, F. Soil microbial community composition and respiration along an experimental precipitation gradient in a semiarid steppe. Sci. Rep. 2016, 6, 24317. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.L.C.; Gherardi, L.A.; de Tomasel, C.M.; Andriuzzi, W.S.; Ankrom, K.E.; Shaw, E.A.; Bach, E.M.; Sala, O.E.; Wall, D.H. Drought suppresses soil predators and promotes root herbivores in mesic, but not in xeric grasslands. Proc. Natl. Acad. Sci. USA 2019, 116, 12883–12888. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Wu, S.; Baur, L.; Patton, M.T. Soil nematode assemblages respond to interacting environmental changes. Oecologia 2023, 202, 481–495. [Google Scholar] [CrossRef]
- Cui, S.; Han, X.; Xiao, Y.; Wu, P.; Zhang, S. Increase in rainfall intensity promotes soil nematode diversity but offset by nitrogen addition in a temperate grassland. Sci. Total Environ. 2022, 825, 154039. [Google Scholar] [CrossRef]
- Xiong, D.; Wei, C.; Wang, X.; Lü, X. Spatial patterns and ecological drivers of soil nematode β-diversity in natural grasslands vary among vegetation types and trophic position. J. Anim. Ecol. 2021, 90, 1367–1378. [Google Scholar] [CrossRef]
- Mezeli, M.M.; Page, S.; George, T.S.; Neilson, R.; Mead, A.; Blackwell, M.S.; Haygarth, P.M. Using a meta-analysis approach to understand complexity in soil biodiversity and phosphorus acquisition in plants. Soil Biol. Biochem. 2020, 142, 107695. [Google Scholar] [CrossRef]
- Zhou, H.; Li, L.; Liu, Y. Biological soil crust development affects bacterial communities in the community in alpine sandy areas. Front. Microbiol. 2023, 14, 1106739. [Google Scholar] [CrossRef]
- Irshad, U.; Yergeau, E. Bacterial subspecies variation and nematode grazing change P dynamics in the wheat rhizosphere. Front. Microbiol. 2018, 9, 1990. [Google Scholar] [CrossRef]
- Chen, J.; Gao, Y.; Zeng, Y.; Huang, M.; Yang, X.; Ochoa-Hueso, R.; Sun, W.; Yang, T. Reduced Precipitation Frequency Decreases the Stability of the Soil Organic Carbon Pool by Altering Microbial Communities in Degraded Grasslands. Agronomy 2025, 15, 977. [Google Scholar] [CrossRef]


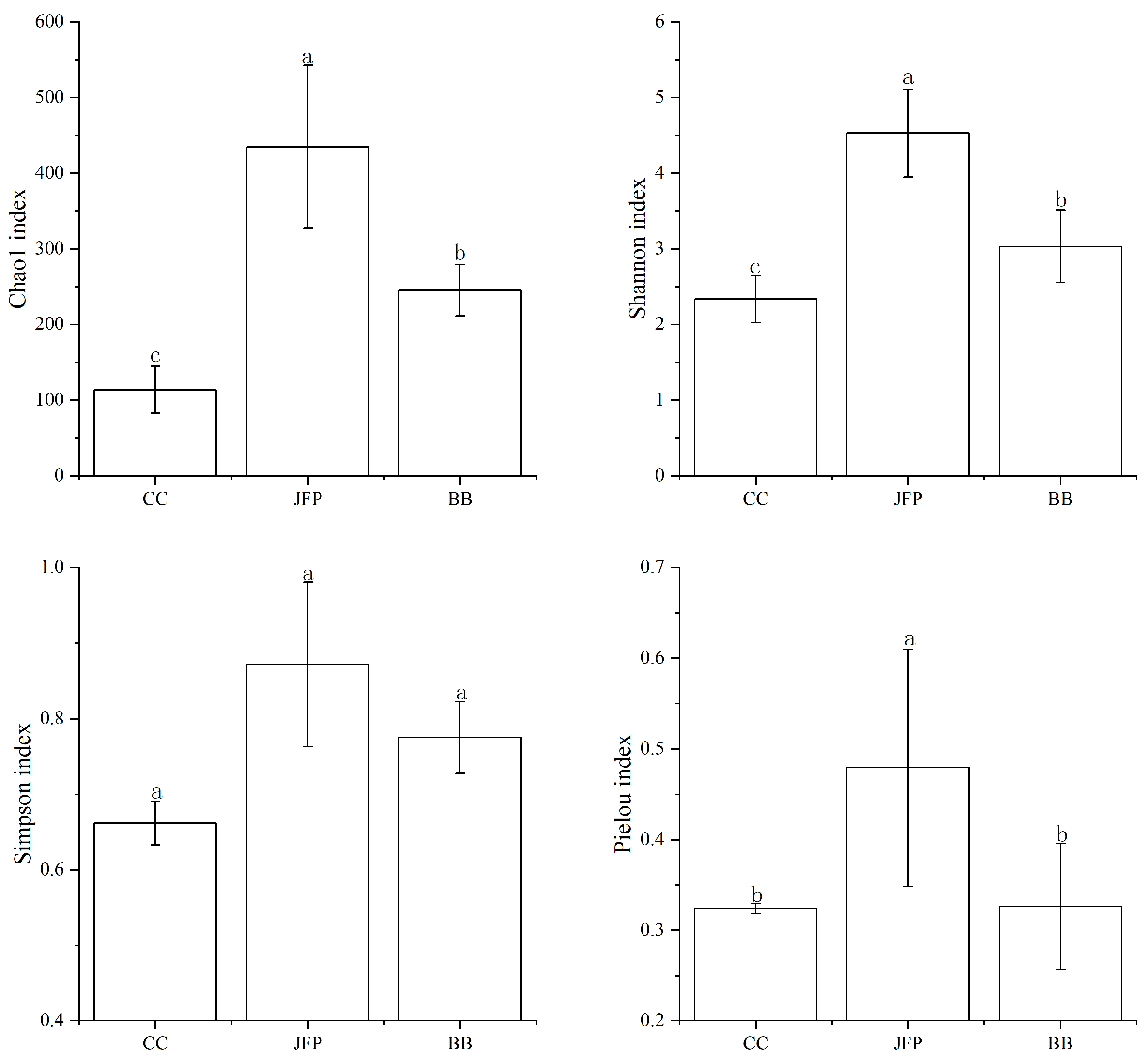




| Sampling Site | Annual Precipitation (mm) | Slope Aspect | Stand Age (a) | Altitude (m) | Slope Gradient (°) | Canopy Density (%) |
|---|---|---|---|---|---|---|
| Changcheng | 400~410 | Shady slope | 30 | 1505.7 | 23 | 30 |
| Jinfoping | 440~445 | Shady slope | 30 | 1331.6 | 20 | 75 |
| Baibao | 460~470 | Shady slope | 30 | 1484.1 | 25 | 85 |
| Soil Physicochemical Indicator | Changcheng (400–410 mm) | Jinfoping (440–445 mm) | Baibao (460–470 mm) |
|---|---|---|---|
| NH4+-N (mg kg−1) | 0.46 ± 0.14 a | 0.58 ± 0.1 a | 0.55 ± 0.12 a |
| NO3−-N (mg kg−1) | 0.43 ± 0.19 b | 0.63 ± 0.05 a | 1.84 ± 1.31 a |
| AN (mg kg−1) | 38.55 ± 10 a | 72.3 ± 38.86 a | 68.85 ± 26.44 b |
| SOM (g kg−1) | 13.41 ± 4.87 a | 11.98 ± 4.58 a | 11.46 ± 2.33 a |
| pH | 8.27 ± 0.02 a | 8.06 ± 0.03 c | 8.16 ± 0.07 b |
| EC (μS cm−1) | 68 ± 6.2 a | 73 ± 0.38 a | 100.13 ± 12.61 b |
| AP (mg kg−1) | 12.34 ± 9.54 a | 17.99 ± 12.39 a | 14.11 ± 12.06 a |
| AK (mg kg−1) | 97.94 ± 11.53 a | 170.61 ± 65.83 b | 127.06 ± 51.92 ab |
| TN (g kg−1) | 0.63 ± 0.25 b | 0.97 ± 0.6 b | 2.31 ± 0.45 a |
| TP (g kg−1) | 0.7 ± 0.14 a | 0.84 ± 0.44 a | 0.76 ± 0.19 a |
| CAT (ml g−1) | 2.76 ± 1.12 a | 2.8 ± 0.87 a | 2.91 ± 0.43 a |
| ALP (mg g−1 d−1) | 0.33 ± 0.1 b | 0.43 ± 0.07 a | 0.49 ± 0.07 a |
| URE (mg g−1 d−1) | 0.3 ± 0.14 a | 0.21 ± 0.04 b | 0.41 ± 0.1 a |
| SWC (%) | 8.13 ± 0.58 a | 15.74 ± 4.26 b | 19.54 ± 1.48 b |
| BD (g·cm−3) | 1.4 ± 0.04 a | 1.29 ± 0.06 b | 1.19 ± 0.09 c |
| MaxWHC (%) | 32.26 ± 0.76 c | 37.78 ± 3.24 b | 44.73 ± 6.12 a |
| CWHC (%) | 28.59 ± 0.46 b | 34.15 ± 2.08 a | 37.01 ± 2.73 a |
| NCP (%) | 5.12 ± 0.68 b | 4.62 ± 1.69 b | 8.95 ± 3.67 a |
| CP (%) | 39.9 ± 1.15 b | 44.06 ± 1.28 a | 43.31 ± 1.6 a |
| TPR (%) | 45.02 ± 1.38 c | 48.68 ± 1.96 b | 52.27 ± 3.55 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Shi, J.; Qiang, F.; Liu, C.; Ai, N. Characteristics of Soil Nematode Communities in Pure Populus hopeiensis Forests in the Loess Hilly Region and Their Responses to Precipitation. Agronomy 2025, 15, 1341. https://doi.org/10.3390/agronomy15061341
Hu Y, Shi J, Qiang F, Liu C, Ai N. Characteristics of Soil Nematode Communities in Pure Populus hopeiensis Forests in the Loess Hilly Region and Their Responses to Precipitation. Agronomy. 2025; 15(6):1341. https://doi.org/10.3390/agronomy15061341
Chicago/Turabian StyleHu, Yani, Jiahao Shi, Fangfang Qiang, Changhai Liu, and Ning Ai. 2025. "Characteristics of Soil Nematode Communities in Pure Populus hopeiensis Forests in the Loess Hilly Region and Their Responses to Precipitation" Agronomy 15, no. 6: 1341. https://doi.org/10.3390/agronomy15061341
APA StyleHu, Y., Shi, J., Qiang, F., Liu, C., & Ai, N. (2025). Characteristics of Soil Nematode Communities in Pure Populus hopeiensis Forests in the Loess Hilly Region and Their Responses to Precipitation. Agronomy, 15(6), 1341. https://doi.org/10.3390/agronomy15061341







