Phenotyping Common Bean Under Drought Stress: High-Throughput Approaches for Enhanced Drought Tolerance
Abstract
1. Introduction
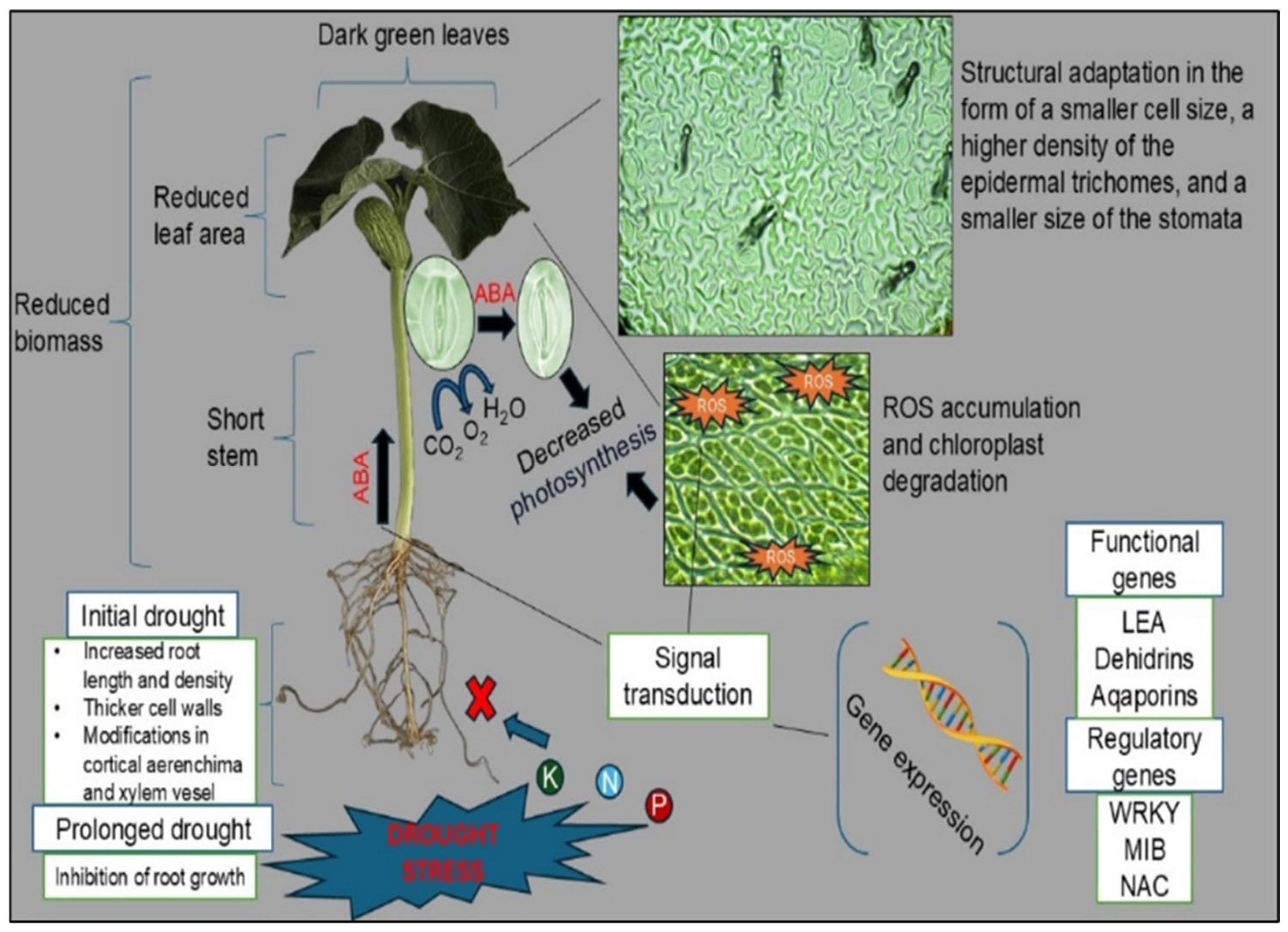
2. Key Developmental, Morphological, Physiological, and Molecular Responses to Drought Stress in Common Bean
2.1. Root Morphology
2.2. Stem Morphology
2.3. Leaf Morphology
2.4. Generative Organs
2.5. Stomatal Conductance and Transpiration
2.6. Photosynthetic Pigments
2.7. Light Reaction and Calvin Cycle
2.8. Osmotic Adjustment
2.9. Antioxidant Activity
2.10. Phytohormones
2.11. Molecular Response
2.12. Bean Phenology
3. The Role of High-Throughput Phenotyping Techniques in Common Bean Breeding for Drought Tolerance
3.1. RGB Imaging
3.2. Multispectral and Hyperspectral Imaging
| Index | Abbreviation | Equation | Utilization | Reference |
|---|---|---|---|---|
| Anthocyanin Index | ARI | ARI = (RGreen)−1 − (RFarRed)−1 | Assessment of anthocyanin content | [174] |
| Chlorophyll Index | CHI | CHI = (RChl)−1 − (RNIR)−1 | Assessment of chlorophyll content | [151] |
| Drought Severity Index | DSI | Calculation is based on the model which uses relatively fine-scale (1 km resolution) NDVI inputs from Moderate Resolution Imaging Spectroradiometer (MODIS) | Agricultural drought monitoring and early warning | [175] |
| Green Normalized Difference Vegetation Index | GNDVI | GNDVI = (RNIR − RGreen)/(RNIR + RGreen) | Vegetation health assessment and yield prediction | [176] |
| Modified Chlorophyll Absorption in Reflectance Index | MCARI1 | MCAR1 = 1.2 × (2.5 × (RNIR − RRed) − 1.3 × (RNIR − RGreen)) | Assessment of vegetation health, biomass, and chlorophyll content | [177] |
| Moisture Stress Index | MSI | MSI = 1600 nm/820 nm | Determines leaf and canopy water content | [178] |
| Normalized Difference Drought Index | NDDI | NDDI = NDVI − NDWI/NDVI + NDWI | More sensitive indicator of drought than the NDVI | [179] |
| Normalized Difference Water Index | NDWI | NDWI = (860 nm − 1240 nm)/(860 nm + 1240 nm) | Monitoring changes in the water content of plants | [180] |
| Optimized Soil-Adjusted Vegetation Index | OSAVI | OSAVI = RNIR − RRed/RNIR + RRed + 0.16 | Assessment of vegetation health | [181] |
| Normalized Difference Vegetation Index | NDVI | NDVI = (RNIR − RRed)/(RNIR + RRed) | Assessment of vegetation, phenophase, and biomass | [163] |
| Water Band Index | WBI | WBI = 950 nm/900 nm | Assessment of water stress and water use efficiency | [165] |
3.3. Chlorophyll Fluorescence Imaging
| Abbreviation | Trait | Equation |
|---|---|---|
| Fv/Fm | Maximum Efficiency of Photosystem II | Fv/Fm = (Fm − F0)/Fm [186] |
| Fq’/Fm’ | Effective Quantum Yield of Photosystem II | Fq’/Fm’ = (Fm’ − Fs’)/Fm’ [187] |
| rETR | Relative Electron Transport Rate | ETR = Fq’/Fm’ × PPFD × (0.5) [187] |
| NPQ | Non-Photochemical Quenching | NPQ = (Fm − Fm’)/Fm’ [188] |
| qP | Coefficient of Photochemical Quenching | qP = (Fm’ − Fs)/Fv [189] |
| qN | Coefficient of Non-Photochemical Quenching | qN = 1 − (Fm’ − Fo’)/(Fm − Fo) [189] |
3.4. Three-Dimensional (3D) Imaging
3.5. Thermal Imaging
3.6. Root Phenotyping
4. Conclusion and Perspectives: Towards an Effective Phenotyping Strategy for Drought Tolerance in Common Bean
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| APX | Ascorbate peroxidase |
| ARI | Anthocyanin Index |
| ATP | Adenosine triphosphate |
| CAT | Catalase |
| CF | Chlorophyll fluorescence |
| CHI | Chlorophyll Index |
| CT | X-ray computed tomography |
| CYPs | Cytochrome P450 |
| DHAR | Dehydroascorbate reductase |
| DSI | Drought Severity Index |
| ETC | Electron transport chain |
| F0 | Minimum chlorophyll fluorescence of dark-adapted plants |
| F0′ | Minimum fluorescence yield of illuminated plant |
| Fm | Maximum chlorophyll fluorescence of dark-adapted plants |
| Fm’ | Maximum chlorophyll fluorescence of light-adapted plants |
| Fq’/Fm’ | Effective Quantum Yield of Photosystem II |
| FS’ | Steady-state fluorescence yield |
| Fv/Fm | Maximum Efficiency of Photosystem II |
| GNDVI | Green Normalized Difference Vegetation Index |
| GPX | Glutathione peroxidase |
| GR | Glutathione reductase |
| GSTs | Glutathione S-transferase |
| GWAS | Genome-wide association studies |
| HNBs | Hyperspectral narrowbands |
| HTP | Hight-throughput phenotyping |
| HVIs | Hyperspectral vegetation indices |
| IRGA | Infrared gas analyzer |
| JA | Jasmonic acid |
| LAI | Leaf area index |
| LEADER | Leaf Element Accumulation from Deep Roots |
| LHC | Light-harvesting complex |
| LiDAR | Light Detection and Ranging |
| LWIR | Long-wave infrared |
| MAPK | Mitogen-activated protein kinase |
| MCARI1 | Modified Chlorophyll Absorption in Reflectance Index |
| MDHAR | Monodehydroascorbate reductase |
| MRI | Magnetic resonance imaging |
| MSI | Moisture Stress Index |
| MVS | Multi-view stereo |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NCED | 9-cis-epoxycarotenoid dioxygenase |
| NDDI | Normalized Difference Drought Index |
| NDVI | Normalized Difference Vegetation Index |
| NDWI | Normalized Difference Water Index |
| NO | Nitric oxide |
| NPQ | Non-photochemical quenching |
| ɸno | Quantum yield of non-regulated non-photochemical energy loss in PSII |
| OEC | Oxygen-evolving complex |
| OEE | Oxygen-evolving enhancer |
| PAR | Photosynthetically active radiation |
| POD | Peroxidase |
| PYR | Pyrabactin resistance |
| qP | Coefficient of photochemical quenching |
| qN | Coefficient of non-photochemical quenching |
| rETR | Relative electron transport rate |
| RGB | Red–green–blue |
| ROS | Reactive oxygen species |
| RuBisCO | Ribulose-1,5-diphosphate carboxylase/oxygenase |
| RuBP | Ribulose bisphosphate |
| SfM | Structure-from-motion |
| SNP | Single-nucleotide polymorphism |
| SOD | Superoxide dismutase |
| TRX | Thioredoxin |
| UAV | Unmanned aerial vehicle |
References
- Gholinia, A.; Abbaszadeh, P. Agricultural Drought Monitoring: A Comparative Review of Conventional and Satellite-Based Indices. Atmosphere 2024, 15, 1129. [Google Scholar] [CrossRef]
- Vidak, M.; Malešević, S.; Grdiša, M.; Šatović, Z.; Lazarević, B.; Carović-Stanko, K. Phenotypic Diversity among Croatian Common Bean (Phaseolus vulgaris L.). Landraces. Agric. Conspec. Sci. 2015, 80, 133–137. [Google Scholar]
- Carović-Stanko, K.; Liber, Z.; Vidak, M.; Barešić, A.; Grdiša, M.; Lazarević, B.; Šatović, Z. Genetic Diversity of Croatian Common Bean Landraces. Front. Plant Sci. 2017, 8, 604. [Google Scholar] [CrossRef] [PubMed]
- Scatolini, P.; Vaquero-Piñeiro, C.; Cavazza, F.; Zucaro, R. Do Irrigation Water Requirements Affect Crops’ Economic Values? Water 2024, 16, 77. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Blair, M.W.; Acosta-Gallegos, J.A. Phenotyping Common Beans for Adaptation to Drought. Front. Physiol. 2013, 4, 35. [Google Scholar] [CrossRef]
- Geleta, R.J.; Roro, A.G.; Terfa, M.T. Phenotypic and Yield Responses of Common Bean (Phaseolus vulgaris L.) Varieties to Different Soil Moisture Levels. BMC Plant Biol. 2024, 24, 242. [Google Scholar] [CrossRef]
- Uebersax, M.A.; Cichy, K.A.; Gomez, F.E.; Porch, T.G.; Heitholt, J.; Osorno, J.M.; Kamfwa, K.; Snapp, S.S.; Bales, S. Dry Beans (Phaseolus vulgaris L.) as a Vital Component of Sustainable Agriculture and Food Security—A Review. Legum. Sci. 2023, 5, e155. [Google Scholar] [CrossRef]
- Yavas, I.; Jamal, M.A.; Ul Din, K.; Ali, S.; Hussain, S.; Farooq, M. Drought-Induced Changes in Leaf Morphology and Anatomy: Overview, Implications and Perspectives. Pol. J. Environ. Stud. 2023, 33, 1517–1530. [Google Scholar] [CrossRef]
- Walter, A.; Liebisch, F.; Hund, A. Plant Phenotyping: From Bean Weighing to Image Analysis. Plant Methods 2015, 11, 14. [Google Scholar] [CrossRef]
- Pabuayon, I.L.B.; Sun, Y.; Guo, W.; Ritchie, G.L. High-Throughput Phenotyping in Cotton: A Review. J. Cott. Res. 2019, 2, 18. [Google Scholar] [CrossRef]
- Shakoor, N.; Lee, S.; Mockler, T.C. High Throughput Phenotyping to Accelerate Crop Breeding and Monitoring of Diseases in the Field. Curr. Opin. Plant Biol. 2017, 38, 184–192. [Google Scholar] [CrossRef]
- Lipovac, A.; Bezdan, A.; Moravčević, D.; Djurović, N.; Ćosić, M.; Benka, P.; Stričević, R. Correlation between Ground Measurements and UAV Sensed Vegetation Indices for Yield Prediction of Common Bean Grown under Different Irrigation Treatments and Sowing Periods. Water 2022, 14, 3786. [Google Scholar] [CrossRef]
- Mulugeta Aneley, G.; Haas, M.; Köhl, K. LIDAR-Based Phenotyping for Drought Response and Drought Tolerance in Potato. Potato Res. 2022, 66, 1225–1256. [Google Scholar] [CrossRef]
- Verheyen, J.; Dhondt, S.; Abbeloos, R.; Eeckhout, J.; Janssens, S.; Leyns, F.; Scheldeman, X.; Storme, V.; Vandelook, F. High-Throughput Phenotyping Reveals Multiple Drought Responses of Wild and Cultivated Phaseolinae Beans. bioRxiv 2024, 15, 1385985. [Google Scholar] [CrossRef] [PubMed]
- De Souza, T.K.G. Obtaining Water Stress Index for Bean Crop Using Thermal Images. Master’s Thesis, University of Brasilia, Brasilia, Brazil, 2023. [Google Scholar]
- Omari, M.K.; Lee, J.; Faqeerzada, M.A.; Joshi, R.; Park, E.; Cho, B.-K. Digital Image-Based Plant Phenotyping: A Review. Korean J. Agric. Sci. 2020, 47, 119–130. [Google Scholar] [CrossRef]
- Gehan, M.A.; Kellogg, E.A. High-Throughput Phenotyping. Am. J. Bot. 2017, 104, 505–508. [Google Scholar] [CrossRef]
- Javornik, T.; Carović-Stanko, K.; Gunjača, J.; Vidak, M.; Lazarević, B. Monitoring Drought Stress in Common Bean Using Chlorophyll Fluorescence and Multispectral Imaging. Plants 2023, 12, 1386. [Google Scholar] [CrossRef]
- Jangra, S.; Chaudhary, V.; Yadav, R.C.; Yadav, N.R. High-Throughput Phenotyping: A Platform to Accelerate Crop Improvement. Phenomics 2021, 1, 31–53. [Google Scholar] [CrossRef]
- Polania, J.; Rao, I.M.; Cajiao, C.; Rivera, M.; Raatz, B.; Beebe, S. Physiological Traits Associated with Drought Resistance in Andean and Mesoamerican Genotypes of Common Bean (Phaseolus vulgaris L.). Euphytica 2016, 210, 17–29. [Google Scholar] [CrossRef]
- Vidak, M.; Lazarević, B.; Gunjača, J.; Carović-Stanko, K. New Age of Common Bean. In Production and Utilization of Legumes—Progress and Prospects; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Federici, C.T.; Ehdaie, B.; Waines, J.G. Domesticated and Wild Tepary Bean: Field Performance with and without Drought-Stress. Agron. J. 1990, 82, 896–900. [Google Scholar] [CrossRef]
- Rodriguez, D.F.C.; Urban, M.O.; Santaella, M.; Gereda, J.M.; Contreras, A.D.; Wenzl, P. Using Phenomics to Identify and Integrate Traits of Interest for Better-Performing Common Beans: A Validation Study on an Interspecific Hybrid and Its Acutifolii Parents. Front. Plant Sci. 2022, 13, 1008666. [Google Scholar] [CrossRef] [PubMed]
- Polania, J.A.; Salazar-Chavarría, V.; Gonzalez-Lemes, I.; Acosta-Maspons, A.; Chater, C.C.C.; Covarrubias, A.A. Contrasting Phaseolus Crop Water Use Patterns and Stomatal Dynamics in Response to Terminal Drought. Front. Plant Sci. 2022, 13, 894657. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.S.; Hussain, A.; Hussain, S.J.; Wani, O.A.; Zahid Nabi, S.; Dar, N.A.; Baloch, F.S.; Mansoor, S. Plant Drought Stress Tolerance: Understanding Its Physiological, Biochemical and Molecular Mechanisms. Biotechnol. Biotechnol. Equip. 2021, 35, 1912–1925. [Google Scholar] [CrossRef]
- Tardieu, F.; Draye, X.; Javaux, M. Root Water Uptake and Ideotypes of the Root System: Whole-Plant Controls Matter. Vadose Zone J. 2017, 16, 1–10. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Global Synthesis of Drought Effects on Cereal, Legume, Tuber and Root Crops Production: A Review. Agric. Water Manag. 2017, 179, 18–33. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Devi, M.J.; Polania, J. Common Beans, Biodiversity, and Multiple Stresses: Challenges of Drought Resistance in Tropical Soils. Crop Pasture Sci. 2014, 65, 667. [Google Scholar] [CrossRef]
- Riyaz, I.; Shafi, S.; Zaffar, A.; Wani, M.A.; Zargar, S.M.; Djanaguiraman, M.; Prasad, P.V.V.; Sofi, P.A. Differential Spatial Plasticity Response in Common Bean (Phaseolus vulgaris L.) Root Architecture under Water Stress Is Driven by Increased Root Diameter, Surface Area and Volume at Deeper Layers. Discov. Plants 2024, 1, 6. [Google Scholar] [CrossRef]
- Purushothaman, R.; Zaman-Allah, M.; Mallikarjuna, N.; Pannirselvam, R.; Krishnamurthy, L.; Laxmipathi Gowda, C.L. Root Anatomical Traits and Their Possible Contribution to Drought Tolerance in Grain Legumes. Plant Prod. Sci. 2013, 16, 1–8. [Google Scholar] [CrossRef]
- Saeed, N.; Maqbool, N.; Haseeb, M.; Sadiq, R. Morpho-Anatomical Changes in Roots of Chickpea (Cicer arietinum L.) under Drought Stress Condition. J. Agric. Sci. Technol. B 2016, 6, 1–9. [Google Scholar] [CrossRef][Green Version]
- Lynch, J.P.; Chimungu, J.G.; Brown, K.M. Root Anatomical Phenes Associated with Water Acquisition from Drying Soil: Targets for Crop Improvement. J. Exp. Bot. 2014, 65, 6155–6166. [Google Scholar] [CrossRef]
- Sponchiado, B.N.; White, J.W.; Castillo, J.A.; Jones, P.G. Root Growth of Four Common Bean Cultivars in Relation to Drought Tolerance in Environments with Contrasting Soil Types. Exp. Agric. 1989, 25, 249–257. [Google Scholar] [CrossRef]
- White, J.W.; Castillo, J.A. Relative Effect of Root and Shoot Genotypes on Yield of Common Bean under Drought Stress. Crop Sci. 1989, 29, 360–362. [Google Scholar] [CrossRef]
- Widuri, L.I.; Lakitan, B.; Sodikin, E.; Hasmeda, M.; Meihana, M.; Kartika, K.; Siaga, E. Shoot and Root Growth in Common Bean (Phaseolus vulgaris L.) Exposed to Gradual Drought Stress. Agrivita 2018, 40, 442–452. [Google Scholar] [CrossRef]
- Gilbert, M.E.; Medina, V. Drought Adaptation Mechanisms Should Guide Experimental Design. Trends Plant Sci. 2016, 21, 639–647. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Mukeshimana, G.; Lasley, A.L.; Loescher, W.H.; Kelly, J.D. Identification of Shoot Traits Related to Drought Tolerance in Common Bean Seedlings. J. Am. Soc. Hortic. Sci. 2014, 139, 299–309. [Google Scholar] [CrossRef]
- Emam, Y.; Shekoofa, A.; Salehi, F.; Jalali, A.H.; Pessarakli, M. Drought Stress Effects on Two Common Bean Cultivars with Contrasting Growth Habits. Arch. Agron. Soil Sci. 2012, 58, 527–534. [Google Scholar] [CrossRef]
- Yasar, F.; Uzal, O.; Yasar, O.; Ellialtioglu, S.S. Root, Stem, and Leaf Ion Accumulation in Drought Stressed Green Bean (Phaseolus vulgaris L.) Genotypes Treated with Peg-6000. Fresenius Environ. Bull. 2014, 23, 2656–2662. [Google Scholar]
- Ohashi, Y.; Nakayama, N.; Saneoka, H.; Mohapatra, P.K.; Fujita, K. Differences in the Responses of Stem Diameter and Pod Thickness to Drought Stress during the Grain Filling Stage in Soybean Plants. Acta Physiol. Plant. 2009, 31, 271–277. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Sytar, O. Osmotic Adjustment and Plant Adaptation to Drought Stress. In Drought Stress Tolerance in Plants, Vol 1: Physiology and Biochemistry; Springer: Berlin/Heidelberg, Germany, 2016; pp. 105–143. [Google Scholar]
- Anjum, S.A.; Ashraf, U.; Zohaib, A.; Tanveer, M.; Naeem, M.; Ali, I.; Tabassum, T.; Nazir, U. Growth and Developmental Responses of Crop Plants under Drought Stress: A Review. Zemdirb. Agric. 2017, 104, 267–276. [Google Scholar] [CrossRef]
- Nuñez Barrios, A.; Hoogenboom, G.; Nesmith, D.S. Drought Sress and the Distribution of Vegetative and Reproductive Traits of a Bean Cultivar. Sci. Agric. 2005, 62, 18–22. [Google Scholar] [CrossRef]
- Ibrahim, S.; Desoky, E.; Elrys, A. Influencing of Water Stress and Micronutrients on Physio-Chemical Attributes, Yield and Anatomical Features of Common Bean Plants (Phaseolus vulgaris L.). Egypt. J. Agron. 2017, 39, 251–265. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General Mechanisms of Drought Response and Their Application in Drought Resistance Improvement in Plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.; Comstock, J.P.; Ehleringer, J.R. Variation in Leaf Movements among Common Bean Cultivars. Crop Sci. 1994, 34, 1273–1278. [Google Scholar] [CrossRef]
- Pastenes, C.; Pimentel, P.; Lillo, J. Leaf Movements and Photoinhibition in Relation to Water Stress in Field-Grown Beans. J. Exp. Bot. 2005, 56, 425–433. [Google Scholar] [CrossRef]
- Stenglein, S.A.; Arambarri, A.M.; Vizgarra, O.N.; Balatti, P.A. Micromorphological Variability of Leaf Epidermis in Mesoamerican Common Bean (Phaseolus vulgaris, Leguminosae). Aust. J. Bot. 2004, 52, 73–80. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Liakopoulos, G.; Bresta, P.; Nikolopoulos, D. The Optical Properties of Leaf Structural Elements and Their Contribution to Photosynthetic Performance and Photoprotection. Plants 2021, 10, 1455. [Google Scholar] [CrossRef]
- Fang, X.; Turner, N.C.; Yan, G.; Li, F.; Siddique, K.H.M. Flower Numbers, Pod Production, Pollen Viability, and Pistil Function Are Reduced and Flower and Pod Abortion Increased in Chickpea (Cicer arietinum L.) under Terminal Drought. J. Exp. Bot. 2010, 61, 335–345. [Google Scholar] [CrossRef]
- Phillips, B.B.; Shaw, R.F.; Holland, M.J.; Fry, E.L.; Bardgett, R.D.; Bullock, J.M.; Osborne, J.L. Drought Reduces Floral Resources for Pollinators. Glob. Change Biol. 2018, 24, 3226–3235. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research Progress and Perspective on Drought Stress in Legumes: A Review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef]
- Soureshjani, H.K.; Nezami, A.; Kafi, M.; Tadayon, M. Responses of Two Common Bean (Phaseolus vulgaris L.) Genotypes to Deficit Irrigation. Agric. Water Manag. 2019, 213, 270–279. [Google Scholar] [CrossRef]
- Tan, M.; Liao, F.; Hou, L.; Wang, J.; Wei, L.; Jian, H.; Xu, X.; Li, J.; Liu, L. Genome-Wide Association Analysis of Seed Germination Percentage and Germination Index in Brassica napus L. under Salt and Drought Stresses. Euphytica 2017, 213, 40. [Google Scholar] [CrossRef]
- Wu, L.; Chang, Y.; Wang, L.; Wang, S.; Wu, J. Genome-Wide Association Analysis of Drought Resistance Based on Seed Germination Vigor and Germination Rate at the Bud Stage in Common Bean. Agron. J. 2021, 113, 2980–2990. [Google Scholar] [CrossRef]
- Buckley, T.N. How Do Stomata Respond to Water Status? N. Phytol. 2019, 224, 21–36. [Google Scholar] [CrossRef]
- Murtaza, G.; Rasool, F.; Habib, R.; Javed, T.; Sardar, K.; Ayub, M.M.; Ayub, M.A.; Rasool, A. A Review of Morphological, Physiological and Biochemical Responses of Plants under Drought Stress Conditions. Imp. J. Interdiscip. Res. 2016, 2, 1600–1606. [Google Scholar]
- Lawson, T.; Blatt, M.R. Stomatal Size, Speed, and Responsiveness Impact on Photosynthesis and Water Use Efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S. Identification of Drought Tolerant Genotypes Using Physiological Traits in Soybean. Physiol. Mol. Biol. Plants 2019, 25, 697–711. [Google Scholar] [CrossRef]
- Gonçalves, J.G.R.; Andrade, E.R.d.; Silva, D.A.d.; Esteves, J.A.D.F.; Chiorato, A.F.; Carbonell, S.A.M. Drought Tolerance Evaluated in Common Bean Genotypes. Ciência Agrotecnologia 2019, 43, e001719. [Google Scholar] [CrossRef]
- Polania, J.A.; Poschenrieder, C.; Beebe, S.; Rao, I.M. Effective Use of Water and Increased Dry Matter Partitioned to Grain Contribute to Yield of Common Bean Improved for Drought Resistance. Front. Plant Sci. 2016, 7, 660. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Havaux, M. Sensing β-Carotene Oxidation in Photosystem II to Master Plant Stress Tolerance. N. Phytol. 2019, 223, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. Agron. Sustain. Dev. 2009, 29, 153–188. [Google Scholar] [CrossRef]
- Khayatnezhad, M.; Gholamin, R.; Jamaati-e-Somarin, S.; Zabihi-e-Mahmoodabad, R. The Leaf Chlorophyll Content and Stress Resistance Relationship Considering in Corn Cultivars (Zea mays). Adv. Environ. Biol. 2011, 5, 118–122. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Darkwa, K.; Ambachew, D.; Mohammed, H.; Asfaw, A.; Blair, M.W. Evaluation of Common Bean (Phaseolus vulgaris L.) Genotypes for Drought Stress Adaptation in Ethiopia. Crop J. 2016, 4, 367–376. [Google Scholar] [CrossRef]
- Mathobo, R.; Marais, D.; Steyn, J.M. The Effect of Drought Stress on Yield, Leaf Gaseous Exchange and Chlorophyll Fluorescence of Dry Beans (Phaseolus vulgaris L.). Agric. Water Manag. 2017, 180, 118–125. [Google Scholar] [CrossRef]
- Sánchez-Reinoso, A.D.; Ligarreto-Moreno, G.A.; Restrepo-Díaz, H. Physiological and Biochemical Responses of Common Bush Bean to Drought. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 393–401. [Google Scholar] [CrossRef]
- Asfaw, A.; Blair, M.W.; Struik, P.C. Multienvironment Quantitative Trait Loci Analysis for Photosynthate Acquisition, Accumulation, and Remobilization Traits in Common Bean Under Drought Stress. G3 Genes|Genomes|Genet. 2012, 2, 579–595. [Google Scholar] [CrossRef]
- Rasti Sani, M.; Ganjeali, A.; Lahouti, M.; Mousavi Kouhi, S.M. Morphological and Physiological Responses of Two Common Bean Cultivars to Drought Stress. J. Plant Process Funct. 2018, 6, 37–46. [Google Scholar]
- Procházková, D.; Wilhelmová, N. The Capacity of Antioxidant Protection during Modulated Ageing of Bean (Phaseolus vulgaris L.) Cotyledons. 1. The Antioxidant Enzyme Activities. Cell Biochem. Funct. 2007, 25, 87–95. [Google Scholar] [CrossRef]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulías, J.; Flexas, J. Regulation of Photosynthesis of C3 Plants in Response to Progressive Drought: Stomatal Conductance as a Reference Parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Zahra, N.; Hafeez, M.B.; Kausar, A.; Al Zeidi, M.; Asekova, S.; Siddique, K.H.M.; Farooq, M. Plant Photosynthetic Responses under Drought Stress: Effects and Management. J. Agron. Crop Sci. 2023, 209, 651–672. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant Adaptation to Drought Stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef]
- Ort, D.R. When There Is Too Much Light. Plant Physiol. 2001, 125, 29–32. [Google Scholar] [CrossRef]
- Asada, K. The Water–Water Cycle as Alternative Photon and Electron Sinks. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2000, 355, 1419–1431. [Google Scholar] [CrossRef]
- Mladenov, P.; Aziz, S.; Topalova, E.; Renaut, J.; Planchon, S.; Raina, A.; Tomlekova, N. Physiological Responses of Common Bean Genotypes to Drought Stress. Agronomy 2023, 13, 1022. [Google Scholar] [CrossRef]
- Zlatev, Z.S. Drought-Induced Changes and Recovery of Photosynthesis in Two Bean Cultivars (Phaseolus vulgaris L.). Emir. J. Food Agric. 2013, 25, 1014. [Google Scholar] [CrossRef]
- Zadražnik, T.; Moen, A.; Šuštar-Vozlič, J. Chloroplast Proteins Involved in Drought Stress Response in Selected Cultivars of Common Bean (Phaseolus vulgaris L.). 3 Biotech 2019, 9, 331. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, J. Effects of Water Stress on Photosystem II Photochemistry and Its Thermostability in Wheat Plants. J. Exp. Bot. 1999, 50, 1199–1206. [Google Scholar] [CrossRef]
- Han, Q.; Kang, G.; Guo, T. Proteomic Analysis of Spring Freeze-Stress Responsive Proteins in Leaves of Bread Wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2013, 63, 236–244. [Google Scholar] [CrossRef]
- Lawlor, D.W. Limitation to Photosynthesis in Water-Stressed Leaves: Stomata vs. Metabolism and the Role of ATP. Ann. Bot. 2002, 89, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, Y.Y.; Bai, Y.W.; Camberato, J.J.; Xue, J.Q.; Zhang, R.H. Effects of Drought Stress on the Photosynthesis in Maize. Russ. J. Plant Physiol. 2018, 65, 849–856. [Google Scholar] [CrossRef]
- Zadražnik, T.; Hollung, K.; Egge-Jacobsen, W.; Meglič, V.; Šuštar-Vozlič, J. Differential Proteomic Analysis of Drought Stress Response in Leaves of Common Bean (Phaseolus vulgaris L.). J. Proteom. 2013, 78, 254–272. [Google Scholar] [CrossRef]
- Dias, M.C.; Brüggemann, W. Limitations of Photosynthesis in Phaseolus vulgaris under Drought Stress: Gas Exchange, Chlorophyll Fluorescence and Calvin Cycle Enzymes. Photosynthetica 2010, 48, 96–102. [Google Scholar] [CrossRef]
- Boroujerdnia, M.; Bihamta, Ř.R.; Said, A.; Abdossi, V. Expression Profile of Some Important Genes Related to Carbohydrates Metabolism under Drought Stress in Bean (Phaseolus vulgaris L.). Iran. J. Genet. Plant Breed. 2020, 9, 94–106. [Google Scholar]
- Blum, A. Osmotic Adjustment Is a Prime Drought Stress Adaptive Engine in Support of Plant Production. Plant. Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, Distribution and Roles of Osmoprotective Compounds Accumulated in Halophytes under Abiotic Stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Khatun, M.; Sarkar, S.; Era, F.M.; Islam, A.K.M.M.; Anwar, M.P.; Fahad, S.; Datta, R.; Islam, A.K.M.A. Drought Stress in Grain Legumes: Effects, Tolerance Mechanisms and Management. Agronomy 2021, 11, 2374. [Google Scholar] [CrossRef]
- Amede, T.; Schubert, S.; Stahr, K. Mechanisms of Drought Resistance in Grain Legumes I: Osmotic Adjustment. SINET Ethiop. J. Sci. 2003, 26, 37–46. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Al Shiblawi, F.R.; Sentenac, H. Roles and Transport of Sodium and Potassium in Plants. In The Alkali Metal Ions: Their Role for Life; Springer: Berlin/Heidelberg, Germany, 2016; pp. 291–324. [Google Scholar]
- Mansour, M.M.F.; Salama, K.H.A. Proline and Abiotic Stresses: Responses and Adaptation. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives II: Mechanisms of Adaptation and Stress Amelioration; Springer: Berlin/Heidelberg, Germany, 2020; pp. 357–397. [Google Scholar]
- Sánchez-Reinoso, A.D.; Ligarreto-Moreno, G.A.; Restrepo-Díaz, H. Drought-Tolerant Common Bush Bean Physiological Parameters as Indicators to Identify Susceptibility. HortScience 2019, 54, 2091–2098. [Google Scholar] [CrossRef]
- Osmolovskaya, N.; Shumilina, J.; Kim, A.; Didio, A.; Grishina, T.; Bilova, T.; Keltsieva, O.A.; Zhukov, V.; Tikhonovich, I.; Tarakhovskaya, E.; et al. Methodology of Drought Stress Research: Experimental Setup and Physiological Characterization. Int. J. Mol. Sci. 2018, 19, 4089. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-Related Redox Regulation and Signaling in Plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative Modifications to Cellular Components in Plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Hussain, S.; Rao, M.J.; Anjum, M.A.; Ejaz, S.; Zakir, I.; Ali, M.A.; Ahmad, N.; Ahmad, S. Oxidative Stress and Antioxidant Defense in Plants under Drought Conditions. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Springer: Berlin/Heidelberg, Germany, 2019; pp. 207–219. [Google Scholar]
- AL-Aloosy, Y.A.M.; AL-Tameemi, A.J.; Jumaa, S.S. The Role of Enzymatic and Non-Enzymatic Antioxidants in Facing the Environmental Stresses on Plant: A Review. Plant Arch. 2019, 19, 1057–1060. [Google Scholar]
- López, C.M.; Pineda, M.; Alamillo, J.M. Differential Regulation of Drought Responses in Two Phaseolus vulgaris Genotypes. Plants 2020, 9, 1815. [Google Scholar] [CrossRef]
- Zlatev, Z.S.; Lidon, F.C.; Ramalho, J.C.; Yordanov, I.T. Comparison of Resistance to Drought of Three Bean Cultivars. Biol. Plant. 2006, 50, 389–394. [Google Scholar] [CrossRef]
- Mombeni, M.; Abbasi, A. Biochemical Responses of Some Common Bean (Phaseolus vulgaris L.) Genotypes to Drought Stress. J. Agric. Sci. Technol. 2019, 21, 407–421. [Google Scholar]
- Guan, Z.; Chai, T.; Zhang, Y.; Xu, J.; Wei, W. Enhancement of Cd Tolerance in Transgenic Tobacco Plants Overexpressing a Cd-Induced Catalase CDNA. Chemosphere 2009, 76, 623–630. [Google Scholar] [CrossRef]
- Abass, S.M.; Mohamed, H.I. Alleviation of Adverse Effects of Drought Stress on Common Bean (Phaseolus vulgaris L.) by Exogenous Application of Hydrogen Peroxide. Bangladesh J. Bot. 2011, 40, 75–83. [Google Scholar] [CrossRef]
- Brookbank, B.P.; Patel, J.; Gazzarrini, S.; Nambara, E. Role of Basal ABA in Plant Growth and Development. Genes 2021, 12, 1936. [Google Scholar] [CrossRef] [PubMed]
- Schachtman, D.P.; Goodger, J.Q.D. Chemical Root to Shoot Signaling under Drought. Trends Plant Sci. 2008, 13, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, A.; Chatterjee, A.; Kumar, R.; Singh, M.; Naik, P.S. Physiological and Biochemical Basis of Drought Tolerance in Vegetables. Veg. Sci. 2011, 38, 1–16. [Google Scholar]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.H.; Lee, S.C. Function of ABA in Stomatal Defense against Biotic and Drought Stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones Regulate Accumulation of Osmolytes Under Abiotic Stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Szarejko, I. Open or Close the Gate—Stomata Action under the Control of Phytohormones in Drought Stress Conditions. Front. Plant Sci. 2013, 4, 138. [Google Scholar] [CrossRef]
- Tiwari, S.; Lata, C.; Chauhan, P.S.; Prasad, V.; Prasad, M. A Functional Genomic Perspective on Drought Signalling and Its Crosstalk with Phytohormone-Mediated Signalling Pathways in Plants. Curr. Genom. 2017, 18, 469–482. [Google Scholar] [CrossRef]
- de Ollas, C.; Dodd, I.C. Physiological Impacts of ABA–JA Interactions under Water-Limitation. Plant Mol. Biol. 2016, 91, 641–650. [Google Scholar] [CrossRef]
- Müller, M. Foes or Friends: Aba and Ethylene Interaction under Abiotic Stress. Plants 2021, 10, 448. [Google Scholar] [CrossRef]
- Margay, A.R.; Mehmood, A.; Bashir, L. Review on Hormonal Regulation of Drought Stress Response in Plants. Int. J. Plant Soil Sci. 2024, 36, 902–916. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, A.; Ramakrishnan, M.; Van Ha, C.; Zheng, B.; Bhardwaj, M.; Tran, L.-S.P. Roles of Abscisic Acid and Auxin in Plants during Drought: A Molecular Point of View. Plant Physiol. Biochem. 2023, 204, 108129. [Google Scholar] [CrossRef] [PubMed]
- WANG, Q. Effects of Drought Stress on Endogenous Hormones and Osmotic Regulatory Substances of Common Bean (Phaseolus vulgaris L.) at Seedling Stage. Appl. Ecol. Environ. Res. 2019, 17, 4447–4457. [Google Scholar] [CrossRef]
- Subramani, M.; Urrea, C.A.; Habib, R.; Bhide, K.; Thimmapuram, J.; Kalavacharla, V. Comparative Transcriptome Analysis of Tolerant and Sensitive Genotypes of Common Bean (Phaseolus vulgaris L.) in Response to Terminal Drought Stress. Plants 2023, 12, 210. [Google Scholar] [CrossRef]
- Labastida, D.; Ingvarsson, P.K.; Rendón-Anaya, M. Dissecting the Genetic Basis of Drought Responses in Common Bean Using Natural Variation. Front. Plant Sci. 2023, 14, 1143873. [Google Scholar] [CrossRef]
- Liu, G.; Li, B.; Li, X.; Wei, Y.; He, C.; Shi, H. MaWRKY80 Positively Regulates Plant Drought Stress Resistance through Modulation of Abscisic Acid and Redox Metabolism. Plant Physiol. Biochem. 2020, 156, 155–166. [Google Scholar] [CrossRef]
- Martínez-Barradas, V.; Galbiati, M.; Barco-Rubio, F.; Paolo, D.; Espinoza, C.; Cominelli, E.; Arce-Johnson, P. PvMYB60 Gene, a Candidate for Drought Tolerance Improvement in Common Bean in a Climate Change Context. Biol. Res. 2024, 57, 52. [Google Scholar] [CrossRef]
- Abobatta, W.F. Plant Responses and Tolerance to Combined Salt and Drought Stress. In Salt and Drought Stress Tolerance in Plants: Signaling Networks and Adaptive Mechanisms; Springer: Berlin/Heidelberg, Germany, 2020; pp. 17–52. [Google Scholar]
- Zhao, Y.; Chan, Z.; Gao, J.; Xing, L.; Cao, M.; Yu, C.; Hu, Y.; You, J.; Shi, H.; Zhu, Y.; et al. ABA Receptor PYL9 Promotes Drought Resistance and Leaf Senescence. Proc. Natl. Acad. Sci. USA 2016, 113, 1949–1954. [Google Scholar] [CrossRef]
- González, R.M.; Iusem, N.D. Twenty Years of Research on Asr (ABA-Stress-Ripening) Genes and Proteins. Planta 2014, 239, 941–949. [Google Scholar] [CrossRef]
- Chai, T.Y.; Zhang, Y.X. Acta Botanica Sinica Gene Expression Analysis of a Proline-Rich Protein from Bean under Biotic and Abiotic Stress. Acta Bot. Sin. 1999, 41, 111–113. [Google Scholar]
- Aroca, R.; Ruiz-Lozano, J.M. Regulation of Root Water Uptake under Drought Stress Conditions. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Springer: Berlin/Heidelberg, Germany, 2013; pp. 113–127. [Google Scholar] [CrossRef]
- Zupin, M.; Sedlar, A.; Kidrič, M.; Meglič, V. Drought-Induced Expression of Aquaporin Genes in Leaves of Two Common Bean Cultivars Differing in Tolerance to Drought Stress. J. Plant Res. 2017, 130, 735–745. [Google Scholar] [CrossRef]
- Ponce, T.P.; Bugança, M.D.S.; da Silva, V.S.; de Souza, R.F.; Moda-Cirino, V.; Tomaz, J.P. Differential Gene Expression in Contrasting Common Bean Cultivars for Drought Tolerance during an Extended Dry Period. Genes 2024, 15, 935. [Google Scholar] [CrossRef]
- Zadražnik, T.; Egge-Jacobsen, W.; Meglič, V.; Šuštar-Vozlič, J. Proteomic Analysis of Common Bean Stem under Drought Stress Using In-Gel Stable Isotope Labeling. J. Plant Physiol. 2017, 209, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Moloi, S.J.; Ngara, R. The Roles of Plant Proteases and Protease Inhibitors in Drought Response: A Review. Front. Plant Sci. 2023, 14, 1165845. [Google Scholar] [CrossRef]
- Diaz, S.; Ariza-Suarez, D.; Izquierdo, P.; Lobaton, J.D.; de la Hoz, J.F.; Acevedo, F.; Duitama, J.; Guerrero, A.F.; Cajiao, C.; Mayor, V.; et al. Genetic Mapping for Agronomic Traits in a MAGIC Population of Common Bean (Phaseolus vulgaris L.) under Drought Conditions. BMC Genom. 2020, 21, 799. [Google Scholar] [CrossRef]
- Duc, G.; Agrama, H.; Bao, S.; Berger, J.; Bourion, V.; De Ron, A.M.; Gowda, C.L.L.; Mikic, A.; Millot, D.; Singh, K.B.; et al. Breeding Annual Grain Legumes for Sustainable Agriculture: New Methods to Approach Complex Traits and Target New Cultivar Ideotypes. Crit. Rev. Plant Sci. 2015, 34, 381–411. [Google Scholar] [CrossRef]
- Xu, Y. Envirotyping for Deciphering Environmental Impacts on Crop Plants. Theor. Appl. Genet. 2016, 129, 653–673. [Google Scholar] [CrossRef]
- Costa, C.; Schurr, U.; Loreto, F.; Menesatti, P.; Carpentier, S. Plant Phenotyping Research Trends, a Science Mapping Approach. Front. Plant Sci. 2019, 9, 1933. [Google Scholar] [CrossRef]
- Li, D.; Quan, C.; Song, Z.; Li, X.; Yu, G.; Li, C.; Muhammad, A. High-Throughput Plant Phenotyping Platform (HT3P) as a Novel Tool for Estimating Agronomic Traits from the Lab to the Field. Front. Bioeng. Biotechnol. 2021, 8, 623705. [Google Scholar] [CrossRef]
- Junker, A.; Muraya, M.M.; Weigelt-Fischer, K.; Arana-Ceballos, F.; Klukas, C.; Melchinger, A.E.; Meyer, R.C.; Riewe, D.; Altmann, T. Optimizing Experimental Procedures for Quantitative Evaluation of Crop Plant Performance in High Throughput Phenotyping Systems. Front. Plant Sci. 2015, 5, 770. [Google Scholar] [CrossRef]
- Elmasry, G.; Mandour, N.; Al-Rejaie, S.; Belin, E.; Rousseau, D. Recent Applications of Multispectral Imaging in Seed Phenotyping and Quality Monitoring—An Overview. Sensors 2019, 19, 1090. [Google Scholar] [CrossRef]
- Nikolopoulos, D.; Bresta, P.; Daliani, V.; Haghiou, V.; Darra, N.; Liati, M.; Mavrogianni, E.; Papanastasiou, A.; Porfyraki, T.; Psaroudi, V.; et al. Leaf Anatomy Affects Optical Properties and Enhances Photosynthetic Performance under Oblique Light. Plant. Cell Environ. 2024, 47, 1471–1485. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; Mangina, E.; Negrão, S. Advancements in Imaging Sensors and AI for Plant Stress Detection: A Systematic Literature Review. Plant Phenomics 2024, 6, 0153. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Feng, H.; Zhang, X.; Zhang, J.; Doonan, J.H.; Batchelor, W.D.; Xiong, L.; Yan, J. Crop Phenomics and High-Throughput Phenotyping: Past Decades, Current Challenges, and Future Perspectives. Mol. Plant 2020, 13, 187–214. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Guo, Z.; Huang, C.; Duan, L.; Chen, G.; Jiang, N.; Fang, W.; Feng, H.; Xie, W.; Lian, X. Combining High-Throughput Phenotyping and Genome-Wide Association Studies to Reveal Natural Genetic Variation in Rice. Nat. Commun. 2014, 5, 5087. [Google Scholar] [CrossRef]
- Souza, A.; Yang, Y. High-Throughput Corn Image Segmentation and Trait Extraction Using Chlorophyll Fluorescence Images. Plant Phenomics 2021, 2021, 9792582. [Google Scholar] [CrossRef]
- Al-Tamimi, N.; Langan, P.; Bernád, V.; Walsh, J.; Mangina, E.; Negrão, S. Capturing Crop Adaptation to Abiotic Stress Using Image-Based Technologies. Open Biol. 2022, 12, 210353. [Google Scholar] [CrossRef]
- Leal-Delgado, R.; Peña-Valdivia, C.B.; García-Nava, R.; García-Esteva, A.; Martínez-Barajas, E.; Padilla-Chacón, D. Phenotypical, Physiological and Biochemical Traits of the Vegetative Growth of Wild Tepary Bean (Phaseolus acutifolius) under Restricted Water Conditions. S. Afr. J. Plant Soil 2019, 36, 261–270. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Huang, D. A Review of Imaging Techniques for Plant Phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef]
- Deng, L.; Mao, Z.; Li, X.; Hu, Z.; Duan, F.; Yan, Y. UAV-Based Multispectral Remote Sensing for Precision Agriculture: A Comparison between Different Cameras. ISPRS J. Photogramm. Remote Sens. 2018, 146, 124–136. [Google Scholar] [CrossRef]
- Zhu, L.; Suomalainen, J.; Liu, J.; Hyyppä, J.; Kaartinen, H.; Haggren, H. A Review: Remote Sensing Sensors. In Multi-Purposeful Application of Geospatial Data; InTechOpen: London, UK, 2018. [Google Scholar]
- Peng, Y.; Dallas, M.M.; Ascencio-Ibáñez, J.T.; Hoyer, J.S.; Legg, J.; Hanley-Bowdoin, L.; Grieve, B.; Yin, H. Early Detection of Plant Virus Infection Using Multispectral Imaging and Spatial–Spectral Machine Learning. Sci. Rep. 2022, 12, 3113. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Chen, D.; Huang, Y.; Kong, W.; Yuan, L.; Ye, H.; Huang, W. Assessment of Leaf Chlorophyll Content Models for Winter Wheat Using Landsat-8 Multispectral Remote Sensing Data. Remote Sens. 2020, 12, 2574. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between Leaf Chlorophyll Content and Spectral Reflectance and Algorithms for Non-Destructive Chlorophyll Assessment in Higher Plant Leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Zahir, S.A.D.M.; Omar, A.F.; Jamlos, M.F.; Azmi, M.A.M.; Muncan, J. A Review of Visible and Near-Infrared (Vis-NIR) Spectroscopy Application in Plant Stress Detection. Sens. Actuators A Phys. 2022, 338, 113468. [Google Scholar] [CrossRef]
- Falcioni, R.; Antunes, W.C.; Demattê, J.A.M.; Nanni, M.R. Reflectance Spectroscopy for the Classification and Prediction of Pigments in Agronomic Crops. Plants 2023, 12, 2347. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Al-Suhaibani, N.A.; Hassan, W.M.; Dewir, Y.H.; Elsayed, S.; Al-Ashkar, I.; Abdella, K.A.; Schmidhalter, U. Evaluation of Wavelengths and Spectral Reflectance Indices for High-Throughput Assessment of Growth, Water Relations and Ion Contents of Wheat Irrigated with Saline Water. Agric. Water Manag. 2019, 212, 358–377. [Google Scholar] [CrossRef]
- Boochs, F.; Kupfer, G.; Dockter, K.; Kühbauch, W. Shape of the Red Edge as Vitality Indicator for Plants. Remote Sens. 1990, 11, 1741–1753. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A.K. Red Edge Shift and Biochemical Content in Grass Canopies. ISPRS J. Photogramm. Remote Sens. 2007, 62, 34–42. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Mariotto, I.; Gumma, M.K.; Middleton, E.M.; Landis, D.R.; Huemmrich, K.F. Selection of Hyperspectral Narrowbands (Hnbs) and Composition of Hyperspectral Twoband Vegetation Indices (HVIS) for Biophysical Characterization and Discrimination of Crop Types Using Field Reflectance and Hyperion/EO-1 Data. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2013, 6, 427–439. [Google Scholar] [CrossRef]
- Avgoustaki, D.D.; Avgoustakis, I.; Miralles, C.C.; Sohn, J.; Xydis, G. Autonomous Mobile Robot with Attached Multispectral Camera to Monitor the Development of Crops and Detect Nutrient and Water Deficiencies in Vertical Farms. Agronomy 2022, 12, 2691. [Google Scholar] [CrossRef]
- Liu, H.; Bruning, B.; Garnett, T.; Berger, B. Hyperspectral Imaging and 3D Technologies for Plant Phenotyping: From Satellite to Close-Range Sensing. Comput. Electron. Agric. 2020, 175, 105621. [Google Scholar] [CrossRef]
- Kitić, G.; Tagarakis, A.; Cselyuszka, N.; Panić, M.; Birgermajer, S.; Sakulski, D.; Matović, J. A New Low-Cost Portable Multispectral Optical Device for Precise Plant Status Assessment. Comput. Electron. Agric. 2019, 162, 300–308. [Google Scholar] [CrossRef]
- Wójtowicz, M.; Wójtowicz, A.; Piekarczyk, J. Application of Remote Sensing Methods in Agriculture. Commun. Biometry Crop Sci. 2016, 11, 31–50. [Google Scholar]
- Adão, T.; Hruška, J.; Pádua, L.; Bessa, J.; Peres, E.; Morais, R.; Sousa, J. Hyperspectral Imaging: A Review on UAV-Based Sensors, Data Processing and Applications for Agriculture and Forestry. Remote Sens. 2017, 9, 1110. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. NASA Spec. Publ. 1974, 351, 309. [Google Scholar]
- El-Hendawy, S.E.; Hassan, W.M.; Al-Suhaibani, N.A.; Schmidhalter, U. Spectral Assessment of Drought Tolerance Indices and Grain Yield in Advanced Spring Wheat Lines Grown under Full and Limited Water Irrigation. Agric. Water Manag. 2017, 182, 1–12. [Google Scholar] [CrossRef]
- Penuelas, J.; Gamon, J.A.; Griffin, K.L.; Field, C.B. Assessing Community Type, Plant Biomass, Pigment Composition, and Photosynthetic Efficiency of Aquatic Vegetation from Spectral Reflectance. Remote Sens. Environ. 1993, 46, 110–118. [Google Scholar] [CrossRef]
- Tayade, R.; Yoon, J.; Lay, L.; Khan, A.L.; Yoon, Y.; Kim, Y. Utilization of Spectral Indices for High-Throughput Phenotyping. Plants 2022, 11, 1712. [Google Scholar] [CrossRef]
- Khuimphukhieo, I.; Bhandari, M.; Enciso, J.; da Silva, J.A. Assessing Drought Stress of Sugarcane Cultivars Using Unmanned Vehicle System (UAS)-Based Vegetation Indices and Physiological Parameters. Remote Sens. 2024, 16, 1433. [Google Scholar] [CrossRef]
- Berhan Demisse, G.; Venkata Suryabhagavan, K.; Melese Eshetie, S.; Suryabhagavan, K.V. Evaluation of Vegetation Indices for Agricultural Drought Monitoring in East Amhara, Ethiopia. Artic. Int. J. Sci. Res. 2016, 5, 535–540. [Google Scholar]
- Du, T.L.T.; Bui, D.D.; Nguyen, M.D.; Lee, H. Satellite-Based, Multi-Indices for Evaluation of Agricultural Droughts in a Highly Dynamic Tropical Catchment, Central Vietnam. Water 2018, 10, 659. [Google Scholar] [CrossRef]
- Lazarević, B.; Kontek, M.; Carović-Stanko, K.; Clifton-Brown, J.; Al Hassan, M.; Trindade, L.M.; Jurišić, V. Multispectral Image Analysis Detects Differences in Drought Responses in Novel Seeded Miscanthus Sinensis Hybrids. GCB Bioenergy 2022, 14, 1219–1234. [Google Scholar] [CrossRef]
- Afshar, M.H.; Al-Yaari, A.; Yilmaz, M.T. Comparative Evaluation of Microwave L-Band VOD and Optical NDVI for Agriculture Drought Detection over Central Europe. Remote Sens. 2021, 13, 1251. [Google Scholar] [CrossRef]
- Sankaran, S.; Zhou, J.; Khot, L.R.; Trapp, J.J.; Mndolwa, E.; Miklas, P.N. High-Throughput Field Phenotyping in Dry Bean Using Small Unmanned Aerial Vehicle Based Multispectral Imagery. Comput. Electron. Agric. 2018, 151, 84–92. [Google Scholar] [CrossRef]
- Behmann, J.; Mahlein, A.-K.; Paulus, S.; Dupuis, J.; Kuhlmann, H.; Oerke, E.-C.; Plümer, L. Generation and Application of Hyperspectral 3D Plant Models: Methods and Challenges. Mach. Vis. Appl. 2016, 27, 611–624. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical Properties and Nondestructive Estimation of Anthocyanin Content in Plant Leaves. Photochem. Photobiol. 2001, 74, 38. [Google Scholar] [CrossRef]
- Mu, Q.; Zhao, M.; Kimball, J.S.; McDowell, N.G.; Running, S.W. A Remotely Sensed Global Terrestrial Drought Severity Index. Bull. Am. Meteorol. Soc. 2013, 94, 83–98. [Google Scholar] [CrossRef]
- Omer, G.; Mutanga, O.; Abdel-Rahman, E.M.; Peerbhay, K.; Adam, E. Mapping Leaf Nitrogen and Carbon Concentrations of Intact and Fragmented Indigenous Forest Ecosystems Using Empirical Modeling Techniques and WorldView-2 Data. ISPRS J. Photogramm. Remote Sens. 2017, 131, 26–39. [Google Scholar] [CrossRef]
- Haboudane, D. Hyperspectral Vegetation Indices and Novel Algorithms for Predicting Green LAI of Crop Canopies: Modeling and Validation in the Context of Precision Agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Hunt, E.R., Jr.; Rock, B.N. Detection of Changes in Leaf Water Content Using Near-and Middle-Infrared Reflectances. Remote Sens. Environ. 1989, 30, 43–54. [Google Scholar]
- Renza, D.; Martinez, E.; Arquero, A.; Sanchez, J. Drought Estimation Maps by Means of Multidate Landsat Fused Images. In Proceedings of the 30th EARSeL Symposium, Paris, France, 31 May–3 June 2010; pp. 775–782. [Google Scholar]
- Zarco-Tejada, P.J.; Rueda, C.A.; Ustin, S.L. Water Content Estimation in Vegetation with MODIS Reflectance Data and Model Inversion Methods. Remote Sens. Environ. 2003, 85, 109–124. [Google Scholar] [CrossRef]
- Zou, X.; Mõttus, M. Sensitivity of Common Vegetation Indices to the Canopy Structure of Field Crops. Remote Sens. 2017, 9, 994. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P. Frequently Asked Questions about Chlorophyll Fluorescence, the Sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef]
- Govindjee. Chlorophyll a Fluorescence: A Bit of Basics and History. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2004; pp. 1–41. ISBN 978-1-4020-3218-9. [Google Scholar]
- Lazarević, B. Chlorophyll Fluorescence Imaging in Assessing Crop Abiotic Stress. In Chlorophyll a Fluorescence Measurements in Croati·First Twenty Years; Agricultural Institute Osijek: Osijek, Croatia, 2023; pp. 75–86. [Google Scholar]
- Ohashi, Y.; Nakayama, N.; Saneoka, H.; Fujita, K. Effects of Drought Stress on Photosynthetic Gas Exchange, Chlorophyll Fluorescence and Stem Diameter of Soybean Plants. Biol. Plant. 2006, 50, 138–141. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W.L. Quenching of Chlorophyll Fluorescence and Primary Photochemistry in Chloroplasts by Dibromothymoquinone. Biochim. Biophys. Acta (BBA) Bioenerg. 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The Relationship between the Quantum Yield of Photosynthetic Electron Transport and Quenching of Chlorophyll Fluorescence. Biochim. Biophys. Acta (BBA) Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the Xanthophyll Cycle in Photoprotection Elucidated by Measurements of Light-Induced Absorbance Changes, Fluorescence and Photosynthesis in Leaves of Hedera Canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous Recording of Photochemical and Non-Photochemical Chlorophyll Fluorescence Quenching with a New Type of Modulation Fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- Souza, R.P.; Machado, E.C.; Silva, J.A.B.; Lagôa, A.; Silveira, J.A.G. Photosynthetic Gas Exchange, Chlorophyll Fluorescence and Some Associated Metabolic Changes in Cowpea (Vigna unguiculata) during Water Stress and Recovery. Environ. Exp. Bot. 2004, 51, 45–56. [Google Scholar] [CrossRef]
- Saglam, A.; Saruhan, N.; Terzi, R.; Kadioglu, A. The Relations between Antioxidant Enzymes and Chlorophyll Fluorescence Parameters in Common Bean Cultivars Differing in Sensitivity to Drought Stress. Russ. J. Plant Physiol. 2011, 58, 60–68. [Google Scholar] [CrossRef]
- Sánchez-Reinoso, A.D.; Ligarreto-Moreno, G.A.; Restrepo-Díaz, H. Chlorophyll α Fluorescence Parameters as an Indicator to Identify Drought Susceptibility in Common Bush Bean. Agronomy 2019, 9, 526. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Humplík, J.F.; Lazár, D.; Husičková, A.; Spíchal, L. Automated Phenotyping of Plant Shoots Using Imaging Methods for Analysis of Plant Stress Responses–A Review. Plant Methods 2015, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Minervini, M.; Scharr, H.; Tsaftaris, S.A. Image Analysis: The New Bottleneck in Plant Phenotyping [Applications Corner]. IEEE Signal Process. Mag. 2015, 32, 126–131. [Google Scholar] [CrossRef]
- Pieruschka, R.; Schurr, U. Plant Phenotyping: Past, Present, and Future. Plant Phenomics 2019, 2019, 7507131. [Google Scholar] [CrossRef]
- Harandi, N.; Vandenberghe, B.; Vankerschaver, J.; Depuydt, S.; Van Messem, A. How to Make Sense of 3D Representations for Plant Phenotyping: A Compendium of Processing and Analysis Techniques. Plant Methods 2023, 19, 60. [Google Scholar] [CrossRef]
- Paturkar, A.; Sen Gupta, G.; Bailey, D. Making Use of 3D Models for Plant Physiognomic Analysis: A Review. Remote Sens. 2021, 13, 2232. [Google Scholar] [CrossRef]
- Lazarević, B.; Šatović, Z.; Nimac, A.; Vidak, M.; Gunjača, J.; Politeo, O.; Carović-Stanko, K. Application of Phenotyping Methods in Detection of Drought and Salinity Stress in Basil (Ocimum basilicum L.). Front. Plant Sci. 2021, 12, 629441. [Google Scholar] [CrossRef]
- Vollmer, M. Fundamentals of Thermal Imaging. In Thermal Cameras in Science Education; Springer: Berlin/Heidelberg, Germany, 2022; pp. 7–25. [Google Scholar]
- Sarawade, A.A.; Charniya, N.N. Infrared Thermography and Its Applications: A Review. In Proceedings of the 3rd International Conference on Communication and Electronics Systems (ICCES), Coimbatore, India, 28–29 October 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 280–285. [Google Scholar]
- Prashar, A.; Jones, H.G. Assessing Drought Responses Using Thermal Infrared Imaging. Methods Mol. Biol. 2016, 1398, 209–219. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.B.; Reginato, R.J.; Pinter, P.J., Jr. Canopy Temperature as a Crop Water Stress Indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Pradawet, C.; Khongdee, N.; Pansak, W.; Spreer, W.; Hilger, T.; Cadisch, G. Thermal Imaging for Assessment of Maize Water Stress and Yield Prediction under Drought Conditions. J. Agron. Crop Sci. 2023, 209, 56–70. [Google Scholar] [CrossRef]
- Bian, J.; Zhang, Z.; Chen, J.; Chen, H.; Cui, C.; Li, X.; Chen, S.; Fu, Q. Simplified Evaluation of Cotton Water Stress Using High Resolution Unmanned Aerial Vehicle Thermal Imagery. Remote Sens. 2019, 11, 267. [Google Scholar] [CrossRef]
- Biju, S.; Fuentes, S.; Gupta, D. The Use of Infrared Thermal Imaging as a Non-Destructive Screening Tool for Identifying Drought-Tolerant Lentil Genotypes. Plant Physiol. Biochem. 2018, 127, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.M.; Grant, O.M.; Chaves, M.M. Thermography to Explore Plant–Environment Interactions. J. Exp. Bot. 2013, 64, 3937–3949. [Google Scholar] [CrossRef] [PubMed]
- Sweet, K.J.; Peak, D.; Mott, K.A. Stomatal Heterogeneity in Responses to Humidity and Temperature: Testing a Mechanistic Model. Plant. Cell Environ. 2017, 40, 2771–2779. [Google Scholar] [CrossRef]
- Siddiqui, Z.S.; Umar, M.; Kwon, T.-R.; Park, S.C. Phenotyping through Infrared Thermography in Stress Environment. In Sabkha Ecosystems: Volume VI: Asia/Pacific; Springer: Berlin/Heidelberg, Germany, 2019; pp. 239–251. [Google Scholar]
- Toro, G.; Flexas, J.; Escalona, J.M. Contrasting Leaf Porometer and Infra-Red Gas Analyser Methodologies: An Old Paradigm about the Stomatal Conductance Measurement. Theor. Exp. Plant Physiol. 2019, 31, 483–492. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Namieśnik, J. Moving Your Laboratories to the Field–Advantages and Limitations of the Use of Field Portable Instruments in Environmental Sample Analysis. Environ. Res. 2015, 140, 593–603. [Google Scholar] [CrossRef]
- Zhu, J.; Ingram, P.A.; Benfey, P.N.; Elich, T. From Lab to Field, New Approaches to Phenotyping Root System Architecture. Curr. Opin. Plant Biol. 2011, 14, 310–317. [Google Scholar] [CrossRef]
- Strock, C.F.; Burridge, J.; Massas, A.S.F.; Beaver, J.; Beebe, S.; Camilo, S.A.; Fourie, D.; Jochua, C.; Miguel, M.; Miklas, P.N.; et al. Seedling Root Architecture and Its Relationship with Seed Yield across Diverse Environments in Phaseolus vulgaris. Field Crops Res. 2019, 237, 53–64. [Google Scholar] [CrossRef]
- Casto, A.L.; Schuhl, H.; Tovar, J.C.; Wang, Q.; Bart, R.S.; Fahlgren, N.; Gehan, M.A. Picturing the Future of Food. Plant Phenome J. 2021, 4, e20014. [Google Scholar] [CrossRef]
- Teixeira, A.; Da Silva, D.A.; Gonçalves, J.G.R.; Esteves, J.A.F.; Carbonell, S.A.M.; Chiorato, A.F. Root Characterization of Bean Genotypes (Phaseolus vulgaris) under Drought Stress. Genet. Mol. Res. 2019, 18, GMR18086. [Google Scholar] [CrossRef]
- Kumar, J.; Sen Gupta, D.; Djalovic, I.; Kumar, S.; Siddique, K.H.M. Root-Omics for Drought Tolerance in Cool-Season Grain Legumes. Physiol. Plant. 2021, 172, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Hund, A.; Trachsel, S.; Stamp, P. Growth of Axile and Lateral Roots of Maize: I Development of a Phenotying Platform. Plant Soil 2009, 325, 335–349. [Google Scholar] [CrossRef]
- Wasaya, A.; Zhang, X.; Fang, Q.; Yan, Z. Root Phenotyping for Drought Tolerance: A Review. Agronomy 2018, 8, 241. [Google Scholar] [CrossRef]
- Tracy, S.R.; Black, C.R.; Roberts, J.A.; Dodd, I.C.; Mooney, S.J. Using X-Ray Computed Tomography to Explore the Role of Abscisic Acid in Moderating the Impact of Soil Compaction on Root System Architecture. Environ. Exp. Bot. 2015, 110, 11–18. [Google Scholar] [CrossRef]
- van Dusschoten, D.; Metzner, R.; Kochs, J.; Postma, J.A.; Pflugfelder, D.; Bühler, J.; Schurr, U.; Jahnke, S. Quantitative 3D Analysis of Plant Roots Growing in Soil Using Magnetic Resonance Imaging. Plant Physiol. 2016, 170, 1176–1188. [Google Scholar] [CrossRef]
- Jeudy, C.; Adrian, M.; Baussard, C.; Bernard, C.; Bernaud, E.; Bourion, V.; Busset, H.; Cabrera-Bosquet, L.; Cointault, F.; Han, S. RhizoTubes as a New Tool for High Throughput Imaging of Plant Root Development and Architecture: Test, Comparison with Pot Grown Plants and Validation. Plant Methods 2016, 12, 31. [Google Scholar] [CrossRef]
- Wasson, A.P.; Nagel, K.A.; Tracy, S.; Watt, M. Beyond Digging: Noninvasive Root and Rhizosphere Phenotyping. Trends Plant Sci. 2020, 25, 119–120. [Google Scholar] [CrossRef]
- Hanlon, M.T.; Brown, K.M.; Lynch, J.P. LEADER (Leaf Element Accumulation from DEep Roots): A Nondestructive Phenotyping Platform to Estimate Rooting Depth in the Field. Crop Sci. 2024, 64, 333–353. [Google Scholar] [CrossRef]




| Environment | Media | Methods | Advantages | Disadvantages |
|---|---|---|---|---|
| Field Phenotyping | Soil | Shovelomics, soil coring/washing/breaking, trenching, minirhizotrons | - Plants grow under natural environmental conditions - Provides the most representative physiological response - Reflects realistic root development | - Labor-intensive - Requires multiple steps such as soil riddling and washing - Potential root damage and loss - Repeated measurements on the same plant are not possible - High variability due to soil and climate differences |
| Laboratory/Greenhouse Phenotyping | Substrate based systems | Pots, tubes, boxes, rhizoboxes, root chambers | - Easier experimental control and monitoring - Allows for more measurements in less time - Requires less root preparation before measurement - Lower risk of root damage during handling and extraction | - Root growth is restricted by container size - Possible root breakage during extraction and washing - Growth conditions are not fully representative of field conditions |
| Soilless, transparent systems | Agar plates, gel-based systems, “pouch-and-wick” systems, hydroponics, aeroponics | - Provides easy root access - Eliminates the need for extensive root cleaning - Allows for repeated measurements on the same plant - Highly reproducible | - Lacks natural environmental influences on root development - Requires further validation for physiological relevance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javornik, T.; Carović-Stanko, K.; Gunjača, J.; Šatović, Z.; Vidak, M.; Safner, T.; Lazarević, B. Phenotyping Common Bean Under Drought Stress: High-Throughput Approaches for Enhanced Drought Tolerance. Agronomy 2025, 15, 1344. https://doi.org/10.3390/agronomy15061344
Javornik T, Carović-Stanko K, Gunjača J, Šatović Z, Vidak M, Safner T, Lazarević B. Phenotyping Common Bean Under Drought Stress: High-Throughput Approaches for Enhanced Drought Tolerance. Agronomy. 2025; 15(6):1344. https://doi.org/10.3390/agronomy15061344
Chicago/Turabian StyleJavornik, Tomislav, Klaudija Carović-Stanko, Jerko Gunjača, Zlatko Šatović, Monika Vidak, Toni Safner, and Boris Lazarević. 2025. "Phenotyping Common Bean Under Drought Stress: High-Throughput Approaches for Enhanced Drought Tolerance" Agronomy 15, no. 6: 1344. https://doi.org/10.3390/agronomy15061344
APA StyleJavornik, T., Carović-Stanko, K., Gunjača, J., Šatović, Z., Vidak, M., Safner, T., & Lazarević, B. (2025). Phenotyping Common Bean Under Drought Stress: High-Throughput Approaches for Enhanced Drought Tolerance. Agronomy, 15(6), 1344. https://doi.org/10.3390/agronomy15061344









